Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Phytochemical Screening
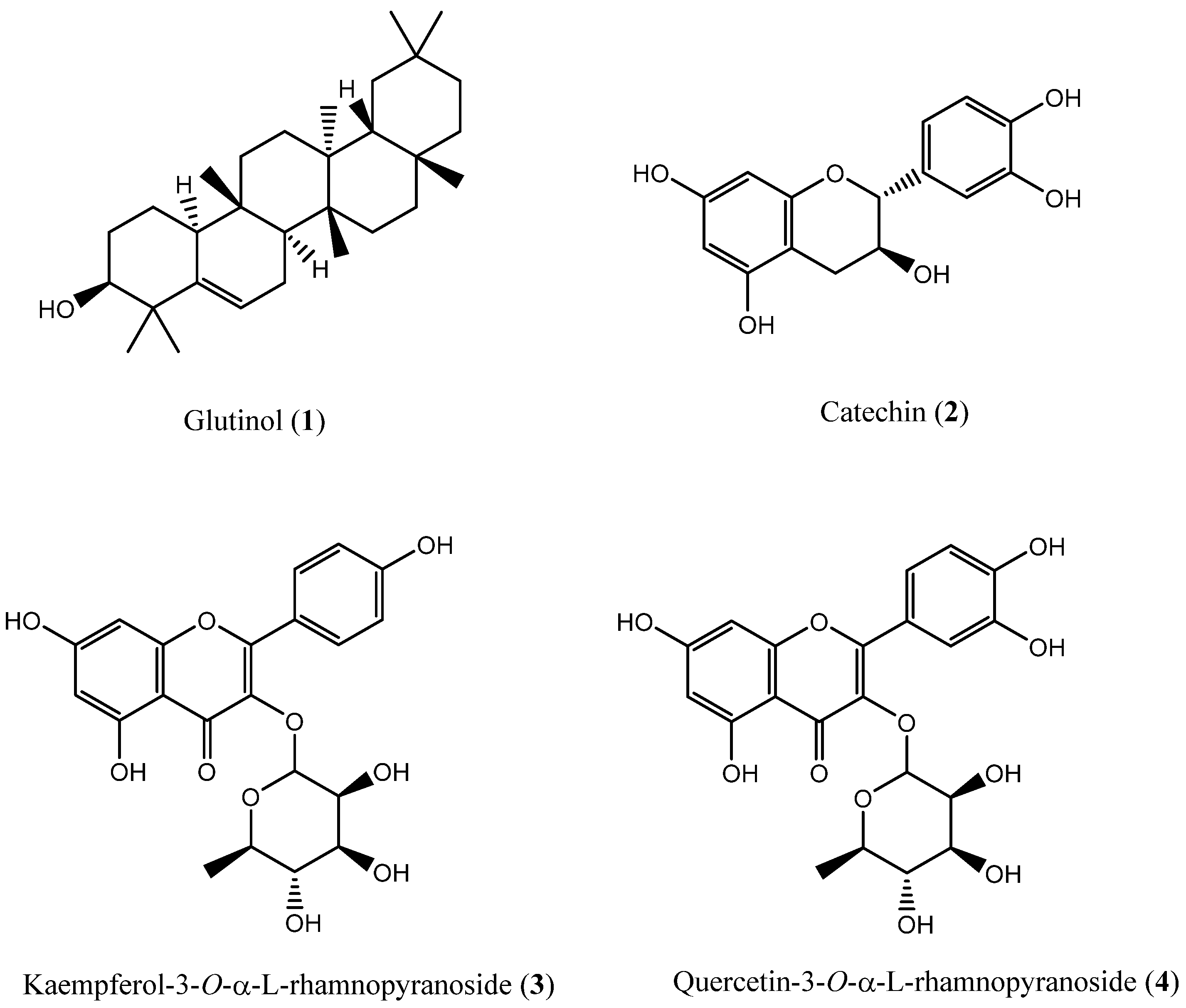
2.2. Isolation of Chemical Constituents
2.3. Antioxidant Activity
2.3.1. Free Radical Scavenging Activity (DPPH•)
2.3.2. Ferrous Ion Chelating Assay (FIC)
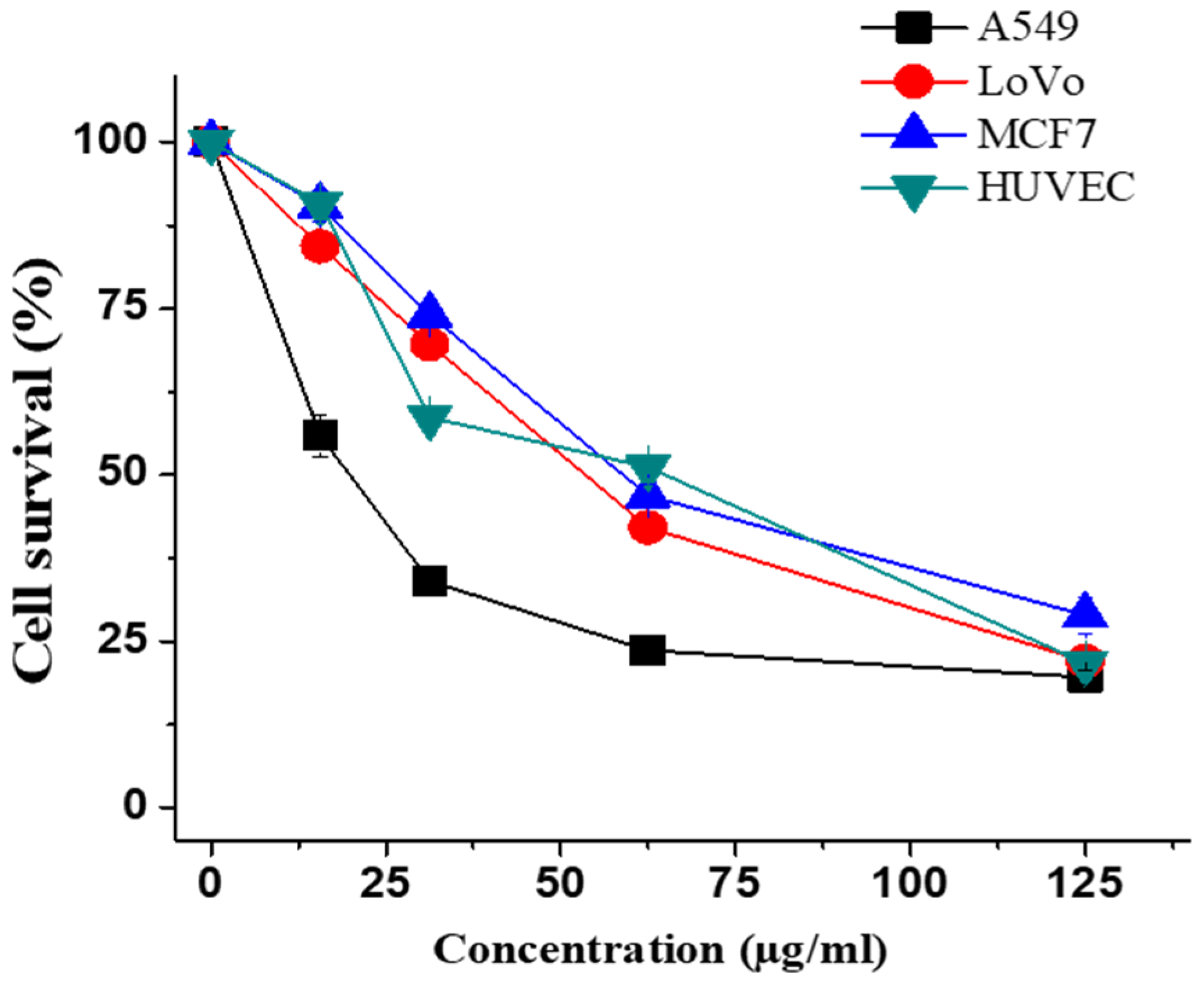
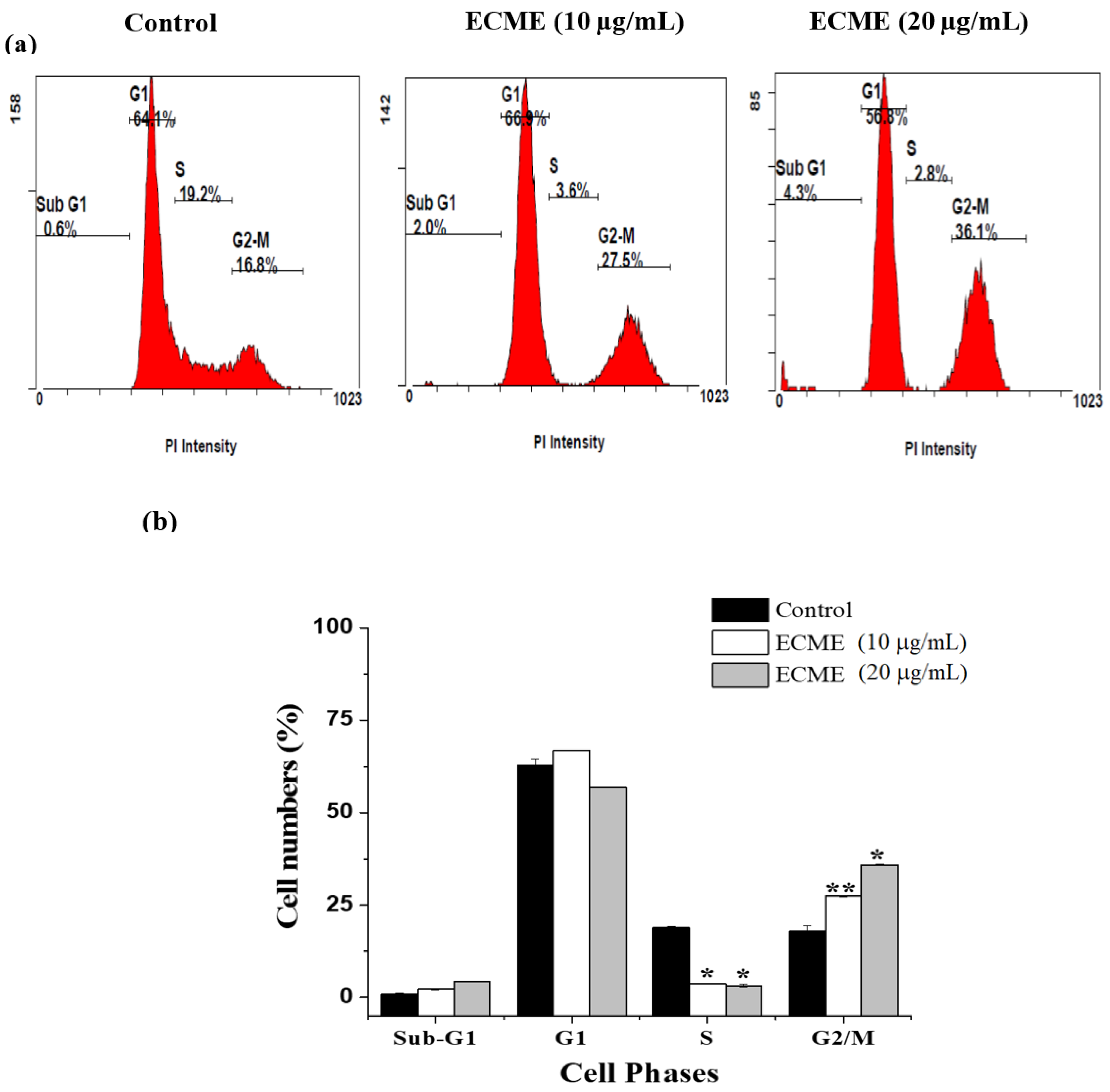
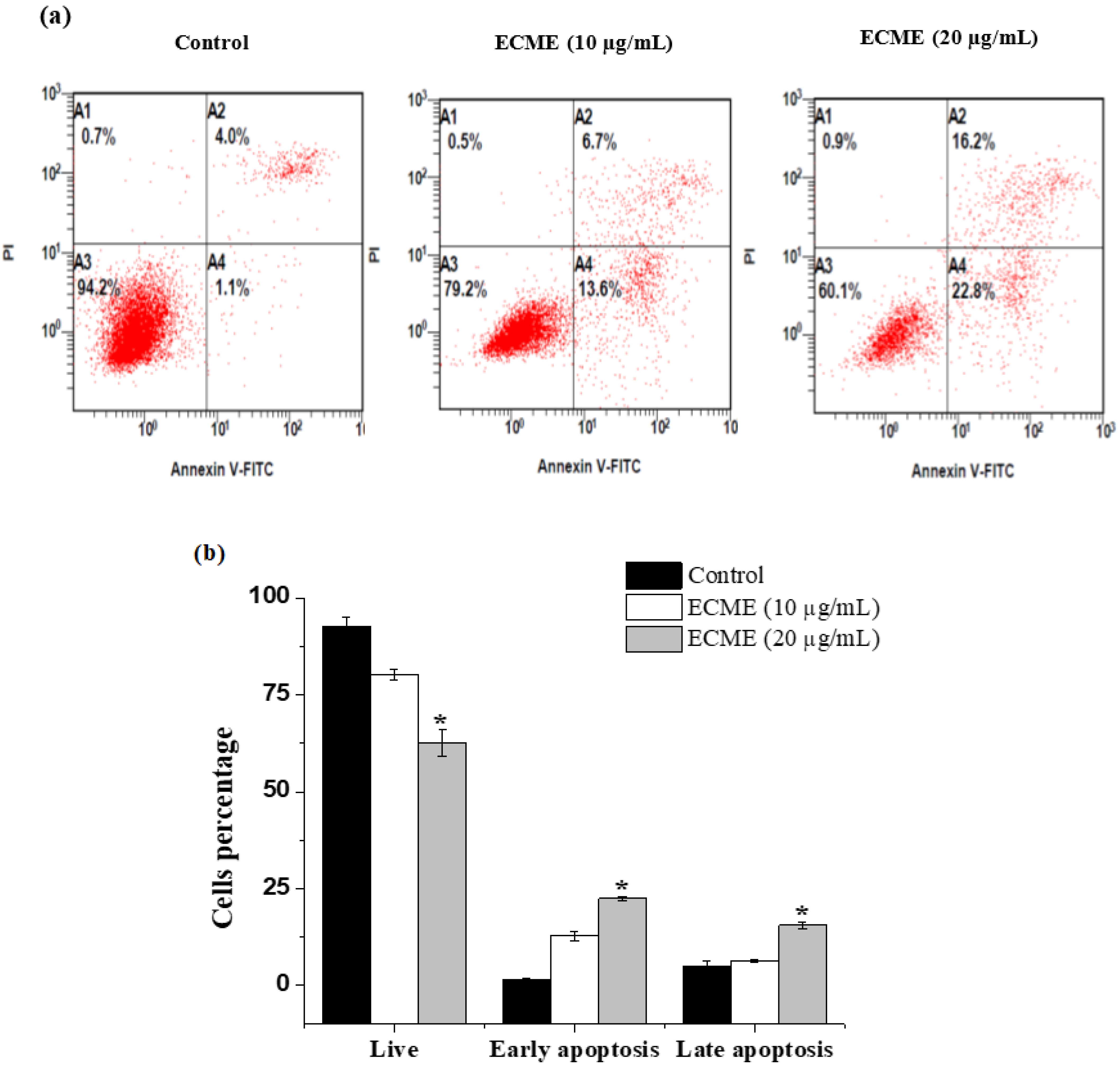
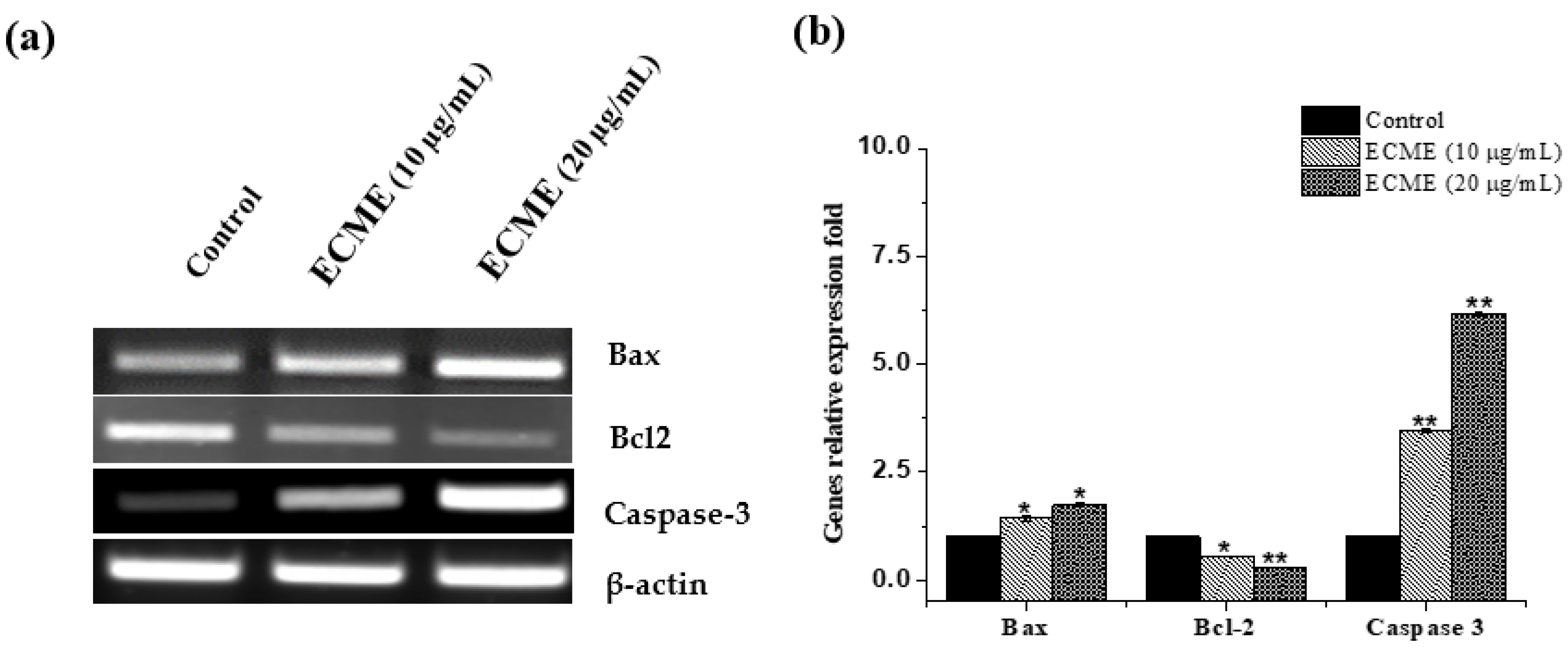
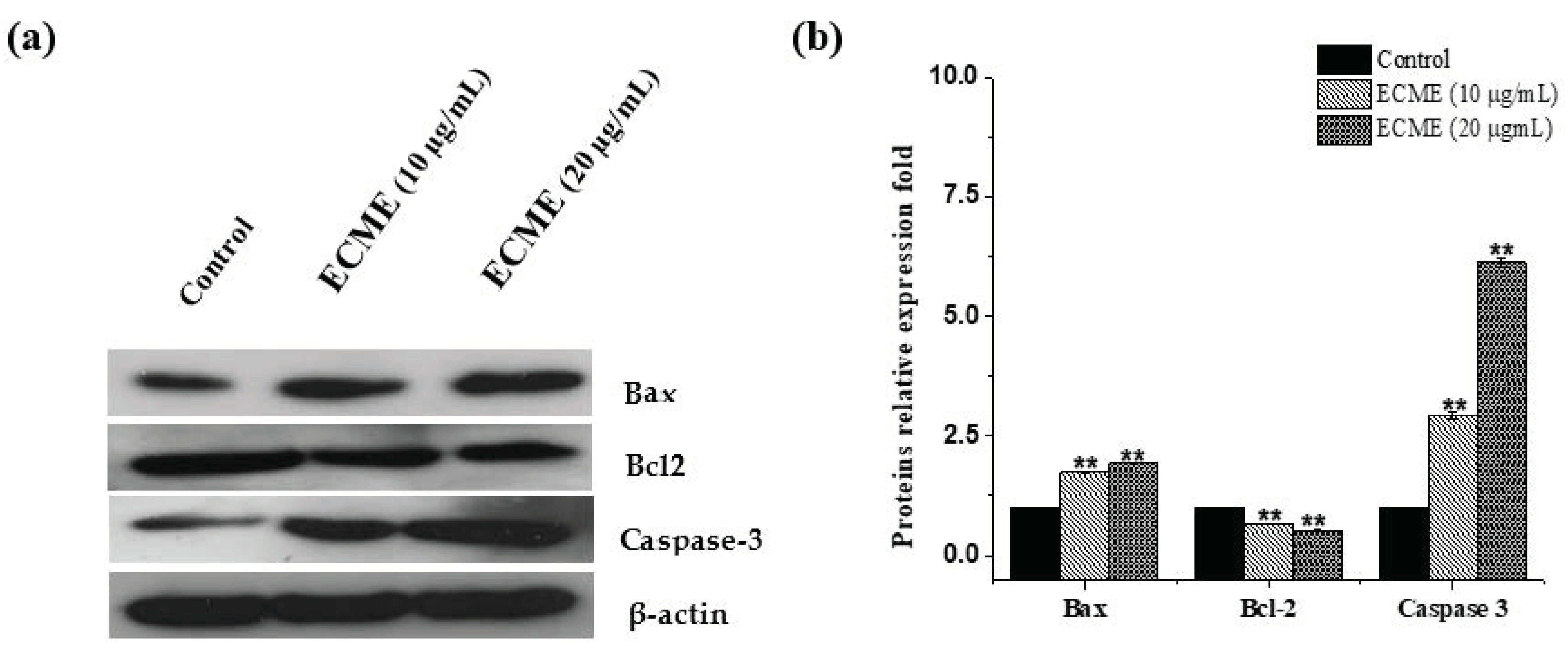
2.4. Antiproliferative and Apoptotic Activity of ECME
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extract
3.3. Preliminary Phytochemical Screening
3.4. Isolation of Chemical Constituents
Spectral Analysis of Isolated Compounds
3.5. Antioxidant Activity
3.5.1. DPPH• Assay
3.5.2. Ferrous Ion Chelating Assay (FIC)
3.6. Cytotoxicity Assay
3.7. Cell Cycle Analysis
3.8. Annexin V-FITC/PI Apoptosis Detection
3.9. RNA Extraction and RT-PCR
3.10. Western Blot
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECME | E. cactus methanolic extract. |
| MeOH | Methanol. |
| DMSO | Dimethyl sulfoxide. |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl. |
| FIC | ferrous ion chelating. |
| BHT | Butylated hydroxytoluene. |
| ETDA | Ethylenediaminetetraacetate. |
| MTT | 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide. |
| MCF7 | Michigan Cancer Foundation-7. |
| V-FITC | V-fluorescein Isothiocyanate. |
| RT-PCR | Reverse transcription polymerase chain reaction. |
References
- Horn, J.W.; Xi, Z.; Riina, R.; Peirson, J.A.; Yang, Y.; Dorsey, B.L.; Berry, P.E.; Davis, C.C.; Wurdack, K.J. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 2014, 68, 3485–3504. [Google Scholar] [CrossRef] [PubMed]
