Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass
Abstract
1. Introduction
2. Results
2.1. Proximate Composition
2.2. Mineral Composition
2.3. Non-Nutritional Compounds
2.4. Polyphenols Profilere
2.5. Other Chemicals
2.6. Correlations between Quality Parameters
2.7. Volatiles Profile Analysis
3. Discussion
4. Materials and Methods
4.1. Plants Materials
4.2. Chemical Reagents
4.3. Nutritional Characteristics
4.3.1. Proximate Composition
4.3.2. Mineral Composition
4.4. Non-Nutritional Compounds
4.4.1. Total Antioxidants
4.4.2. Total Phenolic Content
4.4.3. Polyphenols Profile by HPLC
4.4.4. Chlorophylls: a, b, Total
4.5. Other Chemicals: Nitrates, Ph and Total Acidity
4.6. Volatiles Profile Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldron, A.; Miller, D.; Redding, D.; Mooers, A.; Kuhn, T.; Nibbelink, N.; Roberts, T.; Tobias, J.; Gittleman, J. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef]
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. J. Sci. Food Agric. 2008, 88, 294–304. [Google Scholar] [CrossRef]
- Chatzopoulou, E.; Carocho, M.; Di Gioia, F.; Petropoulos, S.A. The beneficial health effects of vegetables and wild edible greens: The case of the mediterranean diet and its sustainability. Appl. Sci. 2020, 10, 9144. [Google Scholar] [CrossRef]
- Khoury, C.; Bjorkman, A.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.; Struik, P. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Caetano, C.M.; Peña, C.R.D.; Maigual, J.J.L.; Vasquez, D.L.N.; Nunes, D.C.; Pazdiora, B.R. Mejoramiento participativo: Herramienta para la conservación de cultivos subutilizados y olvidados. Acta Agron. 2015, 64, 307–327. [Google Scholar] [CrossRef][Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Clapp, J.; Moseley, W.G. This food crisis is different: COVID-19 and the fragility of the neoliberal food security order. J. Peasant Stud. 2020, 47, 1393–1417. [Google Scholar] [CrossRef]
- Rizou, M.; Galanakis, I.; Aldawoud, T.; Galanakis, C. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Pardo-de-Santayana, M.; Tardio, J.; Blanco, E.; Carvalho, A.M.; Lastra, J.J.; San Miguel, E.; Morales, R. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): A comparative study. J. Ethnobiol. Ethnomed. 2007, 3, 27. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Barata, A.M. The Consumption of Wild Edible Plants. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; John Wiley & Sons: Chichester, UK; Hoboken, NJ, USA, 2017; pp. 159–198. [Google Scholar]
- Beilin, K.O. Messages from the Underground: Interspecies Memory in Times of the Climate Change. 452 °F. Rev. Teoría Lit. Lit. Comp. 2019, 21, 35–54. [Google Scholar]
- de Aquino, D.R.M.; Flores, M.D.S.A. Plantas alimentícias não convencionais em Belém, Pará: Conhecimento, usos e segurança alimentar. Novos Cad. NAEA 2021, 24, 73–97. [Google Scholar] [CrossRef]
- Disciglio, G.; Tarantino, A.; Frabboni, L.; Gagliardi, A.; Giuliani, M.M.; Tarantino, E.; Gatta, G. Qualitative characterization of cultivated and wild edible plants: Mineral elements, phenols content and antioxidant capacity. Ital. J. Agron. 2017, 12, 383–394. [Google Scholar] [CrossRef]
- Bello, O.M.; Jagaba, S.M.; Bello, O.E.; Ogbesejana, A.B.; Dada, O.; Adetunji, C.C.; Abubakar, S.M. Phytochemistry, pharmacology and perceived health uses of non-cultivated vegetable Cyphostemma adenocaule (Steud. ex A. Rich.) Desc. ex Wild and R.B. Drumm: A review. Sci. Afr. 2019, 2, e00053. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Blanco-Salas, J.; Gutierrez-Garcia, L.; Labrador-Moreno, J.; Ruiz-Tellez, T. Wild Plants Potentially Used in Human Food in the Protected Area “Sierra Grande de Hornachos” of Extremadura (Spain). Sustainability 2019, 11, 456. [Google Scholar] [CrossRef]
- Minde, J.; Venkataramana, P.; Matemu, A. Dolichos Lablab-an underutilized crop with futurepotentials for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, A.O.; Ijatuyi, E.J.; Ogunniyi, A.I.; Aremu, A.O. Exploring the Resource Value of Transvaal Red MilkWood (Mimusops zeyheri) for Food Security and Sustainability: An Appraisal of Existing Evidence. Plants 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; La Rosa, A.; Garfi, G.; Marceno, C.; Gristina, A.S.; Carimi, F.; Guarino, R. An updated checklist of the Sicilian native edible plants: Preserving the traditional ecological knowledge of century-old agro-pastoral landscapes. Front. Plant Sci. 2020, 11, 308. [Google Scholar] [CrossRef]
