Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety
Abstract
:1. Introduction
2. Results
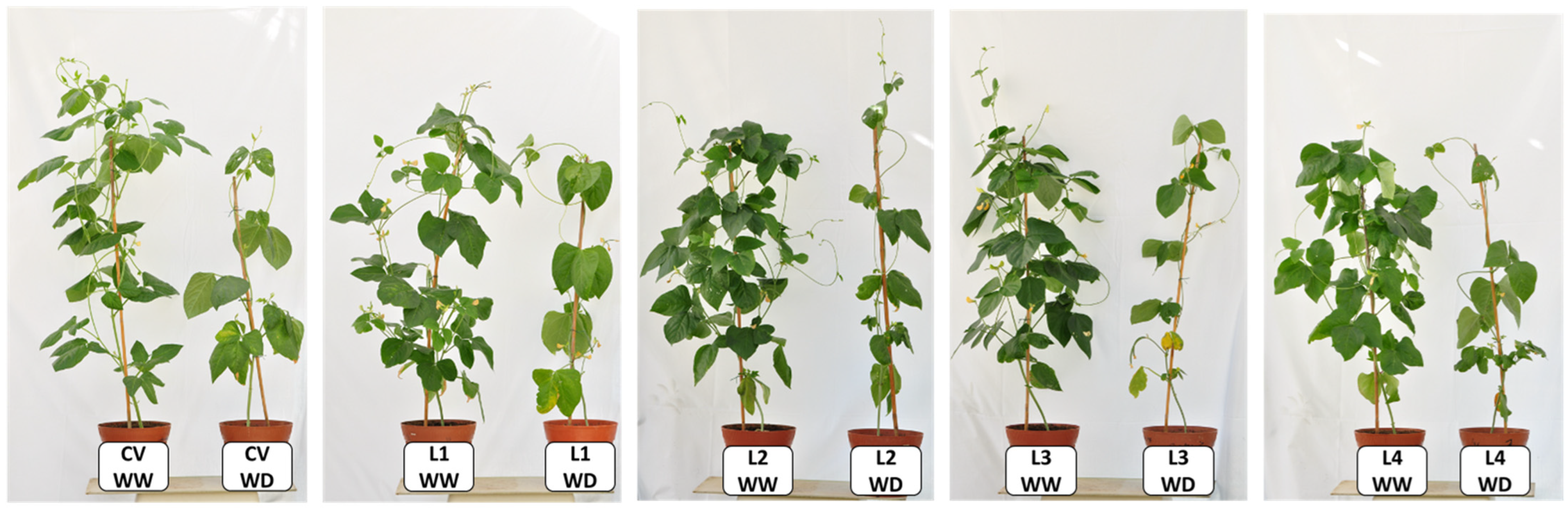
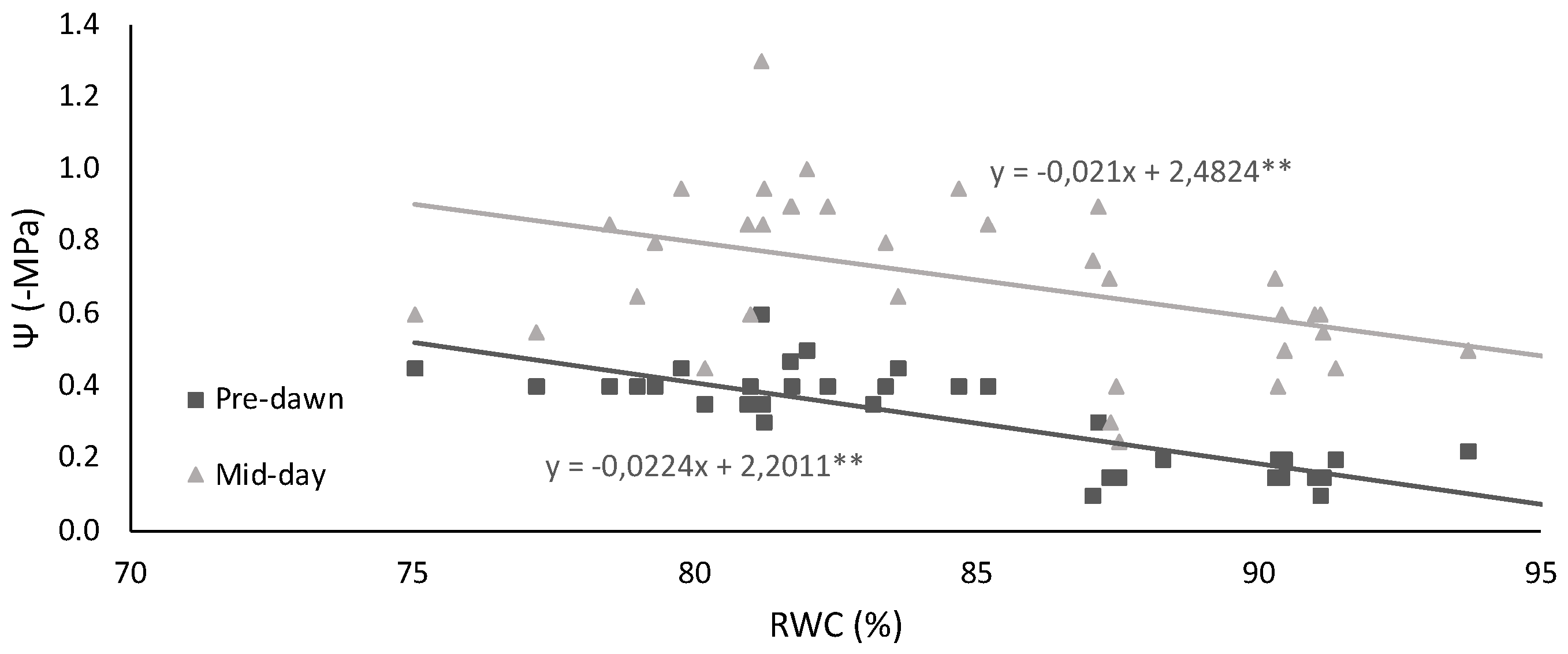
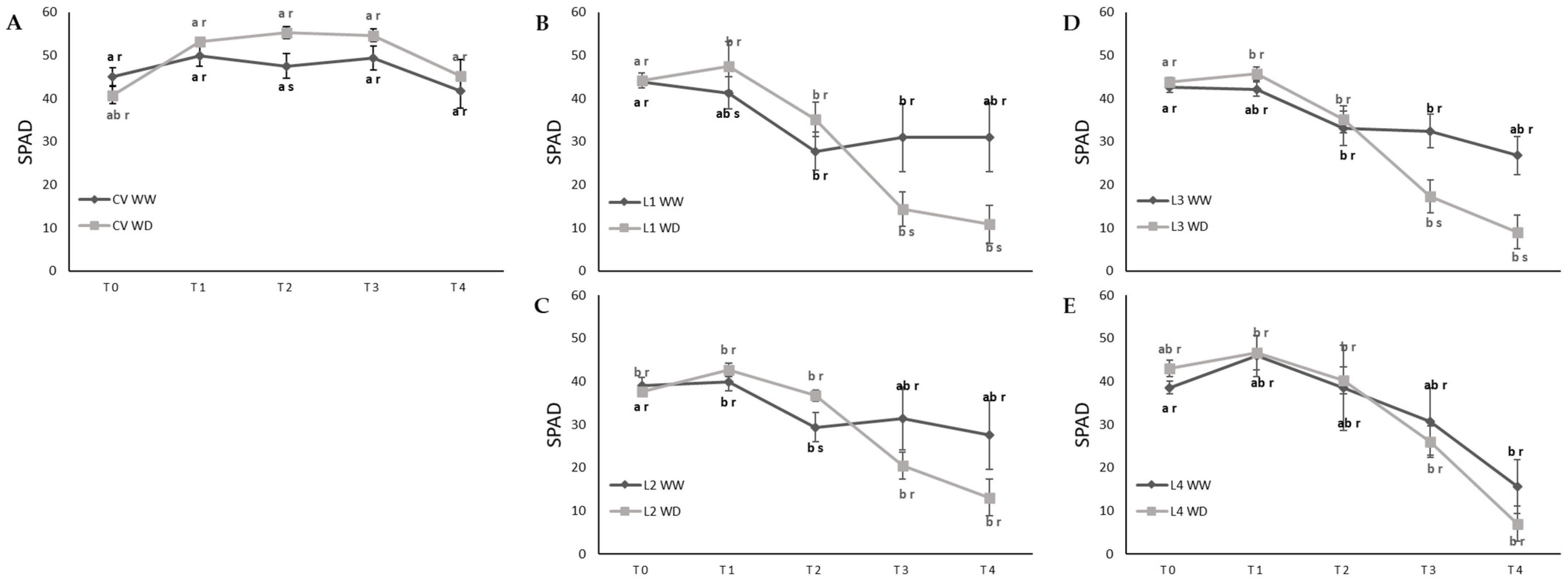
2.1. Leaf Water Status and Membrane Injury Index
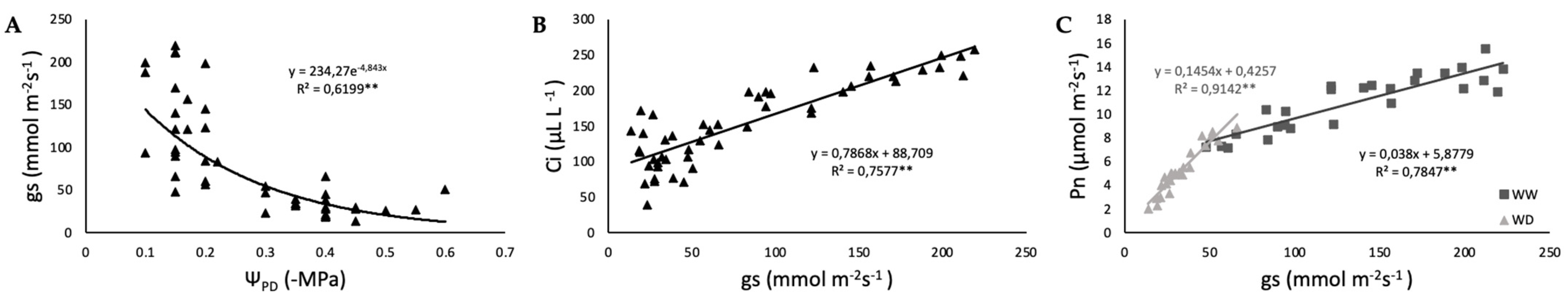
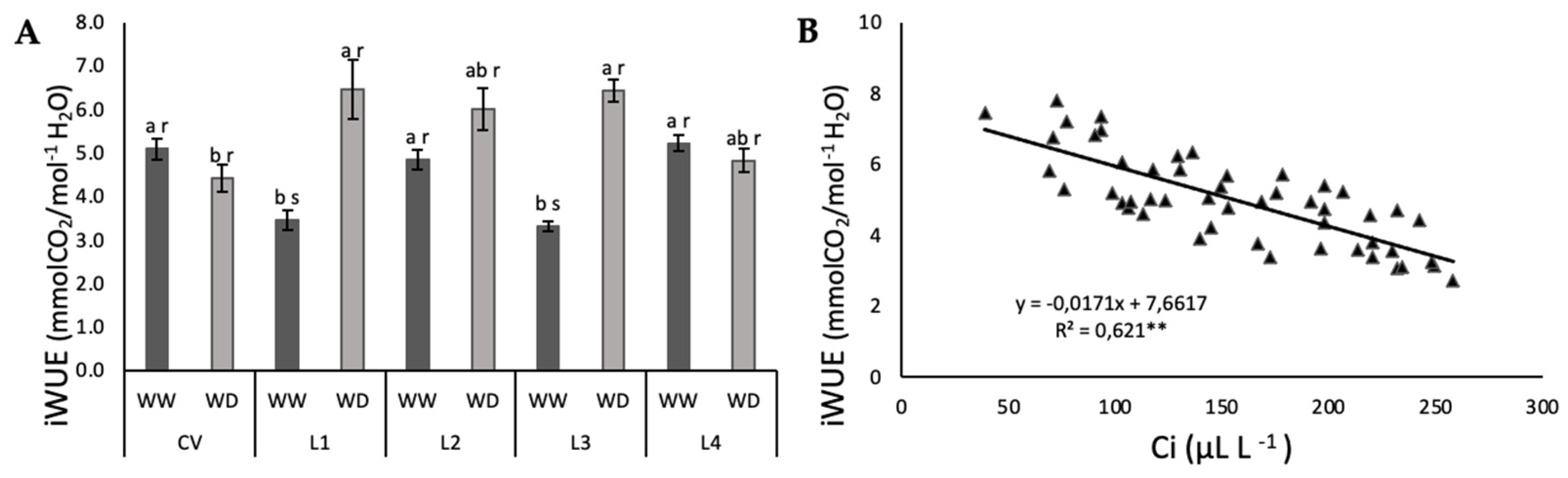
2.2. Photosynthetic Function and iWUE
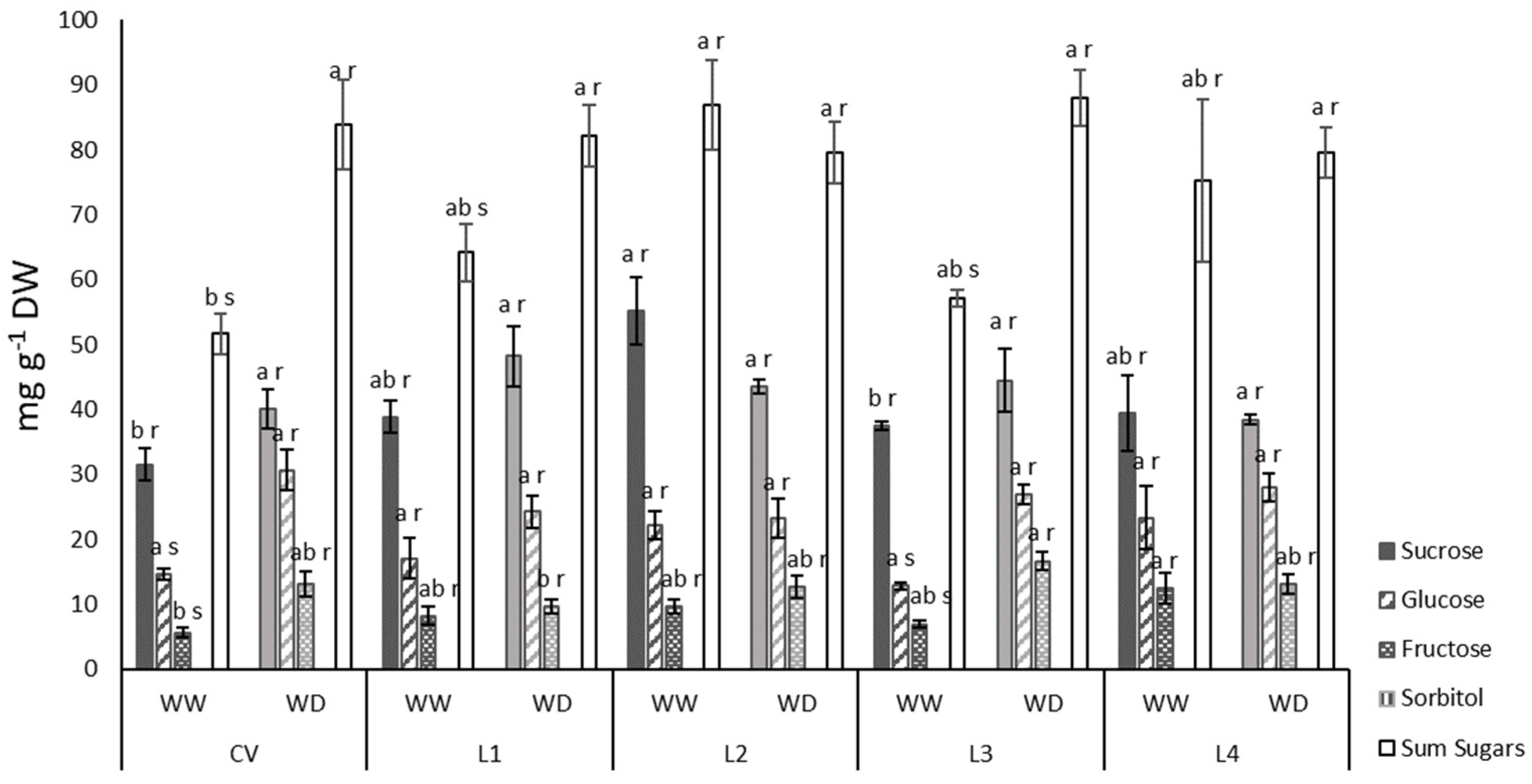
2.3. Leaf Sugar Content and Yield
3. Discussion
4. Materials and Methods
4.1. Field Capacity and Water Deficit Induction
4.2. Relative Water Content
4.3. Leaf Water Potential
4.4. SPAD Measurements
4.5. Leaf Pigments
4.6. Electrolyte Leakage Test
4.7. Gas Exchange Measurements
4.8. Chlorophyl Fluorescence Measurements
4.9. Yield
4.10. Soluble Sugars Determination
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, P.H.; Vance, C.P. Legumes: Importance and constrains to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Oritz, R. Cowpeas from Nigeria: A silent food revolution. Outlook Agric. 1998, 27, 125–128. [Google Scholar] [CrossRef]
- Tariku, S. Breeding cowpea Vigna unguiculata L. Walp for quality traits. Ann. Rev. Res. 2018, 3, 45–50. [Google Scholar] [CrossRef]
- Dadson, R.B.; Hashem, F.M.; Javaid, I.; Allen, A.L.; Devine, T.E. Effect of water stress on yield of cowpea (Vigna unguiculata L. Walp.) genotypes in the Delmarva region of the United States. J. Agron. Crop. Sci. 2005, 191, 210–217. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acuña, H.; Inostrosza, L.; Sanchez, M.P.; Tapia, G. Drought-tolerant naturalized populations of Lotus tenuis for constrained environments. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2010, 60, 174–181. [Google Scholar] [CrossRef]
