Persistence of the Strictly Endemic Plants of Forest Margins: The Case of Cirsium alpis-lunae in the Northern Apennines (Italy)
Abstract
:1. Introduction
2. Results
2.1. Population Size and Structure
2.2. Relathionships betwenn C. alpis-lunae and Environmental Parameters
3. Discussion
4. Materials and Methods
4.1. Study Species
4.2. Data Collection and Preparation
4.3. Data Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001; p. 713. [Google Scholar]
- Menges, E.S. Population viability analysis in plants: Challenges and opportunities. Trends Ecol. Evol. 2000, 15, 51–56. [Google Scholar] [CrossRef]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis; Sinauer Associates: Sunderland, MA, USA, 2002; p. 480. [Google Scholar]
- Silvertown, J.; Charlesworth, D. Plant Population Biology, 4th ed.; Blackwell Science Ltd.: Oxford, UK, 2006; p. 346. [Google Scholar]
- Menges, E.S.; Smith, S.A.; Weekley, C.W. Adaptive introductions: How multiple experiments and comparisons to wild populations provide insights into requirements for long-term introduction success of an endangered shrub. Plant Divers. 2016, 38, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Sulis, E.; Bacchetta, G.; Cogoni, D.; Fenu, G. From global to local scale: Where is the best for conservation purpose? Biodivers. Conserv. 2021, 30, 183–200. [Google Scholar] [CrossRef]
- Oostermeijer, J.G.B.; Luijten, S.H.; den Nijs, J.C.M. Integrating demographic and genetic approaches in plant conservation. Biol. Conserv. 2003, 113, 389–398. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [Green Version]
- Kew, B.G. The State of the World’s Plants Report; Royal Botanic Gardens, Kew: Richmond, UK, 2020. [Google Scholar]
- Fenu, G.; Cogoni, D.; Pinna, M.S.; Bacchetta, G. Threatened Sardinian vascular flora: A synthesis of 10 years of monitoring activities. Plant Biosyst. 2015, 149, 473–482. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Liu, C.; Zhang, J.; Lu, X.; Ma, K. Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 2016, 198, 104–112. [Google Scholar] [CrossRef]
- Schemske, D.W.; Husband, B.C.; Ruckelshaus, M.H.; Goodwillie, C.; Parker, I.M.; Bishop, J.G. Evaluating approaches to the conservation of rare and endangered plants. Ecology 1994, 75, 584–606. [Google Scholar] [CrossRef] [Green Version]
- Nantel, P.L.; Jones, J.; Drake, C. Viability of multiple populations across the range of a species at risk: The case of Pitcher’s thistle, Cirsium pitcheri, in Canada. Glob. Ecol. Conserv. 2018, 16, e00445. [Google Scholar] [CrossRef]
- Cogoni, D.; Sulis, E.; Bacchetta, G.; Fenu, G. The unpredictable fate of the single population of a threatened endemic Mediterranean plant. Biodivers. Conserv. 2019, 28, 1799–1813. [Google Scholar] [CrossRef]
- Rees, M.; Rose, K.E. Evolution of flowering strategies in Oenothera glazioviana: An integral projection model approach. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L. Flowering life-history strategies differ between the native and introduced ranges of a monocarpic perennial. Am. Nat. 2009, 174, 660–672. [Google Scholar] [CrossRef]
- Coulson, T. Integral projections models, their construction and use in posing hypotheses in ecology. Oikos 2012, 121, 1337–1350. [Google Scholar] [CrossRef]
- Dibner, R.R.; Peterson, M.L.; Louthan, A.M.; Doak, D.F. Multiple mechanisms confer stability to isolated populations of a rare endemic plant. Ecol. Monog. 2019, 89, e01360. [Google Scholar] [CrossRef]
- Belaid, A.H.; Maurice, S.; Fréville, H.; Carbonell, D.; Imbert, E. Predicting population viability of the narrow endemic Mediterranean plant Centaurea corymbosa under climate change. Biol. Conserv. 2018, 223, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Sulis, E.; Bacchetta, G.; Cogoni, D.; Fenu, G. Short-term population dynamics of Helianthemum caput-felis, a perennial Mediterranean coastal plant: A key element for an effective conservation programme. Syst. Biodivers. 2018, 16, 774–783. [Google Scholar] [CrossRef]
- Sulis, E.; Bacchetta, G.; Cogoni, D.; Gargano, D.; Fenu, G. Assessing the global conservation status of the rock rose Helianthemum caput-felis. Oryx 2020, 54, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Valli, A.-T.; Koumandou, V.L.; Iatrou, G.; Andreou, M.; Papasotiropoulos, V.; Trigas, P. Conservation biology of threatened Mediterranean chasmophytes: The case of Asperula naufraga endemic to Zakynthos island (Ionian islands, Greece). PLoS ONE 2021, 16, e0246706. [Google Scholar] [CrossRef]
