Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review
Abstract
:1. Introduction
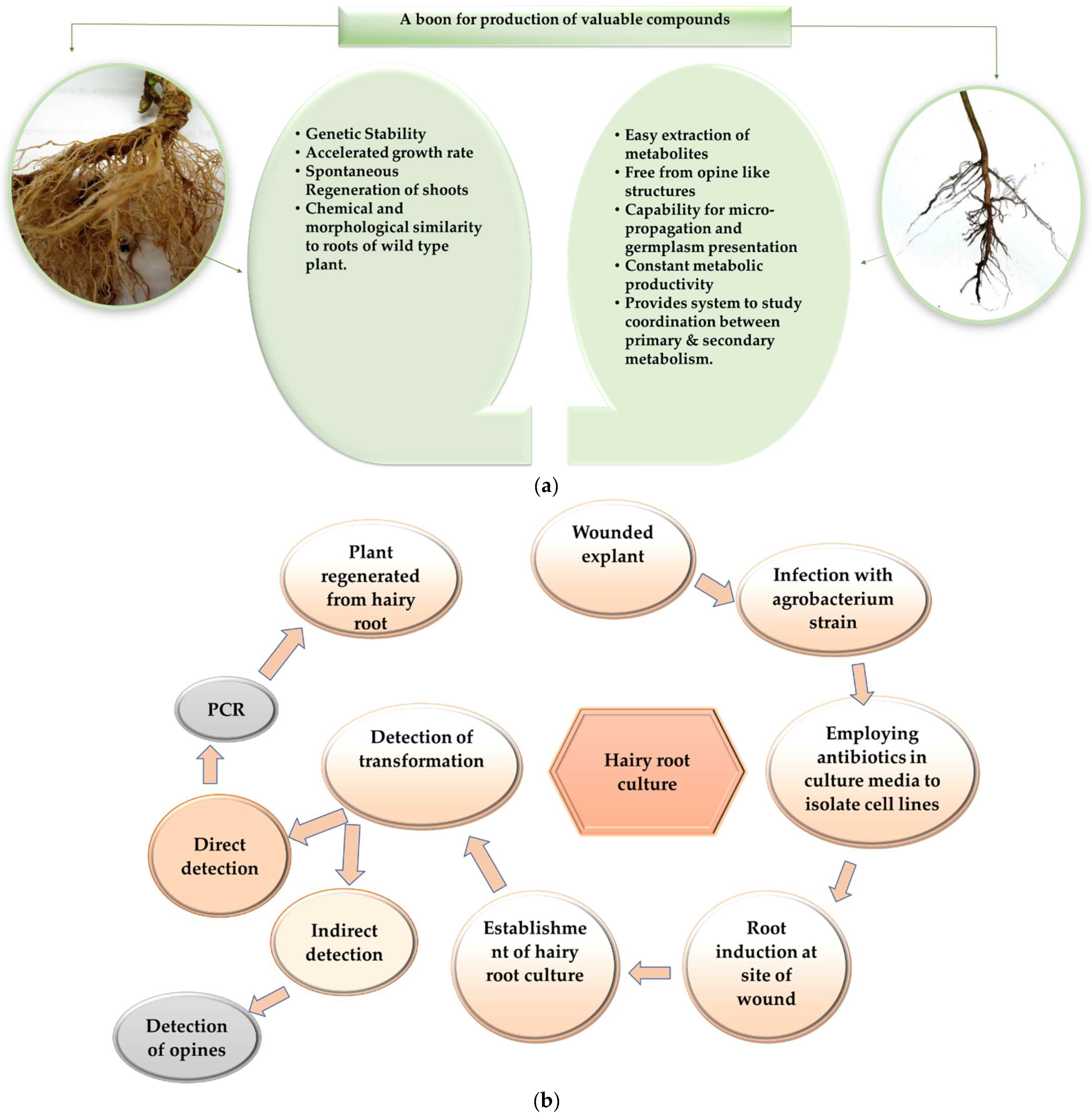
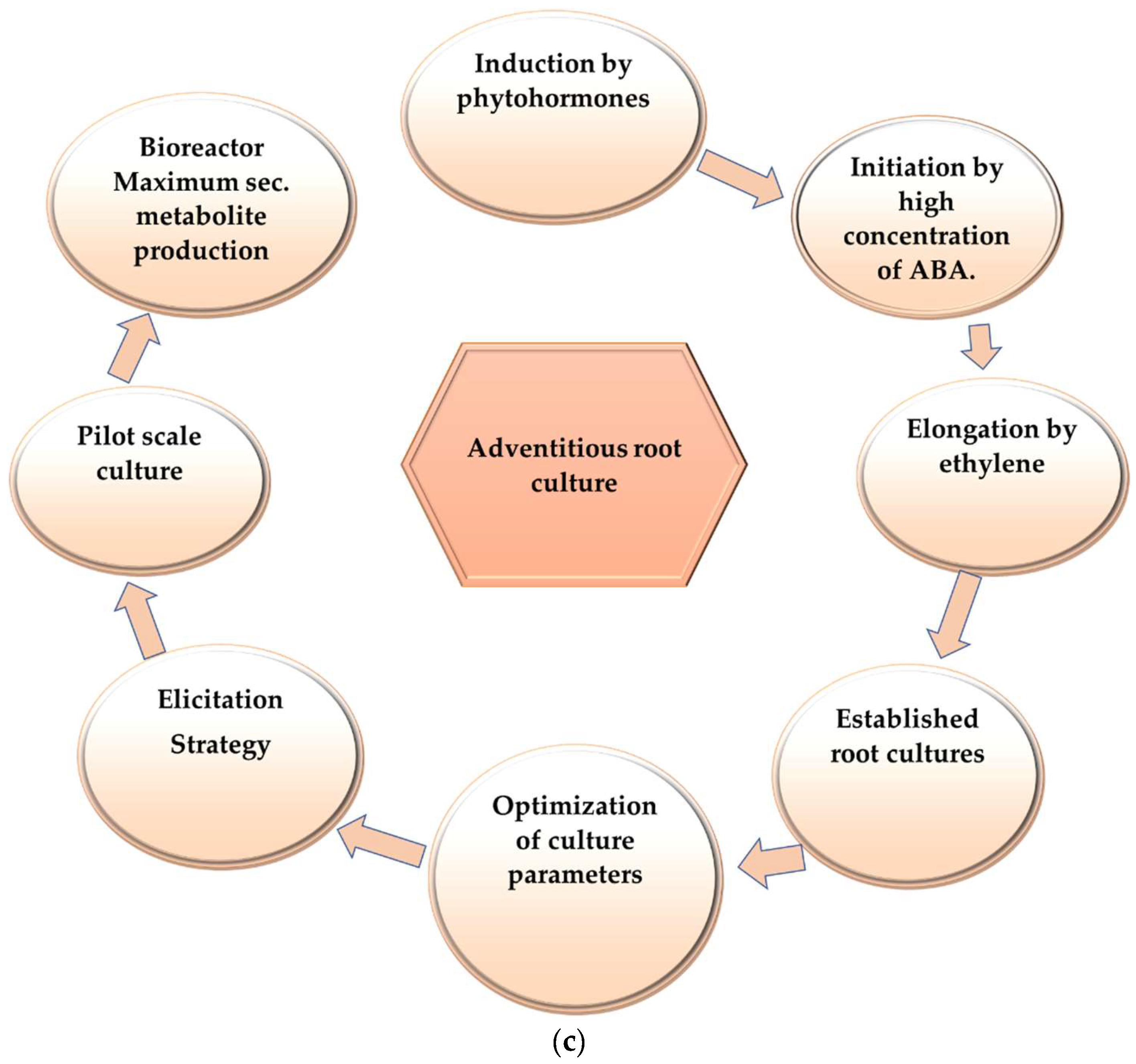
2. Adventitious Root Culture
3. Hairy Root Culture
4. Hairy Root vs. Adventitious Root Culture
5. Media Properties and Culture Condition Effects
6. Role of Plant Growth Regulator
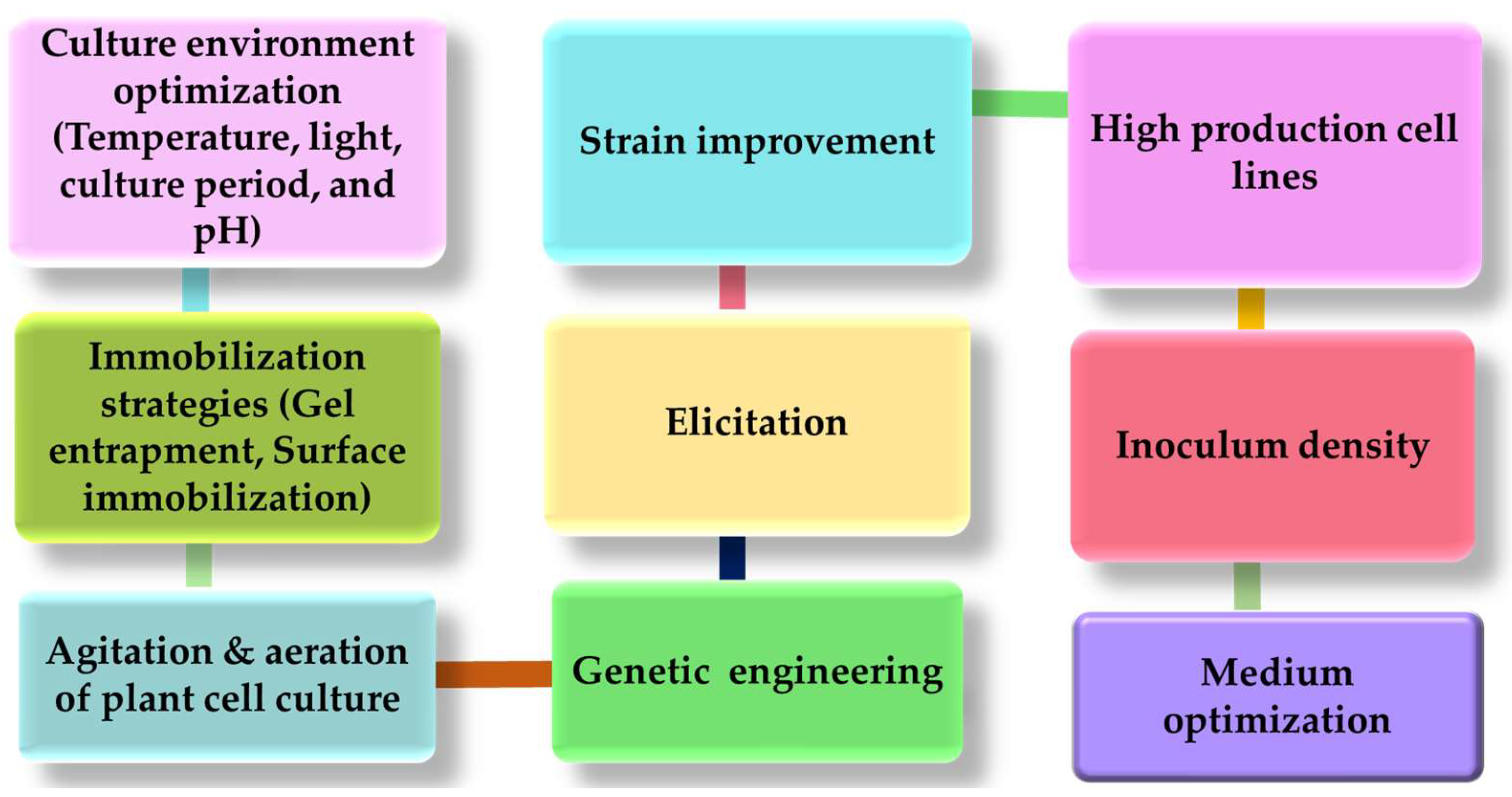
7. Optimization Strategies to Improve Secondary Metabolite Production
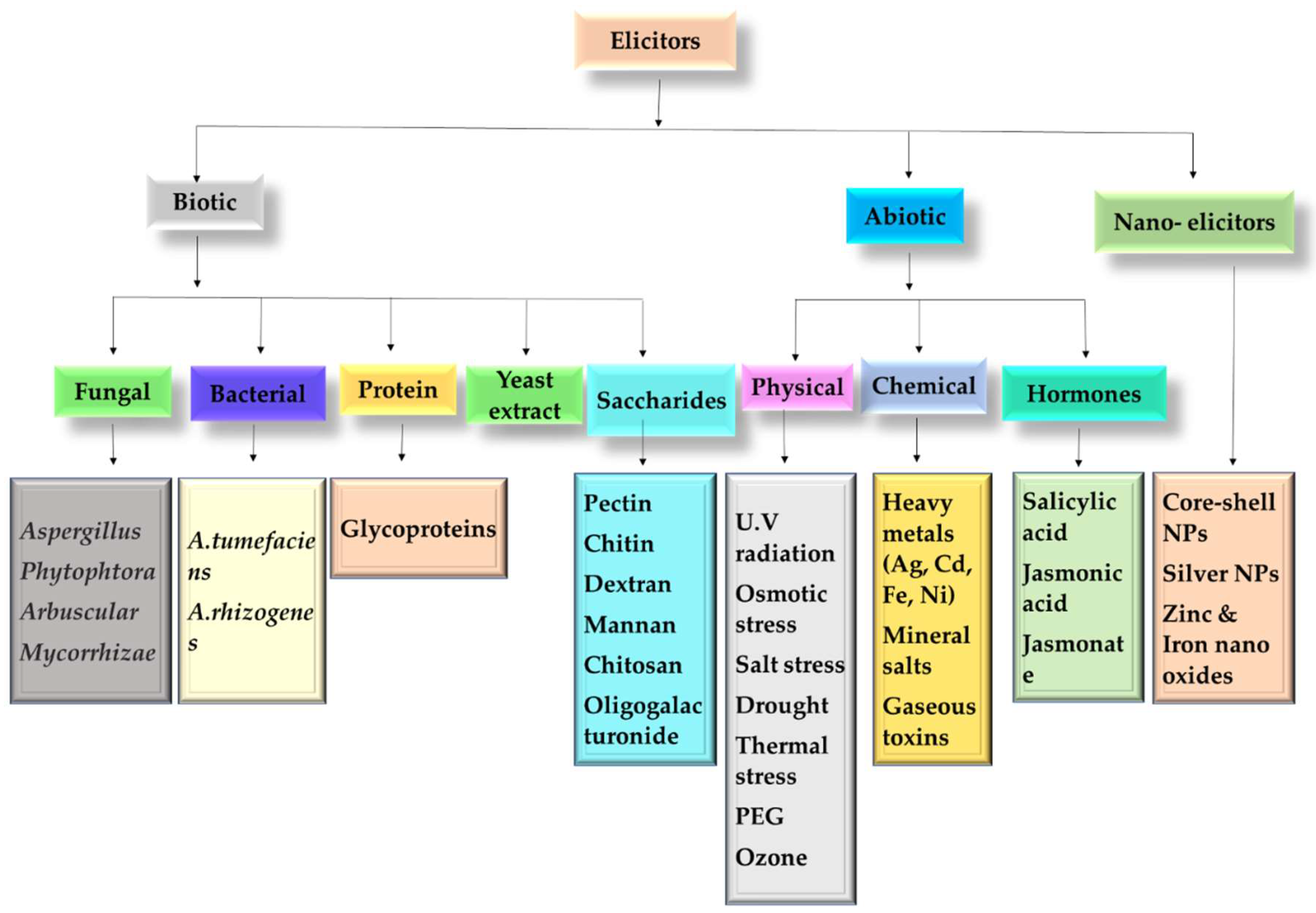
8. Elicitation
8.1. Biotic Elicitors
8.2. Abiotic Elicitors
8.3. Nano-Elicitors
9. Scale-Up of Culture Process
10. Case Studies
10.1. Polygonum multiflorum
10.2. Withania somnifera
10.3. Echinacea purpurea
10.4. Ajuga bracteosa
| Plant | Explant Used | Valuable Compound | Culture Conditions and PGRs | Elicitation Strategy | Increase in Yield of Valuable Compound | Bioreactor Employed | Optimization Strategy of Culture Conditions and Bioreactor | Reference |
|---|---|---|---|---|---|---|---|---|
| Ajuga bracteosa Wall. ex Benth. | Leaf explants | poly phenols flavonoids | MS media + (1.5) NAA | Different Spectral lights | Enhanced production of polyphenols (44.2 mg) and flavonoids (2.51 mg) were observed in presence of blue light. | _ | pH 5.6–5.8 photoperiod (16 h light and 8 h dark) under cool-white light (~100 μmol/m2/s) Temperature 25 ± 2 °C | [123] |
| Allamanda cathartica L. | Nodal segment | iridoids | ½ MS media + 0.1 μM IAA, 0.5 μM IBA, 1.0 μM NAA | - | Total yield of iridoid glycoside content was highest in S3 sample 5.53 ± 0.03% treated with 0.5 M IBA + 4 percent sucrose+ 120 mM NaCl. | - | Sucrose 3% NACL supplementation Culture period: 4 weeks pH 5.8 relative humidity: 55–60% | [128] |
| Angelica gigas N. | Seeds | decursin & decursinol angelate | MS media + 1.0 mg/L IBA+ 0.6 g/L caseine hydrolysate | yeast extract, chitin, MeJA, SA, and copper | 1.5-fold increase in plant yield. 1.7-fold increase in production of decursin. | _ | Sucrose: 30 g/L pH: 5.7 Subculturing, every 21 days. | [129] |
| Artemisia amygdalina D. | Leaf explant | Essential oils | MS media + 1.0 mg/L α-naphthalene acetic acid (NAA) | methyl jasmonate | (Me-J: 0.5 mg/L) resulted in the higher production of total phenolic content (3.6 mg), total flavonoid content (2.3 mg), and phenylalanine ammonia-lyase (4.8 U/g× FW). | _ | light intensity: ~40 µM/m2 s Humidity: 70% Sucrose: 3% | [130] |
| Artimisia scoparia Waldst. and Kit. | Leaf explant | DPPH | MS media + 1.0 mg/L PAA and NAA | MeJA | 87% antioxidant potential was achieved in presence of 0.5 mg/L MeJA. | _ | Relative humidity: 70% light intensity of ~40 μM/m2 s sucrose: 3% pH: 5.8 Culture period: 44 days | [130] |
| Asphodeloideae L. | Young shoots | Aloe-emodin and chrysophanol | MS liquid media with 0.3 mg/L IBA | salicylic acid, methyl jasmonate, and ethephon | Aloe-emodin increased 10–11-fold by SA treatment. Chrysophanol was increased by 5–13-folds by SA treatment. | _ | 30 g/L sucrose pH 5.8 constant light conditions (light intensity: 7 mE/m2 s) culture period: 3 weeks | [31] |
| Astragalus membranaceus L. | Roots | Calycosin-7-O-β-d-glucoside (CG) | MS media + 7 mg/L IBA | MeJA L-phenylalanine | 2.02-fold increase in CG content, with 200 µM MeJA for 8 days. 3.12-fold increase in CG by feeding MeJA-elicited CG with l-phenylalanine. | _ | Sucrose: 30 g/L PEG treatment | [131] |
| Astragalus membranaceus var. mongholicus F. | Shoot | calycosin-7-O-β-D-glucoside and astragaloside Ⅳ | MS media + 1 mg/L NAA | Green leaf volatiles (hexanal, hexanol, and E-2-hexenal) | Treatments with hexanol and E-2-hexenal significantly increased the content of astragaloside Ⅳ by 81.67% and 81.41%. Treatments with hexanal and hexanol increased the CG content up to 56.07% (0.57 ± 0.05 mg/g) and 65.18% (0.60 ± 0.02 mg/g), respectively | - | Sucrose: 30 g/L Photo period: 16 h light/8 h dark Culture period: 4 weeks | [132] |
| Boesenbergia rotunda L. | Bud explant | pinostrobin | ½ MS media + 2.0 mg/L NAA | - | High pinostrobin production (3.54 mg/g) obtained with 50 g/L of sucrose concentration. | - | Sucrose: 50 g/L pH: 5.75 to 5.80 Culture period: 8 weeks | [27] |
| Celastrus paniculatus W. | Leaf explants | Celastrol | MS media + 0.3 mg/L IAA | Silver nanoparticles and acetosyringone | Celastrol increased 1.87-fold by treatment with 10 µg/mL of AgNPs (48 h exposure). | _ | 3% sucrose pH 5.8 Culture period: 2 weeks | [133] |
| Clitoria teretea L. | Seeds | pentacyclic triterpenoid, saponins, flavonoids, anthocyanins | ½ MS media + 2.50 mg/L NAA, 2.50 mg/L (4-chloroindole-3-acetic acid) 4-Cl-IAA | - | Taraxasterol content was detected with peak area 72.01% and 6.35% respectively. | - | Sucrose: 3% Photoperiod: 16/8 h Light intensity: 32.5 µE/m2 s Culture period: 6 weeks | [134] |
| Codonopsis lanceolata S.Z. | Leaf explant | flavonoids, total phenolic compound, and DPPH. | MS media + 1.0 mg/L IBA | MeJA and SA | By treatment with 20 µM MeJA, DPPH scavenging activity was 24.2. Flavonoids: 38.45 mg/g of C. lanceolata Phenolic content: 74.53 mg/g | _ | Culture period: 5 weeks Sucrose: 30 g/L | [135] |
| Curculigo orchioides G. | Leaf explants | Phenolics and Curculigoside | MS media + 3.0 mg/L NAA in liquid culture | _ | Adventitious roots grown in modified ¾ strength of MS medium showed the highest amount of curculigoside (76.521 µg/Treatment). | _ | ¾ strength of MS medium 4% (w/v) of sucrose | [136] |
| Decalepis salicifolia (Bedd. ex Hook.f.) | Leaf | Vanillin isomer: 2-hydroxy-4-methoxybenzaldehyde (2H4MB), | WPM liquid media + 0.5 mg/L NAA, 1.0 mg/L Kinetin, 0.3 mg/L IBA | - | The total production of 2H4MB was 4.9-fold higher in adventitious root culture (139.54 μg) as compared to field-grown plants (28.62 μg). | - | Sucrose: 5% pH: 7.0 culture period: 60 days | [128] |
| Echinacea purpurea L. | roots | caffeic acid derivatives caftaric acid, chlorogenic acid, and cichoric acid. | MS media + 2 mg/L IBA | _ | A 10-fold increase in biomass and secondary compounds. the concentration of cichoric acid was the highest (26.64 mg/g dry weight) | Balloon-type bubble bioreactor. | Sucrose: 5% inoculum density: 7 g/L FW aeration rate: 0.1 vvm | [114] |
| Echinecea pallida N. | Phenolics, flavonoids | ¾ MS media + 1 mg/L IBA | - | ARs were cultured in a 5-L air-lift bioreactor the bioactive compounds (53.5 mg/g phenolics and 37.6 mg/g flavonoids) reached the maximum values, and the productivities of phenolics and flavonoids were 398.7 and 280.4 mg/L. | Air-lift balloon type bioreactors | sucrose: 50 g/L pH: 5.8 nitrogen: 45 mM Phosphorus: 1.56 Mm Culture period: 30 days | [137] | |
| Eleutherococcus koreanum M. | Seed-derived plants | eleutherosides B&E, chlorogenic acid, total phenolics, and flavonoids | MS media + 5 mg/L 0.01 mg/L TDZ | MeJA and SA | At 303.93 mg/L of MeJA production of targeted bioactive compounds was 37.77%. High concentrations of MeJA and SA increased DPPH activity and H2O2 content in the roots. | Airlift bio- reactors | (HN4: NO3-, 5: 25) Sucrose: 30 g/L Gelrite: 2.3 g/L density of AR = 5 g/L aeration volume = 0.1 vvm | [84] |
| Eurycoma longifolia J. | Leaf | Eurycomanone and polysaccharides | ¾ MS media + 3 mg/L IBA | - | 8.8 mg/Leurycomanone and 2.4 g/L polysaccharides obtained after 40 days of culture in Bubble column bioreactor | Bubble column bioreactor | Sucrose: 40 g/L inoculation density: 5 g/L Aeration rate: 0.05 vvm Culture period: 40 days | [138] |