- Prenner, G.; Rudall, P.J. Comparative ontogeny of the cyathium in Euphorbia (Euphorbiaceae) and its allies: Exploring the organ-flower-inflorescence boundary. Am. J. Bot. 2007, 94, 1612–1629. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, R.l.; Frodin, D.G.; Radcliffe-Smith, A. World Checklist and Bibliography of Euphorbiaceae (with Pandaceae); Royal Botanic Gardens, Kew: Richmond, UK, 2000; Volume 2. [Google Scholar]
- Rizk, A.-F.M. The chemical constituents and economic plants of the Euphorbiaceae. Bot. J. Linn. Soc. 1987, 94, 293–326. [Google Scholar] [CrossRef]
- Ernst, M.; Nothias, L.F.; van der Hooft, J.J.J.; Silva, R.R.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Martinez-Swatson, K.; Hassemer, G.; Funez, L.A.; Simonsen, H.T.; et al. Assessing Specialized Metabolite Diversity in the Cosmopolitan Plant Genus Euphorbia L. Front. Plant Sci. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Nilsson, N.; Simonsen, H.T.; Horn, J.W.; Ronsted, N. Evolutionary prediction of medicinal properties in the genus Euphorbia L. Sci. Rep. 2016, 6, 30531. [Google Scholar] [CrossRef] [Green Version]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A Review of the Ethnomedicinal Uses, Biological Activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-Derived Natural Products with Potential for Use in Health Maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Aya, T.; Kinoshita, T.; Imai, S.; Koizumi, S.; Mizuno, F.; Osato, T.; Satoh, C.; Oikawa, T.; Kuzumaki, N.; Ohigashi, H.; et al. Chromosome translocation and c-MYC activation by Epstein-Barr virus and Euphorbia tirucalli in B lymphocytes. Lancet 1991, 337, 1190. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Hecker, E. On the active principles of the Euphorbiaceae, XII. Highly unsaturated irritant diterpene esters from Euphorbia tirucalli originating from Madagascar. J. Nat. Prod. 1986, 49, 386–397. [Google Scholar] [CrossRef]
- Prakash, E.; Gupta, D.K. Cytotoxic Activities of Extracts of Medicinal Plants of Euphorbiacae Family Studied on Seven Human Cancer Cell lines. Univers. J. Plant Sci. 2013, 1, 113–117. [Google Scholar] [CrossRef]