- Torija-Isasa, M.; Matallana-González, M. A Historical Perspective of Wild Plant Foods in the Mediterranean Area. In Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Propertie. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- García-Herrera, P.; Morales, P.; Cámara, M.; Fernández-Ruiz, V.; Tardío, J.; Sánchez-Mata, M.C. Nutritional and phytochemical composition of Mediterranean wild vegetables after culinary treatment. Foods 2020, 9, 1761. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P. High contents of nonextractable polyphenols in fruit suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.G.G.; Perea, J.M.; Anta, R.M.O. Los alimentos funcionales en el contexto de la dieta mediterránea. Mediterráneo Económico 2015, 27, 139–160. [Google Scholar]
- Morales, R.; Tardío, J.; Aceituno, L.; Molina, M.; Pardo de Santayana, M. Biodiversidad y Etnobotánica en España. In Memorias Real Sociedad, Española de Historia Natural; Viejo Montesinos, J.L., Ed.; Real Sociedad Española de Historia Natural: Madrid, Spain, 2011; pp. 157–208. [Google Scholar]
- Poonia, A.; Upadhayay, A. Chenopodium album Linn: Review of nutritive value and biological properties. J. Food Sci. Technol. 2015, 52, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Tardío, J.; de Cortes Sánchez-Mata, M.; Morales, R.; Molina, M.; García-Herrera, P.; Boussalah, N. Ethnobotanical and food composition monographs of selected Mediterranean wild edible plant. In Mediterranean Wild Edible Plants; Sánchez-Mata, M., Tardío, J., Eds.; Springer Science: New York, NY, USA, 2016; pp. 273–470. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Lukasiak, J.; Wolska, J. Garden nasturtium (Tropaeolum majus L.) a source of mineral elements and bioactive compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Falasca, L.; Cinquanta, L. Bioactive compounds in wild Asteraceae edible plants consumed in the Mediterranean Diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef]
- Fukalova Fukalova, T.; García Martínez, M.D.; Raigón, M.D. Five undervalued edible species inherent to autumn-winter season: Nutritional composition, bioactive constituents and volatiles profile. Peer J. 2021, 9, e12488. [Google Scholar] [CrossRef]
- Rocchetti, G.; Braceschi, G.P.; Odello, L.; Bertuzzi, T.; Trevisan, M.; Lucini, L. Identification of markers of sensory quality in ground coffee: An untargeted metabolomics approach. Metabolomics 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.D.S.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of edible plants: Effects of boiling on nutritional, antioxidant, and physicochemical properties. Foods 2020, 9, 599. [Google Scholar] [CrossRef]
- Lara, C.D.; Boettler, B.; Ovando, M. Diagnóstico del pápaloquelite en México Porophyllum ruderale (Jacq.) Cass. var. macrocephalum (DC.) Cronq. In Red Quelites; Márquez Ortíz, L., Ed.; Universidad Autónoma Chapingo: Chapingo, México, 2011; pp. 1–61. [Google Scholar]
- USDA: U.S. Department of Agriculture. National Nutrient Database for Standard Reference. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169274/nutrients (accessed on 5 July 2021).
- Mangoba, P.M.A. Prospecção de Características Fitoquímicas, Antibacterianas e Físico-Químicas de Portulaca Oleracea L. (Beldroega). Dissertation Thesis, Porto Alegre, Brazil, 2015. Available online: https://lume.ufrgs.br/handle/10183/115207 (accessed on 9 October 2021).
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Carbajal Azcona, Á. Manual de Nutrición y Dietética; Departamento de Nutrición, Facultad de Farmacia: Madrid, Spain, 2013; pp. 1–367. [Google Scholar]
- BEDCA: Base de Datos Española de Composición de Alimentos. Available online: https://www.bedca.net/bdpub/index.php (accessed on 4 November 2021).
- Kamal-Uddin, K.; Quan, L.; Haasan, M.; Motmainna, M.; Selamat, M. Purslane: A perspective plant source of nutrition and antioxidant. Plant Arch. 2020, 20, 1624–1630. [Google Scholar]
- Aberoumand, A. Protein, fat, calories, minerals, phytic acid and phenolic in some plant foods based diet. J. Food Process Technol. 2011, 2, 1–4. [Google Scholar] [CrossRef]
- Darmon, N.; Darmon, M.; Maillot, M.; Drewnowski, A. A nutrient density standard for vegetables and fruits: Nutrients per calorie and nutrients per unit cost. J. Am. Diet. Assoc. 2005, 105, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Comparison of the proximate composition, vitamins (ascorbic acid, α-tocopherol and retinol), anti-nutrients (phytate and oxalate) and the GC-MS analysis of the essential oil of the root and leaf of Rumex crispus L. Plants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- Moscuzza, N. Verdolaga una alternativa saludable. Master’s Thesis, Universidad FASTA, Mar de Plata, Argentina, 2016. Available online: http://redi.ufasta.edu.ar:8080/xmlui/handle/123456789/1271 (accessed on 9 October 2021).