| Fagonia indica L. | callus explants | Gallic acid Rutin Myricetin Catechin Caffeic acid Apigenin | MS media + (0.5, 1.0 or 2.0 mg/L) IBA, IAA or NAA | MeJA PAA | By 0.5 mg/L MeJA treatment, maximum Total Phenolic Content (TPC; 6.0 mg GAE/g of dry weight) and Total Flavonoid Content (TFC; 5.0 mg QE/g of dry weight) were achieved. Gallic acid (148.0 ± 4.8 μg/mg of dry weight) Rutin (122.3 ± 3.8 μg/mg of dry weight) Apigenin (25.3 ± 0.6 μg/mg of dry weight), Caffeic acid (25.3 ± 0.6 μg/mg of dry weight) and Catechin (9.4 ± 0.07 μg/mg dry weight). | _ | Sucrose: 3% MS salts: 0.44% Culture period: 33 days relative humidity: 70% irradiance: 35–45 μ mol/m2 s pH: 5.6–5.8 | [139] |
| Gentiana scabra B. | Leaf | secoiridoids | MS media + 3.0 mg/L NAA and 0.25 mg/L TDZ | - | HPLC revealed Maximal gentiopicroside (25.59 ± 0.65 mg/g dry weight), swertiamarin (1.61 ± 0.04 mg/g dry weight) and sweroside (4.42 ± 0.11 mg/g dry weight) levels after 4 weeks culture | - | Phytohormones: 30 mg/L α-naphthalene acetic acid and 0.25 mg/L thidiazuron. Photoperiod: 16/8-h Culture period: 8 weeks | [140] |
| Ginseng C.A.Mey. and Echinacea L. | roots | ginsenosides and caffeic acid derivatives | MS media + 25 µM IBA | MeJA | Higher production of ginsenosides and caffeic acid derivates was achieved by the establishment of co-cultures with higher inoculum proportion of ginseng to Echinacea, ensued by elicitation 200 µM MeJA. | Air-Lift bioreactors | Co-culture system Inoculum proportion of Ginseng to Echinacea (4:1 and 3:2) Sucrose: 50 g/L | [83] |
| Glycyrrhiza uralensis DC. | root | Flavonoids glycyrrhizic acid glycyrrhetinic acid polysaccharide | MS media +1 mg/L IBA | 10 kDa protein fragments | 10 kDa protein fragments increased the Flavonoids, glycyrrhizic acid, glycyrrhetinic acid, and polysaccharide by up to 2.27-fold, 2.64-fold, 2.70-fold, and 2.32-fold, respectively as compared to control roots. | _ | Sucrose: 30 g/L Culture period: 35 days | [141] |
| Gynura procumbens L. | Leaf explant | flavonoid | MS media + 5 mg/L IBA | _ | Biomass yield of adventitious roots of G. procumbens in temporary immersion bioreactor increased by 5 folds. Isoflavon was detected in adventitious roots at low sucrose treatment. volatile compound and adipic acid were found in all treatments. | Temporary Immersion Bioreactors | various concentrations of sucrose (1, 3, and 5%) various immersion frequency (15 min each 12 h; 5 min each 3 h). Culture period: 21 days | [142] |
| Gynura procumbens L. | Young leaves and internodes | Phenolic compounds and flavonoids | MS media +5 mg/L IBA | _ | The greater yield of Biomass (75.38 ± 0.95 g/L), Total phenolic production (27.98 mg/dry weight), and Flavonoid production rates (256.24 mg/dry weight) were achieved from adventitious roots culture in the BTBB. | Balloon-Type Bubble Bioreactor (BTBB). | aeration rate: 0.15 vvm. inoculum density 3 g/L Sucrose: 30 g/L Culture period: 28 days. | [143] |
| Hybanthus enneaspermus L. | Leaf | L-Dopa | MS media + 0.5 mg/L Indole-3-butyric acid (IBA) | SA, Yeast extract, MeJA, AgNO3 | Among the different elicitors tested, exposure to SA at 100 µM dosage for 6 h enhanced L-dopa yield 12.64 mg/g dry weight (dry weight) when compared to control culture | - | sucrose: 3% pH: 5.8 exposure times (2–8 h) elicitation period: 6 h culture period: 30 days | [144] |
| Hypericum perforatum L. | roots | Naphthodianthrone derivatives | MS media + 1 mg/L of IBA | Different radiation treatment | hypericins production was enhanced by red light. four-weeks grown roots treated with one-week blue light was an effective stimulator for increasing total phenolic compounds and hypericins. | - | Sucrose: 30 g/L the photon flux density of 50 µmol/m2 s | [145] |
| Morinda coreia Buch.-Ham. | Leaf | Anthraquinones and phenolic compounds | ½ MS media+ 1.0 mg/L of Indole-3 butyric acid (IBA) | chitosan | On treatment with 0.4 mg/mL chitosan amount of anthraquinones (292.038 mg/g dry weight) and phenolics (86.8 mg/g dry weight) increased till 4th day of the elicitation | - | Two-phase and two-stage culture system ½ MS media Sucrose: (1.5%). chitosan (0.2, 0.4 and 0.8 mg/mL), growth ratio (5.082), fresh weight (1.568 g) and dry weight (0.163 g) of AR were recorded maximum with the concentration of 1.0 mg/L IBA. | [146] |
| Oldenlandia umbellate L. | shoots | Anthraquinones (AQ) | MS media+ 7.5 μM IBA 1 μM IAA | yeast extract, pectin, xylan, α-keto glutaric acid and L- phenylalanine and piroxicam | Treatment with 50 mg/L pectin, resulted in 2.19-fold increase in AQ production. | _ | Culture period: 60 days Sucrose: 3% pH: 5.8 | [147] |
| Oplopanax elatus N. | Seeds | flavonoids and anthraquinone | MS Media+ 5 mg/L IBA | MeJA | At 200 µM, MeJA significantly increased the contents of quercetin, aloe-emodin, rhein, and emodin, while 225 µM was the optimal concentration for kaempferide accumulation. | Air-lift balloon type bioreactor | Sucrose: 30 g/L pH: 5.8 Culture period: 30 days | [148,149] |
| Panax gingseng C.A.Mey. | Root | Ginsenosides | MS media+ 5 mg/L indole butyric acid | Fungal elicitor | The maximum ginsenoside content reached 29.6 mg/g dry weight when 30-day-old ARs were treated with 200 mg/L fungal elicitor for 8 days | Balloon-type Air-lift bioreactor | Sucrose: 30 g/L Culture period: 30 days 200 mg/L fungal elicitor was selected to treat 30-day-old ARs for 2, 4, 6, 8, and 10 days. | [150] |
| Panax quinquefolius L. | Root | Ginsenosides | MS media + 1 mg/L 2, 4-D, 0.25 mg/L kinetin | Pathogenic fungal elicitors | The maximum ginsenoside production (276.0 mg/L) was achieved with the A. panax (4 mg/L) extract. | Balloon-type airlift bioreactor | sucrose: 50 g/L pH: 5.8 ¾ MS medium supplemented with 5 mg/L IBA air volume: 100 mL/min Culture period: 30 days Elicitation period: 8 days | [90] |
| Panax vietnamensis Ha et Grushv. | Leaf | saponins | Modified MS Media + 5 and 7 mg/L IBA +0.5 or 1 mg/L of single BA, Kin and TDZ | JA, SA, YA and Chitosan (CHN) | Saponins maximum productivity was observed in 150 mg/L YE. | Bubble bioreactor | Sucrose: 30 g/L Ratio of NH4+NO3 7.19: 18.50 mM/mM pH: 5.8 culture period of 56 days | [151] |
| Perovskia abrotanoides Karel. | Young leaves | Cryptotanshinone tanshinone IIA | MS media + 2 mg/L (NAA) | yeast extract (YE), (MeJA), AgNO3, and sorbitol | Increased cryptotanshinone and tanshinone IIA production was achieved with 200 mg/L YE and 25 µM AgNO3 | _ | 3% sucrose pH 5.8 ± 0.1 Culture period: 3 weeks (dark) | [152] |
| Plumbago indica L. | Young leaf | plumbagin | Gamborg’s B5 liquid media + 0.1 mg/L NAA | Chitosan + Diaion®HP-20 addition | Plumagin increased upto 6.6-fold by chitosan treatment for 72 h. The sequential addition of Diaion®HP-20 (10 g/L) to the root cultures after the chitosan treatment for 48 h increased the plumbagin production up to 19.93 mg/gdry weight, which was 1.2- and 10-fold higher than the chitosan treated and untreated root cultures respectively. | - | Sucrose: 20 g/L chitosan concentration: 150 mg/L optimal contact period: 72 h Culture period: 20 days | [153] |
| Plumbago rosea L. | Leaf explants | Plumbagin | MS media + 1.5 mg/L IAA + 1 mg/L IBA | Jasmonic acid yeast extract and sodium salicylate | 50 µM jasmonic acid for three days increased plumbagin content in roots to 1.23% dry weight. | _ | 3/4th strength MS liquid media Sucrose: 30% Root inoculum: 2 g/L | [154] |
| Polygonum Multiflorum Thunb. | Leaf | Phenolics and flavonoids | Full-strength MS media + 2 mg/L | Methyl jasmonate (MeJA) and salicylic acid (SA) | Total phenolic compounds increased by 53.08 mg·g−1 dry weight and total flavonoids increased by 25.10 mg/g dry weight | Air lift bioreactor | sucrose: 5% Culture period: 4 weeks Aeration rate: 0.1 vvm (air volume flow per unit of culture volume per minute) | [87] |
