Grain Composition and Quality in Portuguese Triticum aestivum Germplasm Subjected to Heat Stress after Anthesis
Abstract
1. Introduction
2. Results
2.1. Test Weight (TW) and Thousand-Kernel Weight (TKW)
2.2. Soluble Sugars Composition
2.3. Protein Content, SDS Sedimentation Test, and Ash Content
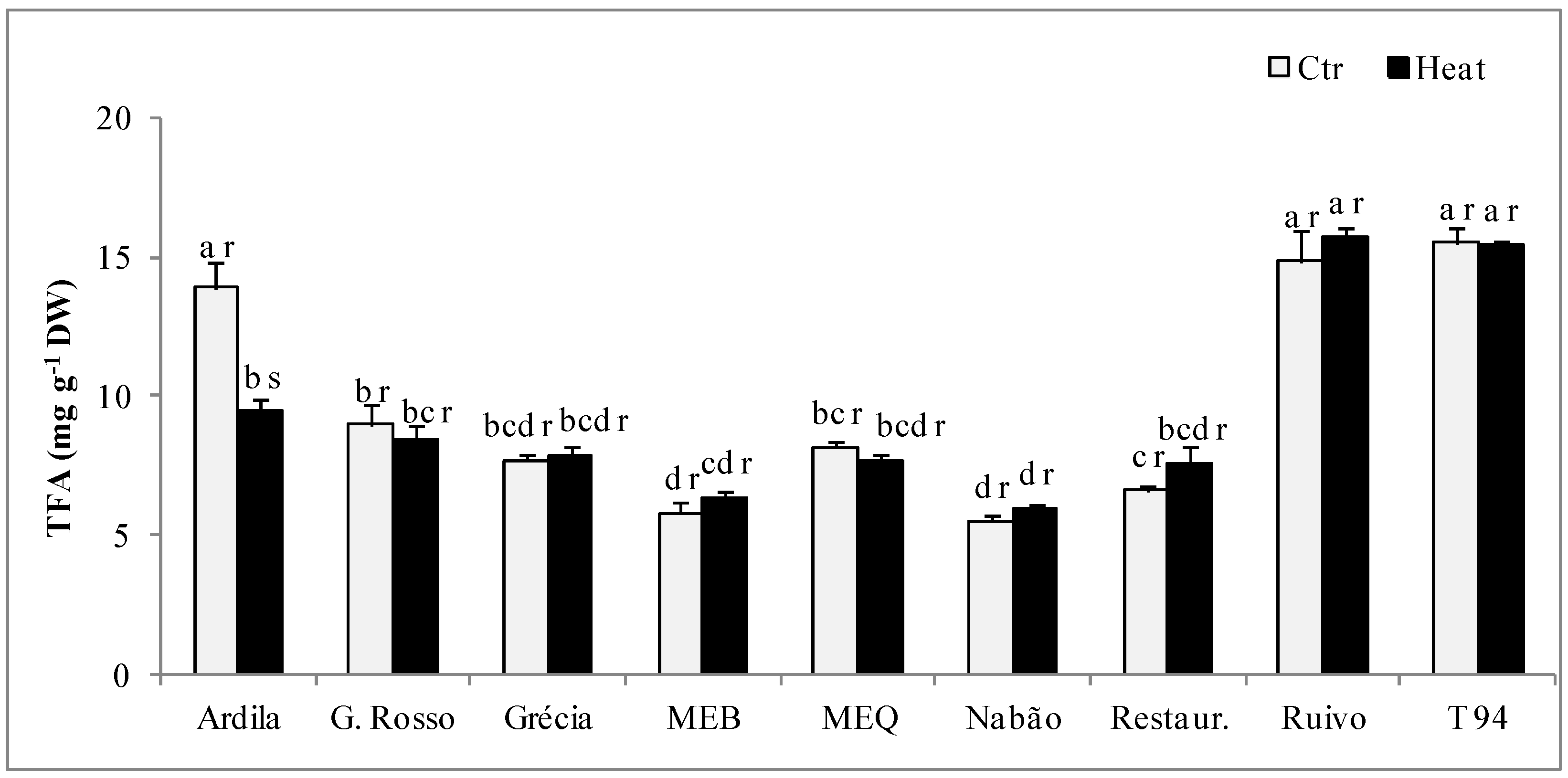
2.4. Lipid Profile
2.5. Color Measurements
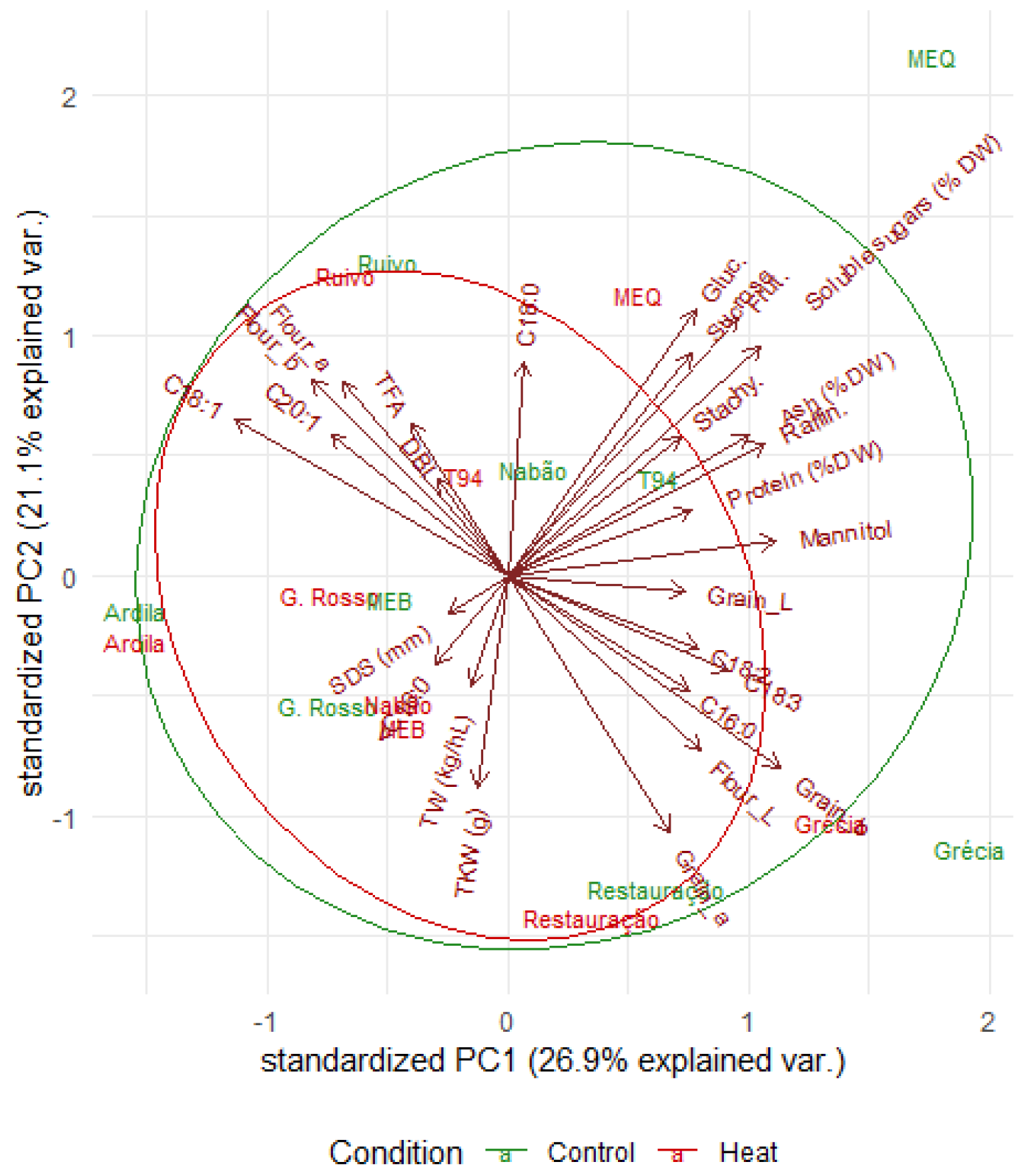
2.6. Principal Component Analysis of Grain Composition and Quality Parameters for Control and Heat Treatments
3. Discussion
4. Materials and Methods
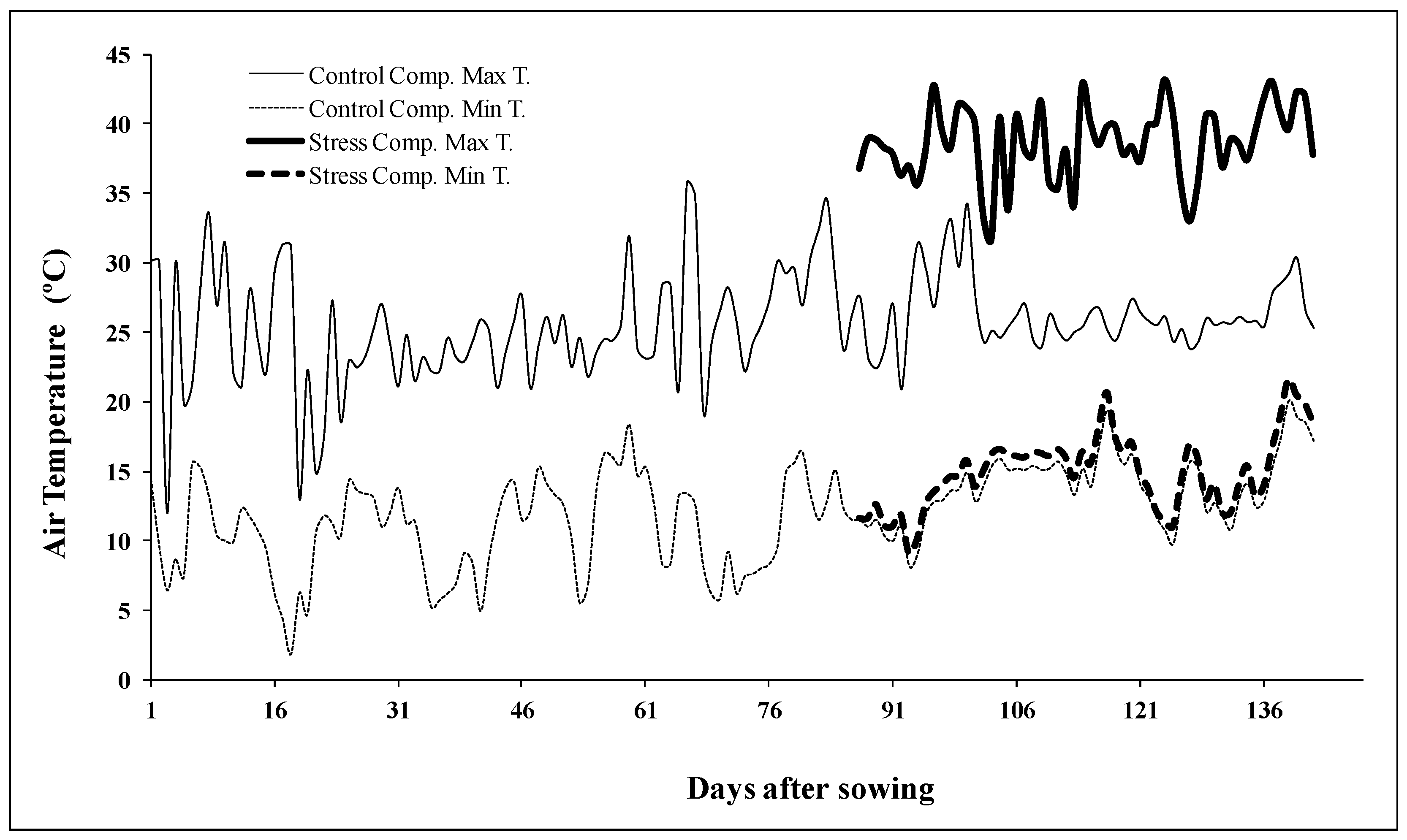
4.1. Plant Material and Experimental Design
4.2. Test Weight (TW), Thousand-Kernel Weight (TKW), and Obtention of Wholemeal Flour
4.3. Soluble Sugars Composition
4.4. Lipid Profile
4.5. Protein Content, SDS Sedimentation Test, and Ash Content
4.6. Color Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melis, S.; Delcour, J. Impact of wheat endogenous lipids on the quality of fresh bread: Key terms, concepts, and underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3715. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kumar, R.R.; Goswami, S.; Shamim, M.; Mishra, U.; Jain, M.; Singh, K.; Singh, J.P.; Dubey, K.; Singh, S.; Rai, G.K.; et al. Biochemical defense response: Characterizing the plasticity of source and sink in spring wheat under terminal heat stress. Front. Plant Sci. 2017, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, R.; Tiwari, R.; Kumar, R.; Gupta, V.K. Wheat Responses and Tolerance to Terminal Heat Stress: A Review. In Wheat Production in Changing Environments; Hasanuzzaman, M., Nahar, K., Hossain, M., Eds.; Springer Nature: Singapore, 2019; p. 149. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.; Welti, R. Alterations in wheat pollen lipidome during high day and night temperature stress. Plant Cell Environ. 2018, 41, 1749. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Bagulho, A.S.; Lidon, F.C. Ultrastructureand and biochemical traits of bread and durum wheat grains under heat stress. Braz. J. Plant. Physiol. 2008, 20, 323. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.; Mannino, G.; Fagoonee, S.; Luzza, F.; Musarella, C. Ancient wheats: Beneficial effects on insulin resistance. Minerva Medica. 2020, 112, 641. [Google Scholar] [CrossRef]
- Maçãs, B.; Gomes, M.C.; Dias, A.S.; Coutinho, J. The tolerance of durum wheat to high temperatures during grain filling. In Durum Wheat Improvement in the Mediterranean Region: New Challenges; Royo, C., Nachit, M.M., Di Fonzo, N., Araus, J.L., Eds.; Options Méditerranéennes: Série A. Séminaires Méditerranéens; n. 40; CIHEAM: Zaragoza, Spain, 2000; pp. 257–261. Available online: http://om.ciheam.org/article.php?IDPDF=600039 (accessed on 15 November 2021).
- Pinheiro, N.; Rita, C.; Almeida, A.S.; Coutinho, J.; Gomes, C.; Maçãs, B. Durum wheat breeding in Mediterranean environments—Influence of climatic variables on quality traits. Emir. J. Food Agric. 2013, 25, 962. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C. Evaluation of grain filling rate and duration in bread and durum wheat, under heat stress after anthesis. J. Agron. Crop Sci. 2009, 195, 137. [Google Scholar] [CrossRef]
