Inhibition of Human Monoamine Oxidases A and B by Specialized Metabolites Present in Fresh Common Fruits and Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fruit and Vegetables Sampling
2.3. Fresh Juice Preparation
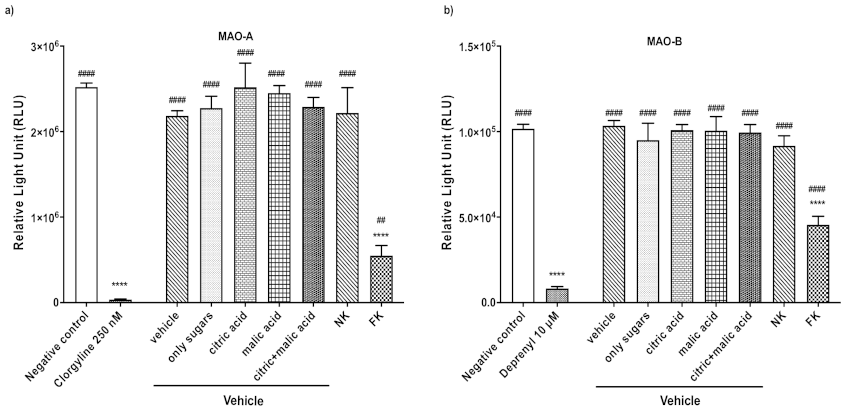
2.4. MAO-Glo Assay
2.5. NMR Spectroscopy
2.6. Protein Concentration Determination
2.7. UPLC-MS Analysis
2.8. Quinic Acid Quantification
2.9. Data Analysis
3. Results
3.1. Kiwifruit as a Model Fruit for MAO Inhibition Assay Optimization and Activity
3.2. Common Fruits and Vegetables Inhibit Human MAO-A and -B
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of Health Survey for England data. J. Epidemiol. Community Health 2014, 68, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Nooyens, A.C.; Bueno-de-Mesquita, H.B.; van Boxtel, M.P.; van Gelder, B.M.; Verhagen, H.; Verschuren, W.M. Fruit and vegetable intake and cognitive decline in middle-aged men and women: The Doetinchem Cohort Study. Br. J. Nutr. 2011, 106, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Henriquez-Sanchez, P.; Ruiz-Canela, M.; Lahortiga, F.; Molero, P.; Toledo, E.; Martinez-Gonzalez, M.A. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; O’Keeffe, M.; Elia, C.; Karamanos, A.; Goff, L.M.; Maynard, M.; Cruickshank, J.K.; Harding, S. Fruit and vegetable consumption and mental health across adolescence: Evidence from a diverse urban British cohort study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Conner, T.S.; Brookie, K.L.; Richardson, A.C.; Polak, M.A. On carrots and curiosity: Eating fruit and vegetables is associated with greater flourishing in daily life. Br. J. Health Psychol. 2015, 20, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Conner, T.S.; Brookie, K.L.; Carr, A.C.; Mainvil, L.A.; Vissers, M.C.M. Let them eat fruit! The effect of fruit and vegetable consumption on psychological well-being in young adults: A randomized controlled trial. PLoS ONE 2017, 12, e0171206. [Google Scholar] [CrossRef]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Vissers, M.C. Mood improvement in young adult males following supplementation with gold kiwifruit, a high-vitamin C food. J. Nutr. Sci. 2013, 2, e24. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P.E. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef]
- Casedas, G.; Les, F.; Gomez-Serranillos, M.P.; Smith, C.; Lopez, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef]
- Muzammil, M.; Satish, S.; Praveenkumar, C.; Salyan, T.; Shabaraya, A. A study on anti-depressant activity of fresh fruit juice of actinidia deliciosa in experimental mice. Int. J. Pharma Chem. Res. 2019, 5, 5. [Google Scholar]