| Prunella vulgaris L. | Leaf | Phenolics and flavonoids | MS + 0.5 mg/L NAA | - | Higher TPC (0.995 GAE mg/g-DRB) and TFC (6.615 RE mg/g- DRB) were observed in 0.5 mg/L NAA treated cultures. | - | sucrose: 30 g/L Photoperiod: 16/8 h Light intensity: 40 mol/m2 s Culture period: 49 days | [155] |
| Stevia rebudiana (Bertoni) Bertoni | Plantlets | Polyphenolics and Steviol Glycosides | MS media + gibberellic acid (GA3; 0.5, 1.0, 1.5 and 2.0 mg/L) and 0.5 mg/L NAA | Gibberellic acid (GA3) | The highest TFC accumulation was shown by 2.0 mg/L of GA3, as compared to the control culture (4.74 mg QE/g dry weight on day 30 and maximum stevioside content (7.13 mg/g dry weight) w GA3, as compared to the control culture 3.39 mg/g dry weight. | - | 2.0 mg/L of GA3 was optimum concentration for maximum biomass biosynthesis (13.12 g/flask) noticed in exponential phase on 27th day of culture. Culture period: 30 days | [156] |
| Talinum paniculatum Ruiz and Pav | shoot | Saponin | MS media + 10 µM IBA | MeJA and SA | By treatment with 0.2 mM MeJA and SA, saponin production increased by 1.5 and 1.3-fold. | _ | Culture period: 28 days Sucrose: 30 g/L | [157] |
| Talinum paniculatum Ruiz and Pav | Leaf explants | saponin | MS media + 2 mg/L IBA | _ | Saponin content was increased by combination of aeration rate of 0.5 vvm and inoculum density of 1 g/400 mL | Balloon type bubble bioreactor | Aeration rate: 0.25, 0.5 and 0.75 vvm Inoculum density: 0.5, 1, 2 g/400 mL. Culture period: 14 days | [22] |
| Tripterygium wilfordii Hook. f., | Leaf | celastrol | 1/2 MS media + 0.25 mg/L indole-3-butyric Acid and 0.25 mg/L naphthylacetic acid | Methyl jasmonate (MeJA) and salicylic acid (SA) | 100 μM MeJA significantly increased celastrol content in adventitious roots to 6321.27 μg/g dry weight | - | Sucrose: 30 g/L Photoperiod: 16/8 h (day/night) Culture period: 6 weeks | [158] |
| Withania somnifera (L.) Dunal | Leaf | Withanolides | ½ MS media + 0.5 mg/L IBA,0.1 mg/L IAA | Methyl jasmonate and salicylic acid | 150 μM SA for 4 h elicitor exposure period resulted in the increase production of withanolide A (48-fold), B (29-fold), withaferin A (20-fold), withanone (37-fold 12-deoxy withastramonolide (nine-fold), withanoside V (seven-fold), withanoside IV (nine-fold) | - | culture age: 30 days old elicitation period: 6 h Culture period: 50 days. biomass: kolli hills variety maximum fresh weight (11.70 g), dry weight (1.90 g) Cumbum variety maximum fresh weight (11.40 g), dry weight (1.85 g) | [159] |
| Plant Species | Strain | Explant | Valuable Compound | Media + PGRs | Elicitation Strategy | Increase in Yield of Valuable Compound | Bioreactor Employed | Optimization Strategy for Culture Conditions and Bioreactor | References |
|---|---|---|---|---|---|---|---|---|---|
| Ajuga bracteosa Wall. ex Benth. | A4, LBA-9402 and ARqua1. | Leaves | phytoecdysteroids | MS media no PGRs | MeJA and coronatine (Cor) | In comparison with the unelicited hairy roots, MeJA doubled phytoecdysteriod content i.e., 8356 µg/g after 14 days of Elicitation. | _ | Sucrose: 30 g/L Solidifying agent:0.8% phytagel | [127] |
| Artemisia annua L. | LBA 9402 | seedlings | artemisinin | MS media with no PGRs | MeJA Fungal elicitors: Alternaria alternate, Curvularia limata, Fusarium solani, and Piriformospora indica | By using P. indica artemisinin production was increased by 1.97 times. By using combination of MeJA and cell homogenate of P.indica artemisnin production was enhanced by 2.44 times. | _ | Sucrose: 30 g/L | [160] |
| Astragulas membranceus L. | Agrobacterium rhizogenes LBA9402 | Leaf | Phytoalexins | MS media with no PGRs | Chitosan | Treatment with 100 mg/L of chitosan increased yields of formononetin and calycosin by 12.45- and 6.17-fold. | _ | Elicitor exposure time: 24 h. Chitosan: 100 mg/L Culture period: 34 days | [161] |
| Celastrus paniculatus W. | MTCC532 | Leaf explant | Celastrol | MS media + 0.3 mg/L IAA | Silver nanoparticles and acetosyringone | Celastrol increased 2.24-fold by treatment with 10 µg mL−1 of AgNPs (48 h exposure) | _ | 3% sucrose pH 5.8 Culture period: 2 weeks | [133] |
| Echinacea purpurea L. | ATCC 43,057 | leaf explants | Phenolics Flavonoids caffeic acid derivatives | MS media no PGRs | 24-epibrassinolide and l-phenylalanine | 1.0 mg/L 24-eBL gave maximum production of phenolics, total flavonoids, cichoric acid, caftaric acid, Echinacoside, and p-coumaric acid. | _ | Culture period:21 Days Sucrose: 50 g/L | [115] |
| Eurycoma longifolia J. | A. rhizogenes strain A4 | Root | 9-methoxycanthin-6-one compound | (MS) basal media with no PGRs | MeJA and SA. | 0.1 mM MeJA increased production of 9-methoxycanthin-6-one up to three folds as compared to control. | _ | pH:4.9 Culture period:12 weeks inoculum size: 0.2 g hairy root in 50 mL of MS basal media | [162] |
| Gentiana scabra B. | A. rhizogenes strains ATCC15834 | Leaf | Iridoids and secoiridoids | B5 media + 1.0 mg/L NAA, (TDZ), zeatin, IBA | Acetosyringone | Loganic acid increased 6.6- fold in the presence of zeatin (1 mg/L) and gentiopicroside accumulation was 1.8- fold higher in the presence of NAA, 1 mg/L and 1.0 mg/L NAA yield 1.4- and 2.5- fold higher gentiopicroside and swertiamarin. | _ | Co-cultivation period: 48 h. Culture period:8 weeks pH:5.7 ± 0.1 N6, WPM, MS and B5 media were tested. B5 liquid media was most suitable | [140] |
| Glycyrrhiza glabra L. | A4 | Shoots | glycyrrhizin | ½ MS medium + 0.1 mg/L IAA | PEG, CdCl2, cellulase, and mannan | 1% PEG enhanced glycyrrhizin yield up to 5.4-fold. 200 µg m/L cellulase enhanced glycyrrhizin yield up to 8.6-fold. 10 mg/L mannan enhanced the yield of glycyrrhizin up to 7.8-fold. | _ | 16 h light/8 h dark period. 100 µM acetosyringone | [163] |
| Hybanthus enneaspermus L. | A. rhizogenes strains A4, A4T, 8196 and LBA 9402 | Leaf or internode explants | coumarin | MS media+ 0.25 mg/L, 16N-benzyladenine (BA+ 0.1 mg/L (IAA) | Acetosyringone | Coumarin accumulation increased three folds in the superior rhizoclone of A4 origin (A4-HRL-2B7) (3.25 mg/g d.wt. extract) as compared to that in natural roots. | _ | Photoperiod: 16 h Culture period: 4 weeks | [164] |
| Panax ginseng C.A.Mey. | Agrobacterium rhizogenes | Root | ginsenosides | ½ MS media + no PGRs | Tween 80 | Coumarin accumulation increased three folds in the superior rhizoclone of A4 origin (A4-HRL-2B7) (3.25 mg/g d.wt. extract) as compared to that in natural roots. | _ | sucrose: 3% inoculum length: 20 mm long Tween 80: 1.2% w/v Culture period: 4 weeks | [165] |
| Panax quinquefolius L. | A.rhizogenes ATCC 15834 | seedlings | ginsenosides | Gamborg media + no PGRs | Yeast extract | (3 days time of exposure and 50 mg/L of YE) increased total ginsenoside content up to 32.25 mg/g D.W | Nutrient sprinkle Bioreactor | Sucrose: 30 g/L YE: 50 mg/L Elicitation period:3 and 7 days Incubation period: 5 weeks | [166] |
| Panax vietnamensis Ha et Grushv. | Rhizobium rhizogenes ATCC 15,834 strain | shoot explants | Ocotillol-type ginsenosides | ½ MS media + no PGRs | _ | With culture conditions the PPD contents evaluated at 0.57% dry weight, the PPT at 0.028% dry weight, and the OCT at 4.3% dry weight in hairy roots. | _ | Co-cultivation system sucrose: 3% (w/v) culture period: 90 days | [167] |
| Perovskia abrotanoides Karel. | ATCC15834 TR105, and R1000 | Seedlings and nodes | Cryptotanshinone and tanshinone IIA | MS media No PGR | Acetosyringone | Transformation frequency increased by up to 60.99% when 100 µM of acetosyringone was used. cryptotanshinone and tanshinone IIA levels were 53.17 ± 0.26 and 14.48 ± 0.30 µg/g dry weight, respectively in hairy roots induced by TR105. | _ | Half strength MS media Sucrose:3% | [152] |