- Valli, A.-T.; Chondrogiannis, C.; Grammatikopoulos, G.; Iatrou, G.; Tigas, P. Conservation of Micromeria browiczii (Lamiaceae), Endemic to Zakynthos Island (Ionian Islands, Greece). Plants 2021, 10, 778. [Google Scholar] [CrossRef]
- Cursach, J.; Besnard, A.; Rita, J.; Fréville, H. Demographic variation and conservation of the narrow endemic plant Ranunculus weyleri. Acta Oecol. 2013, 53, 102–109. [Google Scholar] [CrossRef]
- Marrero, M.V.; Oostermeijer, G.; Nogales, M.; Van Hengstum, T.; Saro, I.; Carqué, E.; Sosa, P.A.; Bañares, Á. Comprehensive population viability study of a rare endemic shrub from the high mountain zone of the Canary Islands and its conservation implications. J. Nat. Conserv. 2019, 47, 65–76. [Google Scholar] [CrossRef]
- Gauthier, P.; Pons, V.; Fisogni, A.; Murru, V.; Berjano, R.; Dessena, S.; Maccioni, A.; Chelo, C.; de Manincor, N.; Doncieux, A.; et al. Assessing vulnerability of listed Mediterranean plants based on population monitoring. J. Nat. Conserv. 2019, 52, 125758. [Google Scholar] [CrossRef]
- Cogoni, D.; Fenu, G.; Dessì, C.; Deidda, A.; Giotta, C.; Piccitto, M.; Bacchetta, G. Importance of Plants with Extremely Small Populations (PSESPs) in Endemic-Rich Areas, Elements Often Forgotten in Conservation Strategies. Plants 2021, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Risser, P.G. The status of the science examining ecotones. BioScience 1995, 45, 318–325. [Google Scholar] [CrossRef]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Menke, S.; Böhning-Gaese, K.; Schleuning, M. Plant–frugivore networks are less specialized and more robust at forest–farmland edges than in the interior of a tropical forest. Oikos 2012, 121, 1553–1566. [Google Scholar] [CrossRef]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Luizão, F.J. Litter production and litter nutrient concentrations in a fragmented Amazonian landscape. Ecol. Appl. 2004, 14, 884–892. [Google Scholar] [CrossRef]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.O.; De Pauw, K.; Diekmann, M.; Gasperini, C.; et al. Microclimatic edge-to-interior gradients of European deciduous forests. Agric. For. Meteorol. 2021, 311, 108699. [Google Scholar] [CrossRef]
- Caruso, C.M.; Peterson, S.B.; Ridley, C.E. Natural selection on floral traits of Lobelia (Lobeliaceae): Spatial and temporal variation. Am. J. Bot. 2003, 90, 1333–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargano, D.; Fenu, G.; Bernardo, L. Local shifts in floral biotic interactions in habitat edges and their effect on quantity and quality of plant offspring. AoB Plants 2017, 9, plx031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P.-A. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Schmidt, M.; Jochheim, H.; Kersebaum, K.-C.; Lischeid, G.; Nendel, C. Gradients of microclimate, carbon and nitrogen in transition zones of fragmented landscapes—A review. Agric. For. Meteorol. 2017, 232, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Matlack, G.R. Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv. 1993, 66, 185–194. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Payne, G.W.; Van Elswijk, M. Microclimate gradients across a forest edge. N. Zealand J. Ecol. 2000, 24, 111–121. [Google Scholar]
- De Smedt, P.; Baeten, L.; Proesmans, W.; Van de Poel, S.; Van Keer, J.; Giffard, B.; Martin, L.; Vanhulle, R.; Brunet, J.; Cousins, S.A.O.; et al. Strength of forest edge effects on litter-dwelling macro-arthropods across Europe is influenced by forest age and edge properties. Divers. Distrib. 2019, 25, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Govaert, S.; Meeussen, C.; Vanneste, T.; Bollmann, K.; Brunet, J.; Cousins, S.A.O.; Diekmann, M.; Graae, B.J.; Hedwall, P.-O.; Heinken, T.; et al. Edge influence on understorey plant communities depends on forest management. J. Veg. Sci. 2020, 31, 281–292. [Google Scholar] [CrossRef]
- De Pauw, K.; Meeussen, C.; Govaert, S.; Sanczuk, P.; Vanneste, T.; Bernhardt-Römermann, M.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.; et al. Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 2021, 109, 2629–2648. [Google Scholar] [CrossRef]
- López-Barrera, F.; Manson, R.H.; González-Espinosa, M.; Newton, A.C. Effects of the type of montane forest edge on oak seedling establishment along forest-edge-exterior gradients. For. Ecol. Manag. 2006, 225, 234–244. [Google Scholar] [CrossRef]
- Hadley, A.S.; Betts, M.G. The effects of landscape fragmentation on pollination dynamics: Absence of evidence not evidence of absence. Biol. Rev. 2012, 87, 526–544. [Google Scholar] [CrossRef] [PubMed]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 8 September 2021).