- Tipton, K.F. 90 years of monoamine oxidase: Some progress and some confusion. J. Neural. Transm. 2018, 125, 1519–1551. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.; Andres, N.; Andrade, C.; Ojuel, J.; Eriksson, K.; Mahy, N. Biphasic and region-specific MAO-B response to aging in normal human brain. Neurobiol. Aging 1997, 18, 497–507. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural. Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Mazzio, E.; Deiab, S.; Park, K.; Soliman, K.F. High throughput screening to identify natural human monoamine oxidase B inhibitors. Phytother. Res. 2013, 27, 818–828. [Google Scholar] [CrossRef]
- Carradori, S.; D’Ascenzio, M.; Chimenti, P.; Secci, D.; Bolasco, A. Selective MAO-B inhibitors: A lesson from natural products. Mol. Divers. 2014, 18, 219–243. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sanchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A. Quercetin as the active principle of hypericum h ircinum exerts a selective inhibitory activity against MAO-A: Extraction, Biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Olsen, H.T.; Stafford, G.I.; van Staden, J.; Christensen, S.B.; Jager, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Hou, W.C.; Lin, R.D.; Chen, C.T.; Lee, M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005, 100, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Sloley, B.D.; Urichuk, L.J.; Morley, P.; Durkin, J.; Shan, J.J.; Pang, P.K.T.; Coutts, R.T. Identification of kaempferol as a monoamine oxidase inhibitor and potential neuroprotectant in extracts of Ginkgo biloba leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit. Contam. 2004, 21, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006, 78, 795–802. [Google Scholar] [CrossRef]
- Lee, S.A.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Oh, G.J.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005, 53, 832–835. [Google Scholar] [CrossRef]
- Dreiseitel, A.; Korte, G.; Schreier, P.; Oehme, A.; Locher, S.; Domani, M.; Hajak, G.; Sand, P.G. Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol. Res. 2009, 59, 306–311. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Commisso, M.; Negri, S.; Bianconi, M.; Gambini, S.; Avesani, S.; Ceoldo, S.; Avesani, L.; Guzzo, F. Untargeted and Targeted Metabolomics and Tryptophan Decarboxylase In Vivo Characterization Provide Novel Insight on the Development of Kiwifruits (Actinidia deliciosa). Int. J. Mol. Sci. 2019, 20, 897. [Google Scholar] [CrossRef]
- Toffali, K.; Ceoldo, S.; Stocchero, M.; Levi, M.; Guzzo, F. Carrot-specific features of the phenylpropanoid pathway identified by feeding cultured cells with defined intermediates. Plant Sci. 2013, 209, 81–92. [Google Scholar] [CrossRef]