- Inès, S.; Talbi, O.; Nasreddine, Y.; Rouached, A.; Gharred, J.; Jdey, A.; Hanana, M.; Abdelly, C. Drought tolerance traits in Medicago species: A review. In Arid Land Research and Management; Taylor and Francis: Abingdon, UK, 2021; Volume 35. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of water deficit on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Baath, G.S.; Rocateli, A.C.; Kakani, V.G.; Singh, H.; Northup, B.K.; Gowda, P.H.; Katta, J.R. Growth and physiological responses of three warm-season legumes to water stress. Sci. Rep. 2020, 10, 12233. [Google Scholar] [CrossRef]
- Nunes, C.; Araujo, S.S.; Silva, J.M.; Fevereiro, M.P.S.; Silva, A.B. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ. Exp. Bot. 2008, 63, 289–296. [Google Scholar] [CrossRef]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A legume crop for a challenging environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Su, M.; Chen, Y.; Gao, N.; Jiao, C.; Sun, Z.; Li, F.; Wang, C. Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biologia 2013, 68, 231–240. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef]
- Pinheiro, C.; Passarinho, J.A.; Ricardo, C.P. Effect of drought and rewatering on the metabolism of Lupinus albus organs. J. Plant Physiol. 2004, 161, 1203–1210. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [Green Version]
- Lazaridi, E.; Ntatsi, G.; Savvas, D.; Bebeli, P.J. Diversity in cowpea (Vigna unguiculata (L.) Walp.) local populations from Greece. Genet. Resour. Crop Evol. 2016, 64, 1529–1551. [Google Scholar] [CrossRef]