- Abela-Hofbauerová, I.; Münzbergová, Z. Increased performance of Cirsium arvense from the invasive range. Flora 2011, 206, 1012–1019. [Google Scholar] [CrossRef]
- Jolls, C.L.; Marik, J.E.; Hamzé, S.I.; Havens, K. Population viability analysis and the effects of light availability and litter on populations of Cirsium pitcheri, a rare, monocarpic perennial of Great Lakes shorelines. Biol. Conserv. 2015, 187, 82–90. [Google Scholar] [CrossRef]
- Román, J.F.C.; Hernández-Lambraño, R.E.; de la Cruz, D.R.; Agudo, J.Á.S. Analysis of the adaptative strategy of Cirsium vulgare (Savi) Ten. in the Colonization of New Territories. Sustainability 2021, 13, 2384. [Google Scholar] [CrossRef]
- Bullock, J.M.; Clear Hill, B.; Silvertown, J. Demography of Cirsium Vulgare in a Grazing Experiment. J. Ecol. 1994, 82, 101–111. [Google Scholar] [CrossRef]
- Klinkhamer, P.G.L.; De Jong, T.J.; Van Der Meijden, E. Production, dispersal and predation of seeds in the biennial Cirsium Vulgare. J. Ecol. 1988, 76, 403–414. [Google Scholar] [CrossRef]
- Gillman, M.; Bullock, J.M.; Silvertown, J.; Hill, B.C. A density-dependent model of Cirsium vulgare population dynamics using field-estimated parameter values. Oecologia 1993, 96, 282–289. [Google Scholar] [CrossRef]
- Sheidai, M.; Zanganeh, S.; Haji-Ramezanali, R.; Nouroozi, M.; Noormohammadi, Z.; Ghsemzadeh-Baraki, S. Genetic diversity and population structure in four Cirsium (Asteraceae) species. Biologia 2013, 68, 384–397. [Google Scholar] [CrossRef]
- Brilli-Cattarini, A.J.B.; Gubellini, L. Una nuova specie di Cirsium (Compositae, Asteroideae, Cynareae) dell’Appennino etrusco meridionale. Webbia 1991, 46, 7–17. [Google Scholar] [CrossRef]
- Raffaelli, M.; Rizzotto, M. Contributo alla conoscenza della flora dell’Alpe della Luna (Appennino Aretino, Toscana). Webbia 1991, 46, 19–79. [Google Scholar] [CrossRef]