- Dias, A.S.; Barreiro, M.G.; Campos, P.S.; Ramalho, J.C.; Lidon, F.C. Wheat cellular membrane thermotolerance under heat stress. J. Agron. Crop. Sci. 2010, 196, 100. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Semedo, J.N.; Pais, I.; Oliveira, M.; Passarinho, M.; Ramalho, J.C. Heat tolerance evaluation of Portuguese old bread wheat varieties. Emir. J. Food Agric. 2014, 26, 170. [Google Scholar] [CrossRef]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11. [Google Scholar] [CrossRef] [PubMed]
- Dunford, N.T. Germ oils from different sources. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 8, pp. 195–231. [Google Scholar]
- Jenner, C.F. Effects of exposure of wheat ears to high temperature on dry matter accumulation and carbohydrate metabolism in the grain of two cultivars. II. Carry-Over effects. Aust. J. Plant Phy. 1991, 18, 179. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extreme on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2019, 49, 184. [Google Scholar] [CrossRef]
- INSA 2021—Detalhe de Alimento, Farinha de Trigo Integral—Macroconstituintes. Available online: http://www2.insa.pt/sites/INSA/Portugues/AreasCientificas/AlimentNutricao/AplicacoesOnline/TabelaAlimentos/PesquisaOnline/Paginas/DetalheAlimento.aspx?ID=IS416 (accessed on 4 November 2021).