- Tosti, N.; Negri, V. On-going on-farm microevolutionary processes in neighbouring cowpea landraces revealed by molecular markers. Theor. Appl. Genet. 2005, 110, 1275–1283. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transpot regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Pushpavalli, R.; Zaman-Allah, M.; Turner, N.C.; Baddam, R.; Rao, M.V.; Vadez, V. Higher flower and seed number leads to higher yield under water stress conditions imposed during reproduction in chickpea. Funct. Plant Biol. 2015, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Suliman, A.S.H. Effect of water stress applied at different stages of growth on seed yield and water-use efficiency of cowpea. Agric. Biol. J. North Amer. 2010, 1, 534–540. [Google Scholar]
- Delmer, D.P. Agriculture in the developing world: Connecting innovations in plant research to downstream applications. Proc. Natl. Acad. Sci. USA 2005, 102, 15739–15746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC; Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J.; Hanson, C.E. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; p. 976. [Google Scholar]
- IPCC; Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Farooq, M.; Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Frahm, M.A.; Rosas, J.C.; Mayek-Pérez, N.; López-Salinas, E.; Acosta-Gallegos, J.A.; Kelly, J.D. Breeding beans for resistance to terminal drought in the lowland tropics. Euphytica 2004, 136, 223–232. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Torres, A.M.; Avila, C.M.; Gutierrez, N.; Palomino, C.; Moreno, M.T.; Cubero, J.I. Marker-assisted selection in faba bean (Vicia faba L.). Field Crop Res. 2010, 115, 243–252. [Google Scholar] [CrossRef]
- Amede, T.; Schubert, S. Mechanisms of drought resistance in grain legumes isosmotic adjustment. Ethiop. J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Novick, K.A.; Konings, A.G.; Gentine, P. Beyond soil water potential: An expanded view on isohydricity including land–atmosphere interactions and phenology. Plant Cell Environ. 