| Plumbago indica L. | ATCC 15834 | Leaf explant | Plumbagin | MS media with no PGRs | Yeast carbohydrate fraction Chitosan Manganese chloride Copper chloride and MeJA | Plumbagin production was enhanced to 1.2–2-fold by treatment with Yeast carbohydrate fraction, chitosan, manganese chloride, copper chloride and MeJA. With 20 days old bioreactor-culture, and exposure of chitosan (200 mg/L) and methyl jasmonate (80 μM) plumbagin production was enhanced to 13.16 ± 1.72 mg g−l dry weight. | Bioreactor with continuous air supply | Bioreactor was maintained in dark at 25 ± 2 °C Sucrose: 3% | [168] |
| Polygonum multiflorum Thunb. | A. rhizogenes strain KCCM 11879 | Leaf | phenolic compounds | MS media + no PGRs | MeJA | Exposure to 50 μM methyl jasmonate for 5 days increased levels of phenolic compounds more than 2.5-fold. | _ | Sucrose: 3% Inoculum density: 0.5 g/100 mL Culture period: 21 days | [88] |
| Prunella vulgaris L., | A. rhizogenes (ATCC15834) | Leaf | Rosmarinic acid | MS media + no PGRs | Ethephon and SA | Rosmarinic acid accumulation increased by 1.66-fold 8 days after Eth elicitation and 1.48-fold 2 days post-SA addition. | _ | Sucrose: 3% Elicitation period:8 days Culture period: 30 days | [169] |
| Stevia rebudiana (Bertoni) Bertoni | A4 strain | Nodal explant | Steviosides | MS media + BAP (0.5–2.0 mg/L) 0.5 mg NAA | Light | Stevioside contents in the SRA4 HR clone on day 75th increased 0.247 ± 0.011 to 1.72 ± 0.052 mg/g dry weight in the root tissues and 0.097 ± 0.072 to 2.12 ± 0.06 mg/L in the media under light conditions | _ | co-cultivation period:2–3 days culture period: 75 days agitation rate: 80 rpm ½ MS Media +1.0 mg/LBAP + 0.1 mg/L NAA | [170] |
| Talinum paniculatum Ruiz and Pav | LB510 | Leaves | saponin | MS media with no PGRs | - | MS medium supplemented with 5% sucrose and 2.0 strength potassium nitrate of MS, produced the maximum saponin content. | balloon-type bubble bioreactor | Inoculum density: 2 g/400 mL Aeration rate: 0.25 vvm Culture period:14 days | [171] |
| Tripterygium wilfordii Hook. F., | A.rhizogenes ATCC15834 | Root | wilforgine and wilforine | MS media + no PGRs | - | 10:50 mM NH4+/NO3− and 0.3125 mM phosphate increased wilforgine and wilforine production by 42% and 48%. | _ | Incubation period: 7 to 42 days. sucrose: 30 g/L NH4+/NO3: 10:50 mM Phosphate:0.3125 mM pH: 5.8 | [158] |
| Valerian jatamansi Jones. | R1601 | Young Leaves | Valtrate | MS media No PGR | MeJA JA SA | By treatment with 100 mg/L MeJA, production of Valtrate was increased to a level of 3.63 times, which was higher than non-elicited control. | _ | pH: 5.9 Subculturing of hairy roots after every 5 weeks. | [172] |
| Withania sominefera (L.) Dunal | Agrobacterium tumefaciens C58C1 (pRiA4) | Leaf | Withaferin A | ½ MS media + no PGRs | _ | WFA in THRs contain 1.51-fold more WFA (330 ± 0.87 µg/g dry weight (dry weight)) than AHRs (218 ± 0.17 µg/g dry weight) | _ | culture period: 40 days biomass doubling time of THRs and AHRs: 18 and 30 days | [173] |
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, J.L.; Li, J.; Li, J.X.; Liu, S.J.; Huang, L.Q.; Gao, W.Y. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Kundu, S.; Salma, U.; Gantait, S. Cryopreservation of Medicinal Herbs: Major Breakthroughs, Hurdles and Future. In Biotechnological Approaches for Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 353–381. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.W.; Murthy, H.N.; Jeong, C.S.; Hahn, E.J.; Paek, K.Y. Organic germanium stimulates the growth of ginseng adventitious roots and ginsenoside production. Process. Biochem. 2005, 40, 2959–2961. [Google Scholar] [CrossRef]
- Sui, Q.; Jiang, C.; Yu, D.; Chen, M.; Zhang, J.; Wang, Y.; Wei, Y. Performance of a sequencing-batch membrane bioreactor (SMBR) with an automatic control strategy treating high-strength swine wastewater. J. Hazard. Mater. 2018, 342, 210–219. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N.; Hahn, E.J.; Zhong, J.J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv. Biochem. Eng. Biotechnol. 2009, 113, 151–176. [Google Scholar]
- Sivakumar, G.; Yu, K.; Paek, K. Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng. Life Sci. 2005, 5, 333–342. [Google Scholar] [CrossRef]
- Habibi, P.; De Sa, M.F.G.; Makhzoum, A.; Malik, S.; da Silva, A.L.L.; Hefferon, K.; Soccol, C.R. Bioengineering hairy roots: Phytoremediation, secondary metabolism, molecular pharming, plant-plant interactions and biofuels. Sustain. Agric. Rev. 2017, 22, 213. [Google Scholar]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [Green Version]
- Ncube, E.N.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Metabolite profiling of the undifferentiated cultured cells and differentiated leaf tissues of Centella asiatica. Plant Cell Tissue Organ Cult. 2017, 129, 431–443. [Google Scholar] [CrossRef]
- Deepthi, S.; Satheeshkumar, K. Effects of major nutrients, growth regulators and inoculum size on enhanced growth and camptothecin production in adventitious root cultures of Ophiorrhiza mungos L. Biochem. Eng. J. 2017, 117, 198–209. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Paek, K.Y. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem. Rev. 2016, 15, 129–145. [Google Scholar] [CrossRef]
- Gantet, P.; Imbault, N.; Thiersault, M.; Doireau, P. Necessity of a functional octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol. 1998, 39, 220–225. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Curtis, W.R. Characterization of fluid-flow resistance in root cultures with a convective flow tubular bioreactor. Biotechnol. Bioeng. 1998, 60, 375–384. [Google Scholar] [CrossRef]
- Sudha, C.G.; Seeni, S. Establishment and analysis of fast-growing normal root culture of Decalepis arayalpathra, a rare endemic medicinal plant. Curr. Sci. 2001, 81, 371–374. [Google Scholar]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Harnessing the potential of hairy roots: Dawn of a new era. TRENDS Biotechnol. 2006, 24, 403–409. [Google Scholar] [CrossRef]
- Liu, C.Z.; Abbasi, B.H.; Gao, M.; Murch, S.J.; Saxena, P.K. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J. Agric. Food Chem. 2006, 54, 8456–8460. [Google Scholar] [CrossRef]
- Pistelli, L.; Giovannini, A.; Ruffoni, B.; Bertoli, A.; Pistelli, L. Hairy root cultures for secondary metabolites production. Adv. Exp. Med. Biol. 2010, 698, 167–184. [Google Scholar]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea biotechnology: Advances, commercialization and future considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Roy, A. Hairy Root Culture an Alternative for Bioactive Compound Production from Medicinal Plants. Curr. Pharm. Biotechnol. 2021, 22, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.P.; Han, J.; Zhang, J.F.; Zhu, C.S.; Zhang, X. A MDR transporter contributes to the different extracellular production of sesquiterpene pyridine alkaloids between adventitious root and hairy root liquid cultures of Tripterygium wilfordii Hook. f. Plant Mol. Biol. 2017, 95, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Son, S.H.; Yun, S.R.; Kwon, O.W.; Seon, J.H.; Paek, K.Y. Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tissue Organ Cult. 2000, 62, 187–193. [Google Scholar] [CrossRef]
- Baque, M.A.; Moh, S.H.; Lee, E.J.; Zhong, J.J.; Paek, K.Y. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, S.J.; Kang, Y.M.; Jung, H.Y.; Prasad, D.T.; Kim, S.W.; Choi, M.S. Production of ginkgolides and bilobalide from optimized theGinkgo biloba cell culture. Biotechnol. Bioprocess Eng. 2004, 9, 41–46. [Google Scholar] [CrossRef]