- Costa, M.G.; Souza, E.L.; Stanford, T.L.M.; Andrade, S.A.C. Technological quality of national and imported wheat grain and wheat flours. Ciênc. Tecnol. Aliment. 2008, 28, 220–225. [Google Scholar] [CrossRef]
- Struyf, N.; Van der Maelen, E.; Hemdane, S.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Bread dough and baker’s yeast: An uplifting synergy. Compr. Rev. Food Sci. Food Saf. 2017, 16, 850. [Google Scholar] [CrossRef]
- Barnes, P. Cereal Lipids. Nut. Food Sci. 1984, 84, 15. [Google Scholar] [CrossRef]
- Williams, M.; Shewry, P.R.; Lawlor, D.W.; Harwood, J.L. The effects of elevated temperature and atmospheric carbon dioxide concentration on the quality of grain lipids in wheat (Triticum aestivum L.) grown at two levels of nitrogen application. Plant Cell Environ. 1995, 18, 999. [Google Scholar] [CrossRef]
- Konopka, I.; Grabinski, J.; Skrajda, M.; Dabrowski, G.; Tanska, M.; Podolska, G. Variation of wheat grain lipid fraction and its antioxidative status under the impact of delayed sowing. J. Cereal Sci. 2017, 76, 56. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937. [Google Scholar] [CrossRef]
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and fatty acids in italian durum wheat (Triticum durum Desf.) cultivars. Foods 2019, 8, 223. [Google Scholar] [CrossRef]
- Beleggia, R.; Rau, D.; Laidò, G.; Platania, C.; Nigro, F.; Fragasso, M.; De Vita, P.; Scossa, F.; Fernie, A.R.; Nikoloski, Z.; et al. Evolutionary metabolomics reveals domestication-associated changes in tetraploid wheat kernels. Mol. Biol. Evol. 2016, 33, 1740. [Google Scholar] [CrossRef]
- Savatier, F. La Domestication du blé a Diminué sa Teneur en Bons Acides Gras. Pour la Science. 2016. Available online: https://www.pourlascience.fr/sd/agronomie/la-domestication-du-ble-a-diminue-sa-teneur-en-bons-acides-gras-12407.php (accessed on 13 December 2021).
- Guarienti, E.M. Qualidade industrial de trigo. Passo Fundo Embrapa-CNPT 1996, 27, 36. [Google Scholar]
- Williams, P.; El-Haramein, F.J.; Nakkouc, H.; Rihawi, S. Crop quality evaluation methods and guidelines. ICARDA 1988, 2, 145. [Google Scholar]
- Gwirtz, J.A.; Eustace, W.D.; Curran, S.P. Effect of Kernel Size and Test Weight on the Kansas State University (KSU) Pilot Mill. Assoc. Oper. Millers Bull. 1996, 6731–6741. [Google Scholar]
- Kesavan, M.; Song, J.T.; Seo, H.S. Seed size: A priority trait in cereal crops. Physiol. Plant. 2013, 147, 113. [Google Scholar] [CrossRef] [PubMed]
- Konopka, I. The effect of water stress on wheat kernel size, color and protein composition. Pol. J. Nat. Sci. 2007, 22, 157. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Jin, M.; Yu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Pre-Anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331. [Google Scholar] [CrossRef]
- Hurkman, W.; McCue, K.; Altenbach, S.; Korn, A.; Tanaka, C.; Kothari, K.; Johnson, E.; Bechtel, D.; Wilson, J.; Anderson, O. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873. [Google Scholar] [CrossRef]
- Weichert, N.; Saalbach, I.; Weichert, H.; Kohl, S.; Erban, A.; Kopka, J.; Hause, B.; Varshney, A.; Sreenivasulu, N.; Strickert, M.; et al. Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiol. 2010, 152, 698. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178. [Google Scholar] [CrossRef]
- Muir, J.G.; Gibson, P.R. The low FODMAP diet for treatment of irritable bowel syndrome and other gastrointestinal disorders. J. Gastroenterol. Hepatol. 2013, 9, 450–452. [Google Scholar]
- Corbellini, M.; Canevar, M.G.; Mazza, L.; Ciaffi, M.; Lafiandra, D.; Borghi, B. Effect of the duration and intensity of heat shock during grain filling on dry matter and protein accumulation, technological quality and protein composition in bread and durum wheat. Aust. J. Plant Physiol. 1997, 24, 245. [Google Scholar] [CrossRef]
- Bagulho, A.S.; Monho, A.; Almeida, A.S.; Costa, R.; Moreira, J.; Pais, I.; Scotti, P.; Coutinho, J.; Maçãs, B. Technological value of blends (bread wheat flour and durum wheat semolina) for bread manufacture. Emir. J. Food Agric. 2016, 28, 389. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Masure, H.G.; Delcour, J.A. Native and enzymatically modified wheat (Triticum aestivum L.) endogenous lipids in bread making: A focus on gas cell stabilization mechanisms. Food Chem. 2015, 172, 613. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.; Shewry, P.R.; et al. Distribution of lipids in the grain of wheat (cv. Hereward) determined by lipidomic analysis of milling and pearling fractions. J. Agric. Food Chem. 2015, 63, 10705. [Google Scholar] [CrossRef]
- Barthole, G.; Lepiniec, L.; Rogowsky, P.M.; Baud, S. Controlling lipid accumulation in cereal grains. Plant Sci. 2012, 185, 33. [Google Scholar] [CrossRef]
- Vasconcelos, J.C. Trigos Portugueses ou de há Muito Cultivados no País; Serviço de Publicidade e Biblioteca da Direcção Geral da Acção Social Agrária: Lisboa, Portugal, 1933; pp. 1–150. [Google Scholar]
- Medlicott, A.P.; Thompson, A.K. Analysis of sugar and organic acids in ripening mango fruits (Mangifera indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food. Agric. 1985, 36, 561. [Google Scholar] [CrossRef]
- Megahad, O.A.; Kinawy, O.S.E. Studies on the extraction of wheat germ oil by commercial hexane. Grasas y Aceites 2002, 53, 414. [Google Scholar] [CrossRef]
- Mazliak, P. Plant membrane lipids: Changes and alterations during aging and senescence. In Postharvest Physiology and Crop Preservation; Lieberman, M., Ed.; Plenum Press: New York, NY, USA, 1983; p. 123. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Dick, J.W.; Quick, J.S. A modified screening test for rapid estimation of gluten strength in early-generation durum wheat breeding lines. Cereal Chem. 1983, 60, 315–318. [Google Scholar]
- Instituto Português da Qualidade. IPQ NP Cereais e Derivados Determinação do Teor de Cinza a 900 °C; Instituto Português da Qualidade: Lisbon, Portugal, 1993. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 17 January 2022).