- Chalabi, M.; Khademi, F.; Yarani, R.; Mostafaie, A. Proteolytic activities of kiwifruit actinidin (Actinidia deliciosa cv. Hayward) on different fibrous and globular proteins: A comparative study of actinidin with papain. Appl. Biochem. Biotechnol. 2014, 172, 4025–4037. [Google Scholar] [CrossRef]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic Amines in Plant-Origin Foods: Are they Frequently Underestimated in Low-Histamine Diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef]
- Butnariu, M.; Butu, A. Chemical composition of Vegetables and their products. Handb. Food Chem. 2015, 1, 627–692. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, K.; Wang, Z.; Zhu, K.; Tan, X.; Cao, J. A Review of Plant Vacuoles: Formation, Located Proteins, and Functions. Plants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Genard, M.; Lobit, P.; Mbeguie, A.M.D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Mei, Y.; Pan, D.; Jiang, Y.; Zhang, W.; Yao, X.; Dai, Y.; Yu, Y.; Yao, X. Target discovery of chlorogenic acid derivatives from the flower buds of Lonicera macranthoides and their MAO B inhibitory mechanism. Fitoterapia 2019, 134, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Zhao, J.; Xing, X.; Zhang, C.; Meng, H. Neuroprotective Effects of D-(−)-Quinic Acid on Aluminum Chloride-Induced Dementia in Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 5602597. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, X.; Zheng, Z.; Liu, S.; Xing, J.; Liu, Z.; Zhou, H. Chemical characterization of small-molecule inhibitors of monoamine oxidase B synthesized from the Acanthopanax senticosus root with affinity ultrafiltration mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8694. [Google Scholar] [CrossRef]
- Andrade, J.M.; Biegelmeyer, R.; Dresch, R.R.; Maurmann, N.; Pranke, P.; Henriques, A.T. In vitro Antioxidant and Enzymatic Approaches to Evaluate Neuroprotector Potential of Blechnum Extracts without Cytotoxicity to Human Stem Cells. Pharmacogn. Mag. 2016, 12, 171–177. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Hellin, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsogon, A.; Araujo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390. [Google Scholar] [CrossRef]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Huang, W.Y.; Xiong, X.Q.; Freedman, N.D.; Cross, A.J.; et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef]
- Hagl, S.; Deusser, H.; Soyalan, B.; Janzowski, C.; Will, F.; Dietrich, H.; Albert, F.W.; Rohner, S.; Richling, E. Colonic availability of polyphenols and D-(−)-quinic acid after apple smoothie consumption. Mol. Nutr. Food Res. 2011, 55, 368–377. [Google Scholar] [CrossRef]
- Lintas, C.; Adorisio, S.; Cappelloni, M.; Monastra, E. Composition and Nutritional-Evaluation of Kiwifruit Grown in Italy. N. Zealand J. Crop. Hortic. Sci. 1991, 19, 341–344. [Google Scholar] [CrossRef][Green Version]
