Sprouts and Microgreens—Novel Food Sources for Healthy Diets
Abstract
:1. Introduction
2. Crops Commonly Used for Microscale Vegetable Production

- Seed activation through imbibition, favourable temperature, oxygen, light, or darkness
- Enhanced respiration and metabolic activities
- Enzymes mobilize stored seed reserves and convert starch to sugar
- Hydrolysis of storage proteins, release of essential amino acids
- Accumulation of phenolic compounds with antioxidant ability
- Accumulation of vitamins (C, folate, thiamin, pyridoxin, tocopherols, niacin, etc.).
- Phytate, oxalate, and tannin degradation, leading to enhanced palatability, improved bioaccessibility of iron and calcium, and enhanced digestibility of proteins.

- Photosynthetic activity in microgreens further enhances vitamin C, phylloquinone, and tocopherol accumulation compared to sprouts
- Accumulation of carotenoids is often higher than in mature vegetables
- Increased accumulation of chlorophyll and phenolic compounds with antioxidant ability, compared to sprouts
- Often higher content of macro- and micronutrients and lower content of nitrate in microgreens compared to the adult growth stage
- Biofortification with specific elements (iodine, iron, zinc, selenium) made easy in hydroponic systems
- Microgreens are consumed raw, hence thermolabile ascorbic acid content can be fully utilized, unlike in cooked mature vegetables.
2.1. Bioactive Composition and Potential Health Effects of Brassica Microscale Vegetables
2.2. Consumer Acceptance of Sprouts and Microgreens and Nutritional Profile of Microscale Vegetables
3. Underutilized Species with Potential for Microscale Vegetable Production to Enhance Nutrition Security
| Family | Species | Type of Plant Material | Secondary Metabolites | References |
|---|---|---|---|---|
| Amaranthaceae | Amaranthus caudatus (foxtail amaranth) | old varieties | High total phenolics, total betalain, and total flavonoid content | [83] |
| Amaranthus cruentus (red amaranth) | old varieties | Amaranth sprouts are a good source of anthocyanins and total phenolics with high antioxidant activity | [83,84] | |
| Amaranthus hypochondriacus (Prince’s feather) | ornamental | Good source of antioxidants, especially the leaves | [83] | |
| Amaranthus tricolor (edible amaranth) | landrace | A genebank accession (VI044470) consistently received the highest ratings for appearance, texture, taste, and general acceptability at the sprout, microgreen, and fully grown stage compared to commercial cultivars | [45] | |
| Atriplex hortensis (red orach) | under-utilized | Ascorbic acid content | [69] | |
| Chenopodium album (pigweed) | under-utilized | Antioxidant activity and total phenolic content are enhanced in germinated C. album seeds | [85] | |
| Chenopodium quinoa (quinoa) | old variety | Quinoa sprouts are a good source of anthocyanins and total phenolics with high antioxidant activity | [84] | |
| Apiaceae | Anethum graveolens (dill) | under-utilized | Total phenolic and total flavonoid content; antioxidant activity | [86] |
| Coriandrum sativum (coriander) | under-utilized | A strong influence of the substrate on the content of carotenoids and total phenolics | [87] | |
| Araliaceae | Panax ginseng (Korean ginseng) | under-utilized medicinal plant | Ginsenosides (triterpene glycoside saponin) | [81,82] |
| Asteraceae | Artemisia dracunculus (tarragon) | aromatic herb | N/A; red and blue LED exposure enhances germination and growth of tarragon sprouts | [88] |
| Cichorium intybus (chicory) | medicinal herb | Total phenolics, tocopherols, anthocyanins, high levels of carotenoids | [89] | |
| Taraxacum officinale (common dandelion) | wild plants | Anthocyanins and carotenoids; high Fe content | [33] | |
| Basellaceae | Basella alba (Malabar spinach) | underutilized vegetable | High ascorbic acid and total phenolic content | [90] |
| Boraginaceae | Borago officinalis (borage) | medicinal herb | Total phenolic and carotenoid content, antioxidant capacity | [91] |
| Phacelia tanacetifolia (phacelia) | wildflower | Total phenolics, flavonoids, and antioxidant activity | [80] | |
| Brassicaceae | Brassica oleracea var. italica (broccoli) | landrace | (1) High polyphenol content in broccoli landrace; (2) highest vitamin C content found in microgreens of broccoli landrace | [76] |