- Sharma, V.; Pracheta. Anti-carcinogenic potential of Euphorbia neriifolia leaves and isolated flavonoid against N-nitrosodiethylamine-induced renal carcinogenesis in mice. Indian J. Biochem. Biophys. 2013, 50, 521–528. [Google Scholar] [PubMed]
- Fayed, A.A.; Al-Zahrani, D.A. Three New Spiny Euphorbia (Euphorbiaceae) Species from Western Saudi Arabia. Edinb. J. Bot. 2007, 64, 117–129. [Google Scholar] [CrossRef]
- Al- Hajj, M.M.A.; Al-Shamahy, H.A.; Alkhatib, B.Y.; Moharram, B.A. In Vitro Anti-Leishmanial Activity against Cutaneous Leishmania Parasites and Preliminary Phytochemical Analysis of Four Yemeni Medicinal Plants. Univers. J. Pharm. Res. 2018, 3, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Al-Faifi, Z.I. In vitro Anticancer, Antioxidant and Antimicrobial Activities of Crude Methanolic Extract of Euphorbia cactus Ehrenb Plant. Int. J. Pharmacol. 2019, 15, 907–915. [Google Scholar] [CrossRef]
- Madureira, A.M.; Ascenso, J.R.; Valdeira, L.; Duarte, A.; Frade, J.P.; Freitas, G.; Ferreira, M.J. Evaluation of the antiviral and antimicrobial activities of triterpenes isolated from Euphorbia segetalis. Nat. Prod. Res. 2003, 17, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; Abou-Hussein, D.; Petereit, F. Chemical Constituents from the Leaves of Euphorbia ammak Growing in Saudi Arabia. Pharmacogn. Res. 2015, 7, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Uysal, A.; Aktumsek, A.; Mocan, A.; Mollica, A.; Locatelli, M.; Custodio, L.; Neng, N.R.; Nogueira, J.M.F.; Aumeeruddy-Elalfi, Z.; et al. Euphorbia denticulata Lam.: A promising source of phyto-pharmaceuticals for the development of novel functional formulations. Biomed. Pharm. 2017, 87, 27–36. [Google Scholar] [CrossRef]
- Majid, M.; Khan, M.R.; Shah, N.A.; Ul Haq, I.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complementary Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef] [Green Version]