- Martinez Pomier, K.; Ahmed, R.; Melacini, G. Catechins as Tools to Understand the Molecular Basis of Neurodegeneration. Molecules 2020, 25, 3571. [Google Scholar] [CrossRef]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by beta-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; Leon, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.H.; Belouahem-Abed, D.; et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 Value | |||
|---|---|---|---|---|
| MAO-A a | MAO-B a | |||
| (mg/mL) b | M c | (mg/mL) b | M c | |
| Clorgyline (MAO-A control) | 5.49 × 10−6 ± 2.4 × 10−7 | 0.0178 ± 0.0007 µM | - | - |
| Deprenyl (MAO-B control) | - | - | 1.96 × 10−5 ± 8.2 × 10−7 | 0.1048 ± 0.004 µM |
| Kiwifruit b | 86 ± 2.6 | - | 122.9 ± 5.5 | - |
| D-(−)-Quinic acid | - | - | 6.60 ± 0.25 | 34.37 ± 1.307 mM |
| Caffeic acid | 0.49 ± 0.02 | 2.747 ± 0.126 mM | 0.08 ± 0.005 | 0.439 ± 0.03 mM |
| Catechin | 1.88 ± 0.04 | 6.464 ± 0.121 mM | 1.17 ± 0.04 | 4.029 ± 0.122 mM |
| Esculin | 3.93 ± 0.15 | 11.55 ± 0.4305 mM | 4.10 ± 0.13 | 12.06 ± 0.3721 mM |
| Sample or Compound Tested | IC50 Values (mg/mL) a | |
|---|---|---|
| MAO-A b | MAO-B b | |
| Clorgyline | 5.49 × 10−6 ± 2.4 × 10−7 | - |
| R-(−)-Deprenyl | - | 1.96 × 10−5 ± 8.2 × 10−7 |
| Kiwifruit (Actinidia deliciosa) | 86.0 ± 2.6 | 122.9 ± 5.5 |
| Apple (Malus domestica) cv. golden delicious | 186.5 ± 5.9 | ND |
| Apple (Malus domestica) cv. prussian | 159.0 ± 5.2 | 100.7 ± 5.3 |
| Carrot (Daucus carota) | 79.7 ± 4.0 | 61.5 ± 1.7 |
| Cherry (Prunus avium) cv. black star | 79.0 ± 1.9 | 84.9 ± 2.7 |
| Cherry (Prunus avium) cv. roana | 52.5 ± 0.8 | 56.3 ± 2.0 |
| Cucumber (Cucumis sativus) | 15.7 ± 0.5 | 20.8 ± 0.7 |
| Fennel (Foeniculum vulgare) | 47.1 ± 2.4 | 66.7 ± 1.1 |
| Lettuce (Lactuca sativa) | 69.0 ± 2.4 | 69.8 ± 1.9 |
| Nectarine (Prunus persica) | 133.9 ± 4.7 | 155.5 ± 7.0 |
| Onion (Allium cepa) | 93.0 ± 3.7 | 113.7 ± 3.4 |
| Peach (Prunus persica) | 103.7 ± 2.7 | 98.3 ± 5.0 |
| Pear (Pyrus communis) | 106.3 ± 3.0 | 97.0 ± 3.0 |
| Red chicory (Cichorium intybus) | 13.8 ± 0.3 | 29.3 ± 0.6 |
| Ripe bell pepper (Capsicum annuum) | 59.0 ± 2.7 | 38.7 ± 1.9 |
| Shallot (Allium ascalonicum) | 48.2 ± 1.9 | 47.8 ± 1.7 |
| Tomato (Solanum lycopersicum) | 81.0 ± 2.8 | 103.5 ± 3.0 |
| Unripe bell pepper (Capsicum annuum) | 74.8 ± 4.4 | 43.6 ± 3.3 |
| Species | Id | Rt (min) | m/z (-) Detected | m/z (-) Expected | Elemental Formula | Putative Identification | Fragments | Main Adduct |
|---|---|---|---|---|---|---|---|---|
| DC, FV, AD, LS, CI, MDG, MDP, PC, PPN, PP, RCA, UCA, SL | 1 | 0.80 | 533.174 | 533.171 | C18H32O15 | glucose–glucose–rhamnose | fa | |
| DC, AC, AA, FV, AD, LS, CI, MDG, MDP, PC, PPN, PP, RCA, UCA, SL | 2 | 0.80 | 387.115 | 387.113 | C12H22O11 | sucrose | fa | |
| DC, CS, FV, CI, MDP, PP, UCA | 3 | 0.75 | 96.961 | 96.969 | H3PO4 | phosphate | M-1H+ | |