| Brassica oleracea var. acephala (kale) | landrace | (1) Higher content of flavonoids (quercetin and kaempferol derivatives) in traditional cultivars than in modern cultivars (hybrids); (2) among 8 cultivars, higher concentrations of lutein and β-carotene were found in old cultivars | [49] | |
| Sinapis arvensis (field mustard) | under-utilized | Carotenoids and anthocyanins | [33] | |
| Wasabi japonica (wasabi) | under-utilized | Ascorbic acid, β-carotene, lutein/zeaxanthin content | [69] | |
| Convolvulaceae | Ipomoea aquatica (water spinach) | under-utilized | High total phenolics and total flavonoid content; high antioxidant activity | [89,90] |
| Cucurbitaceae | Cucumis sativus (cucumber) | under-utilized | High ascorbic acid content | [90] |
| Cucurbita moschata pumpkin) | under-utilized | High total phenolics and total flavonoids content | [90] | |
| Lagenaria siceraria (bottle gourd) | under-utilized | High total phenolics content; high antioxidant activity; high Cu and Fe levels | [90] | |
| Fabaceae | Glycine max (soybean) | landrace | Nutrient and antioxidant contents of soybean sprouts were superior to mungbean sprouts | [92] |
| Medicago intertexta (hedgehog medick) | wild species | Total phenolic and flavonoid contents, antioxidant, and antidiabetic activities | [93] | |
| Medicago polymorpha (bur clover) | wild, invasive species | Total phenolic and flavonoid contents, antioxidant, and antidiabetic activities | [93] | |
| Melilotus indicus (annual yellow sweet clover) | wild species | Total phenolic and flavonoid contents, antioxidant, and antidiabetic activities | [93] | |
| Vigna radiata (mungbean) | landrace | (1) Old mungbean accessions were superior in protein, calcium (Ca), iron (Fe), zinc (Zn), carotenoid, and vitamin C content compared to improved mungbean lines at the fully mature stage; (2) compared to commercial mungbean varieties, a landrace from Taiwan (VI000323) showed the highest levels of caffeic acid and kaempferol at the sprouting and fully mature stage | [92] | |
| Lamiaceae | Ocimum basilicum (Sweet basil) | culinary herb | High phylloquinone and total phenolics concentration | [39] |
| Ocimum x africanum (lemon basil) | culinary herb | Total phenolic and total flavonoid content; antioxidant activity | [86] | |
| Ocimum sanctum (sacred basil) | medicinal herb | Total phenolic and total flavonoid content; antioxidant activity | [86] | |
| Salvia hispanica (chia) | under-utilized | Total phenolics, flavonoids, antioxidant activity. | [80] | |
| Linaceae | Linum flavum (golden flax) | under-utilized | Total phenolics, flavonoids, antioxidant activity. | [80] |
| Malvaceae | Corchorus olitorius (jute mallow) | under-utilized | High ascorbic acid and total phenolics content; high antioxidant activity | [90] |
| Hibiscus subdariffa (red roselle) | under-utilized culinary herb | Anthocyanins, flavonoids, and phenolic acids contribute to the antioxidative activity | [65] | |
| Onagraceae | Oenothera biennis (evening primrose) | under-utilized | Total phenolics, flavonoids, antioxidant activity | [80] |
| Plantaginaceae | Plantago coronopus (buck’s-horn plantain) | wild herb | Total phenolics, flavonoids, and antioxidant activity | [94] |
| Polygonaceae | Rumex acetosa (sorrel) | wild herb | Total phenolics, flavonoids, and antioxidant activity | [94] |
| Portulacaceae | Portulaca oleracea (purslane) | wild herb | Total phenolics, flavonoids, and antioxidant activity | [94] |
| Rosaceae | Sanguisorba minor (salad burnet) | under-utilized | Carotenoids and anthocyanins; high amounts of Mg, P, Zn, Mn, and Mo | [33] |
4. Variation of Nutritional Value and Content of Phytochemicals According to Plant Growth Stages
| Family | Species | Secondary Metabolites | Reference |
|---|---|---|---|
| Amaranthaceae | Amaranthus caudatus (foxtail amaranth) | Amaranth sprouts showed significantly higher contents of total flavonoids, rutin, amaranthine, and iso-amaranthine than ungerminated seeds. | [83] |
| Amaranthus cruentus (red amaranth) | Amaranth sprouts have a significantly higher antioxidant activity than seeds, which may be a result of the difference in the content of polyphenols, anthocyanins, and other compounds. | [84] | |