- Rédei, D.; Kúsz, N.; Szabó, M.; Pinke, G.; Zupkó, I.; Hohmann, J. First phytochemical investigation of secondary metabolites of Euphorbia davidii Subils. and antiproliferative activity of its extracts. Acta Biol. Hung. 2015, 66, 464–467. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.F.; Hamed, A.R.; Ibrahim, M.A.A.; Talat, Z.; Reda, E.H.; Abdel-Azim, N.S.; Hammouda, F.M.; Nakamura, S.; Matsuda, H.; Haggag, E.G.; et al. Euphosantianane A(-)D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamoud, G.A.; Saud Orfali, R.; Perveen, S.; Mizuno, K.; Takeda, Y.; Nehira, T.; Masuda, K.; Sugimoto, S.; Yamano, Y.; Otsuka, H.; et al. Lasianosides A-E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity. Molecules 2019, 24, 3995. [Google Scholar] [CrossRef] [Green Version]
- Diplock, A.T. Chapter 4—Antioxidants and free radical scavengers. In New Comprehensive Biochemistry; Rice-Evans, C.A., Burdon, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 28, pp. 113–130. [Google Scholar]
- Al-Yousef, H.M.; Fantoukh, O.I.; El-Sayed, M.A.; Amina, M.; Adel, R.; Hassan, W.H.B.; Abdelaziz, S. Metabolic profiling and biological activities of the aerial parts of Micromeria imbricata Forssk. growing in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 5609–5616. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, I.; Untea, A.E.; Saracila, M. In Vitro Antioxidant Properties of Berry Leaves and Their Inhibitory Effect on Lipid Peroxidation of Thigh Meat from Broiler Chickens. Eur. J. Lipid Sci. Technol. 2020, 122, 1900384. [Google Scholar] [CrossRef]
- Budi, H.S.; Pebriani, I. The Difference of Temperature and Storage Time on the Antioxidant Activity of Curcuma Ethanol Extract (Curcuma zanthorrhiza) using the DPPH. Syst. Rev. Pharm. 2020, 11, 324–331. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.M.; Santacruz-Ortega, H.; Navarro, R.E.; Sotelo-Mundo, R.R.; Gonzalez-Aguilar, G.A. A (1)H NMR Investigation of the Interaction between Phenolic Acids Found in Mango (Manguifera indica cv Ataulfo) and Papaya (Carica papaya cv Maradol) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) Free Radicals. PLoS ONE 2015, 10, e0140242. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kilic, O.; Buldurun, K. Chemical constituent and radical scavenging antioxidant activity of Anthemis kotschyana Boiss. Nat. Prod. Res. 2020, 35, 4794–4797. [Google Scholar] [CrossRef]
- Barla, A.; Ozturk, M.; Kultur, S.; Oksuz, S. Screening of antioxidant activity of three Euphorbia species from Turkey. Fitoterapia 2007, 78, 423–425. [Google Scholar] [CrossRef]
- Battu, G.R.; Ethadi, S.R.; Veda Priya, G.; Swathi Priya, K.; Chandrika, K.; Venkateswara Rao, A.; Reddy, S.O. Evaluation of antioxidant and anti-inflammatory activity of Euphorbia heyneana Spreng. Asian Pac. J. Trop. Biomed. 2011, 1, S191–S194. [Google Scholar] [CrossRef]
- Ben Jannet, S.; Hymery, N.; Bourgou, S.; Jdey, A.; Lachaal, M.; Magne, C.; Ksouri, R. Antioxidant and selective anticancer activities of two Euphorbia species in human acute myeloid leukemia. Biomed. Pharm. 2017, 90, 375–385. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.-H.; Lee, S.-J.; Oh, S.J.; Lee, T.-H.; Jeong, D.K. Evaluation of the antioxidant, anti-inflammatory, and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef] [Green Version]
- Abdelhakim, S.; Rima, B.; Abdelouahab, B. Heavy Metals Chelating Ability and Antioxidant Activity of Phragmites australis Stems Extracts. J. Ecol. Eng. 2019, 20, 116–123. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Zhu, X.; Lin, T.; Hao, D.; Xu, J. Antioxidant activities of Clerodendrum cyrtophyllum Turcz leaf extracts and their major components. PLoS ONE 2020, 15, e0234435. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Chang, C.J.; Wan, C.F.; Chen, C.P.; Chiu, Y.H.; Leu, Y.L.; Peng, K.C. Euphorbia formosana root extract induces apoptosis by caspase-dependent cell death via Fas and mitochondrial pathway in THP-1 human leukemic cells. Molecules 2013, 18, 1949–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, B.; Vuong, Q.V.; Chalmers, A.C.; Goldsmith, C.D.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, Antioxidant and Anti-Cancer Properties of Euphorbia tirucalli Methanolic and Aqueous Extracts. Antioxidants 2015, 4, 647–661. [Google Scholar] [CrossRef]
- Cheng, J.; Han, W.; Wang, Z.; Shao, Y.; Wang, Y.; Zhang, Y.; Li, Z.; Xu, X.; Zhang, Y. Hepatocellular Carcinoma Growth Is Inhibited by Euphorbia helioscopia L. Extract in Nude Mice Xenografts. Biomed. Res. Int. 2015, 2015, 601015. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Friedrich, U.; Jenett-Siems, K. Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia 2005, 76, 355–358. [Google Scholar] [CrossRef]
- Magozwi, D.K.; Peter, X.; Langat, M.K.; Mhlanga, R.; Vukea, N.; Mare, J.-A.d.l.; Siwe-Noundou, X.; Krause, R.W.M.; Tembu, V.J. In vitro cytotoxic effects of chemical constituents of Euphorbia grandicornis Blanc against breast cancer cells. Sci. Afr. 2021, 14, e01002. [Google Scholar] [CrossRef]
- Chen, T.; Stephens, P.A.; Middleton, F.K.; Curtin, N.J. Targeting the S and G2 checkpoint to treat cancer. Drug Discov. Today 2012, 17, 194–202. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.; Ein Oon, C.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekofteh, N.; Ghafourian Boroujerdnia, M.; Khosravi, N.; Kalantar, K.; Malek-Hosseini, S.; Namdari, H.; Amirghofran, Z. Apoptosis-inducing Effect of the Hexane Extracts from Three Native Iranian Euphorbia Plants. Int. J. Cancer Manag. 2017, 10, e8306. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.; Kaur, M.; Kaur, H. Phytochemical screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–116. [Google Scholar]