- Gonnelli, V. Segnalazioni Floristiche Italiane: 805 Cirsium alpis-lunae Br-Catt & Gubell., Specie nuova per l’Emilia Romagna, ulteriori dati distributivi in Italia. Inform. Bot. Ital. 1995, 27, 277–278. [Google Scholar]
- Viciani, D.; Gabellini, A.; Gonnelli, V.; De Dominicis, V. La vegetazione della Riserva Naturale Alpe della Luna (Arezzo, Toscana) ed i suoi aspetti di interesse botanico-conservazionistico. Webbia 2002, 57, 153–170. [Google Scholar] [CrossRef]
- Viciani, D.; Gonnelli, V.; Sirotti, M.; Agostini, N. An annotated check-list of the vascular flora of the “Parco Nazionale delle Foreste Casentinesi, Monte Falterona e Campigna” (Northern Apennines Central Italy). Webbia 2010, 65, 3–131. [Google Scholar] [CrossRef]
- Viciani, D.; Gabellini, A.; Gonnelli, V.; De Dominicis, V. La vegetazione della Riserva Naturale Alta Valle del Tevere—Monte Nero (Arezzo, Toscana) ed i suoi aspetti di interesse botanico-conservazionistico. Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 2004, 109, 11–25. [Google Scholar]
- Conti, F.; Abbate, G.; Alessandrini, A.; Blasi, C. (Eds.) An Annotated Checklist of the Italian Vascular Flora. Ministero dell’Ambiente e della Tutela del Territorio, Direzione Protezione della Natura, Dipartimento di Biologia Vegetale, Università degli Studi di Roma “La Sapienza”; Palombi Editori: Roma, Italy, 2005; p. 429. [Google Scholar]
- Casavecchia, S.; Paradisi, L.; Pesaresi, S.; Biondi, E. Phytosociological study of the eastern slopes of Alpe della Luna (northern Apennines, Italy). Plant Sociol. 2014, 51, 89–136. [Google Scholar] [CrossRef]
- Gennai, M.; Gonnelli, V.; Viciani, D. Cirsium alpis-lunae Brilli-Catt. & Gubellini. Inf. Bot. Ital. 2015, 47, 109–111. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Viciani, D.; Lazzaro, L.; Gonnelli, V.; Lastrucci, L. A new plant community with the strictly endemic Cirsium alpis-lunae (Asteraceae) in the Northern Apennines (Italy) and considerations on the alliances Senecionion samniti and Adenostylion alpinae. Medit. Bot. 2019, 40, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Pollock, L.J.; O’Connor, L.M.J.; Mokany, K.; Rosauer, D.F.; Talluto, M.V.; Thuiller, W. Protecting Biodiversity (in all its complexity): New models and methods. Trends Ecol. Evol. 2020, 35, 1119–1128. [Google Scholar] [CrossRef]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). Summary for Policymakers of the Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean: Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020; p. 464. [Google Scholar]
- Garcıà, M.B. Demographic Viability of a Relict Population of the Critically Endangered Plant Borderea chouardii. Conserv. Biol. 2003, 17, 1672–1680. [Google Scholar] [CrossRef]
- Heywood, V.H.; Iriondo, J.M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- Picó, F.X.; Riba, M. Regional-scale demography of Ramonda myconi: Remnant population dynamics in a preglacial relict species. Plant Ecol. 2002, 161, 1–13. [Google Scholar] [CrossRef]
- García, M.B.; Domingo, D.; Pizarro, M.; Font, X.; Gómez, D.; Ehrlén, J. Rocky habitats as microclimatic refuges for biodiversity A close-up thermal approach. Environ. Exp. Bot. 2020, 170, 103886. [Google Scholar] [CrossRef]
- Fenu, G.; Ferretti, G.; Gennai, M.; Lahora, A.; Mendoza-Fernández, A.J.; Mota, J.; Robles, J.; Serra, L.; Schwarzer, H.; Sánchez-Gómez, P.; et al. Global and Regional IUCN Red List Assessments: 4. Ital. Bot. 2017, 4, 61–71. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Iriondo, J.M. Population dynamics of Aster pyrenaeus Desf., a threatened species of temperate forest edges: A view of meso- and micro-scales. Plant Biosyst. 2014, 148, 645–654. [Google Scholar] [CrossRef]
- Tomimatsu, H.; Ohara, M. Edge effects on recruitment of Trillium camschatcense in small forest fragments. Biol. Conserv. 2004, 117, 509–519. [Google Scholar] [CrossRef]
- Prieto-Benítez, S.; Morente-López, J.; Teso, M.L.R.; Lara-Romero, C.; García-Fernández, A.; Torres, E.; Iriondo, J.M. Evaluating Assisted Gene Flow in Marginal Populations of a High Mountain Species. Front. Ecol. Evol. 2021, 9, 638837. [Google Scholar] [CrossRef]