- Vu, V.Q. Ggbiplot: A Ggplot2 Based Biplot. R Package Version 0. Available online: http://github.com/vqv/ggbiplot (accessed on 14 January 2022).
| Genotypes | Treatment | TW (kg/hL) | TKW (g) |
|---|---|---|---|
| Ardila | Ctr | 77.89 ± 0.65 a r | 38.23 ± 0.61 b r |
| Heat | 75.00 ± 1.24 ab s | 33.57 ± 0.08 bc s | |
| G. Rosso | Ctr | 69.79 ± 0.63 cd r | 46.27 ± 0.84 a r |
| Heat | 71.00 ± 0.89 bc r | 46.57 ± 0.12 a r | |
| Grécia | Ctr | 74.13 ± 0.07 ab s | 33.57 ± 0.66 b r |
| Heat | 77.62 ± 0.70 a r | 36.50 ± 0.07 b r | |
| MEB | Ctr | 66.45 ± 1.13 d r | 25.01 ± 1.38 d s |
| Heat | 63.27 ± 0.48 d s | 37.95 ± 0.96 b r | |
| MEQ | Ctr | 69.00 ± 0.28 cd r | 32.13 ± 3.31 c r |
| Heat | 67.69 ± 0.95 c r | 23.70 ± 1.68 d s | |
| Nabão | Ctr | 71.86 ± 1.75 b s | 25.37 ± 0.35 d r |
| Heat | 77.66 ± 1.58 a r | 28.63 ± 0.28 cd r | |
| Restauração | Ctr | 71.51 ± 0.54 b s | 37.52 ± 0.78 bc r |
| Heat | 74.32 ± 0.44 ab r | 38.10 ± 0.21 b r | |
| Ruivo | Ctr | 72.89 ± 0.32 bc r | 18.45 ± 2.48 e r |
| Heat | 72.49 ± 0.14 b r | 18.92 ± 0.85 e r | |
| T94 | Ctr | 62.62 ± 1.15 d r | 18.87 ± 0.33 e s |
| Heat | 64.37 ± 0.16 d r | 25.74 ± 0.68 d r |
| Genotype | Treat. | Stachy. | Raffin. | Sucrose | Gluc. | Frut. | Mannitol | Total |
|---|---|---|---|---|---|---|---|---|
| Ardila | Ctr | 0.21 ± 0.00 c s | 0.68 ± 0.01 e r | 1.51 ± 0.03 cd r | 0.13 ± 0.01 e r | 0.09 ± 0.00 c r | 0.04 ± 0.00 cd r | 2.66 ± 0.05 e r |
| Heat | 0.24 ± 0.01 b r | 0.76 ± 0.01 e r | 1.44 ± 0.02 d r | 0.14 ± 0.00 c r | 0.07 ± 0.01 c s | 0.04 ± 0.00 b r | 2.70 ± 0.04 d r | |
| G. Rosso | Ctr | 0.30 ± 0.01 b r | 1.00 ± 0.02 cd r | 1.33 ± 0.02 d s | 0.17 ± 0.01 de r | 0.10 ± 0.01 c r | 0.03 ± 0.00 d s | 2.93 ± 0.04 de s |
| Heat | 0.30 ± 0.01 a r | 0.96 ± 0.02 cd s | 1.56 ± 0.04 cd r | 0.17 ± 0.01 bc r | 0.11 ± 0.01 c r | 0.05 ± 0.00 b r | 3.15 ± 0.07 c r | |
| Grécia | Ctr | 0.29 ± 0.01 b r | 1.10 ± 0.03 bc r | 1.85 ± 0.04 b r | 0.18 ± 0.01 cd s | 0.15 ± 0.02 bc r | 0.07 ± 0.00 a r | 3.65 ± 0.04 b r |
| Heat | 0.28 ± 0.00 ab r | 1.01 ± 0.03 b s | 1.86 ± 0.03 a r | 0.21 ± 0.02 ab r | 0.15 ± 0.02 ab r | 0.07 ± 0.00 a r | 3.58 ± 0.05 ab r | |
| MEB | Ctr | 0.22 ± 0.00 c r | 0.94 ± 0.05 d r | 1.64 ± 0.04 c r | 0.20 ± 0.01 cd r | 0.14 ± 0.02 bc r | 0.04 ± 0.00 cd r | 3.18 ± 0.07 cd r |
| Heat | 0.20 ± 0.02 c s | 0.92 ± 0.03 d r | 1.67 ± 0.06 bc r | 0.18 ± 0.01 bc s | 0.12 ± 0.02 bc r | 0.04 ± 0.00 b r | 3.13 ± 0.12 c r | |
| MEQ | Ctr | 0.42 ± 0.02 a r | 1.49 ± 0.08 a r | 2.22 ± 0.03 a r | 0.38 ± 0.01 a r | 0.32 ± 0.01 a r | 0.06 ± 0.00 ab r | 4.89 ± 0.09 a r |
| Heat | 0.32 ± 0.01 a s | 1.26 ± 0.03 a s | 1.82 ± 0.06 ab s | 0.21 ± 0.01 ab s | 0.20 ± 0.04 a s | 0.05 ± 0.00 b r | 3.86 ± 0.06 a r | |
| Nabão | Ctr | 0.24 ± 0.01 c r | 1.23 ± 0.03 b r | 1.82 ± 0.04 b r | 0.20 ± 0.01 cd r | 0.16 ± 0.01 b r | 0.05 ± 0.00 bc r | 3.71 ± 0.08 b r |
| Heat | 0.19 ± 0.00 c s | 1.15 ± 0.02 ab r | 1.58 ± 0.04 cd s | 0.14 ± 0.01 c s | 0.13 ± 0.01 b s | 0.05 ± 0.00 b r | 3.24 ± 0.06 c s | |
| Restauração | Ctr | 0.22 ± 0.01 c r | 1.09 ± 0.02 cd r | 1.48 ± 0.03 cd r | 0.15 ± 0.00 e r | 0.13 ± 0.00 bc r | 0.06 ± 0.01 ab r | 3.13 ± 0.06 cd r |