- Yusuf, N.A.; Rahim, N.S.M.; Azhar, S.Z.A.; Ghani, K.A.; Sommano, S.; Khalid, N. Adventitious root cultures of Boesenbergia rotunda as a source of Pinostrobin. Int. J. Adv. Sci. Eng. IT 2018, 8, 377–383. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, H.G.; Kim, J.C.; Sim, S.J.; Min, J.Y.; Hwang, J.G.; Choi, M.S. Effects of culture media on catechins and caffeine production in adventitious roots of tea tree (Camellia sinensis L.). Agric. Life Sci. Res. 2013, 47, 11–20. [Google Scholar]
- Wu, C.H.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J. Plant Biol. 2007, 50, 636–643. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yang, T.J.; Park, S.U.; Baek, J.H.; Wu, S.; Lim, K.B. Induction and Proliferation of Adventitious Roots from ‘Aloe vera’ Leaf Tissues for ‘In Vitro’ Production of Aloe-Emodin. Plant Omics 2011, 4, 190–194. [Google Scholar]
- Lee, Y.S.; Ju, H.K.; Kim, Y.J.; Lim, T.G.; Uddin, M.R.; Kim, Y.B.; Yang, T.J. Enhancement of anti-inflammatory activity of Aloe vera adventitious root extracts through the alteration of primary and secondary metabolites via salicylic acid elicitation. PLoS ONE 2013, 8, e82479. [Google Scholar] [CrossRef]
- Khalafalla, M.M.; Daffalla, H.M.; El-Shemy, H.A.; Abdellatef, E. Establishment of in vitro fast-growing normal root culture of Vernonia amygdalina-A potent African medicinal plant. Afr. J. Biotechnol. 2009, 8, 5952–5957. [Google Scholar]
- Hussein, S.; Ling, A.P.K.; Ng, T.H.; Ibrahim, R.; Paek, K.Y. Adventitious roots induction of recalcitrant tropical woody plant, Eurycoma longifolia. Rom. Biotechnol. Lett. 2012, 17, 7027. [Google Scholar]
- Hahn, E.J.; Kim, Y.S.; Yu, K.W.; Jeong, C.S.; Paek, K.Y. Adventitious root cultures of Panax ginseng CV Meyer and ginsenoside production through large-scale bioreactor system. J. Plant Biotechnol. 2003, 5, 1–6. [Google Scholar]
- Praveen, N.; Manohar, S.H.; Naik, P.M.; Nayeem, A.; Jeong, J.H.; Murthy, H.N. Production of andrographolide from adventitious root cultures of Andrographis paniculata. Curr. Sci. 2009, 96, 694–697. [Google Scholar]
- Manokari, M.; Shekhawat, M.S. Implications of auxins in induction of adventitious roots from leaf explants of cannon ball tree (Couroupita guianensis Aubl.). World Sci. News 2016, 33, 109–121. [Google Scholar]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baque, M.A.; Hahn, E.J.; Paek, K.Y. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotechnol. Rep. 2010, 4, 109–116. [Google Scholar] [CrossRef]
- Ling, A.K.; Kok, K.M.; Hussein, S.; Ong, S.L. Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am.-Eurasian J. Sustain. Agric. 2009, 3, 493–501. [Google Scholar]
- Lee, E.J.; Mobin, M.; Hahn, E.J.; Paek, K.Y. Effects of sucrose, inoculum density, auxins, and aeration volume on ceil growth of Gymnema sylvestre. J. Plant Biol. 2006, 49, 427–431. [Google Scholar] [CrossRef]
- Wu, C.H.; Dewir, Y.H.; Hahn, E.J.; Paek, K.Y. Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J. Plant Biol. 2006, 49, 193–199. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hahn, E.J.; Yeung, E.C.; Paek, K.Y. Lateral root development and saponin accumulation as affected by IBA or NAA in adventitious root cultures of Panax ginseng CA Meyer. Vitr. Cell. Dev. Biol.-Plant 2003, 39, 245–249. [Google Scholar] [CrossRef]
- Kollárová, K.; Lišková, D.; Kákoniová, D.; Lux, A. Effect of auxins on Karwinskia humboldtiana root cultures. Plant Cell Tissue Organ Cult. 2004, 79, 213–221. [Google Scholar] [CrossRef]
- Zhou, X.; Zhong, J.J. Plant cell culture, secondary product accumulation. Encycl. Ind. Biotechnol. Bioprocess Biosep. Cell Technol. 2010, 7, 1–28. [Google Scholar]
- Rao, R.S.; Ravishankar, A.G. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Liu, C.Z.; Guo, C.; Wang, Y.C.; Ouynag, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process. Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Kakegawa, K.; Hattori, E.; Koike, K.; Takeda, K. Induction of anthocyanin synthesis and related enzyme activities in cell cultures of Centaurea cyanus by UV-light irradiation. Phytochemistry 1991, 30, 2271–2273. [Google Scholar] [CrossRef]
- Mulder-Krieger, T.; Verpoorte, R.; Svendse, A.; Cheffer, J. Production of essential oils and flavours in plant cell and tissue cultures. A review. Plant Cell Tissue Organ Cult. 1988, 13, 85–154. [Google Scholar] [CrossRef]
- Jeong, C.S.; Murthy, H.N.; Hahn, E.J.; Lee, H.L.; Paek, K.Y. Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of ginseng (Panax ginseng CA Meyer). Acta Physiol. Plant. 2009, 31, 219–222. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, K.A.; Bisaria, S.V. Bioprocess. considerations for production of secondary metabolites by plant cell suspension cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Verpoorte, R.; Contin, A.; Memelink, J.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, K.S.; Pyo, B.S.; Hwang, B. Saponin production by hairy root cultures of Panax ginseng CA Meyer: Influence of PGR and polyamines. Biotechnol. Bioprocess Eng. 1999, 4, 309–312. [Google Scholar] [CrossRef]
- Dicosmo, F.; Misawa, M. Plant cell and tissue culture: Alternatives for metabolite production. Biotechnol. Adv. 1995, 13, 425–453. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca2+ signalling in plant immune response: From pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; He, X.; Dixon, R.A. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biol. 2008, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence–A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Peebles, C.A.; Hughes, E.H.; Shanks, J.V.; San, K.Y. Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metab. Eng. 2009, 11, 76–86. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Shibuya, N.; Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.J.; Brodschelm, W.; Spannagl, E.; Zenk, M.H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. USA 1993, 90, 7490–7494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotechnol. 2013, 171, 330–340. [Google Scholar] [CrossRef]
- Anasori, P.; Asghari, G. Effects of light and differentiation on gingerol and zingiberene production in callus culture of Zingiber officinale Rosc. Res. Pharm. Sci. 2008, 3, 59–63. [Google Scholar]
- Kumari, R.S.B.; Agrawal, S.; Singh, N.K.D. Supplemental ultraviolet-B induced changes in essential oil composition and total phenolics of Acorus calamus L. (sweet flag). Ecotoxicol. Environ. Saf. 2009, 72, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ. Exp. Bot. 2007, 59, 109–116. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Yi Li, W.; Wen Wang, J. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Ghanati, F.; Bakhtiarian, S. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop. J. Pharm. Res. 2014, 13, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Sharafi, E.; Fotokian, M.H.; Loo, H. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of John’swort (Hypericum perforatum L). J. Med. Plants By-Prod. 2013, 2, 177–184. [Google Scholar]
- Raei, M.; Angaji, S.A.; Omidi, M.; Khodayari, M. Effect of abiotic elicitors on tissue culture of Aloe vera. Int. J. Biosci. 2014, 5, 74–81. [Google Scholar]
- Zhong, J.J. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv. Biochem. Eng. Biotechnol. 2001, 72, 1–26. [Google Scholar] [PubMed]