- Gentili, R.; Bacchetta, G.; Fenu, G.; Cogoni, D.; Abeli, T.; Rossi, G.; Salvatore, M.C.; Baroni, C.; Citterio, S. From cold to warm-stage refugia for boreo-alpine plants in southern European and Mediterranean mountains: The last chance to survive or an opportunity for speciation? Biodiversity 2015, 16, 247–261. [Google Scholar] [CrossRef]
- Meiners, S.J.; Pickett, S.T.A.; Handel, S.N. Probability of tree seedling establishment changes across a forest-old field edge gradient. Am. J. Bot. 2002, 89, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; Giusso del Galdo, G.P.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A common approach to the conservation of threatened island vascular plants: First results in the Mediterranean Basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Bini, C.; Del Sette, M.; Fastelli, C. Lineamenti ambientali e pedologici dell’Alta Valtiberina. Ecol. Agrar. 1982, 18, 1–55. [Google Scholar]
- Carmignani, L.; Conti, P.; Cornamusini, G.; Pirro, A. Geological map of Tuscany (Italy). J. Maps 2013, 9, 487–497. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 8 February 2020).
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 0.13. 17. 2010. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 8 February 2020).
- Wickham, H. ggplot2. In Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
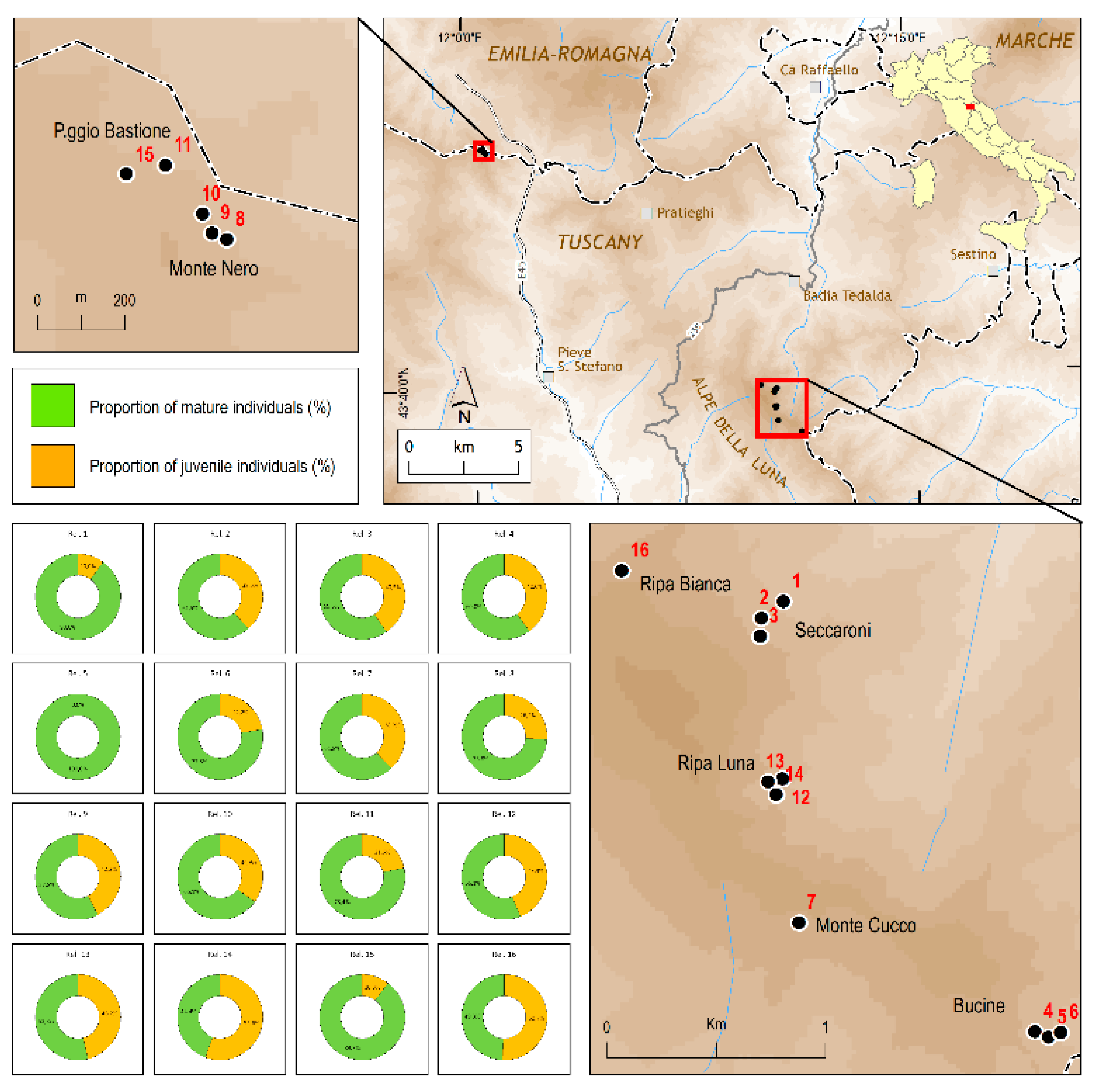
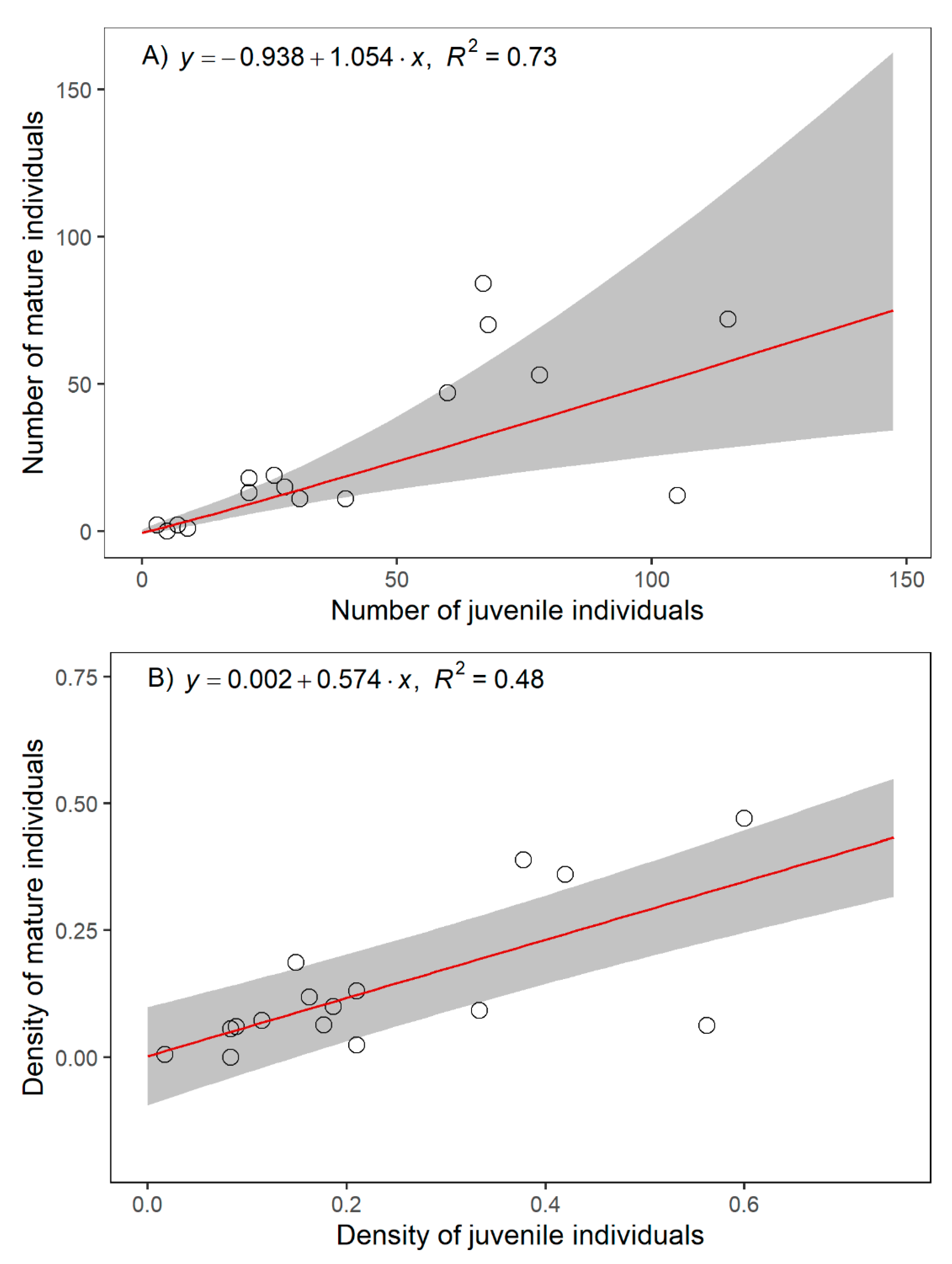
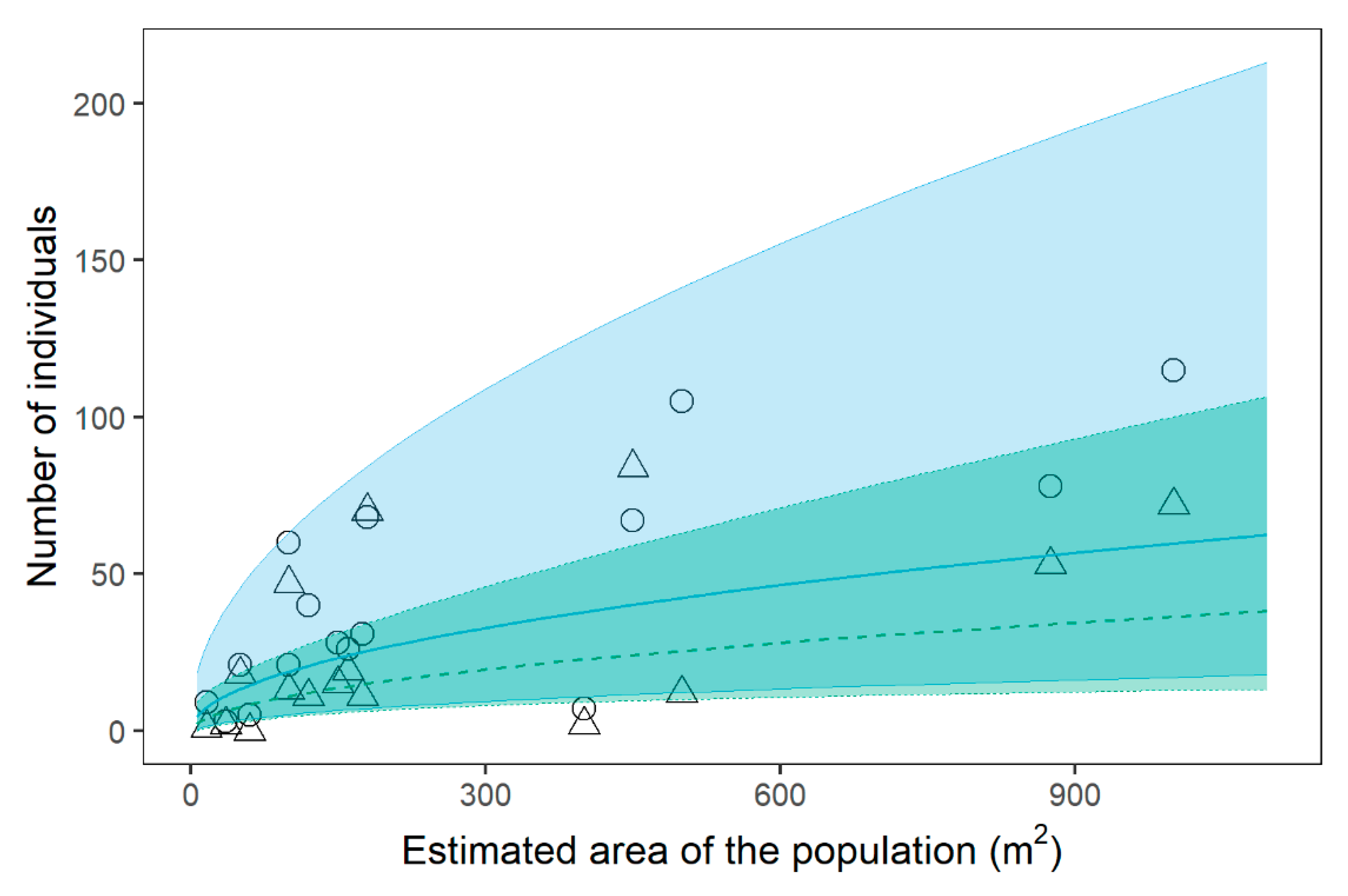
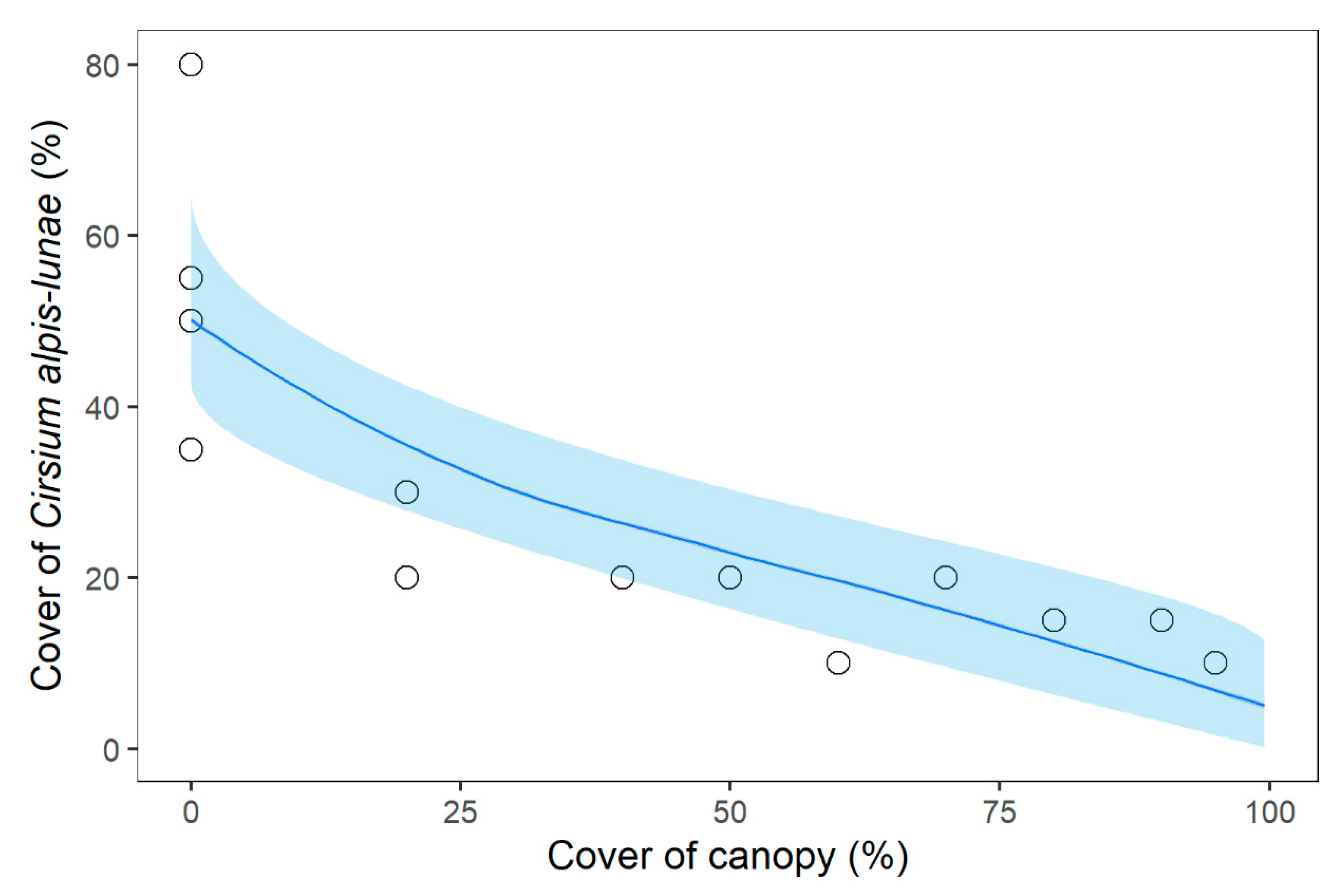
| No. | Locality (Municipality Administrative Province) | Altitude (m a.s.l.) | Slope (%) | Aspect | Estimated Area (m2) | Total n. of Plants | N. of Mature Plants | Recruitment |
|---|---|---|---|---|---|---|---|---|
| 1 | Seccaroni 1 (Alpe della Luna), Badia Tedalda, Arezzo | 1118 | 100 | N-NE | 20 | 10 | 1 | absent |