| Heat | 0.20 ± 0.01 cr | 1.12 ± 0.02 ab r | 1.46 ± 0.06 c r | 0.16 ± 0.01 c r | 0.12 ± 0.01 b r | 0.04 ± 0.00 b s | 3.10 ± 0.09 c r | |
| Ruivo | Ctr | 0.20 ± 0.01 c s | 0.93 ± 0.02 d s | 2.18 ± 0.04 a r | 0.22 ± 0.01 bc r | 0.17 ± 0.01 b r | 0.06 ± 0.00 ab r | 3.76 ± 0.03 b r |
| Heat | 0.25 ± 0.01 b r | 1.08 ± 0.02 bc r | 1.73 ± 0.04 abc s | 0.24 ± 0.01 a r | 0.18 ± 0.01 ab r | 0.05 ± 0.00 b r | 3.54 ± 0.05 b s | |
| T94 | Ctr | 0.22 ± 0.01 c r | 1.04 ± 0.02 cd r | 1.55 ± 0.02 c r | 0.26 ± 0.01 b r | 0.20 ± 0.01 b r | 0.06 ± 0.00 ab r | 3.33 ± 0.05 c r |
| Heat | 0.16 ± 0.01 c s | 0.96 ± 0.01 cd r | 1.58 ± 0.02 cd r | 0.24 ± 0.00 a r | 0.18 ± 0.00 ab r | 0.05 ± 0.00 b r | 3.18 ± 0.03 c r |
| Genotypes | Treatment | Protein (% DW) | SDS (mm) | Ash (% DW) |
|---|---|---|---|---|
| Ardila | Ctr | 17.20 ± 0.21 c s | 29.67 ± 0.33 c r | 2.16 ± 0.15 ab r |
| Heat | 18.23 ± 0.23 ab r | 30.00 ± 0.00 c r | 1.91 ± 0.13 b s | |
| G. Rosso | Ctr | 18.97 ± 12 b r | 53.00 ± 0.58 a r | 1.89 ± 0.13 b r |
| Heat | 17.37 ± 0.67 b s | 52.00 ± 1.00 a r | 1.96 ± 0.05 ab r | |
| Grécia | Ctr | 20.03 ± 0.07 ab r | 22.00 ± 2.65 d r | 2.37 ± 0.07 a r |
| Heat | 17.60 ± 0.17 b s | 24.33 ± 2.19 c r | 2.10 ± 0.04 ab s | |
| MEB | Ctr | 16.97 ± 0.03 c r | 45.00 ± 1.15 ab r | 2.13 ± 0.06 ab r |
| Heat | 16.65 ± 0.14 c r | 47.50 ± 0.29 ab r | 2.04 ± 0.04 ab r | |
| MEQ | Ctr | 19.37 ± 0.33 a r | 41.67 ± 1.86 bc r | 2.41 ± 0.08 a r |
| Heat | 19.20 ± 0.10 a r | 43.67 ± 1.86 b r | 2.33 ± 0.02 a r | |
| Nabão | Ctr | 16.97 ± 0.18 c r | 51.67 ± 0.88 a r | 2.15 ± 0.08 ab r |
| Heat | 16.87 ± 0.28 c r | 53.67 ± 1.45 a r | 1.81 ± 0.13 b s | |
| Restauração | Ctr | 17.20 ± 0.06 c r | 52.331.45 ± a r | 2.15 ± 0.04 ab r |
| Heat | 16.97 ± 0.12 c r | 52.00 ± 0.58 a r | 2.10 ± 0.09 ab r | |
| Ruivo | Ctr | 17.47 ± 0.09 c r | 34.00 ± 1.73 c r | 2.15 ± 0.05 ab r |
| Heat | 16.40 ± 0.25 c s | 31.33 ± 4.37 c r | 2.12 ± 0.08 ab r | |
| T94 | Ctr | 20.35 ± 0.14 a r | 36.50 ± 2.02 c s | 2.14 ± 0.07 ab r |
| Heat | 17.63 ± 0.15 b s | 43.00 ± 1.15 b r | 2.11 ± 0.04 ab r |
| Genotype | Treat. | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | <0.5% | DBI |
|---|---|---|---|---|---|---|---|---|---|
| mol (%) | |||||||||
| Ardila | Ctr | 18.27 ± 0.13 b r | 0.96 ± 0.02 bc r | 15.80 ± 0.05 ab r | 57.83 ± 0.17 abc r | 4.06 ± 0.07 b r | 0.79 ± 0.01 b r | 2.25 ± 0.14 a r | 6.86 ± 0.09 a r |
| Heat | 19.80 ± 0.64 a r | 0.93 ± 0.03 c r | 15.85 ± 0.12 b r | 56.40 ± 0.44 ab s | 3.86 ± 0.04 c r | 0.79 ± 0.01 a r | 2.36 ± 0.15 b r | 6.32 ± 0.22 a r | |
| G. Rosso | Ctr | 21.47 ± 0.89 ab r | 1.06 ± 0.05 a s | 14.20 ± 0.28 cd s | 56.36 ± 0.82 bc r | 3.84 ± 0.06 b r | 0.63 ± 0.06 c r | 2.44 ± 0.39 a s | 5.88 ± 0.30 a r |
| Heat | 19.32 ± 0.49 a r | 1.26 ± 0.08 b r | 15.54 ± 0.23 bc r | 55.62 ± 0.62 b s | 3.70 ± 0.24 c r | 0.66 ± 0.04 bc r | 3.90 ± 0.40 a r | 5.95 ± 0.20 a r | |
| Grécia | Ctr | 23.20 ± 1.11 a r | 0.86 ± 0.03 c r | 11.16 ± 0.53 f s | 57.11 ± 1.41 abc s | 4.76 ± 0.21 a r | 0.62 ± 0.06 c r | 2.30 ± 0.20 a r | 5.58 ± 0.37 a s |