2019, 42, 1802–1815. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Scotti-Campos, P.; Pham-Thi, A.T.; Semedo, J.N.; Pais, I.P.; Ramalho, J.C.; Matos, M.C. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emir. J. Food Agric. 2013, 25, 1002–1013. [Google Scholar] [CrossRef] [Green Version]
- Campos, P.S.; Ramalho, J.; Silva, M.J.; Lauriano, J.A.; Matos, M.C. Effects of drought on photosynthetic performance and water relations of four Vigna genotypes. Photosynthetica 1999, 36, 79–87. [Google Scholar] [CrossRef]
- Blum, A. Towards a conceptual ABA ideotype in plant breeding for water limited environments. Funct. Plant Biol. 2015, 42, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Costa, J.M.; Zarrouka, O.; Pinheiro, C.; Lopes, C.M.; Pereira, J.S. Controlling stomatal aperture in semi-arid regions—The dilemma of saving water or being cool? Plant Sci. J. 2016, 251, 54–64. [Google Scholar] [CrossRef]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol. 2014, 201, 1205–1217. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Nageswara Rao, R.C.; Joshi, Y.C. Moisture deficit-induced changes in leaf-water content, leaf carbon exchange rate and biomass production in groundnut cultivars differing in specific leaf area. Field Crop. Res. 2002, 74, 67–79. [Google Scholar] [CrossRef]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—Not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Lopez, F.B.; Chauhan, Y.S.; Johansen, C. Effects of timing of drought stress on leaf area development and canopy light interception of short-duration pigeonpea. J. Agron. Crop Sci. 1997, 178, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hayatu, M.; Mukhtar, F.B. Physiological responses of some drought resistant cowpea genotypes (Vigna unguiculata (L.) Walp) to water stress. Bayero J. Pure Appl. Sci. 2010, 3, 69–75. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Muchero, W.; Roberts, P.A.; Diop, N.N.; Drabo, I.; Cisse, N.; Close, T.J.; Muranaka, S.; Baukar, O.; Ehlers, J.D. Genetic architecture of delayed senescence, biomass, and grain yield under drought stress in cowpea. PLoS ONE 2013, 8, e70041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumudini, S. Trials and tribulations: A review of the role of assimilate supply in soybean genetic yield improvement. Field Crop. Res. 2002, 75, 211–222. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E.; Ehlers, J.D. Delayed leaf senescence and heat tolerance traits mainly are independently expressed in cowpea. Crop Sci. 2000, 40, 1049–1055. [Google Scholar] [CrossRef]
- Ronghua, L.; Pei-guo, G.; Baum, M.; Grando, S.; Ceccarelli, S. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Hamidou, F.; Zombre, G.; Braconnier, S. Physiological and biochemical responses of cowpea genotypes to water stress under glasshouse and field conditions. J. Agron. Crop Sci. 2007, 193, 229–237. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H.; Laffray, D.; Louguet, P. Comparison of the physiological responses of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Environ. Exp. Bot. 1998, 40, 197–207. [Google Scholar] [CrossRef]
- Iseki, K.; Takahashi, Y.; Muto, C.; Naito, K.; Tomooka, N. Diversity of drought tolerance in the genus vigna. Front. Plant Sci. 2018, 9, 729. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Montville, M.E.; Pinto, J.R. Using container weights to determine irrigation needs: A simple method. Nativ. Plants J. 2015, 16, 67–71. [Google Scholar] [CrossRef]