- Zhong, J.J.; Pan, Z.W.; Wang, Z.Y.; Wu, J.; Chen, F.; Takagi, M.; Yoshida, T. Effect of mixing time on taxoid production using suspension cultures of Taxus chinensis in a centrifugal impeller bioreactor. J. Biosci. Bioeng. 2002, 94, 244–250. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym. Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Chiou, S.Y.; Sung, J.M.; Huang, P.W.; Lin, S.D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Smart, N.J.; Fowler, M.W. Effect of aeration on large-scale cultures of plant cells. Biotechnol. Lett. 1981, 3, 171–176. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.I.; Wang, L.; Huang, D.; Ho, C.T.; Sang, S. Stilbene glucoside from Polygonum multiflorum Thunb.: A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 2010, 58, 2239–2245. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, Y.; Zheng, T.; Li, X.; Zhai, R. Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb. Carbohydr. Polym. 2014, 99, 765–773. [Google Scholar] [CrossRef]
- Ho, T.T.; Jeong, C.S.; Lee, H.; Park, S.Y. Effect of explant type and genotype on the accumulation of bioactive compounds in adventitious root cultures of Polygonum multiflorum. Plant Cell Tissue Organ Cult. 2019, 137, 115–124. [Google Scholar] [CrossRef]
- Han, M.N.; Lu, J.M.; Zhang, G.Y.; Yu, J.; Zhao, R.H. Mechanistic studies on the use of Polygonum multiflorum for the treatment of hair graying. BioMed Res. Int. 2015, 2015, 651048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.H.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Establishment of adventitious root co-culture of Ginseng and Echinacea for the production of secondary metabolites. Acta Physiol. Plant. 2008, 30, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Park, S.Y.; Paek, K.Y. Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tissue Organ Cult. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Murthy, H.N.; Praveen, N. Carbon sources and medium pH affects the growth of Withania somnifera (L.) Dunal adventitious roots and withanolide A production. Nat. Prod. Res. 2013, 27, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Lee, K.J.; Lee, J.D.; Bhushan, S.; Paek, K.Y.; Park, S.Y. Adventitious root culture of Polygonum multiflorum for phenolic compounds and its pilot-scale production in 500 L-tank. Plant Cell Tissue Organ Cult. 2017, 130, 167–181. [Google Scholar] [CrossRef]
- Ho, T.T.; Le, K.C.; Kim, S.W.; Park, S.Y. Culture condition optimization and FT-IR analysis of Polygonum multiflorum Thunb adventitious root cultures grown in an air-lift bioreactor system. Plant Cell Tissue Organ Cult. 2021, 144, 371–381. [Google Scholar]
- Ho, T.T.; Lee, J.D.; Jeong, C.S.; Paek, K.Y.; Park, S.Y. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. Appl. Microbiol. Biotechnol. 2018, 102, 199–209. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, E.H.; Kim, S.H.; Chung, I.M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, W.B.; Li, X.Y.; Piao, X.C.; Jiang, J.; Lian, M.L. Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius during bioreactor culture. Ind. Crop. Prod. 2016, 94, 729–735. [Google Scholar] [CrossRef]
- Huang, B.; Lin, H.; Yan, C.; Qiu, H.; Qiu, L.; Yu, R. Optimal inductive and cultural conditions of Polygonum multiflorum transgenic hairy roots mediated with Agrobacterium rhizogenes R1601 and an analysis of their anthraquinone constituents. Pharmacogn. Mag. 2014, 10, 77. [Google Scholar]
- Murthy, H.N.; Dijkstra, C.; Anthony, P.; White, D.A.; Davey, M.R.; Power, J.B.; Paek, K.Y. Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J. Integr. Plant Biol. 2008, 50, 975–981. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R. Opportunities and challenges in the cultivation of Ashwagandha {Withania somnifera (L.) Du-nal}. J. Pharmacogn. 2012, 3, 88–91. [Google Scholar]
- Kumar, A.; Kaul, M.K.; Bhan, M.K.; Khanna, P.K.; Suri, K.A. Morphological and chemical variation in 25 collections of the Indian medicinal plant, Withania somnifera (L.) Dunal (Solanaceae). Genet. Resour. Crop. Evol. 2007, 54, 655–660. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Sharada, M.; Ahuja, A.; Vij, S.P. Applications of Biotechnology in Indian Ginseng (Ashwagandha): Progress and Prospects. In Recent Advances in Plant Biotechnology and Its Applications; IK International Pvt. Ltd.: New Delhi, India, 2008; pp. 645–667. [Google Scholar]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Sharma, P.K.; Dudhe, R.; Singh, S. Biological activities of Withania somnifera. Ann. Biol. Res. 2010, 1, 56–63. [Google Scholar]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef]
- Kaileh, M.; Berghe, W.V.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; Haegeman, G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef] [Green Version]
- Sivanandhan, G.; Arun, M.; Mayavan, S.; Rajesh, M.; Jeyaraj, M.; Dev, G.K.; Ganapathi, A. Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl. Biochem. Biotechnol. 2012, 168, 681–696. [Google Scholar] [CrossRef]
- Dhar, R.S.; Verma, V.; Suri, K.A.; Sangwan, R.S.; Satti, N.K.; Kumar, A.; Qazi, G.N. Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry 2006, 67, 2269–2276. [Google Scholar] [CrossRef]
- Praveen, N.; Murthy, H.N. Production of withanolide-A from adventitious root cultures of Withania somnifera. Acta Physiol. Plant. 2010, 32, 1017–1022. [Google Scholar] [CrossRef]
- Rangaraju, S.; Lokesha, A.N.; Aswath, C.R. Improved production of withanolides in adventitious root cultures of Withania somnifera by suspension culture method. Biosci. Biotech. Res. Comm. 2019, 12, 73–79. [Google Scholar]
- Rani, G.; Arora, S.; Nagpal, A. Direct rhizogenesis from in vitro leaves of Withania somnifera (L.) Dunal. J. Herbs Spices Med. Plants 2003, 10, 47–54. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Arun, M.; Mayavan, S.; Rajesh, M.; Mariashibu, T.S.; Manickavasagam, M.; Ganapathi, A. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind. Crop. Prod. 2012, 37, 124–129. [Google Scholar] [CrossRef]
- Praveen, N.; Murthy, H.N. Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind. Crop. Prod. 2012, 35, 241–243. [Google Scholar] [CrossRef]
- Pandey, H.; Pandey, P.; Pandey, S.S.; Singh, S.; Banerjee, S. Meeting the challenge of stevioside production in the hairy roots of Stevia rebaudiana by probing the underlying process. Plant Cell Tissue Organ Cult. 2016, 126, 511–521. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Dev, G.K.; Jeyaraj, M.; Rajesh, M.; Arjunan, A.; Muthuselvam, M.; Ganapathi, A. Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult. 2013, 114, 121–129. [Google Scholar] [CrossRef]
- Thilip, C.; Mehaboob, V.M.; Varutharaju, K.; Faizal, K.; Raja, P.; Aslam, A.; Shajahan, A. Elicitation of withaferin-A in hairy root culture of Withania somnifera (L.) Dunal using natural polysaccharides. Biologia 2019, 74, 961–968. [Google Scholar]
- Abbasi, B.H.; Saxena, P.K.; Murch, S.J.; Liu, C.Z. Echinacea biotechnology: Challenges and opportunities. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 481–492. [Google Scholar] [CrossRef]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.A.; Wu, C.H.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Application of an airlift bioreactor system for the production of adventitious root biomass and caffeic acid derivatives of Echinacea purpurea. Biotechnol. Bioprocess Eng. 2009, 14, 91–98. [Google Scholar] [CrossRef]
- Demirci, T.; Akçay, U.Ç.; Baydar, N.G. Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Zhu, L.; Chen, L.; Zhang, Y. Genetic transformation of Echinacea purpurea with Agrobacterium rhizogenes and bioactive ingredient analysis in transformed cultures. Colloids Surf. B Biointerfaces 2006, 53, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Kết, N.V.; Anh, T.T.L. ẢNH HƯỞNG CỦA HÀM LƯỢNG KHOÁNG ĐA LƯỢNG VÀ BỔ SUNG DINH DƯỠNG VÀO GIAI ĐOẠN SAU CỦA QUÁ TRÌNH NUÔI CẤY ĐẾN SỰ SINH TRƯỞNG HUYỀN PHÙ TẾ BÀO SÂM NGỌC LINH. Tạp Chí Khoa Học Đại Học Đà Lạt 2017, 6, 419–430. [Google Scholar] [CrossRef]