| Heat | 19.63 ± 0.56 a s | 0.97 ± 0.01 c r | 12.20 ± 0.17 e r | 59.54 ± 0.63 a r | 4.53 ± 0.06 a r | 0.61 ± 0.04 c r | 2.52 ± 0.47 b r | 6.53 ± 0.28 a r | |
| MEB | Ctr | 20.70 ± 1.00 ab r | 1.06 ± 0.06 a r | 14.74 ± 0.26 bc r | 56.30 ± 0.80 bc r | 3.82 ± 0.07 b r | 0.87 ± 0.05 ab r | 2.51 ± 0.21 a r | 6.02 ± 0.37 a r |
| Heat | 20.96 ± 1.13 a r | 0.96 ± 0.02 c r | 14.36 ± 0.22 bcd r | 56.91 ± 0.61 ab r | 3.88 ± 0.05 c r | 0.85 ± 0.06 ab r | 2.07 ± 0.27 bc r | 6.07 ± 0.30 a r | |
| MEQ | Ctr | 20.70 ± 1.07 ab r | 1.23 ± 0.02 a s | 13.27 ± 0.24 cde r | 58.40 ± 0.74 abc r | 4.12 ± 0.07 b r | 0.59 ± 0.04 c r | 1.69 ± 0.15 ab r | 6.27 ± 0.34 a r |
| Heat | 19.60 ± 1.03 a r | 1.53 ± 0.03 a r | 14.14 ± 0.22 cd r | 58.59 ± 0.75 ab r | 3.98 ± 0.07 b r | 0.62 ± 0.05 c r | 1.50 ± 0.15 c r | 6.56 ± 0.35 a r | |
| Nabão | Ctr | 21.00 ± 0.90 ab r | 1.10 ± 0.04 ab r | 13.03 ± 0.19 de r | 57.81 ± 0.64 abc r | 4.62 ± 0.08 a r | 0.89 ± 0.07 ab r | 1.55 ± 0.08 ab r | 6.30 ± 0.33 a r |
| Heat | 20.52 ± 1.15 a r | 1.00 ± 0.02 c r | 13.43 ± 0.22 de r | 58.38 ± 0.78 ab r | 4.40 ± 0.07 a r | 0.92 ± 0.07 a r | 1.35 ± 0.06 c r | 6.58 ± 0.41 a r | |
| Restauração | Ctr | 20.01 ± 0.86 ab r | 0.95 ± 0.03 bc r | 13.01 ± 0.35 de r | 60.08 ± 0.87 a r | 4.13 ± 0.06 b r | 0.57 ± 0.04 c r | 1.26 ± 0.11 b r | 6.81 ± 0.35 a r |
| Heat | 21.87 ± 1.13 a r | 0.96 ± 0.04 c r | 13.01 ± 0.68 de r | 58.05 ± 1.14 ab r | 4.35 ± 0.20 ab r | 0.60 ± 0.06 c r | 1.16 ± 0.09 c r | 6.17 ± 0.40 a r | |
| Ruivo | Ctr | 20.07 ± 0.87 ab r | 1.06 ± 0.08 a r | 16.92 ± 0.72 a r | 55.69 ± 0.33 c r | 3.97 ± 0.06 b r | 1.09 ± 0.06 a r | 1.20 ± 0.04 b r | 6.56 ± 0.33 a r |
| Heat | 19.68 ± 1.12 a r | 1.09 ± 0.02 bc r | 17.75 ± 0.27 a r | 55.46 ± 0.75 b r | 3.73 ± 0.06 c r | 1.02 ± 0.09 a r | 1.28 ± 0.08 c r | 6.66 ± 0.42 a r | |
| T94 | Ctr | 20.59 ± 0.64 ab r | 0.89 ± 0.02 c s | 12.56 ± 0.12 ef s | 59.35 ± 0.44 ab r | 4.55 ± 0.05 a r | 0.89 ± 0.04 ab r | 1.18 ± 0.02 b r | 6.63 ± 0.23 a r |
| Heat | 19.88 ± 0.81 a r | 1.01 ± 0.02 c r | 13.57 ± 0.19 de r | 59.01 ± 0.54 ab r | 4.44 ± 0.05 a r | 0.90 ± 0.05 ab r | 1.19 ± 0.04 c r | 6.82 ± 0.29 a r | |
| Grain | Flour | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | Treat. | L* | a* | b* | L* | a* | b* |
| Ardila | Ctr | 51.93 ± 0.66 c r | 5.88 ± 0.19 a r | 14.43 ± 0.07 d r | 80.21 ± 0.17 e r | 2.77 ± 0.10 a r | 12.52 ± 0.12 a r |
| Heat | 52.37 ± 0.16 e r | 5.81 ± 0.16 a r | 14.66 ± 0.16 d r | 79.38 ± 0.11 e r | 2.91 ± 0.03 a r | 12.61 ± 0.12 ab r | |
| G. Rosso | Ctr | 55.80 ± 0.37 a s | 5.76 ± 0.19 a r | 16.18 ± 0.11 cd r | 84.59 ± 0.15 c r | 3.00 ± 0.04 a r | 12.78 ± 0.04 a r |
| Heat | 59.88 ± 0.81 a r | 5.47 ± 0.14 a r | 16.33 ± 0.32 c r | 85.61 ± 0.46 b r | 3.06 ± 0.08 a r | 12.88 ± 0.07 a r | |
| Grécia | Ctr | 57.02 ± 0.14 a r | 6.65 ± 0.11 a r | 20.32 ± 0.44 a r | 87.78 ± 0.46 a s | 1.51 ± 0.07 a r | 9.10 ± 0.21 b r |
| Heat | 57.73 ± 0.36 b r | 6.39 ± 0.18 a r | 20.33 ± 0.16 a r | 90.99 ± 3.17 a r | 1.58 ± 0.06 a r | 9.14 ± 0.15 c r | |