- Available online: http://www.portalantioxidantes.com/antioxidantes (accessed on 17 October 2021).
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and mineral composition of three wild leafy species: A comparison between microgreens and baby greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef]
- Youssef, K.M.; Mokhtar, S.M. Effect of drying methods on the antioxidant capacity, color and phytochemicals of Portulaca oleracea L. leaves. J. Nutr. Food Sci. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Marques, E.A.; Oliveira, J.A.; Coelho, A.D.; Salimena, J.P.; Gavilanes, M.L. Porophyllum ruderale (Jacq.) Cass. A review of the last 39 years. Res. Soc. Dev. 2020, 9, e944975215. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Quah, E.P.L. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Oliveira, L.D.L.D.; Carvalho, M.V.D.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Rev. Ceres 2014, 61, 764–779. [Google Scholar] [CrossRef]
- Sdouga, D.; Branca, F.; Kabtni, S.; Di Bella, M.C.; Trifi-Farah, N.; Marghali, S. Morphological Traits and Phenolic Compounds in Tunisian Wild Populations and Cultivated Varieties of Portulaca oleracea L. Agronomy 2020, 10, 948. [Google Scholar] [CrossRef]
- Reyes, L.F.; Villarreal, J.E.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Udeagha, A.U.; Shomkegh, S.A.; Daniel, K.S. An assesesment of leaf chlorophyll concentration of afforestation tree species in South-Eastern, Nigeria. J. For. Environ. Sci. 2016, 32, 205–211. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Katoch, V.; Kumar, S.; Chatterjee, S. Functional relationship of vegetable colors and bioactive compounds: Implications in human health. J. Nutr. Biochem. 2021, 92, 108615. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild edible Swiss chard leaves (Beta vulgaris L. var. cicla): Nutritional, phytochemical composition and biological activities. Food Res. Int. 2019, 119, 612–621. [Google Scholar] [CrossRef]
- Moreno, C.B.; Soto, K.; González, R.D. El consumo de nitrato y su potencial efecto sobre la salud cardiovascular. Rev. Chil. Nutr. 2015, 42, 199–205. [Google Scholar] [CrossRef]
- AESAN: Agencia Española de Seguridad Alimentaria y Nutricion. Available online: http://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/nitratos.htm (accessed on 21 June 2021).
- Sobko, T.; Marcus, C.; Govoni, M.; Kamiya, S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide 2010, 22, 136–140. [Google Scholar] [CrossRef]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide 2014, 38, 45–57. [Google Scholar] [CrossRef]
- Silva, L.F.L.E.; Souza, D.C.; Resende, L.V.; Nassur, R.D.C.M.; Samartini, C.Q.; Gonçalves, W.M. Nutritional evaluation of non-conventional vegetables in Brazil. An. Acad. Bras. Ciênc. 2018, 90, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Marín, O.; Yusà-Pelechà, V.; Villalba-Martín, P.; Perez-Dasí, J.A. Monitoring programme on nitrates in vegetables and vegetable-based baby foods marketed in the Region of Valencia, Spain: Levels and estimated daily intake. Food Addit. Contam. 2010, 27, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Raigón, M.D.; García-Martínez, M.D.; Guerrero, C.; Esteve, P. Actividad de la nitrato reductasa y su relación con los factores productivos en lechuga. In Proceedings of the VII Congreso SEAE Zaragoza, Zaragoza, Spain, 18–23 September 2006. [Google Scholar]
- Devkar, S.T.; Suryapujary, S.M.; Jagtap, S.D.; Katyare, S.S.; Hegde, M.V. Effect of macronutrient deficiency on withanolides content in the roots of Withania somnifera and its correlationship with molybdenum content. Pharm. Biol. 2015, 53, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.N.B.; Vieira, L.R.; Sousa, C.A.F.; Souza, M.T., Jr. Morphological Changes in Portulaca oleracea L. under salt stress. In Proceedings of the Inovagri International Meeting, 4, Congresso Nacional de Irrigação e Drenagem, Fortaleza, Brazil; Simpósio Brasileiro de Salinidade, 3. 2017. Available online: https://www.alice.cnptia.embrapa.br/bitstream/doc/1088965/1/TC4800865.pdf (accessed on 9 October 2021).