- Demirci, T.; Akçay, U.Ç.; Baydar, N.G. Physical and biochemical differences in Agrobacterium rhizogenes-mediated transgenic hairy root lines of Echinacea purpurea. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 875–881. [Google Scholar] [CrossRef]
- Abdoli, M.; Mehrpooya, Z.; Talebian, M.R. Effect of salicylic acid and yeast extract on caffeic acid derivatives production in Echinacea purpurea L. J. Med. Plants 2021, 20, 36–47. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Dandin, V.S.; Paek, K.Y.; Park, S.Y. Biotechnological Production of Useful Phytochemicals from Adventitious Root Cultures. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 469–485. [Google Scholar]
- Aboseidah, A.A.; El-hamahmy, A.E.; Abo-Elsoud, I.; Ali, E.M. Effect of UV-C Radiation on Egyptian Henbane (Hyoscyamus muticus L.) Callus Growth and Biochemical Components. Hortscience J. Suez Canal Univ. 2019, 8, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Hairy Roots Biotechnology Unzipped: A Journey of Reality and Promises. In Hairy Root Cultures Based Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–10. [Google Scholar]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Dilshad, E.; Rani, R.; Khan, R.S. Production of biomass and medicinal metabolites through adventitious roots in Ajuga bracteosa under different spectral lights. J. Photochem. Photobiol. B Biol. 2019, 193, 109–117. [Google Scholar] [CrossRef]
- Hafeez, K.; Andleeb, S.; Ghousa, T.G.; Mustafa, R.; Naseer, A.; Shafique, I.; Akhter, K. Phytochemical screening, alpha-glucosidase inhibition, antibacterial and antioxidant potential of Ajuga bracteosa extracts. Curr. Pharm. Biotechnol. 2017, 18, 336–342. [Google Scholar] [CrossRef]
- Imran, M.; Jan, H.; Faisal, S.; Shah, S.A.; Shah, S.; Khan, M.N.; Syed, S. In vitro examination of anti-parasitic, anti-Alzheimer, insecticidal and cytotoxic potential of Ajuga bracteosa Wallich leaves extracts. Saudi J. Biol. Sci. 2021, 28, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, M.A.; Ullah, N.; Khan, R.S. Impacts of hormonal elicitors and photoperiod regimes on elicitation of bioactive secondary volatiles in cell cultures of Ajuga bracteosa. J. Photochem. Photobiol. B Biol. 2018, 183, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kayani, W.K.; Hasan, H.; Mirza, B. Advances in Genetic Engineering of Ajuga Species. In Biotechnological Approaches for Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 599–629. [Google Scholar]
- Rodrigues, V.; Kumar, A.; Prabhu, K.N.; Pragadheesh, V.S.; Shukla, A.K.; Sundaresan, V. Adventitious root cultures of Decalepis salicifolia for the production of 2-hydroxy-4-methoxybenzaldehyde, a vanillin isomer flavor metabolite. Appl. Microbiol. Biotechnol. 2021, 105, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Nabi, N.; Singh, S.; Saffeullah, P. Responses of in vitro cell cultures to elicitation: Regulatory role of jasmonic acid and methyl jasmonate: A review. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 341–355. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Khan, T.; Jan, A.; Ahmad, P. Iron nano modulated growth and biosynthesis of steviol glycosides in Stevia rebaudiana. Plant Cell Tissue Organ Cult. 2020, 143, 121–130. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging Dis. 2017, 8, 868. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Gao, H.; Zhang, C.; Cao, Q. Enhanced production of calycosin-7-O-β-D-glucoside and astragaloside Ⅳ from adventitious root cultures of Astragalus membranaceus var. mongholicus by green leaf volatiles. Ind. Crop. Prod. 2021, 168, 113598. [Google Scholar] [CrossRef]
- Moola, A.K.; Kumar, T.S.; Kumari, B.R. Enhancement of Celastrol compound by silver nanoparticles and acetosyringone in Celastrus paniculatus Willd. through adventitious and hairy root culture. J. Plant Biochem. Biotechnol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Lee, R.X.; Hassan, Z.; Subramaniam, S.; Chew, B.L. Adventitious root cultures of Clitoria ternatea L. and its potential as a memory enhancer alternative. Plant Biotechnol. Rep. 2021, 15, 163–176. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Ha, Y.; Li, J.; Su, Z.; Quan, X.; Wu, W. Drought stress-induced methyl jasmonate accumulation promotes calycosin-7-O-β-d-glucoside production in Astragalus membranaceus adventitious roots. Plant Cell Tissue Organ Cult. 2021, 147, 561–568. [Google Scholar] [CrossRef]
- Swamy, M.K.; Paramashivaiah, S.; Hiremath, L.; Akhtar, M.S.; Sinniah, U.R. Micropropagation and Conservation of Selected Endangered Anticancer Medicinal Plants from the Western Ghats of India. In Anticancer Plants: Natural Products and Biotechnological Implements; Springer: Berlin/Heidelberg, Germany, 2018; pp. 481–505. [Google Scholar]
- Gao, Y.; Wu, C.H.; Piao, X.C.; Han, L.; Gao, R.; Lian, M.L. Optimization of culture medium components and culture period for production of adventitious roots of Echinacea pallida (Nutt.) Nutt. Plant Cell Tissue Organ Cult. 2018, 135, 299–307. [Google Scholar] [CrossRef]
- Fan, M.Z.; An, X.L.; Cui, X.H.; Jiang, X.L.; Piao, X.C.; Jin, M.Y.; Lian, M.L. Production of eurycomanone and polysaccharides through adventitious root culture of Eurycoma longifolia in a bioreactor. Biochem. Eng. J. 2021, 171, 108013. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, N.; Piao, Z.; Zang, J.; Li, H.; Zhou, R. Establishment of adventitious root cultures and assessment of secoiridoid production in the Chinese medicinal plant Gentiana scabra. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 864–873. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hassan, S.E.D. Harnessing Bacterial Endophytes for Promotion of Plant. Growth and Biotechnological Applications: An Overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, D.Y.; Kristanti, A.N.; Manuhara, Y.S.W. Effect of sucrose and immersion frequency on production of adventitious roots and secondary metabolites of Gynura procumbens (Lour.) Merr in temporary immersion bioreactors. Asian J. Plant Sci. 2017, 16, 24–36. [Google Scholar]
- Kusuma, D.Y.; Kristanti, A.N.; Wibowo, A.T.; Chin, T.B.; Manuhara, Y.S.W. Aeration Volume and Inoculum Density Using in Bioreactor to Optimized Biomass Production and Secondary Metabolites in Gynura procumbens (Lour.) Merr. Adventitious Roots Culture. Syst. Rev. Pharm. 2021, 12, 102–112. [Google Scholar]
- Sathish, S.; Vasudevan, V.; Karthik, S.; Elayaraja, D.; Pavan, G.; Ajithan, C.; Manickavasagam, M. Elicitors induced l-Dopa accumulation in adventitious root cultures of Hybanthus enneaspermus (L.) F. Muell. Vegetos 2020, 33, 304–312. [Google Scholar] [CrossRef]
- Sobhani Najafabadi, A.; Khanahmadi, M.; Ebrahimi, M.; Moradi, K.; Behroozi, P.; Noormohammadi, N. Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal. Behav. 2019, 14, 1640561. [Google Scholar] [CrossRef]
- Kannan, N.; Manokari, M.; Shekhawat, M.S. Enhanced production of anthraquinones and phenolic compounds using chitosan from the adventitious roots of Morinda coreia Buck and Ham. Ind. Crop. Prod. 2020, 148, 112321. [Google Scholar] [CrossRef]