| MEB | Ctr | 53.95 ± 0.48 b r | 5.97 ± 0.20 a r | 16.02 ± 0.57 cd r | 84.43 ± 0.27 c r | 2.36 ± 0.09 a r | 10.66 ± 0.17 ab r |
| Heat | 54.10 ± 0.74 d r | 5.65 ± 0.13 a r | 15.89 ± 0.57 cd r | 85.52 ± 0.49 bc r | 1.39 ± 0.12 a r | 9.97 ± 0.18 c r | |
| MEQ | Ctr | 56.32 ± 0.29 a r | 5.60 ± 0.12 a r | 16.78 ± 0.27 c r | 81.86 ± 0.46 de r | 2.86 ± 0.15 a r | 12.30 ± 0.38 ab r |
| Heat | 54.78 ± 0.56 cd s | 6.11 ± 0.14 a r | 16.22 ± 0.48 cd r | 82.23 ± 0.34 d r | 2.68 ± 0.10 a r | 11.89 ± 0.11 ab r | |
| Nabão | Ctr | 54.14 ± 0.52 b r | 5.57 ± 0.08 a r | 15.14 ± 0.21 d r | 81.30 ± 0.65 de s | 2.56 ± 0.14 a r | 11.71 ± 0.77 ab r |
| Heat | 53.12 ± 0.45 de r | 5.71 ± 0.16 a r | 15.25 ± 0.12 cd r | 83.24 ± 0.08 cd r | 2.02 ± 0.03 a r | 10.41 ± 0.13 bc r | |
| Restauração | Ctr | 55.52 ± 0.47 a r | 7.01 ± 0.27 a r | 18.53 ± 0.37 ab r | 87.02 ± 0.57 ab r | 1.63 ± 0.14 a r | 9.19 ± 0.30 b r |
| Heat | 55.32 ± 0.42 cd r | 6.89 ± 0.22 a r | 18.48 ± 0.35 ab r | 83.74 ± 0.41 b r | 1.59 ± 0.11 a r | 9.16 ± 0.26 c r | |
| Ruivo | Ctr | 53.79 ± 0.70 b s | 5.22 ± 0.14 a r | 14.68 ± 0.48 d r | 83.14 ± 0.25 cd r | 2.36 ± 0.04 a r | 11.05 ± 0.11 ab r |
| Heat | 56.34 ± 0.56 bc r | 5.02 ± 0.26 a r | 15.41 ± 0.29 cd r | 83.47 ± 0.50 bc r | 2.20 ± 0.16 a r | 11.18 ± 0.49 a r | |
| T94 | Ctr | 55.60 ± 0.65 a r | 5.56 ± 0.12 a r | 15.72 ± 0.27 cd r | 85.26 ± 0.48 bc r | 1.92 ± 0.14 a r | 10.13 ± 0.19 b s |
| Heat | 55.68 ± 0.47 cd r | 5.59 ± 0.12 a r | 15.46 ± 0.08 cd r | 84.82 ± 0.22 bc r | 2.43 ± 0.07 a r | 11.63 ± 0.06 a r | |
| Principal Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Proportion of variance | 0.2694 | 0.2109 | 0.1508 |
| Cumulative proportion | 0.2694 | 0.4803 | 0.6311 |
| Standard deviation | 2.697 | 2.3861 | 2.0181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti-Campos, P.; Oliveira, K.; Pais, I.P.; Bagulho, A.S.; Semedo, J.N.; Serra, O.; Simões, F.; Lidon, F.C.; Coutinho, J.; Maçãs, B. Grain Composition and Quality in Portuguese Triticum aestivum Germplasm Subjected to Heat Stress after Anthesis. Plants 2022, 11, 365. https://doi.org/10.3390/plants11030365
Scotti-Campos P, Oliveira K, Pais IP, Bagulho AS, Semedo JN, Serra O, Simões F, Lidon FC, Coutinho J, Maçãs B. Grain Composition and Quality in Portuguese Triticum aestivum Germplasm Subjected to Heat Stress after Anthesis. Plants. 2022; 11(3):365. https://doi.org/10.3390/plants11030365
Chicago/Turabian StyleScotti-Campos, Paula, Karliana Oliveira, Isabel P. Pais, Ana Sofia Bagulho, José N. Semedo, Octávio Serra, Fernanda Simões, Fernando C. Lidon, José Coutinho, and Benvindo Maçãs. 2022. "Grain Composition and Quality in Portuguese Triticum aestivum Germplasm Subjected to Heat Stress after Anthesis" Plants 11, no. 3: 365. https://doi.org/10.3390/plants11030365
APA StyleScotti-Campos, P., Oliveira, K., Pais, I. P., Bagulho, A. S., Semedo, J. N., Serra, O., Simões, F., Lidon, F. C., Coutinho, J., & Maçãs, B. (2022). Grain Composition and Quality in Portuguese Triticum aestivum Germplasm Subjected to Heat Stress after Anthesis. Plants, 11(3), 365. https://doi.org/10.3390/plants11030365