| DC, CS, PAR, FV, AD, LS, CI, MDG, MDP, PC, PPN, PP, SL | 4 | 0.86 | 133.014 | 133.013 | C4H6O5 | malic acid | 115.003 | M-1H+ |
| DC, CS, AC, AA, FV, AD, LS, CI, MDG, MDP, PPN, PP, RCA, UCA, SL | 5 | 1.32 | 191.020 | 191.019 | C6H8O7 | citric acid | 111.008 | M-1H+ |
| DC | 6 | 4.97 | 431.120 | 431.118 | C17H22O10 | sinapic acid hexoside | 222.993 | fa |
| CS | 7 | 3.45 | 315.072 | 315.071 | C13H16O9 | dihydroxybenzoic acid hexoside | 150.016 | M-1H+ |
| PAB, PAR, PPN, PP | 8 | 4.00 | 353.088 | 353.087 | C16H18O9 | caffeoyl quinic acid | 135.034; 179.034 | M-1H+ |
| PAB, PAR | 9 | 4.58 | 337.093 | 337.092 | C16H18O8 | coumaroyl quinic acid | 163 | M-1H+ |
| PAB, PAR | 10 | 4.66 | 477.161 | 477.16 | C19H28O11 | caffeoyl quinic acid derivative | 135.034; 179.034; 353.087 | fa |
| PAB, PAR, SL | 11 | 6.26 | 609.146 | 609.145 | C27H30O16 | quercetin-O-rutinoside | 300.027 | M-1H+ |
| PAR | 12 | 4.72 | 593.151 | 593.15 | C27H31O15 | cyanidin-O-rutinoside | 284.032 | M-1H+ |
| PAR, AD, LS, CI, MDG, MDP, PC, PPN, PP, RCA, UCA, SL | 13 | 0.79 | 191.056 | 191.055 | C7H12O6 | quinic acid | 127.045 | M-1H+ |
| AC, AA | 14 | 1.87 | 873.273 | 873.272 | C30H52O26 | penta-hexose | 827.268 | fa |
| AC, AA | 15 | 2.22 | 873.273 | 873.272 | C30H52O26 | penta-hexose | 827.268 | fa |
| AC, AA, LS | 16 | 2.47 | 1035.327 | 1035.325 | C36H62O31 | hexa-hexose | 989.322 | fa |
| AC, AA | 17 | 2.54 | 1197.379 | 1197.377 | C42H72O36 | hepta-hexose | 1151.374 | fa |
| AC, AA | 18 | 5.64 | 625.141 | 625.14 | C27H30O17 | quercetin-O-dihexoside | 301.034; 464.087 | M-1H+ |
| AC, AA | 19 | 5.83 | 639.156 | 639.156 | C28H32O17 | rhamnetin/isorhamnetin-O-dihexoside | 313.034; 315.049 | M-1H+ |
| AC, AA | 20 | 7.23 | 463.088 | 463.087 | C21H20O12 | quercetin-O-hexoside | 151.002; 178.997; 301.034 | M-1H+ |
| AC, AA | 21 | 7.56 | 477.103 | 477.103 | C22H22O12 | rhamnetin/isorhamnetin-O-hexoside | 314.042 | M-1H+ |
| AC, AA, LS, CI | 22 | 0.82 | 549.166 | 549.166 | C18H32O16 | tri-hexose | fa | |
| AA | 23 | 1.11 | 711.221 | 711.219 | C24H42O21 | tetra-hexose | fa | |
| CI | 24 | 6.58 | 461.072 | 461.072 | C21H18O12 | kaempferol-O-glucuronide | 285.039 | M-1H+ |
| AA | 25 | 2.66 | 1359.432 | 1359.43 | C48H82O41 | octo-hexose | 1313.427 | fa |
| DC, PP | 26 | 3.52 | 329.087 | 329.087 | C14H18O9 | vanillic acid glucoside | 108.021; 123.046; 152.011; 167.034 | |
| DC | 27 | 3.62 | 465.125 | 465.103 | C21H22O12 | taxifolin-O-hexoside | M-1H+ | |
| FV | 28 | 7.06 | 429.140 | 429.118 | C22H22O9 | hydroxy methoxy flavone-O-hexoside | M-1H+ | |
| FV | 29 | 5.70 | 367.103 | 367.102 | C17H20O9 | feruloyl quinic acid | 193.05 | M-1H+ |
| CS, FV, LS | 30 | 2.51 | 312.095 | 312.094 | C10H13N5O4 | adenosine | 134.046 | fa |
| FV | 31 | 2.67 | 282.083 | 282.083 | C10H13N5O5 | guanosine | 133.015; 150.04 | M-1H+ |
| AD | 32 | 3.99 | 341.088 | 341.087 | C15H18O9 | caffeic acid glucoside | 135.034; 179.034 | M-1H+ |