- Krishnan, S.S.; Siril, E. Elicitor mediated adventitious root culture for the large-scale production of anthraquinones from Oldenlandia umbellata L. Ind. Crop. Prod. 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Jiang, X.L.; Piao, X.C.; Gao, R.; Jin, M.Y.; Jiang, J.; Jin, X.H.; Lian, M.L. Improvement of bioactive compound accumulation in adventitious root cultures of an endangered plant species, Oplopanax elatus. Acta Physiol. Plant. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Han, L.; Piao, X.C.; Jiang, J.; Jiang, X.L.; Yin, C.R.; Lian, M.L. A high production of flavonoids and anthraquinones via adventitious root culture of Oplopanax elatus and evaluating antioxidant activity. Plant Cell Tissue Organ Cult. 2019, 137, 173–179. [Google Scholar] [CrossRef]
- Hao, Y.J.; An, X.L.; Sun, H.D.; Piao, X.C.; Gao, R.; Lian, M.L. Ginsenoside synthesis of adventitious roots in Panax ginseng is promoted by fungal suspension homogenate of Alternaria panax and regulated by several signaling molecules. Ind. Crop. Prod. 2020, 150, 112414. [Google Scholar] [CrossRef]
- Linh, N.T.N.; Tam, H.T.; Tung, H.T.; Luan, V.Q.; Hien, V.T.; Loc, N.H.; Nhut, D.T. Improvement of bioactive saponin accumulation in adventitious root cultures of Panax vietnamensis via culture periods and elicitation. Plant Cell Tissue Organ Cult. 2019, 137, 101–113. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Zaker, A.; Abrishamchi, P.; Bahrami, A.R.; Ganjeali, A.; Sodagar, N. Hairy root induction and secondary metabolite production in Perovskia abrotanoides Karel. J. Plant Process. Funct. 2017, 6, 17–26. [Google Scholar]
- Jaisi, A.; Panichayupakaranant, P. Chitosan elicitation and sequential Diaion® HP-20 addition a powerful approach for enhanced plumbagin production in Plumbago indica root cultures. Process. Biochem. 2017, 53, 210–215. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Huo, Y.; Zhang, J.; Zhu, C.; Zhang, X.; Ma, Z. Establishment of adventitious root cultures from leaf explants of Tripterygium wilfordii (thunder god vine) for the production of celastrol. Ind. Crop. Prod. 2020, 155, 112834. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Shujait Ali, S.; Khan, A.; Wei, D.Q. Sustainable production of biomass and industrially important secondary metabolites in cell cultures of selfheal (Prunella vulgaris L.) elicited by silver and gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ali, H.; Khan, H.; Begam, A.; Khan, S.; Ali, S.S.; Abbasi, B.H. Effect of gibberellic acid on production of biomass, polyphenolics and steviol glycosides in adventitious root cultures of Stevia rebaudiana (Bert.). Plants 2020, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Faizal, A.; Sari, A.V. Enhancement of saponin accumulation in adventitious root culture of Javanese ginseng (Talinum paniculatum Gaertn.) through methyl jasmonate and salicylic acid elicitation. Afr. J. Biotechnol. 2019, 18, 130–135. [Google Scholar]
- Zhang, B.; Chen, M.; Pu, S.; Chen, L.; Zhang, X.; Zhang, J.; Zhu, C. Identification of secondary metabolites in Tripterygium wilfordii hairy roots and culture optimization for enhancing wilforgine and wilforine production. Ind. Crop. Prod. 2020, 148, 112276. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Selvaraj, N.; Ganapathi, A.; Lim, Y.P. Withanolide Production in Hairy Root Culture of Withania somnifera (L.) Dunal: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 607–624. [Google Scholar]
- Zhai, X.; Luo, D.; Li, X.; Han, T.; Jia, M.; Kong, Z.; Zheng, C. Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front. Microbiol. 2018, 8, 2694. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.Y.; Jiao, J.; Wang, X.; Liu, J.; Wang, Z.Y.; Fu, Y.J. Chitosan promoting formononetin and calycosin accumulation in Astragalus membranaceus hairy root cultures via mitogen-activated protein kinase signaling cascades. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nazirah, A.; Nor-Hasnida, H.; Ismanizan, I.; Norlia, B.; Abdul-Rashih, A.; Muhammad-Fuad, Y.; Mohd-Saifuldullah, A.W. Production of 9-methoxycanthin-6-one in elicited Eurycoma longifolia hairy root. J. Trop. For. Sci. 2018, 30, 606–614. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, G.; Sharma, S.; Shukla, S.; Misra, P. Elicitation enhanced the yield of glycyrrhizin and antioxidant activities in hairy root cultures of Glycyrrhiza glabra L. J. Plant Growth Regul. 2019, 38, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; Dhooghe, E.; De Keyser, E.; Van Huylenbroeck, J.; Müller, R.; Geelen, D.; Lütken, H. Rhizogenic agrobacteria as an innovative tool for plant breeding: Current achievements and limitations. Appl. Microbiol. Biotechnol. 2020, 104, 2435–2451. [Google Scholar] [CrossRef]
- Murthy, H.N.; Park, S.Y.; Paek, K.Y. Production of Ginsenosides by Hairy Root Cultures of Panax ginseng. In Production of Plant Derived Natural Compounds through Hairy Root Culture; Springer: Cham, Switzerland, 2017; pp. 203–216. [Google Scholar]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Lipert, A.; Szymańska, G. Yeast extract stimulates ginsenoside production in hairy root cultures of American ginseng cultivated in shake flasks and nutrient sprinkle bioreactors. Molecules 2017, 22, 880. [Google Scholar] [CrossRef] [Green Version]
- Gantait, S.; Mitra, M.; Chen, J.-T. Biotechnological interventions for ginsenosides production. Biomolecules 2020, 10, 538. [Google Scholar] [CrossRef] [Green Version]
- Dhiman, N.; Patial, V.; Bhattacharya, A. The Current Status and Future Applications of Hairy Root Cultures. In Biotechnological Approaches for Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 87–155. [Google Scholar]
- Vergara-Martínez, V.M.; Estrada-Soto, S.E.; Valencia-Díaz, S.; Garcia-Sosa, K.; Peña-Rodríguez, L.M.; de Jesús Arellano-García, J.; Perea-Arango, I. Methyl jasmonate enhances ursolic, oleanolic and rosmarinic acid production and sucrose induced biomass accumulation, in hairy roots of Lepechinia caulescens. PeerJ 2021, 9, e11279. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, W.A.; Misra, P.; Atri, N. Withania somnifera: Advances and implementation of molecular and tissue culture techniques to enhance its application. Front. Plant Sci. 2017, 8, 1390. [Google Scholar] [CrossRef] [Green Version]
- Saadah, I.N.; Kristanti, A.N.; Hardjo, P.H.; Manuhara, Y.S.W. Shoots Culture of Gynura procumbens (Lour.) Merr. in Balloon-Type Bubble-Bioreactor Influenced by Sucrose Concentration and Inoculums Density. Asian J. Plant Sci. 2019, 18, 85–90. [Google Scholar] [CrossRef]
- Shuang, Z.; Hong, T. Enhanced production of valtrate in hairy root cultures of Valeriana jatamansi Jones by methyl jasmonate, jasmonic acid and salicylic acid elicitors. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 839–848. [Google Scholar]
- Yousefian, Z.; Hosseini, B.; Rezadoost, H.; Palazón, J.; Mirjalili, M.H. Production of the anticancer compound withaferin a from genetically transformed hairy root cultures of Withania somnifera. Nat. Prod. Commun. 2018, 13, 1934578X1801300806. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants 2022, 11, 439. https://doi.org/10.3390/plants11030439
Hussain MJ, Abbas Y, Nazli N, Fatima S, Drouet S, Hano C, Abbasi BH. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants. 2022; 11(3):439. https://doi.org/10.3390/plants11030439
Chicago/Turabian StyleHussain, Masooma Jawad, Yawar Abbas, Naushaba Nazli, Sara Fatima, Samantha Drouet, Christophe Hano, and Bilal Haider Abbasi. 2022. "Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review" Plants 11, no. 3: 439. https://doi.org/10.3390/plants11030439
APA StyleHussain, M. J., Abbas, Y., Nazli, N., Fatima, S., Drouet, S., Hano, C., & Abbasi, B. H. (2022). Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants, 11(3), 439. https://doi.org/10.3390/plants11030439








