Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato
Abstract
1. Introduction
2. Results
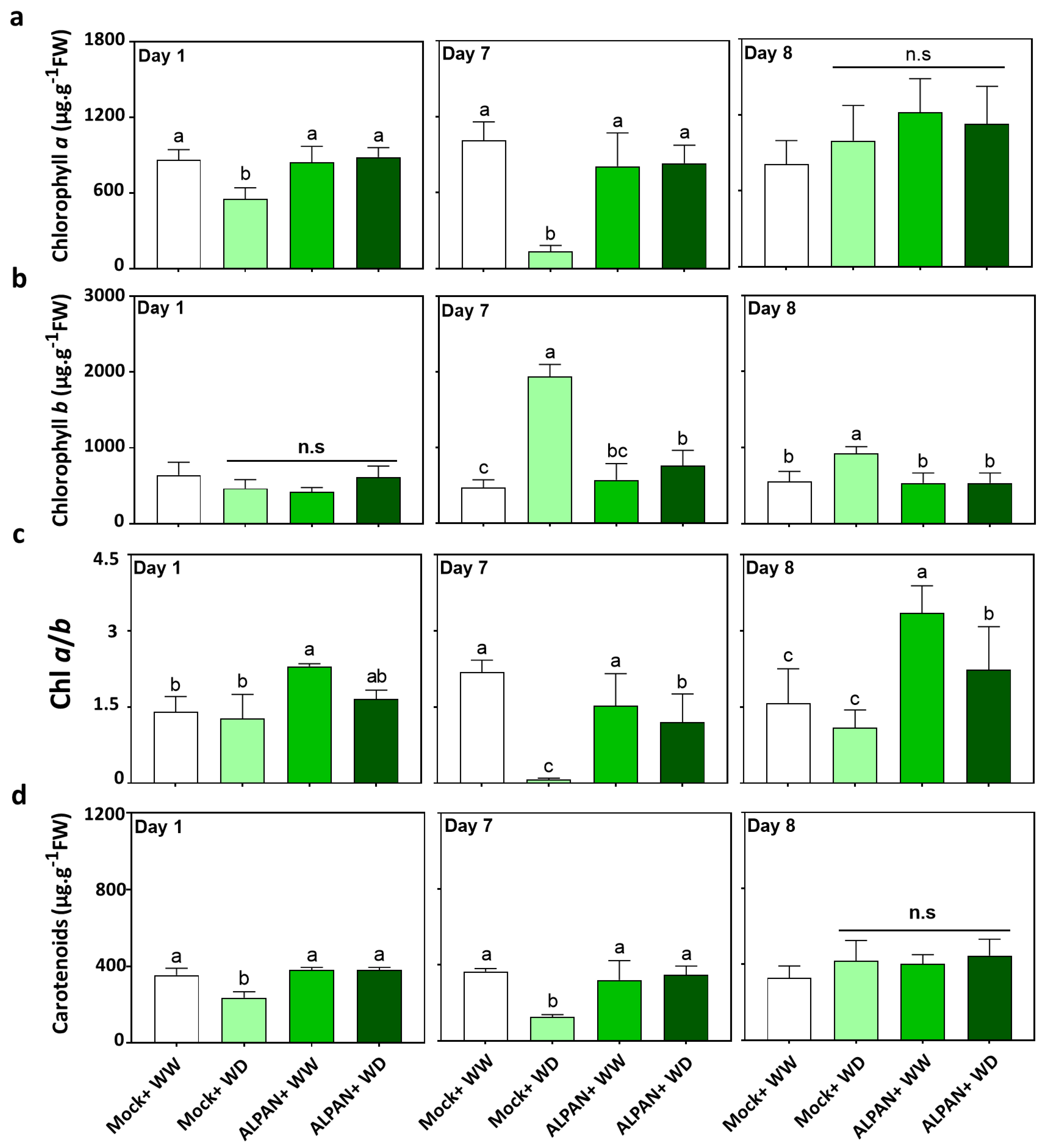
2.1. ALPAN® Treatment Preserved Photosynthesis in Tomato Seedlings under Water Deficit Condition
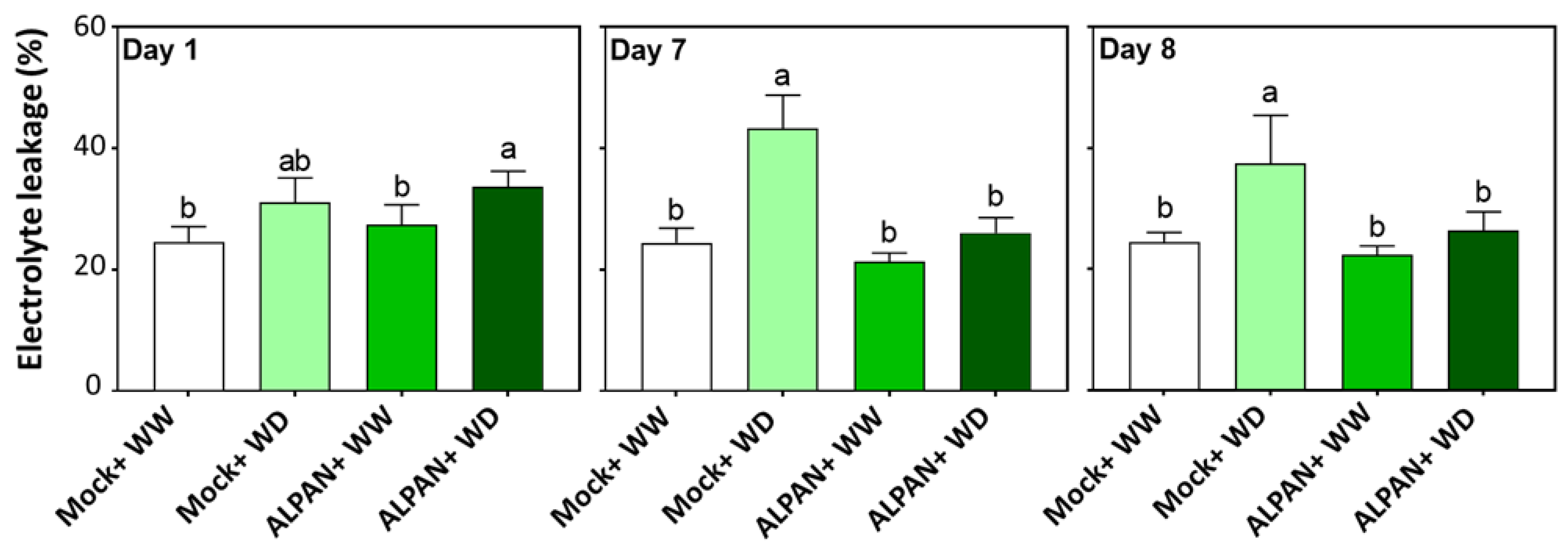
2.2. ALPAN® Treatment Alleviated Cell Damage and Protected Cellular Membranes in Tomato Seedlings under Water Deficit Condition
2.3. ALPAN® Treatment Modulated Proline Levels in Tomato Seedlings under Water Deficit Condition
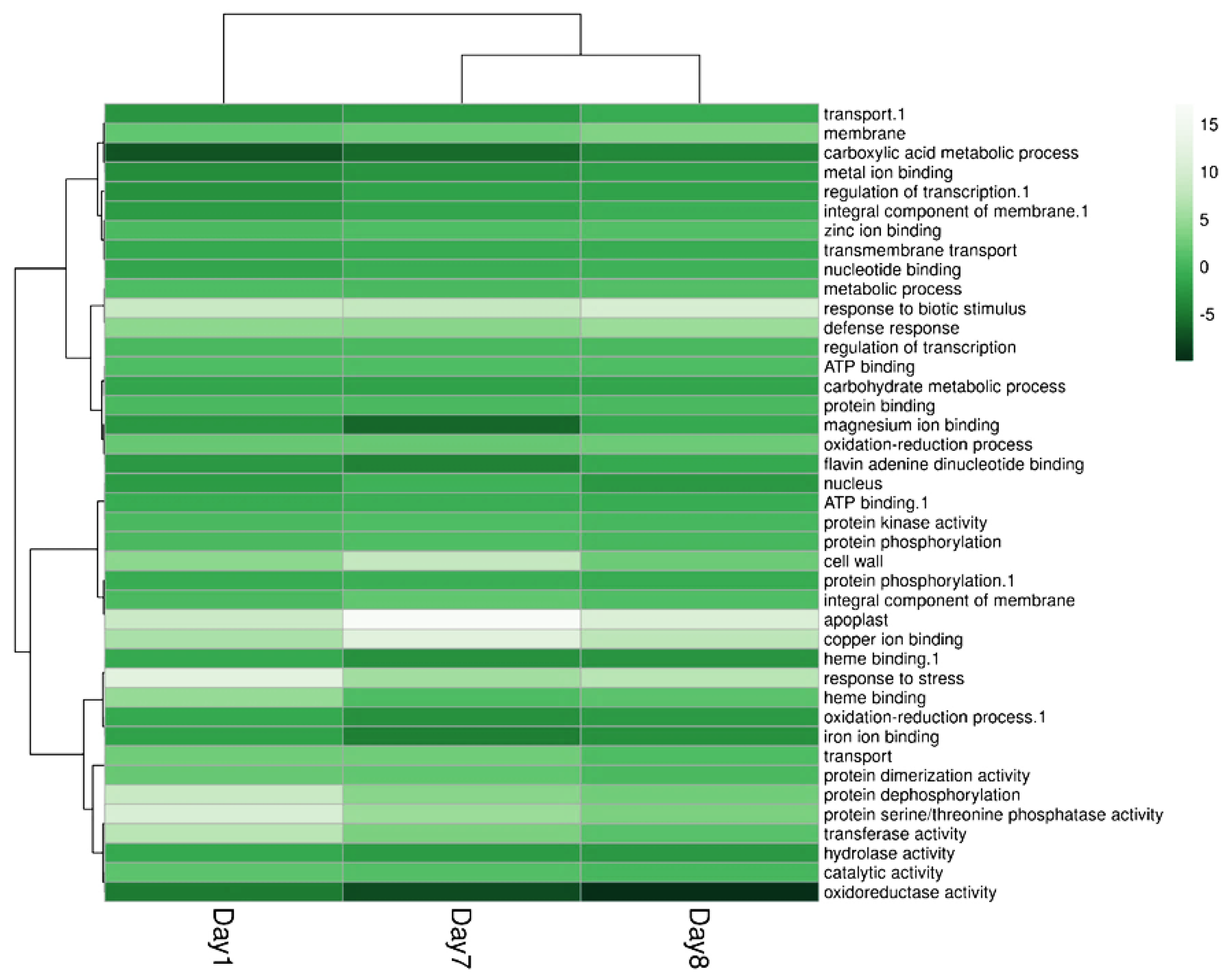
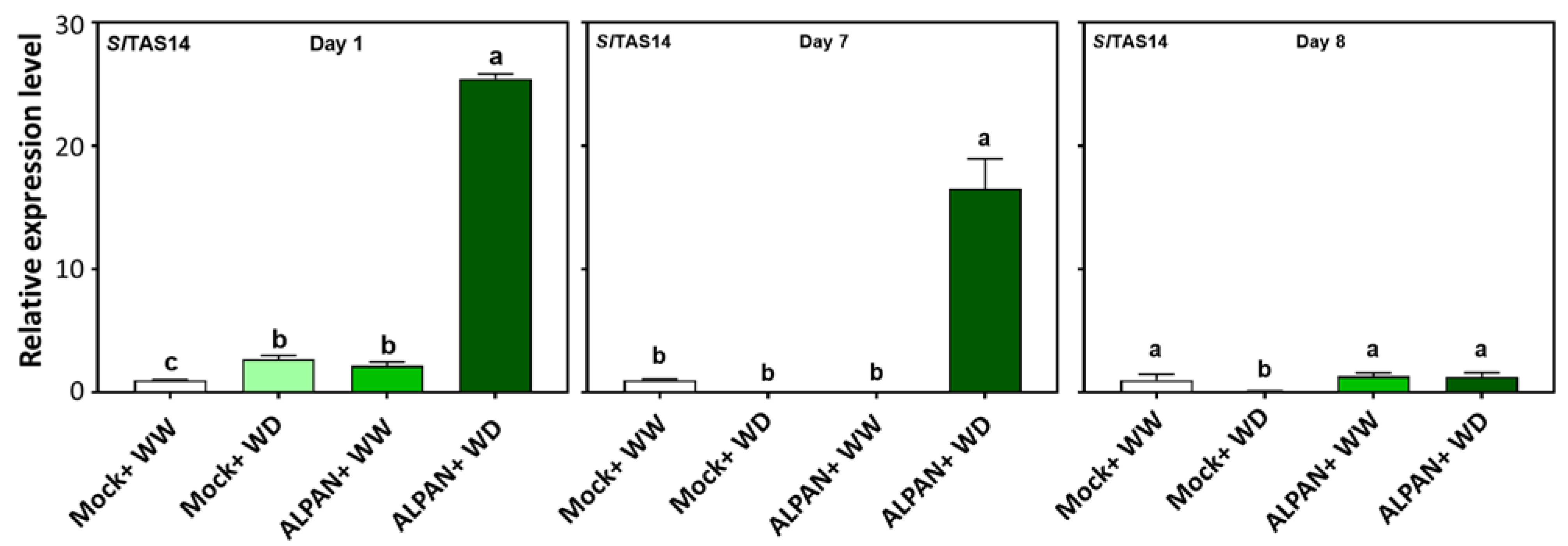
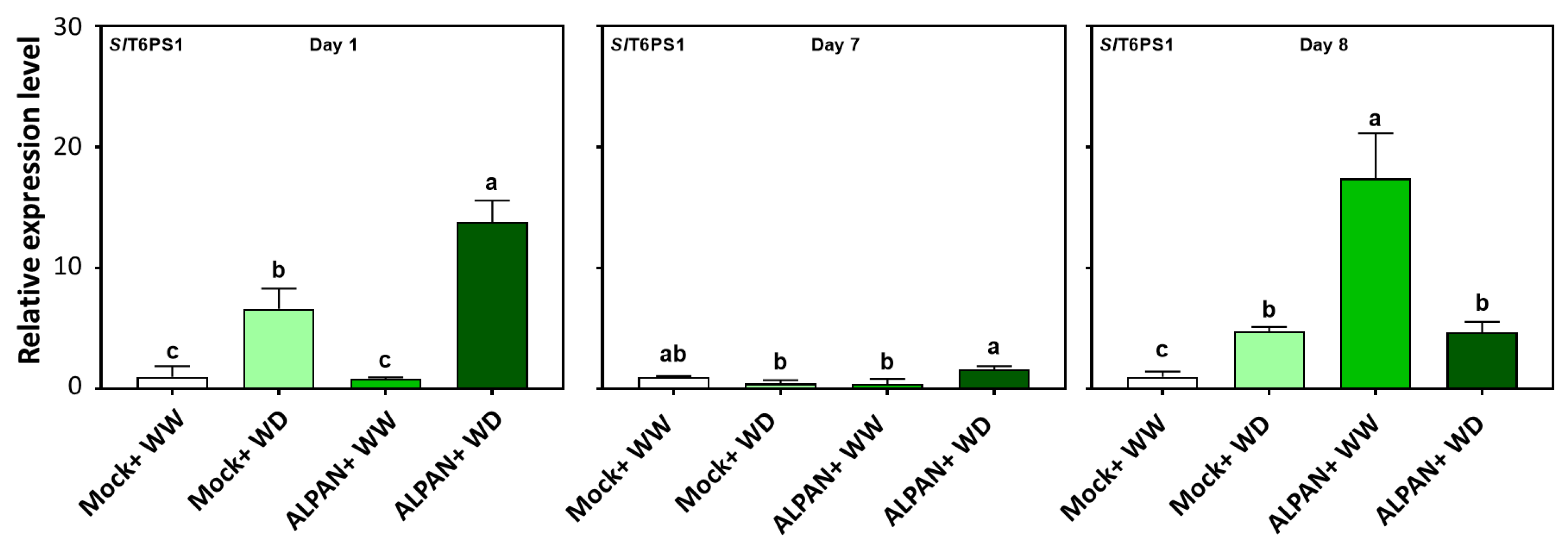
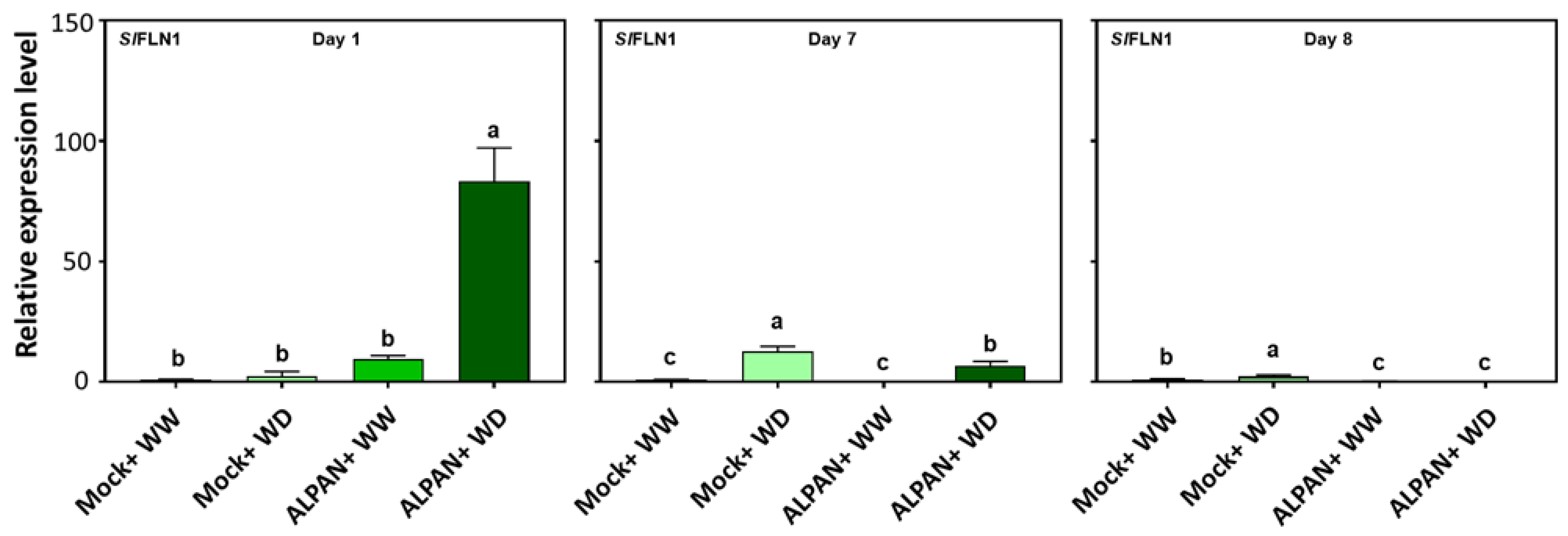
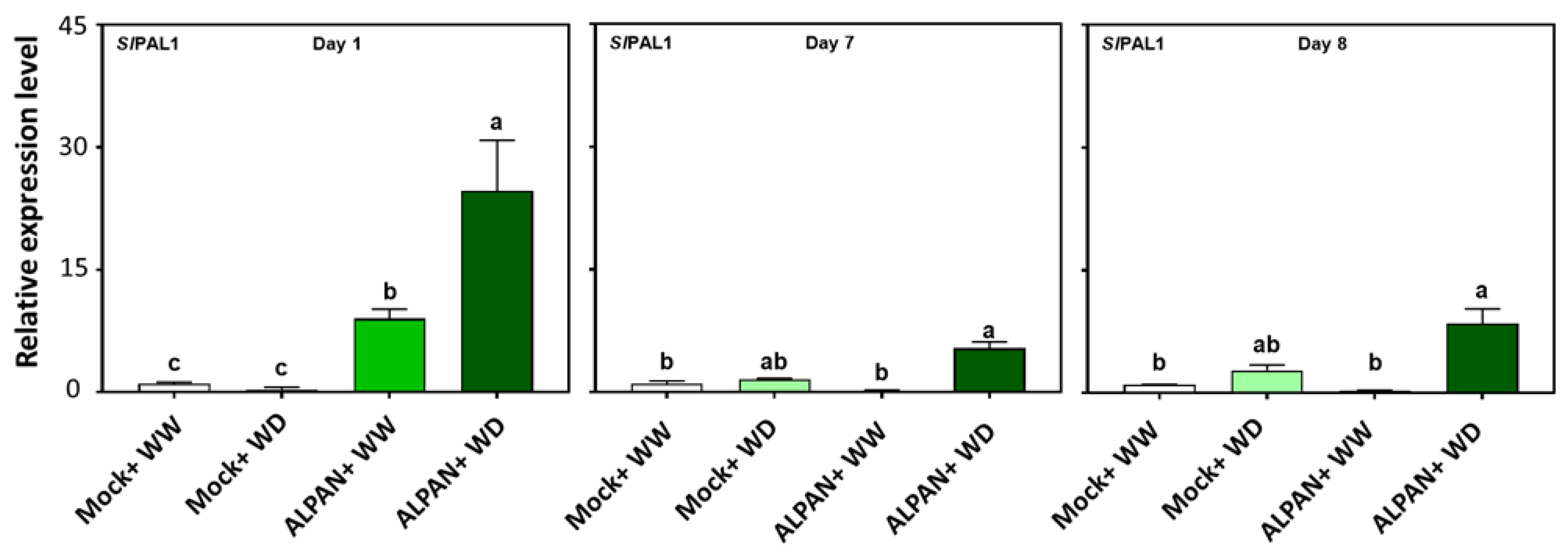
2.4. ALPAN® Treatment Altered the Transcriptome in Tomato Seedlings under Water Deficit Condition
3. Discussion
4. Materials and Methods