- Peñaranda, J.C.; CRodrigo, G.; Ticona-Bustillos, A.R.; Valenzuela, E.; Ramos, S.; San Martin, A.; Ghezzi, F.; Almanza, G.R. Variación en la concentración de flavonoides y clorofila, y cambios en la morfología y anatomía foliar, debidos a radiación visible (PAR) o ultravioleta (UVA, UVB) en Baccharis Latifolia. Rev. Bol. Quim. 2020, 37, 210–222. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, H.; Liu, L.; Zhang, Y.; Xin, X.; Gao, D. Chemical components and antibacterial activity of the essential oil of six pyrrosia species. Chem. Biodivers. 2020, 17, e2000526. [Google Scholar] [CrossRef]
- Misztal, P.K.; Hewitt, C.N.; Wildt, J.; Blande, J.D.; Eller, A.S.; Fares, S.; Goldstein, A.H. Atmospheric benzenoid emissions from plants rival those from fossil fuels. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Baccouri, B.; Temime, S.B.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Application of solid-phase microextraction to the analysis of volatile compounds in virgin olive oils from five new cultivars. Food Chem. 2007, 102, 850–856. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerases essential to the production of the leaf aldehyde in plants. J. Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Zakari, I.S.; N’guessan, A.; Dehaut, A.; Duflos, G. Volatile Compounds Selection via Quantile Correlation and Composite Quantile Correlation: A Whiting Case Study. Open J. Stat. 2016, 6, 995. [Google Scholar] [CrossRef][Green Version]
- Leidinger, W. ¿Por qué pica el ají? Notas químicas sobre el ají. Rev. Quim. PUCP 2020, 34, 22–25. [Google Scholar]
- Megaloudi, F. Wild and cultivated vegetables, herbs and spices in Greek Antiquity (900 B.C. to 400 B.C.). Environ. Archaeol. 2005, 10, 73–82. [Google Scholar] [CrossRef]
- Rzedowski, G.C.; Rzedowski, J. Manual de malezas de la región de Salvatierra, Guanajuato. In Flora del Bajío y de Regiones Adyacente; Centro Regional de Bajío, Ed.; Instituto de Ecología, A.C.: Veracruz, Mexico, 2004; pp. 1–315. [Google Scholar]