| Amaranthus tricolor (edible amaranth) | (1) Mean protein, Fe and Zn content were considerably higher in amaranth sprouts compared with amaranth microgreens; (2) a substantial increase in vitamin C content from amaranth sprouts to microgreens (2.7-fold) and from amaranth microgreens to fully grown leafy amaranth (2.9-fold); (3) α-carotene and β-carotene were detected in all three growth stages and content increased considerably from sprouts to microgreens. | [45] | |
| Chenopodium quinoa (quinoa) | Quinoa sprouts have a significantly higher antioxidant activity than seeds. | [84] | |
| Chenopodium quinoa | Total phenol content and antioxidant activity increase with the sprouting of seeds. | [98] | |
| Chenopodium quinoa | Sprouts have significantly higher antioxidant capacity values after four days of germination than raw seeds; (2) phenolic content values of 4-day-old sprouts are about 2.6 times higher than seeds. | [99] | |
| Spinacia oleracea (spinach) | Higher ascorbic acid and α-tocopherol levels in microgreens compared to the mature stage. | [67] | |
| Asteraceae | Helianthus annuus (sunflower) | Sprouting increased total phenolic and flavonoid levels, as well as the antioxidant activity compared to ungerminated seeds. | [96] |
| Lactuca sativa (lettuce) | Sprouts showed higher amounts of α-tocopherol and carotenoids compared to mature lettuce. | [89] | |
| Lactuca sativa | The average ratio of ten nutrients (P, K, Ca, Mg, S, Mn, Cu, Zn, Na, and Fe) indicated that hydroponically grown lettuce microgreens were 2.7 times more nutrient-rich than mature lettuce. | [104] | |
| Lactuca sativa var. capitata (butterhead lettuce) | The content of essential minerals such as Ca, Mg, Fe, Mn, Zn, Se, and Mo was higher and nitrate content was lower in lettuce microgreens than in mature lettuces. | [106] | |
| Boraginaceae | Phacelia tanacetifolia (phacelia) | TPC and antioxidant activity were higher in sprouts than in ungerminated seeds. | [80] |
| Brassicaceae | Brassica oleracea var. capitata (cabbage) | The average ratio of ten nutrients (P, K, Ca, Mg, S, Mn, Cu, Zn, Na, and Fe) indicated that hydroponically grown cabbage microgreens were 2.9 times more nutrient-rich than mature cabbage. | [104] |
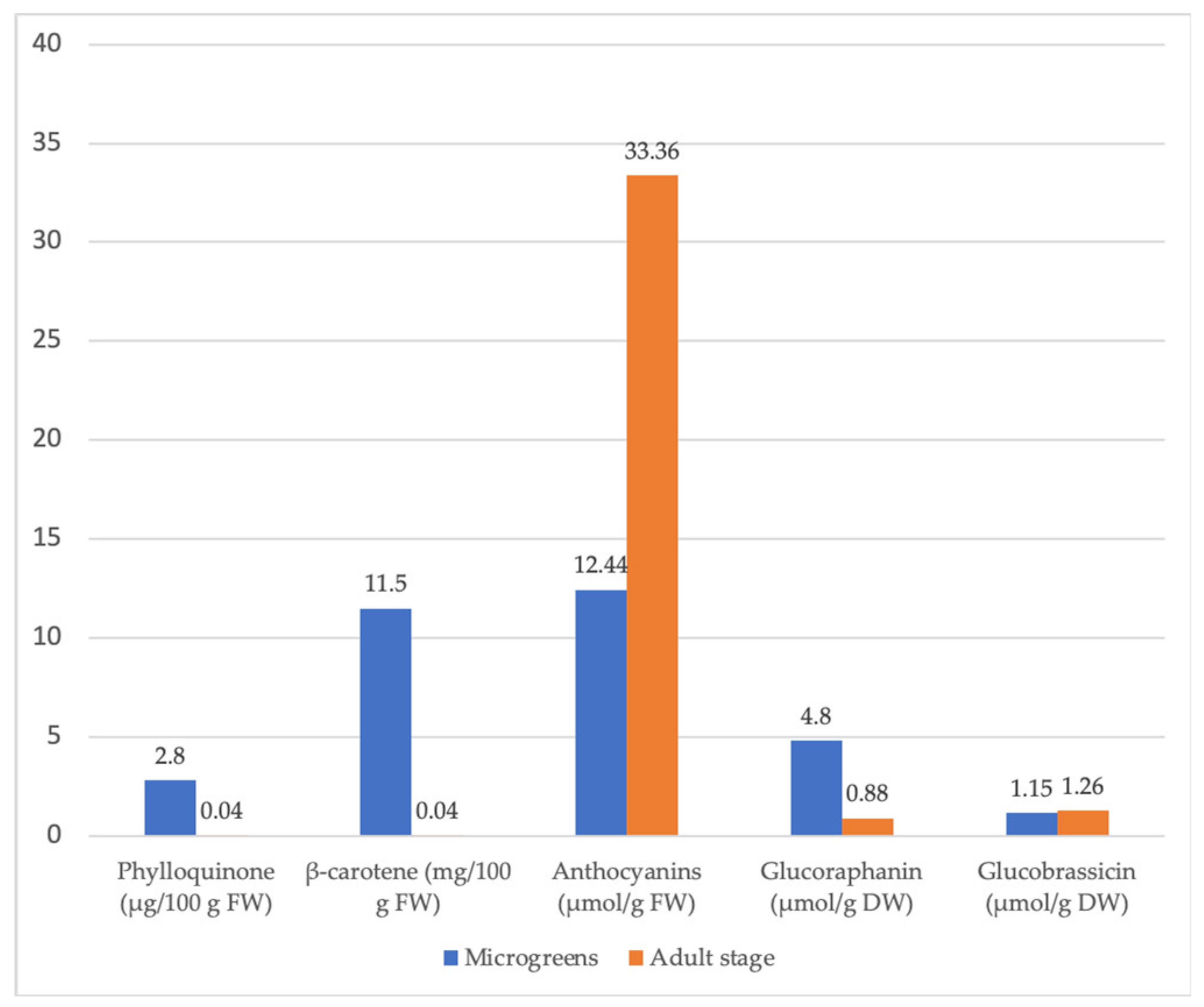
| Brassica oleracea var. capitata | Higher total ascorbic acid, phylloquinone, β-carotene, and glucoraphanin in cabbage microgreens than in mature cabbage. | [95] | |
| Brassica oleracea var. italica (broccoli) | (1) Sprouts showed significantly higher polyphenol values than microgreens and baby leaves; (2) high increments of kaempferol and apigenin in broccoli landrace from the seed to the baby leaves growth stage; (3) antioxidant levels were highest in sprouts and tended to decrease with further growth. | [76] | |
| Brassica oleracea var. italica | Sprouting increased total phenolic and flavonoid levels, as well as the antioxidant activity compared to ungerminated seeds. | [96] | |
| Brassica oleracea var. italica | Health-promoting phytochemicals are more concentrated in cruciferous sprouts (e.g., broccoli and red radish) than in the adult plant edible organs. | [102] | |
| Brassica oleracea var. italica | 3-day-old broccoli sprouts contained a much higher inducer activity of detoxication enzymes than the corresponding mature vegetable. | [61] | |
| Brassica oleracea var. italica | Broccoli sprouts showed higher amounts of α-tocopherol and carotenoids compared to mature broccoli. | [89] | |
| Brassica oleracea var. italica | 10-fold higher content of glucobrassicin in broccoli microgreens compared to the mature stage. | [95] | |
| Brassica oleracea var. acephala (kale) | Sprouts showed significantly higher polyphenol values than microgreens and baby leaves. | [76] | |
| Brassica rapa subsp. chinensis (pak choi) | Decreasing content of 3-butenyl glucosinolates from sprouts to adult leaves. | [49] | |
| Cichorium intybus (chicory) | Sprouts showed higher amounts of α-tocopherol and carotenoids compared to mature chicory. | [89] | |
| Eruca sativa (arugula) | Higher content of total ascorbic acid, phylloquinone, and β-carotene in arugula sprouts compared to the mature stage. | [95] | |
| Raphanus sativus (radish) | Health-promoting phytochemicals are more concentrated in cruciferous sprouts (e.g., broccoli and red radish) than in the respective adult plant edible organs. | [102] | |
| Raphanus sativus | Sprouting increased total phenolic and flavonoid levels and the antioxidant activity compared to ungerminated seeds; radish (and sunflower) sprouts were the richest in phenolic compounds. | [96] | |