| AD | 33 | 4.59 | 341.088 | 341.087 | C15H18O9 | caffeic acid glucoside | 135.034; 179.034 | M-1H+ |
| AD | 34 | 4.25 | 339.071 | 339.071 | C15H16O9 | esculin | 137.024; 177.018 | M-1H+ |
| AC, AA | 35 | 0.78 | 176.038 | 176.038 | C6H11NO3S | alliin/isoalliin | M-1H+ | |
| AC | 36 | 0.73 | 150.022 | 150.022 | C4H9NO3S | methiin | M-1H+ | |
| LS | 37 | 10.40 | 329.232 | 329.232 | C18H34O5 | trihydroxy-octadecenoic acid (oxylipin) | M-1H+ | |
| LS | 38 | 9.82 | 327.217 | 327.217 | C18H32O5 | trihydroxy-octadecadienoic acid (oxylipin) | M-1H+ | |
| LS, CI | 39 | 1.86 | 873.274 | 873.272 | C30H52O26 | penta-hexose | 827.268 | fa |
| LS | 40 | 1.79 | 243.061 | 243.061 | C9H12N2O6 | uridine | M-1H+ | |
| MDG, MDP, PC, PPN, PP | 41 | 4.68 | 353.088 | 353.087 | C16H18O9 | chlorogenic acid | 135.034; 179.034; 191.055 | M-1H+ |
| MDG, MDP | 42 | 5.03 | 577.135 | 577.134 | C30H26O12 | procyanidin P2 type | 289 | M-1H+ |
| MDG, MDP | 43 | 5.45 | 337.092 | 337.092 | C16H18O8 | coumaroyl quinic acid | 163.039; 173.045; 119.049 | M-1H+ |
| MDG, MDP, PC | 44 | 5.35 | 289.071 | 289.071 | C15H14O6 | epicatechin | M-1H+ | |
| MDP | 45 | 5.53 | 865.197 | 865.197 | C45H38O18 | procyanidin P3 type | M-1H+ | |
| MDG, MDP | 46 | 7.12 | 567.173 | 567.171 | C26H32O14 | phloretin 2′-O-xylosyl glucoside | 167.035; 273.075 | M-1H+ |
| MDG, MDP | 47 | 7.05 | 447.092 | 447.092 | C21H20O11 | quercetin-O-desoxyhexoside | M-1H+ | |
| MDG, MDP | 48 | 7.21 | 567.173 | 567.171 | C26H32O14 | phloretin-O-xylosyl glucoside structural isomer | M-1H+ | |
| MDG, MDP | 49 | 7.67 | 435.129 | 435.129 | C21H24O10 | phloretin-O-glucoside | M-1H+ | |
| MDG, MDP | 50 | 4.85 | 353.089 | 353.087 | C16H18O9 | caffeoyl quinic acid | 179.034; 191.055 | M-1H+ |
| PPN, PP | 51 | 4.34 | 577.134 | 577.134 | C30H26O12 | procyanidin P2 type | 289 | M-1H+ |
| PP | 52 | 4.71 | 289.071 | 289.071 | C15H14O6 | catechin | M-1H+ | |
| PC | 53 | 3.49 | 365.134 | 365.134 | C17H22N2O7 | tryptophan glucose | 203.081 | M-1H+ |
| PC | 54 | 3.88 | 447.117 | 447.113 | C18H24O13 | dihydroxybenzoic acid hexose pentose | 153.018 | M-1H+ |
| PC | 55 | 3.97 | 447.117 | 447.113 | C18H24O13 | dihydroxybenzoic acid hexose pentose | 153.018 | M-1H+ |
| PC | 56 | 6.77 | 623.162 | 623.161 | C28H32O16 | isorhamnetin-O-galattosyl rhamnoside | M-1H+ | |
| PC | 57 | 6.83 | 623.162 | 623.161 | C28H32O16 | isorhamnetin-O-rutinoside | M-1H+ | |
| PC | 58 | 7.00 | 477.103 | 477.103 | C22H22O12 | isorhamnetin-3-O-galactoside | 314.0425 | M-1H+ |
| PC | 59 | 7.09 | 477.103 | 477.103 | C22H22O12 | isorhamnetin-3-O-glucoside | 314.0425 | M-1H+ |
| PC | 60 | 7.40 | 519.114 | 519.113 | C24H24O13 | isorhamnetin-3-O-acetyl galactoside | M-1H+ | |
| PC | 61 | 7.51 | 519.114 | 519.113 | C24H24O13 | isorhamnetin-3-O-acetyl glucoside | M-1H+ | |