| 2 | Seccaroni 2 (Alpe della Luna), Badia Tedalda, Arezzo | 1132 | >100 | N | 1000 | 187 | 72 | present |
| 3 | Seccaroni 3 (Alpe della Luna), Badia Tedalda, Arezzo | 1180 | >100 | N | 875 | 131 | 53 | present |
| 4 | Bucine 1 (Alpe della Luna), Badia Tedalda, Arezzo | 1190 | 100 | NE | 36 | 5 | 2 | absent |
| 5 | Bucine 2 (Alpe della Luna), Badia Tedalda, Arezzo | 1170 | 100 | NE | 60 | 5 | 0 | absent |
| 6 | Bucine 3 (Alpe della Luna), Badia Tedalda, Arezzo | 1120 | 100 | NE | 40 | 9 | 2 | absent |
| 7 | Cucco (Alpe della Luna), Sansepolcro, Arezzo | 1270 | 100 | SE | 100 | 34 | 13 | present |
| 8 | Monte Nero 1 (Monte Nero-Poggio Bastione), Verghereto, Forlì-Cesena | 1149 | 75 | NE | 175 | 42 | 11 | present |
| 9 | Monte Nero 2 (Monte Nero-Poggio Bastione), Verghereto, Forlì-Cesena | 1154 | >100 | NE | 160 | 45 | 19 | present |
| 10 | Monte Nero 3 (Monte Nero-Poggio Bastione), Verghereto, Forlì-Cesena | 1148 | 100 | NE | 150 | 43 | 15 | present |
| 11 | Poggio Bastione 1 (Monte Nero-Poggio Bastione), Verghereto, Forlì-Cesena | 1150 | >100 | N | 120 | 51 | 11 | present |
| 12 | Ripa Luna 1 (Alpe della Luna), Sansepolcro, Arezzo | 1125 | 85 | N | 100 | 107 | 47 | present |
| 13 | Ripa Luna 2 (Alpe della Luna), Badia Tedalda, Arezzo | 1145 | 50 | NE | 50 | 39 | 18 | present |
| 14 | Ripa Luna 3 (Alpe della Luna), Badia Tedalda, Arezzo | 1150 | 85 | NNW | 450 | 151 | 84 | present |
| 15 | Poggio Bastione 2 (Monte Nero-Poggio Bastione), Pieve S. Stefano, Arezzo | 1125 | 90 | NE | 500 | 117 | 12 | present |
| 16 | Ripa Bianca (Alpe della Luna), Badia Tedalda, Arezzo | 1270 | 40 | N | 1800 | 138 | 70 | present |
| Response | Variable | Factor Relative Importance w+(j) | Averaged Coefficient | Adjusted SE | z Value | Pr (>|z|) | |
|---|---|---|---|---|---|---|---|
| Abundance of juvenile individuals * | (Intercept) | - | 0.74 | 0.80 | 0.92 | 0.358 | |
| Estimated area + | 1 | 0.49 | 0.09 | 5.66 | <0.001 | *** | |