| Fabaceae | Cicer arietinum (chickpea) | Chickpea microgreens contained higher vitamins and higher antioxidant activity than raw seeds and sprouts. | [42] |
| Trigonella foenum-graecum (fenugreek) | Higher ascorbic acid and α-tocopherol levels in microgreens compared to the mature stage. | [67] | |
| Vigna radiata (mungbean) | Sprouting mungbean seeds enhanced vitamin C content 2.7-fold compared to mature mungbean grain. | [92] | |
| Vigna radiata | Mungbean sprouts showed increased total phenolic (TPC) and total flavonoid (TF) contents and higher antioxidant activity (AA) than ungerminated seeds; radish and sunflower sprouts were superior to mungbean sprouts regarding TPC, TF, and AA levels. | [96] | |
| Vigna radiata | The total phenolics and vitamins content increased in the sequence of raw seeds, sprouts, and microgreens. | [42] | |
| Glycine max (soybean) | (1) Isoflavones were found at high concentrations in soybean sprouts and could easily provide the recommended anticarcinogenic dose range from 1.5 to 2.0 mg/kg of body weight per day; (2) The vegetable soybean stage was nutritionally superior to soybean sprouts in terms of the content of protein (14% increase), Zn (45%), Ca (72%), and Fe (151%). | [92] | |
| Linaceae | Linum usitatissimum (flaxseed) | Microgreens exhibited a higher chlorophyll (+62.6%), carotenoid (+24.4%), and phenol content (+37.8%), as well as higher antioxidant capacity (+25.1%) than sprouts. | [103] |
| Malvaceae | Hibiscus sabdariffa (roselle) | Higher ascorbic acid and α-tocopherol levels in microgreens compared to the mature stage. | [67] |
5. Environmental and Priming Factors That Have an Impact on the Nutrient and Phytochemical Content of Sprouts and Microgreens
5.1. The Effect of Growth Environment and Growing Substrates
5.2. Response to Environmental Stresses
5.3. Seed Priming and Biostimulants
5.4. Biofortification
5.5. Effect of Light in Controlled Environments
5.5.1. Effects of Light Intensity, Exposure Time, and Light Sources
5.5.2. Effects of Light Spectra
6. Microgreen Market Trends and Outlook
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Micha, R.; Mannar, V.; Afshin, A.; Allemandi, L.; Baker, P.; Battersby, J.; Bhutta, Z.; Chen, K.; Corvalan, C.; Di Cesare, M.; et al. 2020 Global Nutrition Report: Action on Equity to End Malnutrition; Development Initiatives: Washington, DC, USA, 2020; Available online: http://eprints.mdx.ac.uk/30645/ (accessed on 12 November 2021).
- Neufeld, L.M.; Hendriks, S.; Hugas, M. Healthy Diet: A Definition for the United Nations Food Systems Summit 2021. A Paper from the Scientific Group of the UN Food Systems Summit. 2021. Available online: https://www.un.org/sites/un2.un.org/files/healthy_diet_scientific_group_march-2021.pdf (accessed on 15 November 2021).
- FAO; IFAD; UNICEF; WFP; WHO. The state of food security and nutrition in the world. In Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021; 240p. [Google Scholar] [CrossRef]
- Herforth, A.; Bai, Y.; Venkat, A.; Mahrt, K.; Ebel, A.; Masters, W. Cost and affordability of healthy diets across and within countries. In Background Paper for The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. Goal 2: Zero Hunger. Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 18 November 2021).
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 18 November 2021).
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [Green Version]
- Fischer, G.C.; Garnett, T. Plates, Pyramids, and Planets: Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT-lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Park, T.; Khoury, C.K.; Béné, C. Changing diets and the transformation of the global food system. Ann. N. Y. Acad. Sci. 2020, 1478, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Padulosi, S.; Sthapit, B.; Lamers, H.; Kennedy, G.; Hunter, D. Horticultural biodiversity to attain sustainable food and nutrition security. Acta Hortic. 2018, 1205, 21–34. [Google Scholar] [CrossRef]
- The Royal Society. Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; The Royal Society: London, UK, 2009; 72p. [Google Scholar]
- World Health Organization (WHO). Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: https://www.who.int/elena/titles/fruit_vegetables_ncds/en/ (accessed on 19 November 2021).