| SL | 62 | 8.19 | 1127.548 | 1127.548 | C51H86O24 | tomatoside A | 1081.875; 919.491 | fa |
| SL | 63 | 6.73 | 1314.597 | 1314.596 | C58H95NO29 | esculeoside A | 1268.591; 1136.548 | fa |
| RCA, UCA | 64 | 9.57 | 1129.528 | 1129.527 | C50H83O25 | triterpenoid saponin | 1083.524; 921.470 | fa |
| CI | 65 | 6.46 | 477.067 | 477.066 | C21H18O13 | quercetin-O-glucuronide | M-1H+ | |
| CI | 66 | 6.79 | 505.096 | 505.098 | C13H22O13 | querceti-O-acetyl-glucoside | 301.035 | M-1H+ |
| CI | 67 | 7.02 | 339.054 | 339.053 | C15H16O7S | deoxylactucin sulphate | M-1H+ | |
| CI | 68 | 7.41 | 259.097 | 259.097 | C15H16O4 | 8-deoxylactucin | 215.106 | M-1H+ |
| DC, CS, AC, AA, FV, AD, LS, CI, RCA, UCA | 69 | 0.72 | 145.061 | 145.061 | C5H10N2O3 | glutamine | M-1H+ | |
| DC, CS, AA, CI, UCA, SL | 70 | 0.72 | 132.030 | 132.029 | C4H7NO4 | aspartic acid | M-1H+ | |
| CS | 71 | 5.43 | 901.241 | 901.24 | C42H46O22 | kaempferol-O-(coumaroyl) glucoside rutinoside | 739.186 | M-1H+ |
| PAB, PAR, MDG, MDP, PC, PPN, PP | 72 | 0.78 | 181.071 | 181.071 | C6H14O6 | sorbitol | M-1H+ | |
| DC, PAB, PAR, FV, AD, PPN, SL | 73 | 0.74 | 195.050 | 195.05 | C6H12O7 | gluconic acid | M-1H+ | |
| RCA, UCA | 74 | 0.80 | 175.024 | 175.024 | C6H8O6 | ascorbic acid | M-1H+ | |
| RCA, UCA, SL | 75 | 1.05 | 175.024 | 175.024 | C6H8O6 | ascorbic acid | M-1H+ | |
| AD, MDG, SL | 76 | 0.73 | 146.042 | 146.45 | C5H8NO4 | glutamic acid | M-1H+ | |
| DC, PAB, PAR, CI, MDG, PC, PPN, RCA, UCA | 77 | 0.73 | 131.045 | 131.045 | C4H8N2O3 | asparagine | M-1H+ | |
| PAB, PAR | 78 | 0.77 | 343.124 | 343.124 | C12H24O11 | sorbitol–glucose | 181.069 | M-1H+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo, C.M.; Gambini, S.; Poletti, S.; Munari, F.; Assfalg, M.; Guzzo, F. Inhibition of Human Monoamine Oxidases A and B by Specialized Metabolites Present in Fresh Common Fruits and Vegetables. Plants 2022, 11, 346. https://doi.org/10.3390/plants11030346
Marzo CM, Gambini S, Poletti S, Munari F, Assfalg M, Guzzo F. Inhibition of Human Monoamine Oxidases A and B by Specialized Metabolites Present in Fresh Common Fruits and Vegetables. Plants. 2022; 11(3):346. https://doi.org/10.3390/plants11030346
Chicago/Turabian StyleMarzo, Claudio Marcello, Sofia Gambini, Stefania Poletti, Francesca Munari, Michael Assfalg, and Flavia Guzzo. 2022. "Inhibition of Human Monoamine Oxidases A and B by Specialized Metabolites Present in Fresh Common Fruits and Vegetables" Plants 11, no. 3: 346. https://doi.org/10.3390/plants11030346
APA StyleMarzo, C. M., Gambini, S., Poletti, S., Munari, F., Assfalg, M., & Guzzo, F. (2022). Inhibition of Human Monoamine Oxidases A and B by Specialized Metabolites Present in Fresh Common Fruits and Vegetables. Plants, 11(3), 346. https://doi.org/10.3390/plants11030346