- Loayza, I.; De Groot, W.; Lorenzo, D.; Dellacassa, E.; Mondello, L.; Dugo, G. Composition of the essential oil of Porophyllum ruderle (Jacq.) Cass. from Bolivia. Flavour Fragr. J. 1999, 14, 393–398. [Google Scholar] [CrossRef]
- Soria García, J.M.; Romo, S.; Palacios Pastor, A.; García Picazo, A.; Aledón Catalá, T.; Calvo García, S. Evaluación de la conservación de los humedales costeros de la Comunidad Valenciana mediante imágenes de Landsat. In Teledetección: Humedales y Espacios Protegidos. XVI; Bustamante, J., Díaz-Delgado, R., Aragonés, D., Afán, I., García, D., Eds.; Asociación Española de Teledetección: Sevilla, Spain, 2015; pp. 354–357. [Google Scholar]
- AOAC. Official Methods of Analysis of Official Analytical Chemists, 18th ed.; Association of Official Analitical Chemist (AOAC International): Washington, DC, USA, 2005. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing capacity, chlorogenic acid content and biological activity in a collection of scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Extraction efficiency and validation of an HPLC method for flavonoid analysis in peppers. Food Chem. 2012, 130, 751–758. [Google Scholar] [CrossRef]
- Hansmann, E. Pigment Analisys. In Handbook of Phycological Methods-Culture Methods and Grow the Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 359–368. [Google Scholar]
- Moreno, E.; Fita, A.; González-Mas, M.C.; Rodríguez-Burruezo, A. HS-SPME study of the volatile fraction of Capsicum accessions and hybrids in different parts of the fruit. Sci. Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
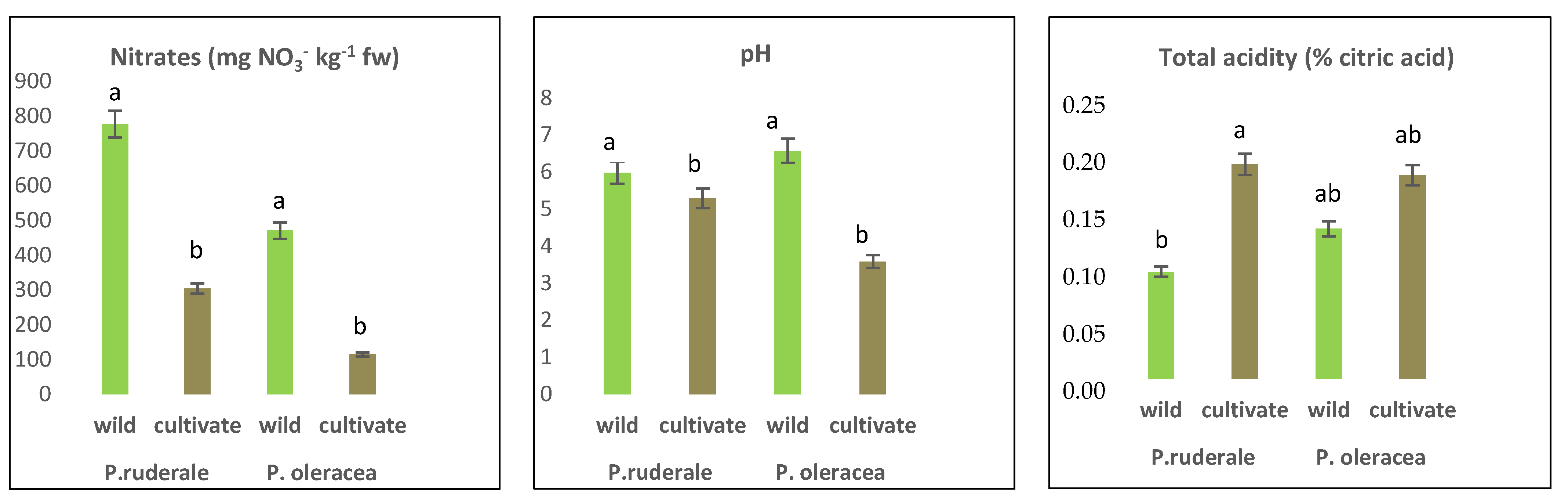

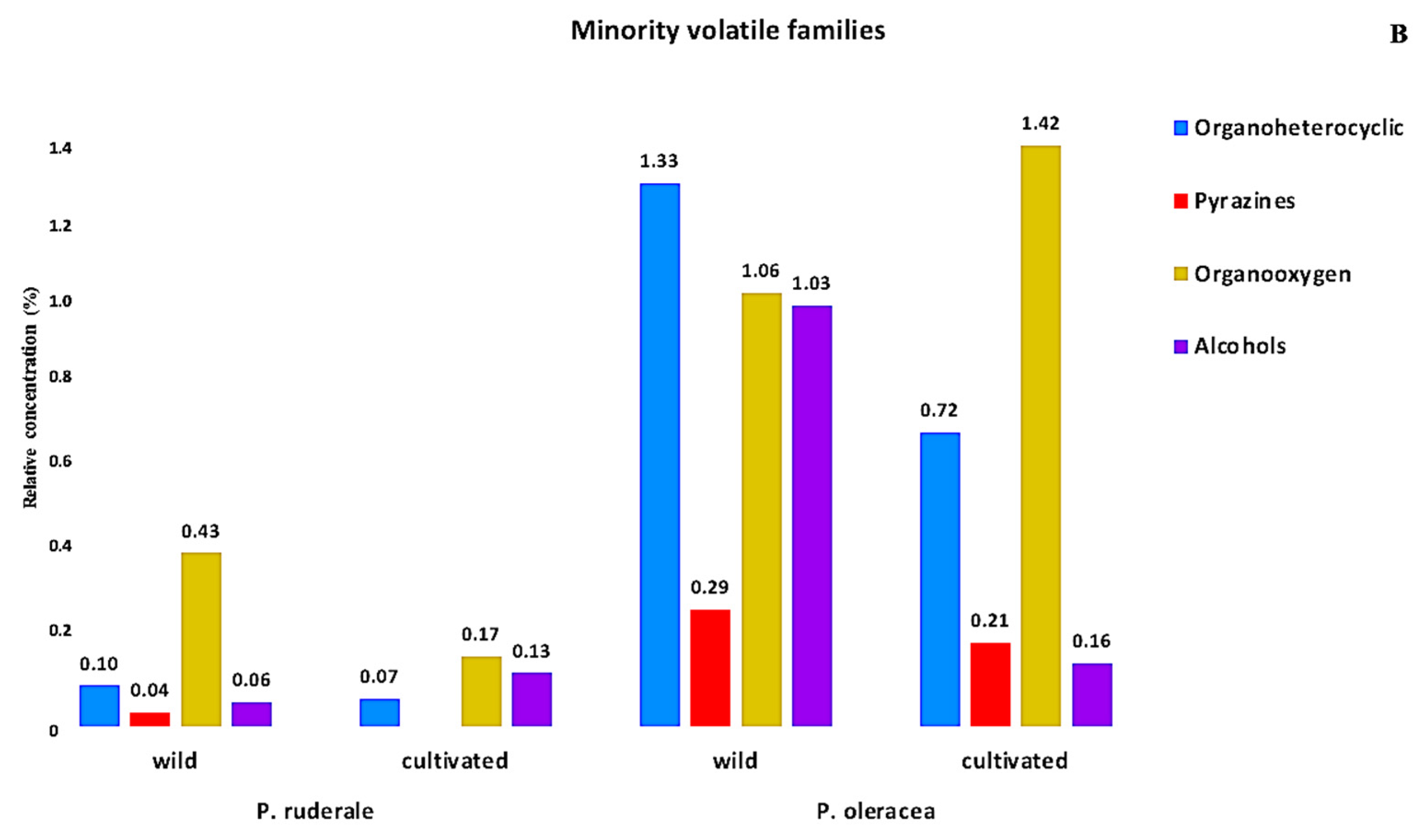
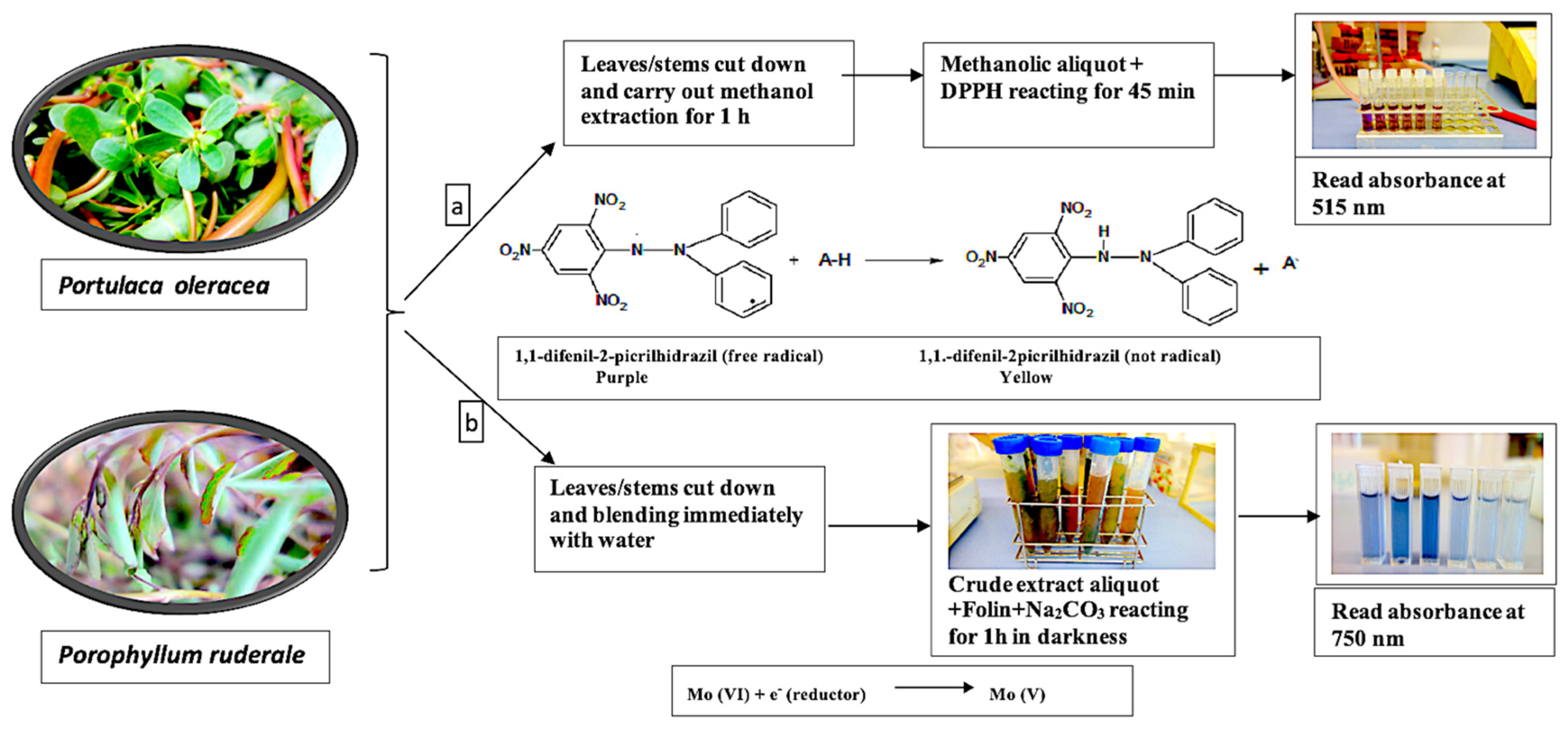
| P. ruderale | P. oleracea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild | CV (%) | Cultivated | CV (%) | p-Value | Wild | CV (%) | Cultivated | CV (%) | p-Value | ||