- Adebayo, S.A.; Shai, L.J.; Eloff, J.N. First isolation of glutinol and a bioactive fraction with good anti-inflammatory activity from n-hexane fraction of Peltophorum africanum leaf. Asian Pac. J. Trop. Med. 2017, 10, 42–46. [Google Scholar] [CrossRef]
- Jung, M.J.; Heo, S.I.; Wang, M.H. Free radical scavenging and total phenolic contents from methanolic extracts of Ulmus davidiana. Food Chem. 2008, 108, 482–487. [Google Scholar] [CrossRef]
- Fukunaga, T.; Nishiya, K.; Kajikawa, I.; Watanabe, Y.; Suzuki, N.; Takeya, K.; Itokawa, H. Chemical Studies on the Constituents of Hyphear tanakae HOSOKAWA from Different Host Trees. Chem. Pharm. Bull. 1988, 36, 1180–1184. [Google Scholar] [CrossRef]
- Nicollier, G.; Thompson, A.C. Flavonoids of Desmanthus illinoensis. J. Nat. Prod. 1983, 46, 112–117. [Google Scholar] [CrossRef]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs--I. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P.S. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Nasr, F.A.; Shahat, A.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Al-Mishari, A.A.; Almoqbil, A.N. Centaurea bruguierana inhibits cell proliferation, causes cell cycle arrest, and induces apoptosis in human MCF-7 breast carcinoma cells. Mol. Biol. Rep. 2020, 47, 6043–6051. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alhawassi, T.; Qamar, W.; El-Gamal, A. Cytotoxic Evaluation and Anti-Angiogenic Effects of Two Furano-Sesquiterpenoids from Commiphora myrrh Resin. Molecules 2020, 25, 1318. [Google Scholar] [CrossRef] [Green Version]
| Phytochemicals | ECME |
|---|---|
| Phenols | +++ |
| Flavonoids | ++ |
| Diterpenes | +++ |
| Sesquiterpenoids | ++ |
| Terpenoids | ++ |
| Anthocyanins | ++ |
| Tannins | ++ |
| Steroids | ++ |
| Cerebrosides | + |
| Anthraquinones | ++ |
| Phloracetophenones | + |
| Glycerols | + |
| Alkaloids | + |
| Carbohydrates | ++ |
| Saponins | - |
| Sample | DPPH• Radical Scavenging | Ferrous Ion Chelating Activity | ||