- Barrs, H.D. Effect of cyclic variations in gas exchange under constant environmental conditions on the ratio of transpiration to net photosynthesis. Physiol. Plant. 1968, 21, 918–929. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Semedo, J.N.; Pais, I.P.; Oliveira, M.; Passarinho, J.; Santos, M.; Almeida, A.S.; Costa, A.R.; Pinheiro, N.; Bagorro, C.; et al. Physiological responses to drought in four developed Triticum aestivum groups. Emir. J. Food Agric. 2015, 27, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef]
- Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Damesin, C.; Lelarge, C. Carbon isotope composition of current year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ. 2003, 26, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.; et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013, 8, e82712. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variety | RWC (%) | I (%) | ΨPD (MPa) | ΨMD (MPa) | σ (ΨPD/ΨMD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| WW | WD | WW | WD | WW | WD | WW | WD | ||
| CV | 88.7 ± 0.70 ar | 79.5 ± 2.67 as | 11.0 ± 0.88 ar | 12.7 ± 0.84 abr | −0.16 ± 0.02 ar | −0.35 ± 0.02 as | −0.64 ± 0.08 br | −0.80 ± 0.06 ar | 0.4064 |
| L1 | 88.2 ± 1.06 ar | 81.3 ± 1.66 as | 8.7 ± 0.50 abs | 10.9 ± 0.53 abr | −0.17 ± 0.01 ar | −0.42 ± 0.02 as | −0.38 ± 0.05 ar | −0.83 ± 0.06 as | 1.6743 |
| L2 | 91.2 ± 0.41 ar | 79.5 ± 1.10 as | 7.3 ± 0.41 bs | 13.3 ± 1.10 ar | −0.16 ± 0.02 ar | −0.39 ± 0.02 as | −0.52 ± 0.05 abr | −0.86 ± 0.08 as | 1.2231 |
| L3 | 89.2 ± 0.92 ar | 83.4 ± 1.11 as | 6.7 ± 0.28 bs | 10.0 ± 0.56 br | −0.14 ± 0.02 ar | −0.43 ± 0.06 as | −0.53 ± 0.03 abr | −0.99 ± 0.10 as | 1.3191 |
| L4 | 90.9 ± 1.27 ar | 80.1 ± 0.67 as | 9.8 ± 0.43 as | 11.5 ± 0.37 abr | −0.17 ± 0.01 ar | −0.42 ± 0.03 as | −0.48 ± 0.06 abr | −0.78 ± 0.08 ar | 1.0585 |