| Nutritional value (g 100 g−1 fw) | Moisture | 84.70 a ± 0.43 | 0.51 | 76.64 b ± 0.02 | 0.03 | 0.0000 | 88.39 a ± 0.24 | 0.27 | 83.12 b ± 1.09 | 1.31 | 0.0014 |
| Dry matter | 15.30 b ± 0.61 | 3.99 | 23.36 a ± 0.17 | 0.73 | 0.0000 | 11.61 b ± 0.37 | 3.19 | 16.88 a ± 0.85 | 5.04 | 0.0014 | |
| Ash | 1.49 b ± 0.02 | 0.28 | 2.33 a ± 0.02 | 0.15 | 0.0020 | 2.62 b ± 0.02 | 0.76 | 3.39 a ± 0.06 | 1.77 | 0.0084 | |
| Crude proteins | 1.89 a ± 0.00 | 0.05 | 1.19 b ± 0.01 | 0.84 | 0.0002 | 1.56 ± 0.00 | 0.06 | 1.49 ± 0.00 | 0.07 | 0.1988 | |
| Fat | 0.66 a ± 0.00 | 0.15 | 0.41 b ± 0.01 | 2.44 | 0.0156 | 0.32 b ± 0.00 | 0.31 | 0.99 a ± 0.00 | 0.10 | 0.0000 | |
| Crude fiber | 5.50 ± 1.88 | 34.18 | 3.57 ± 0.66 | 18.64 | 0.1003 | 2.39 ± 0.01 | 0.42 | 2.60 ± 0.89 | 34.23 | 0.7178 | |
| Carbohyrates | 6.80 b ± 3.49 | 51.32 | 17.55 a ± 0.63 | 3.59 | 0.0008 | 4.72 b ± 0.21 | 4.45 | 8.41 a ± 0.90 | 10.22 | 0.0183 | |
| Energy value (kcal 100 g−1) | 40.70 ± 1.16 | 2.85 | 78.65 ± 0.22 | 0.28 | - | 28.00 ± 0.07 | 0.25 | 48.51 ± 0.30 | 0.62 | - | |
| Minerals (mg 100 g−1 fw) | Calcium | 439.29 b ± 119.86 | 27.29 | 687.49 a ± 19.22 | 2.80 | 0.0240 | 186.67 a ± 28.36 | 15.19 | 110.59 b ± 16.02 | 14.49 | 0.0005 |
| Magnesium | 131.15 b ± 9.22 | 7.03 | 185.54 a ± 21.10 | 11.37 | 0.0150 | 165.33 a ± 9.50 | 5.75 | 91.68 b ± 18.91 | 20.63 | 0.0000 | |
| Potassium | 515.28 ± 49.22 | 9.55 | 477.75 ± 40.99 | 8.58 | 0.3676 | 776.67 a ± 171.50 | 22.08 | 271.91 b ± 34.37 | 12.64 | 0.0000 | |
| Phosphorus | 56.48 b ± 3.31 | 5.86 | 84.57 a ± 3.96 | 4.68 | 0.0007 | 33.67 b ± 0.93 | 2.76 | 58.73 a ± 7.56 | 12.87 | 0.0000 | |
| Sodium | 7.19 ± 1.21 | 16.83 | 8.21 ± 1.60 | 19.49 | 0.4337 | 16.60 a ± 0.03 | 0.18 | 0.81 b ± 0.06 | 7.41 | 0.0000 | |
| Iron | 1.80 ± 0.12 | 6.67 | 1.70 ± 0.17 | 10.00 | 0.4495 | 1.80 ± 0.18 | 10.00 | 1.35 ± 0.18 | 13.33 | 0.1960 | |
| Copper | 0.17 b ± 0.01 | 5.88 | 0.37 a ± 0.01 | 2.70 | 0.0000 | 0.14 b ± 0.01 | 7.14 | 0.36 a ± 0.05 | 13.89 | 0.0000 | |
| Zinc | 0.51 a ± 0.04 | 7.84 | 0.39 b ± 0.03 | 7.69 | 0.0157 | 0.99 b ± 0.06 | 6.06 | 1.08 a ± 0.14 | 12.96 | 0.0011 | |