|---|---|---|---|---|
| Concentration (µg mL−1) | Inhibition (%) | Concentration (µg mL−1) | Inhibition (%) | |
| ECME | 200 | 89.75 ± 0.35 | 3000 | 36.12 ± 0.45 |
| Compound 1 | 200 | 52.34 ± 0.26 | 3000 | 41.23 ± 0.26 |
| Compound 2 | 200 | 49.12 ± 0.34 | 3000 | 53.14 ± 0.22 |
| Compound 3 | 200 | 69.35 ± 0.24 | 3000 | 56.24 ± 0.36 |
| Compound 4 | 200 | 62.21 ± 0.14 | 3000 | 62.45 ± 0.42 |
| BHT | 200 | 41.16 ± 0.36 | - | - |
| EDTA | - | - | 3000 | 95.58 ± 0.45 |
| Cell lines and IC50 (μg/mL) | ||||
|---|---|---|---|---|
| A549 | LoVo | MCF-7 | HUVEC | |
| ECME | 20.1 ± 0.5 | 53.2 ± 0.4 | 58.80 ± 1.83 | 65.26 ± 2.80 |
| Doxorubicin | 1.20 ± 0.02 | 1.30 ± 0.05 | 1.40 ± 0.02 | 4.1 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamoud, G.A.; Fantoukh, O.I.; Amina, M.; Nasr, F.A.; Musayeib, N.M.A.; Ahmed, M.Z.; Noman, O.M.; Al-Sharidah, R.E.; Alasmari, F.; Alqahtani, A.S. Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia. Plants 2022, 11, 681. https://doi.org/10.3390/plants11050681
Al-Hamoud GA, Fantoukh OI, Amina M, Nasr FA, Musayeib NMA, Ahmed MZ, Noman OM, Al-Sharidah RE, Alasmari F, Alqahtani AS. Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia. Plants. 2022; 11(5):681. https://doi.org/10.3390/plants11050681
Chicago/Turabian StyleAl-Hamoud, Gadah A., Omer I. Fantoukh, Musarat Amina, Fahd A. Nasr, Nawal M. Al Musayeib, Mohammad Z. Ahmed, Omar M. Noman, Reem E. Al-Sharidah, Fawaz Alasmari, and Ali S. Alqahtani. 2022. "Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia" Plants 11, no. 5: 681. https://doi.org/10.3390/plants11050681
APA StyleAl-Hamoud, G. A., Fantoukh, O. I., Amina, M., Nasr, F. A., Musayeib, N. M. A., Ahmed, M. Z., Noman, O. M., Al-Sharidah, R. E., Alasmari, F., & Alqahtani, A. S. (2022). Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia. Plants, 11(5), 681. https://doi.org/10.3390/plants11050681