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of Health Survey for England data. J. Epidemiol. Community Health 2014, 68, 856–862. [Google Scholar] [CrossRef]
- Siegel, K.R.; Ali, M.K.; Srinivasiah, A.; Nugent, R.A.; Narayan, K.V. Do we produce enough fruits and vegetables to meet global health need? PLoS ONE 2014, 9, e104059. [Google Scholar] [CrossRef]
- Bahadur, K.K.C.; Dias, G.M.; Veeramani, A.; Swanton, C.J.; Fraser, D.; Steinke, D.; Lee, E.; Wittman, H.; Farber, J.M.; Dunfield, K.; et al. When too much isn’t enough: Does current food production meet global nutritional needs? PLoS ONE 2018, 13, e0205683. [Google Scholar] [CrossRef] [Green Version]
- Mason-D’Croz, D.; Sulser, T.B.; Wiebe, K.; Rosegrant, M.W.; Lowder, S.K.; Nin-Pratt, A.; Willenbockel, D.; Robinson, S.; Zhu, T.; Cenacchi, N.; et al. Agricultural investments and hunger in Africa modeling potential contributions to SDG2–zero hunger. World Dev. 2019, 116, 38–53. [Google Scholar] [CrossRef]
- Mensah, D.O.; Nunes, A.R.; Bockarie, T.; Lillywhite, R.; Oyebode, O. Meat, fruit, and vegetable consumption in sub-Saharan Africa: A systematic review and meta-regression analysis. Nutr. Rev. 2021, 79, 651–692. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts, microgreens, and edible flowers: The potential for high value specialty produce in Asia. In Proceedings of the SEAVEG 2012 High Value Vegetables Southeast Asia Production, Supply Demand, Chiang Mai, Thailand, 16 September 2013; pp. 216–227. [Google Scholar]
- Voinea, L.; Vrânceanu, D.M.; Filip, A.; Popescu, D.V.; Negrea, T.M.; Dina, R. Research on food behavior in Romania from the perspective of supporting healthy eating habits. Sustainability 2019, 11, 5255. [Google Scholar] [CrossRef] [Green Version]
- Ebert, A.W. High value specialty vegetable produce. In Handbook of Vegetables, 1st ed.; Peter, K.V., Hazra, P., Eds.; Studium Press LLC.: Houston, TX, USA, 2015; Chapter 4; Volume 2, pp. 119–143. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and “baby leaf” vegetables. In BT-Minimally Processed Refrigerated Fruits and Vegetables; Food Engineering Series; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Abdallah, M.M.F. Seed sprouts, a pharaoh’s heritage to improve food quality. Arab Univ. J. Agric. Sci. 2008, 16, 469–478. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Sierra, I.; Blazquez, I.; Lambein, F.; Kuo, Y.H. New functional legume foods by germination: Effect on the nutritive value of beans, lentils and peas. Eur. Food Res. Technol. 2002, 215, 472–477. [Google Scholar] [CrossRef]
- Márton, M.; Mandoki, Z.S.; Csapo-Kiss, Z.S.; Csapo, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae 2010, 3, 81–117. [Google Scholar]
- Wanasundara, P.; Shahidi, F.; Brosnan, M.E. Changes in flax (Linum usitatissimum) seed nitrogenous compounds during germination. Food Chem. 1999, 65, 289–295. [Google Scholar] [CrossRef]
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed treatments to advance greenhouse establishment of beet and chard microgreens. J. Hort. Sci. Biotechnol. 2004, 79, 565–570. [Google Scholar] [CrossRef]
- Di Gioia, F.; Mininni, C.; Santamaria, P. How to grow microgreens. In Microgreens: Microgreens: Novel Fresh and Functional Food to Explore All the Value of Biodiversity; Di Gioia, F., Santamaria, P., Eds.; ECO-Logica: Bari, Italy, 2015; pp. 51–79. [Google Scholar]
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and mineral composition of three wild leafy species: A comparison between microgreens and baby greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef] [Green Version]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop; HS1164, rev. 9/2020, IFAS Extension; University of Florida: Gainesville, FL, USA, 2020. [Google Scholar]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonymous. Specialty Greens Pack a Nutritional Punch. Available online: https://agresearchmag.ars.usda.gov/AR/archive/2014/Jan/greens0114.pdf (accessed on 3 December 2021).
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Verlinden, S. Microgreens: Definitions, Product Types, and Production Practices. Hortic. Rev. 2020, 47, 85–124. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Ghani, M.; Kulkarni, K.P.; Song, J.T.; Shannon, J.G.; Lee, J.D. Soybean sprouts: A review of nutrient composition, health benefits and genetic variation. Plant Breed. Biotechnol. 2016, 4, 398–412. [Google Scholar] [CrossRef] [Green Version]
- Butkutė, B.; Taujenis, L.; Norkevičienė, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules 2019, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Kurian, M.S.; Megha, P.R. Assessment of variation in nutrient concentration and antioxidant activity of raw seeds, sprouts and microgreens of Vigna radiata (L.) Wilczek and Cicer arietinum L. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2020; Volume 2263, 030005p. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.A.; Van den Broeck, H.C.; Brouns, F.J.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Pirzadah, T.B.; Malik, B. Pseudocereals as super foods of 21st century: Recent technological interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Ebert, A.W.; Wu, T.H.; Yang, R.Y. Amaranth sprouts and microgreens—A homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum. In Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG 2014), Bangkok, Thailand, 25–27 February 2014; pp. 25–27. [Google Scholar]
- Janovská, D.; Stocková, L.; Stehno, Z. Evaluation of buckwheat sprouts as microgreens. Acta Agric. Slov. 2010, 95, 157. [Google Scholar] [CrossRef] [Green Version]
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, M.; Nie, Z.; Wu, Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and microorganisms boost the nutritional composition of buckwheat (Fagopyrum esculentum Moench) sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods 2021, 10, 427. [Google Scholar] [CrossRef]
- Ng, T.B.; Ng, C.C.W.; Wong, J.H. Health benefits of Brassica species. In Brassicaceae: Characterization, Functional Genomics and Health Benefits; Lang, M., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 1–18. [Google Scholar]
- Sanlier, N.; Guler, S.M. The benefits of Brassica vegetables on human health. J. Hum. Health Res. 2018, 1, 1–13. [Google Scholar]
- Francisco, M.; Velasco, P.; Moreno, D.A.; García-Viguera, C.; Cartea, M.E. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res. Int. 2010, 43, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar] [CrossRef]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, P.; Huang, Q.; Ong, C.N.; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2005, 209, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous Vegetables and Human Cancer Risk: Epidemiologic Evidence and Mechanistic Basis. Pharmacol. Res. 2007, 55, 224. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Srividya, N. Micro-farming of greens: A viable enterprise for enhancing economic, food and nutritional security of farmers. Int. J. Nutr. Agric. Res. 2018, 5, 10–16. [Google Scholar]