| Eastness | 0.14 | −0.06 | 0.21 | 0.31 | 0.758 | ||
| Northness | 0.11 | 0.05 | 0.21 | 0.23 | 0.818 | ||
| Abundance of mature individuals * | (Intercept) | - | 0.29 | 0.95 | 0.30 | 0.763 | |
| Estimated area + | 1 | 0.49 | 0.16 | 3.03 | 0.002 | ** | |
| Eastness | 0.28 | −0.21 | 0.42 | 0.50 | 0.618 | ||
| Density of juvenile individuals | (Intercept) | - | 0.24 | 0.07 | 3.52 | <0.001 | *** |
| Eastness | 0.15 | −0.02 | 0.07 | 0.28 | 0.783 | ||
| Northness | 0.11 | 0.01 | 0.20 | 0.20 | 0.844 | ||
| Density of mature individuals | (Intercept) | - | 0.14 | 0.09 | 1.64 | 0.101 | |
| Northness | 0.13 | 0.01 | 0.05 | 0.23 | 0.822 | ||
| Cover Cirsium £ | (Intercept) | - | 0.76 | 0.21 | 3.52 | <0.001 | *** |
| Canopy cover £ | 1 | −0.41 | 0.08 | 5.03 | <0.001 | *** | |
| Ground cover £ | 0.19 | 0.06 | 0.16 | 0.38 | 0.706 | ||
| Shrubs cover £ | 0.1 | −0.02 | 0.08 | 0.26 | 0.797 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenu, G.; Lazzaro, L.; Lastrucci, L.; Viciani, D. Persistence of the Strictly Endemic Plants of Forest Margins: The Case of Cirsium alpis-lunae in the Northern Apennines (Italy). Plants 2022, 11, 653. https://doi.org/10.3390/plants11050653
Fenu G, Lazzaro L, Lastrucci L, Viciani D. Persistence of the Strictly Endemic Plants of Forest Margins: The Case of Cirsium alpis-lunae in the Northern Apennines (Italy). Plants. 2022; 11(5):653. https://doi.org/10.3390/plants11050653
Chicago/Turabian StyleFenu, Giuseppe, Lorenzo Lazzaro, Lorenzo Lastrucci, and Daniele Viciani. 2022. "Persistence of the Strictly Endemic Plants of Forest Margins: The Case of Cirsium alpis-lunae in the Northern Apennines (Italy)" Plants 11, no. 5: 653. https://doi.org/10.3390/plants11050653
APA StyleFenu, G., Lazzaro, L., Lastrucci, L., & Viciani, D. (2022). Persistence of the Strictly Endemic Plants of Forest Margins: The Case of Cirsium alpis-lunae in the Northern Apennines (Italy). Plants, 11(5), 653. https://doi.org/10.3390/plants11050653