| Bioactive compounds | TAO (µmol TE·100 g−1 fw) | 4645.53 a ± 36.2 | 0.78 | 4392.16 b ± 27.0 | 0.62 | 0.0006 | 7315.0 a ± 386.30 | 5.28 | 4609.98 b ± 168.3 | 3.65 | 0.0004 |
| TPP (mg GAE·100 g−1 fw) | 391.18 ± 141.50 | 36.17 | 316.20 ± 28.90 | 9.14 | 0.4152 | 318.93 a ± 40.20 | 12.60 | 99.09 b ± 35.50 | 35.83 | 0.0021 | |
| Chl a (μg·g−1fw) | 10.45 a ± 0.52 | 4.98 | 5.55 b ± 0.53 | 9.55 | 0.0003 | 3.35 ± 0.11 | 3.28 | 3.37 b ± 0.33 | 9.79 | 0.9434 | |
| Chl b (μg·g−1fw) | 3.55 a ± 0.28 | 7.89 | 1.70 b ± 0.15 | 8.82 | 0.0006 | 1.85 a ± 0.15 | 8.11 | 1.30 b ± 0.13 | 10.00 | 0.0002 | |
| Total Chl (μg·g−1fw) | 14.00 a ± 0.78 | 5.57 | 7.25 b ± 0.67 | 9.24 | 0.0003 | 5.20 ± 0.26 | 5.00 | 4.66 ± 0.44 | 9.44 | 0.1450 | |
| P. ruderale | P. oleracea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild | Cultivated | p-Value | S | Wild | Cultivated | p-Value | S | ||
| Hydroxycinnamic acids (μg·g−1 fw) | Gallic acid | 1.13 ± 0.07 | 0.41 ± 0.07 | 0.0002 | ** | nd | nd | - | - |
| Chlorogenic acid | 798.45 ± 36.52 | 780.08 ± 0.85 | 0.4329 | ns | 6.75 ± 1.09 | 11.38 ± 2.64 | 0.0399 | * | |
| Caffeic acid | 3.93 ± 0.16 | 1.67 ± 0.03 | 0.0000 | ** | 5.72 ± 0.29 | 16.25 ± 0.82 | 0.0000 | ** | |
| p-Coumaric acid | 54.8 ± 2.26 | 175.21 ± 1.28 | 0.0000 | ** | 4.21 ± 0.17 | 17.99 ± 1.19 | 0.0000 | ** | |
| Flavonoids (μg·g−1 fw) | Myricetin | nd | 1.54 ± 0.10 | - | - | 0.95 ± 0.04 | 10.26 ± 0.62 | 0.0000 | ** |
| Rutin | 14.73 ± 0.70 | 15.43 ± 0.46 | 0.2242 | ns | nd | nd | - | - | |
| Quercetin | 40.42 ± 2.44 | 49.95 ± 0.86 | 0.0031 | * | 0.21 ± 0.03 | 0.47 ± 0.01 | 0.0002 | ** | |
| Luteolin | 3.98 ± 0.22 | 7.3 ± 0.13 | 0.0000 | ** | nd | nd | - | - | |
| Kaempferol | 5.84 ± 0.36 | 13.76 ± 0.61 | 0.0000 | ** | 0.09 ± 0.01 | 0.30 ± 0.04 | 0.0006 | ** | |
| Apigenin | 2.48 ± 0.36 | 3.59 ± 0.09 | 0.0068 | * | nd | 0.96 ± 0.06 | - | - | |
| CP | FT | CF | CH | Ca | Mg | K | P | Na | Fe | Cu | Zn | TAO | TPP | TCh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT | 0.879 * | 1 | |||||||||||||