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile Compounds and Total Phenolic Content of Perilla frutescens at Microgreens and Mature Stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, S.; Topalovic, A.; Knezevic, M.; Vajs, V. Free radicals and antioxidants: Antioxidative and other properties of Swiss chard (Beta vulgaris L. subsp. Cicla). Poljopr. Sumar. 2015, 61, 73. [Google Scholar] [CrossRef]
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Garvin, D.F.; Welch, R.M.; Finely, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bradshaw, J.E.; Finlay, M.; Dale, B.; Ramsay, G.; Hammond, J.P.; Broadley, M.R. Relationships between yield and mineral concentrations in potato tubers. Hort Sci. 2009, 44, 6–11. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Lo Scalzo, R.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in Brassica oleracea L. novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the Mediterranean diet and its sustainability. Appl. Sci. 2020, 10, 9144. [Google Scholar] [CrossRef]
- Romojaro, A.; Botella, M.Á.; Obón, C.; Pretel, M.T. Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr. 2013, 64, 944–952. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Gruda, N.S. The potential of introduction of Asian vegetables in Europe. Horticulturae 2020, 6, 38. [Google Scholar] [CrossRef]
- Song, J.S.; Jung, S.; Jee, S.; Yoon, J.W.; Byeon, Y.S.; Park, S.; Kim, S.B. Growth and bioactive phytochemicals of Panax ginseng sprouts grown in an aeroponic system using plasma-treated water as the nitrogen source. Sci. Rep. 2021, 11, 2924. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Physico-chemical, textural, sensory and antioxidant characteristics of gluten–Free cookies made from raw and germinated Chenopodium (Chenopodium album) flour. LWT Food Sci. Technol. 2016, 71, 281–287. [Google Scholar] [CrossRef]
- Harakotr, B.; Srijunteuk, S.; Rithichai, P.; Tabunhan, S. Effects of Light-Emitting Diode Light Irradiance Levels on Yield, Antioxidants and Antioxidant Capacities of Indigenous Vegetable Microgreens. Sci. Technol. Asia 2019, 24, 59–66. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic Constitution, Phytochemical and Macronutrient Content in Three Species of Microgreens as Modulated by Natural Fiber and Synthetic Substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Enache, I.-M.; Livadariu, O. Preliminary results regarding the testing of treatments with light-emitting diode (LED) on the seed germination of Artemisia dracunculus L. Sci. Bull. Ser. F Biotechnol. 2016, 20, 51–56. [Google Scholar]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: Comparison at mature and microgreen stages using chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W.; Chang, C.-H.; Yan, M.-R.; Yang, R.-Y. Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. Food Chem. 2017, 237, 15–22. [Google Scholar] [CrossRef]
- Zrig, A.; Saleh, A.; Hamouda, F.; Okla, M.K.; Al-Qahtani, W.H.; Alwasel, Y.A.; Al-Hashimi, A.; Hegab, M.Y.; Hassan, A.H.; AbdElgawad, H. Impact of Sprouting under Potassium Nitrate Priming on Nitrogen Assimilation and Bioactivity of Three Medicago Species. Plants 2022, 11, 71. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium Biofortification of Three Wild Species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., Grown as Microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Laus, M.; Cataldi, M.; Soccio, M.; Alfarano, M.; Amodio, M.; Colelli, G.; Flagella, Z.; Pastore, D. Effect of germination and sprout storage on antioxidant capacity and phenolic content in quinoa (Chenopodium quinoa Willd). In Proceedings of the SIBV-SIGA Joint Congress Sustainability of Agricultural Environment: Contributions of Plant Genetics and Physiology, Pisa, Italy, 19–22 September 2017. [Google Scholar]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Khosravi, F.; Rastakhiz, N.; Iranmanesh, B.; Olia, S.S.S.J. Determination of Organic Acids in Fruit juices by UPLC. Int. J. Life Sci. 2015, 9, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Puccinelli, M.; Maggini, R.; Angelini, L.G.; Santin, M.; Landi, M.; Tavarini, S.; Castagna, A.; Incrocci, L. Can Light Spectrum Composition Increase Growth and Nutritional Quality of Linum usitatissimum L. Sprouts and Microgreens? Horticulturae 2022, 8, 98. [Google Scholar] [CrossRef]
- Weber, C.F. Nutrient concentration of cabbage and lettuce microgreens grown on vermicompost and hydroponic growing pads. J. Hortic. 2016, 3, 190. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.F. Broccoli microgreens: A mineral-rich crop that can diversify food systems. Front. Nutr. 2017, 4, 7. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Comp. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, Z. Reducing the nitrate content in vegetables through joint regulation of short-distance distribution and long-distance transport. Front. Plant Sci. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Ponnet, K. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS ONE 2020, 15, e0227551. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of Three Brassica L. Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346. [Google Scholar] [CrossRef]
- Matsuo, T.; Asano, T.; Mizuno, Y.; Sato, S.; Fujino, I.; Sadzuka, Y. Water spinach and okra sprouts inhibit cancer cell proliferation. Vitr. Cell. Dev. Biol.-Anim. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khanum, F.; Siddalinga Swamy, M.; Sudarshana Krishna, K.R.; Santhanam, K.; Viswanathan, K.R. Dietary fiber content of commonly fresh and cooked vegetables consumed in India. Plant Foods Hum. Nutr. 2000, 55, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables Through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain Endogenous Selenium and Moderate Salt Stress Work as Synergic Elicitors in the Enrichment of Bioactive Compounds in Maize Sprouts. Agronomy 2020, 10, 735. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp. sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Radovich, T.J.; Kleinhenz, M.D.; Streeter, J.G. Irrigation timing relative to head development influences yield components, sugar levels, and glucosinolate concentrations in cabbage. J. Am. Soc. Hortic. Sci. 2005, 130, 943–949. [Google Scholar] [CrossRef]
- Oh, M.M.; Rajashekar, C.B. Antioxidant content of edible sprouts: Effects of environmental shocks. J. Sci. Food Agric. 2009, 89, 2221–2227. [Google Scholar] [CrossRef]