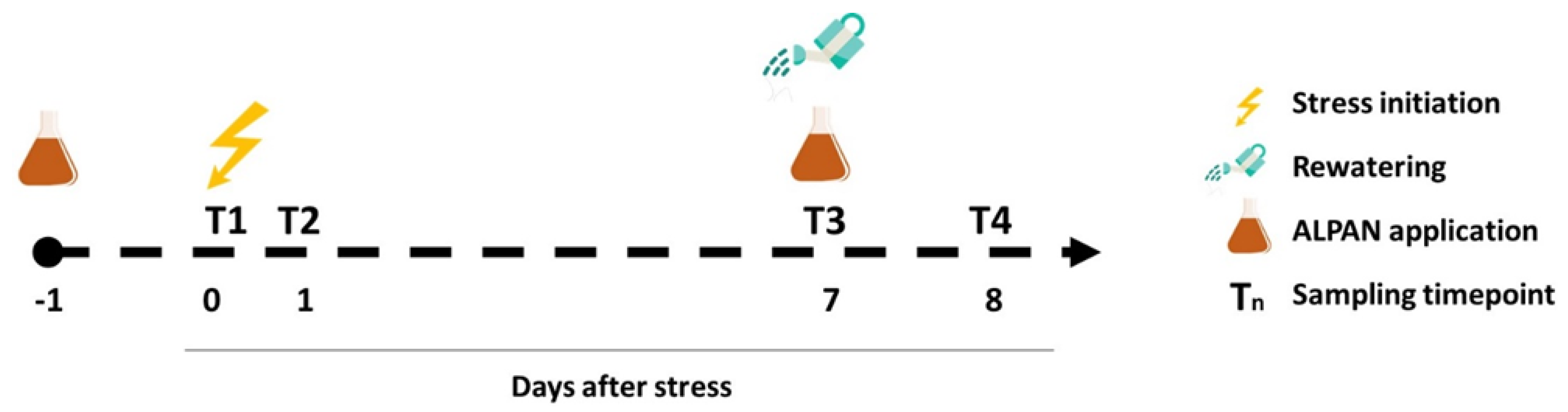
4.1. Plant Material, Growing Conditions, and Experimental Setup
4.2. Chlorophyll and Carotenoids Quantification
4.3. Membrane Integrity Assessment by Conductivity of Electrolyte Leakage
4.4. Lipid Peroxidation by Quantification of Malondialdehyde Levels
4.5. Cellular Proline Quantification
4.6. RNA Isolation, cDNA Library Construction, and Gene Expression Analysis
4.7. RNA-Seq Analysis and Library Construction
4.8. Statistical Analysis
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); United Nations: New York, NY, USA, 2019. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- FAO. The State of Food and Agriculture 2019: Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2020. [Google Scholar]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Keller, F.; Ludlow, M.M. Carbohydrate Metabolism in Drought-Stressed Leaves of Pigeonpea (Cajanus cajan). J. Exp. Bot. 1993, 44, 1351–1359. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Guerra, D.; Crosatti, C.; Khoshro, H.H.; Mastrangelo, A.M.; Mica, E.; Mazzucotelli, E. Post-transcriptional and post-translational regulations of drought and heat response in plants: A spider’s web of mechanisms. Front. Plant Sci. 2015, 6, 57. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N. Role of Plant Nutrients in Plant Growth and Physiology. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapor, 2018; pp. 51–93. [Google Scholar]
- Tavanti, T.R.; de Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.D.S.; da Silva, R.M.; dos Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Shahrajabian, M.; Chaski, C.; Polyzos, N.; Petropoulos, S. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Parra, M.D.M.; del Pozo, O.; Luna, R.; Godoy, J.A.; Pintor-Toro, J. Structure of the dehydrin tas 14 gene of tomato and its developmental and environmental regulation in transgenic tobacco. Plant Mol. Biol. 1996, 32, 453–460. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Mayor, A.; Pineda, B.; Garcia-Abellán, J.O.; Antón, T.; Garcia-Sogo, B.; Sánchez-Bel, P.; Flores, F.B.; Atarés, A.; Angosto, T.; Pintor-Toro, J.A.; et al. Overexpression of dehydrin tas14 gene improves the osmotic stress imposed by drought and salinity in tomato. J. Plant Physiol. 2012, 169, 459–468. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Micallef, M.C.; Marillia, E.-F.; Taylor, D.C.; Grodzinski, B.; Micallef, B.J. Increased mtPDH Activity Through Antisense Inhibition of Mitochondrial Pyruvate Dehydrogenase Kinase Enhances Inflorescence Initiation, and Inflorescence Growth and