| Treatment | Variety | Variety * Treatment | |
|---|---|---|---|
| RWC (%) | *** | n.s. | n.s. |
| I (%) | *** | ** | n.s. |
| ΨPD (MPa) | *** | n.s. | n.s. |
| ΨMD (MPa) | *** | n.s. | n.s. |
| Pn (µmol m−2 s−1) | *** | n.s. | n.s. |
| gs (mmol m−2 s−1) | *** | n.s. | n.s. |
| Ci (µL L−1) | *** | n.s. | n.s. |
| iWUE (mmolCO2/mol−1 H2O) | n.s. | n.s. | * |
| Fv/Fm | *** | * | * |
| Fv’/Fm’ | n.s | * | n.s. |
| Chlorophyll (µg mg−1 DW) | *** | n.s. | ** |
| Carotenoids (µg mg−1 DW) | *** | n.s. | ** |
| Sum Sugars (mg g−1 DW) | * | n.s. | * |
| NPPl (number) | *** | *** | n.s. |
| NGPo (number) | *** | * | n.s. |
| NGPl (number) | *** | *** | ** |
| Weight 10 grains (g) | n.s. | *** | n.s. |
| Temperature (°C) | Relative Humidity (%) | |
|---|---|---|
| Average | 24.6 | 63.2 |
| Maximum | 46.9 | 89.4 |
| Minimum | 13.9 | 22.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, C.; Moreira, R.; Pais, I.; Semedo, J.; Simões, F.; Veloso, M.M.; Scotti-Campos, P. Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants 2022, 11, 593. https://doi.org/10.3390/plants11050593
Nunes C, Moreira R, Pais I, Semedo J, Simões F, Veloso MM, Scotti-Campos P. Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants. 2022; 11(5):593. https://doi.org/10.3390/plants11050593
Chicago/Turabian StyleNunes, Cátia, Rita Moreira, Isabel Pais, José Semedo, Fernanda Simões, Maria Manuela Veloso, and Paula Scotti-Campos. 2022. "Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety" Plants 11, no. 5: 593. https://doi.org/10.3390/plants11050593
APA StyleNunes, C., Moreira, R., Pais, I., Semedo, J., Simões, F., Veloso, M. M., & Scotti-Campos, P. (2022). Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants, 11(5), 593. https://doi.org/10.3390/plants11050593