- Šamec, D.; Ljubej, V.; Redovnikovi’c, I.R.; Fistani’c, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts . Foods 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum LED Lighting as Abiotic Elicitors of Bioactive Compounds in Sprouts, Microgreens, and Baby Leaves—A Comprehensive Review including Their Mode of Action. Foods 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I.; Brown, B.A. UV-B perception and signal transduction. In Light and Plant Development; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 155–182. [Google Scholar]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillejo, N.; Martínez-Zamora, L.; Artés–Hernández, F. Periodical UV-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Baraniak, B.; Karaś, M. Antioxidant activity of polyphenols of adzuki bean (Vigna angularis) germinated in abiotic stress conditions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Świeca, M.; Surdyka, M.; Gawlik-Dziki, U.; Złotek, U.; Baraniak, B. Antioxidant potential of fresh and stored lentil sprouts affected by elicitation with temperature stresses. Int. J. Food Sci. Technol. 2014, 49, 1811–1817. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sugier, D. Improvement of nutraceutical value of broccoli sprouts by natural elicitors. Acta Sci. Pol.-Hortorum Cultus 2013, 12, 129–140. [Google Scholar]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment Reveals an Elevated Level of Glucosinolate Content in CaCl2 Treated Broccoli Microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, W.; Alcazar, J.; Yang, T.; Luo, Y.; Wang, Q.; Chen, P. Effect of preharvest CaCl2 spray and postharvest UV-B radiation on storage quality of broccoli microgreens, a richer source of glucosinolates. J. Food Compos. Anal. 2018, 67, 55–62. [Google Scholar] [CrossRef]
- Mercado, M.F.O.; Fernandez, P.G. Solid matrix priming of soybean seeds. Philipp. J. Crop Sci. 2002, 27, 27–35. [Google Scholar]
- Pandita, V.K.; Anand, A.; Nagarajan, S.; Seth, R.; Sinha, S.N. Solid matrix priming improves seed emergence and crop performance in okra. Seed Sci. Technol. 2010, 38, 665–674. [Google Scholar] [CrossRef]
- Tanha, A.; Golzardi, F.; Mostafavi, K. Seed Priming to Overcome Autotoxicity of Alfalfa (Medicago sativa). World J. Environ. Biosci. 2017, 6, 1–5. [Google Scholar]
- Mondal, S.; Bose, B. An impact of seed priming on disease resistance: A review. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R., Upadhyay, R., Dubey, N., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 193–203. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of seed priming with potassium nitrate on the performance of tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Shehzad, F.; Waseem, M.; Shahid, S.; Hussain, A.I.; Haider, M.Z.; Habib, N.; Hussain, S.M.; Javed, M.T.; Perveen, R. Plant-based biostimulants and plant stress responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 625–661. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zou, L.; Zhong, L.Y.; Peng, L.X.; Ying, P.L.; Tan, M.L.; Zhao, G. Effects of polysaccharide elicitors from endophytic Bionectria pityrodes Fat6 on the growth and flavonoid production in tartary buckwheat sprout cultures. Cereal Res. Commun. 2015, 43, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Briatia, X.; Jomduang, S.; Park, C.H.; Lumyong, S.; Kanpiengjai, A.; Khanongnuch, C. Enhancing growth of buckwheat sprouts and microgreens by endophytic bacterium inoculation. Int. J. Agric. Biol. 2017, 19, 374–380. [Google Scholar] [CrossRef]
- Gerbore, J.; Vallance, J.; Yacoub, A.; Delmotte, F.; Grizard, D.; Regnault-Roger, C.; Rey, P. Characterization of Pythium oligandrum populations that colonize the rhizosphere of vines from the Bordeaux region. FEMS Microbiol. Ecol. 2014, 90, 153–167. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A.; Kroflič, A.; Golob, A.; Maršić, N.K. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Ong, M.M.X.; Lee, Y.Y.; Teo, E.J.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and Tuning Lighting Conditions in Controlled Environment Agriculture: A Review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in ‘Florida Broadleaf’ mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. J. Am. Soc. Hortic. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaityte, A.; Jankauskiene, J.; Viršile, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Sherudilo, E.G.; Rubaeva, A.A.; Titov, A.F. Continuous LED Lighting Enhances Yield and Nutritional Value of Four Genotypes of Brassicaceae Microgreens. Plants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Hornyák, M.; Dziurka, M.; Kula-Maximenko, M.; Pastuszak, J.; Szczerba, A.; Szklarczyk, M.; Płażek, A. Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci. Rep. 2022, 12, 257. [Google Scholar] [CrossRef]
- Fiutak, G.; Michalczyk, M. Effect of artificial light source on pigments, thiocyanates and ascorbic acid content in kale sprouts (Brassica oleracea L. var. Sabellica L.). Food Chem. 2020, 330, 127189. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of light-emitting diodes on the accumulation of glucosinolates and phenolic compounds in sprouting canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to Red, Blue Monochromatic Light Improves the Bioactive Compound Content in Broccoli Sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Samuolienė, G.; Urbonavičiūtė, A.; Brazaitytė, A.; Šabajevienė, G.; Sakalauskaitė, J.; Duchovskis, P. The impact of LED illumination on antioxidant properties of sprouted seeds. Cent. Eur. J. Biol. 2011, 6, 68–74. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Miliauskiene, J.; Vaštakaite-Kairiene, V.; Sutuliene, R.; Laužike, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red LED Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, T.; Pinho, P.; Rantanen, M.; Kariluoto, S.; Lampi, A.; Edelmann, M.; Joensuu, K.; Kauste, K.; Mouhu, K.; Piironen, V.; et al. Effects of LED light spectra on lettuce growth and nutritional composition. Lighting Res. Technol. 2018, 50, 880–893. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Alrifai, O.; Mats, L.; Liu, R.; Hao, X.; Marcone, M.F.; Tsao, R. Effect of combined light-emitting diodes on the accumulation of glucosinolates in Brassica microgreens. Food Prod. Process Nutr. 2021, 3, 30. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. Amber, red and blue LEDs modulate phenolic contents and antioxidant activities in eight Cruciferous microgreens. J. Food Bioact. 2020, 11, 95–109. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; El-Tantawy, A.A.; Moneim, D.A.; Salam, A.A.; Ash-shormillesy, S.M.; Attia, A.; Ali, M.A.; Herranz, R.; El-Esawi, M.A.; et al. Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol. Plant. 2020, 169, 625–638. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative analysis of phenolic compound profiles, antioxidant capacities, and expressions of phenolic biosynthesis-related genes in soybean microgreens grown under different light spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef]
- Mordor Intelligence. Microgreens Market—Growth, Trends, Covid-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/microgreens-market (accessed on 10 December 2021).