Harvest Index at Elevated CO2 in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-Phosphate: Connecting Plant Metabolism and Development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Arsova, B.; Hoja, U.; Wimmelbacher, M.; Greiner, E.; Üstün, S.; Melzer, M.; Petersen, K.; Lein, W.; Börnke, F. Plastidial Thioredoxin z Interacts with Two Fructokinase-Like Proteins in a Thiol-Dependent Manner: Evidence for an Essential Role in Chloroplast Development in Arabidopsis and Nicotiana benthamiana. Plant Cell 2010, 22, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Moles, T.M.; Mariotti, L.; De Pedro, L.F.; Guglielminetti, L.; Picciarelli, P.; Scartazza, A. Drought induced changes of leaf-to-root relationships in two tomato genotypes. Plant Physiol. Biochem. 2018, 128, 24–31. [Google Scholar] [CrossRef]
- Rodrigues, J.; Inzé, D.; Nelissen, H.; Saibo, N.J. Source–Sink Regulation in Crops under Water Deficit. Trends Plant Sci. 2019, 24, 652–663. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell Membrane Stability as a Measure of Drought and Heat Tolerance in Wheat1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Lee, B.-H.; Zhu, J.-K. Phenotypic Analysis of Arabidopsis Mutants: Electrolyte Leakage after Freezing Stress. Cold Spring Harb. Protoc. 2010, 2010, 4970. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.T.O.; Allen, R.L. Dependence of chloroplast pigment synthesis on protein synthesis: Effect of actidione. Biochem. Biophys. Res. Commun. 1965, 21, 523–530. [Google Scholar] [CrossRef]
- Pétriacq, P.; Ton, J.; Patrit, O.; Tcherkez, G.; Gakière, B. NAD Acts as an Integral Regulator of Multiple Defense Layers. Plant Physiol. 2016, 172, 1465–1479. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lacerda, A.L.M.; Fonseca, L.N.; Blawid, R.; Boiteux, L.S.; Ribeiro, S.G.; Brasileiro, A.C.M. Reference Gene Selection for qPCR Analysis in Tomato-Bipartite Begomovirus Interaction and Validation in Additional Tomato-Virus Pathosystems. PLoS ONE 2015, 10, e0136820. [Google Scholar] [CrossRef]



| Locus | Gene | Froward Primer | Reverse Primer | Product Size |
|---|---|---|---|---|
| Solyc01g108020 | SlEF1α | TGGAATTGGAACTGTCCCCG | GAGCTTCGTGGTGCATCTCT | 125 |
| Solyc02g084850 | SlTAS14 | TCCCTACTCCCTGAACCTCC | CAGTCTTGCGCATTTGGTCT | 144 |
| Solyc09g011850 | SlFLN1 | AGTGCAAACAAGGGCTGTCA | TCGGAGCTAAGCAGTGAATCC | 135 |
| Solyc12g009400 | SlPDH1 | TGCTGCACGAGATCCTATCAG | TCATCCGCAAATTCGACAGC | 132 |
| Solyc10g086180 | SlPAL6 | TTTCCAGGGCACTCCCATTG | GTCGTTGACAAGCTCGGAGA | 103 |
| Solyc07g006500 | SlT6PS1 | GGTGTTTGCTCTGTTTATGGTGT | CAGAACAGTCATCAAGATTAAGCAG | 146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamedeh, H.; Antoni, S.; Cocciaglia, L.; Ciccolini, V. Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants 2022, 11, 586. https://doi.org/10.3390/plants11050586
Hamedeh H, Antoni S, Cocciaglia L, Ciccolini V. Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants. 2022; 11(5):586. https://doi.org/10.3390/plants11050586
Chicago/Turabian StyleHamedeh, Haytham, Shaula Antoni, Lorenzo Cocciaglia, and Valentina Ciccolini. 2022. "Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato" Plants 11, no. 5: 586. https://doi.org/10.3390/plants11050586
APA StyleHamedeh, H., Antoni, S., Cocciaglia, L., & Ciccolini, V. (2022). Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants, 11(5), 586. https://doi.org/10.3390/plants11050586