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary assessment of self-produced microgreens as basic ingredients in sweet and savory dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Agrilyst. State of Indoor Farming 2017. Available online: https://www.cropscience.bayer.com/sites/cropscience/files/inline-files/stateofindoorfarming-report-2017.pdf (accessed on 10 December 2021).
- Rajan, P.; Lada, R.R.; MacDonald, M.T. Advancement in indoor vertical farming for microgreen production. Am. J. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Stein, E.W. The Transformative Environmental Effects Large-Scale Indoor Farming May Have on Air, Water, and Soil. Air Soil Water Res. 2021, 14, 1178622121995819. [Google Scholar] [CrossRef]
- ReportLinker. Global Microgreens Market Analysis & Trends—Industry Forecast to 2028. Available online: https://www.reportlinker.com/p06127645/Global-Microgreens-Market-Analysis-Trends-Industry-Forecast-to.html?utm_source=GNW (accessed on 10 December 2021).
- Allied Market Research. Global Microgreens Market—Opportunities and Forecast, 2021–2028. Available online: https://www.alliedmarketresearch.com/microgreens-market-A08733 (accessed on 11 December 2021).


| Crop Group | Family | Species | Common Name | Main Use 1 |
|---|---|---|---|---|
| Legumes | Fabaceae | Arachis hypogaea | peanut | S |
| Cicer arietinum | chickpea | S & M | ||
| Glycine max | soybean | S | ||
| Lens culinaris | lentil | S & M | ||
| Medicago sativa | alfalfa | S & M | ||
| Trifolium repens | clover | S & M | ||
| Vigna angularis | adzuki bean | S (& M) | ||
| Vigna mungo | black gram | S | ||
| Vigna radiata | mungbean | S (& M) | ||
| Vigna unguiculata | cowpea | S | ||
| Cereals | Poaceae | Hordeum vulgare | barley | S & M |
| Zea mays | maize | S & M | ||
| Avena sativa | oat | S &M | ||
| Oryza sativa | rice | S & M | ||
| Secale cereale | rye | S & M | ||
| Triticum aestivum | wheat | S & M | ||
| Zea mays | maize, popcorn | S & M | ||
| Pseudocereals | Amaranthaceae | Amaranthus sp. | amaranth | S & M |
| Chenopodium quinoa | quinoa | S & M | ||
| Polygonaceae | Fagopyrum esculentum | buckwheat | S & M | |
| Oilseeds | Asteraceae | Helianthus annuus | sunflower | S & M |
| Betulaceae | Corylus avellana | hazelnut | S | |
| Linaceae | Linum usitatissimum | linseed, flax | S & M | |
| Pedaliaceae | Sesamum indicum | sesame | S & M | |
| Rosaceae | Prunus amygdalus | almond | S | |
| Vegetables & herbs | Amaranthaceae | Beta vulgaris | beet | S & M |
| Spinacia oleracea | spinach | S & M | ||
| Amaryllidaceae | Allium cepa | onion | S & M | |
| Allium fistulosum | spring onion | S & M | ||
| Allium porrum | leek | S & M | ||
| Allium schoenoprasum | chives | S & M | ||
| Apiaceae | Apium graveolens | celery | S & M | |
| Coriandrum sativum | coriander | S & M | ||
| Daucus carota subsp. sativus | carrot | S & M | ||
| Foeniculum vulgare | fennel | S & M | ||
| Petroselinum crispum | parsley | S & M | ||
| Asteraceae | Lactuca sativa | lettuce | S & M | |
| Brassicaceae | Brassica juncea | purple mustard | S & M | |
| Brassica oleracea, var. alboglabra | Chinese kale | S & M | ||
| Brassica oleracea var. capitata | (red) cabbage | S & M | ||
| Brassica oleracea var. gongylodes | purple kohlrabi | S & M | ||
| Brassica oleracea var. italica | broccoli | S & M | ||
| Brassica rapa var. chinensis | pak choi | S & M | ||
| Brassica rapa var. niposinica | mizuna | S & M | ||
| Brassica rapa var. rapa | turnip | S & M | ||
| Brassica rapa var. rosularis | flat cabbage; tatsoi | S & M | ||
| Eruca sativa | arugula, rocket | S & M | ||
| Lepidium bonariense | peppercress | S & M | ||
| Nasturtium officinale | watercress | S & M | ||
| Raphanus raphanistrum subsp. sativus | daikon, small radish | S & M | ||
| Fabaceae | Trigonella foenum-graecum | fenugreek | S & M | |
| Pisum sativum | garden pea | S & M | ||
| Pisum sativum var. saccharatum | snow peas | S & M | ||
| Lamiaceae | Melissa officinalis | lemon balm | S & M | |
| Ocimum basilicum | sweet basil | S & M | ||
| Perilla frutescens | purple perilla | S & M |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. https://doi.org/10.3390/plants11040571
Ebert AW. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants. 2022; 11(4):571. https://doi.org/10.3390/plants11040571
Chicago/Turabian StyleEbert, Andreas W. 2022. "Sprouts and Microgreens—Novel Food Sources for Healthy Diets" Plants 11, no. 4: 571. https://doi.org/10.3390/plants11040571
APA StyleEbert, A. W. (2022). Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants, 11(4), 571. https://doi.org/10.3390/plants11040571