| CF | 0.685 | 0.798 | 1 | ||||||||||||
| CH | −0.956 * | −0.908 * | −0.856 * | 1 | |||||||||||
| Ca | −0.854 * | −0.740 | −0.792 | 0.914 * | 1 | ||||||||||
| Mg | −0.949 * | −0.836 * | −0.634 | 0.871 * | 0.771 | 1 | |||||||||
| K | 0.465 | 0.298 | 0.625 | −0.541 | −0.439 | −0.481 | 1 | ||||||||
| P | −0.947 * | −0.938 * | −0.832 * | 0.989 * | 0.868 * | 0.840 * | −0.461 | 1 | |||||||
| Na | −0.410 | −0.054 | −0.036 | 0.317 | 0.17 | 0.313 | −0.615 | 0.298 | 1 | ||||||
| Fe | 0.463 | 0.209 | 0.139 | −0.335 | −0.109 | −0.540 | 0.721 | −0.296 | −0.814 * | 1 | |||||
| Cu | −0.983 * | −0.917 * | −0.715 | 0.967 * | 0.834 * | 0.888 * | −0.415 | 0.980 * | 0.385 | −0.386 | 1 | ||||
| Zn | 0.902 * | 0.656 | 0.599 | −0.870 * | −0.940 * | −0.837 * | 0.446 | −0.817 * | −0.395 | 0.321 | −0.848 * | 1 | |||
| TAO | 0.950 * | 0.928 * | 0.812 * | −0.983 * | −0.835 * | −0.842 * | 0.499 | −0.996 * | −0.368 | 0.368 | −0.983 * | 0.802 | 1 | ||
| TPP | 0.430 | 0.438 | −0.137 | −0.244 | 0.045 | −0.391 | −0.210 | −0.342 | −0.326 | 0.373 | −0.475 | 0.124 | 0.386 | 1 | |
| TCh | 0.990 * | 0.836 * | 0.606 | −0.929 * | −0.834 * | −0.908 * | 0.402 | −0.928 * | −0.464 | 0.446 | −0.980 * | 0.908 * | 0.934 * | 0.484 | 1 |
| CP | FT | CF | CH | Ca | Mg | K | P | Na | Fe | Cu | Zn | TAO | TPP | TCh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT | −0.597 | 1 | |||||||||||||
| CF | −0.739 | 0.174 | 1 | ||||||||||||
| CH | −0.517 | 0.880 * | −0.048 | 1 | |||||||||||
| Ca | 0.403 | −0.884 * | −0.034 | −0.808 | 1 | ||||||||||
| Mg | 0.438 | −0.942 * | 0.001 | −0.930 * | 0.956 * | 1 | |||||||||
| K | 0.419 | −0.918 * | −0.166 | −0.787 | 0.954 * | 0.951 * | 1 | ||||||||
| P | −0.704 | 0.947 * | 0.430 | 0.713 | −0.764 | −0.804 | −0.852* | 1 | |||||||
| Na | 0.611 | −0.999 * | −0.185 | −0.889 * | 0.895 * | 0.949 * | 0.924 * | −0.943 * | 1 | ||||||
| Fe | 0.359 | −0.809 | −0.100 | −0.841 * | 0.879 * | 0.929* | 0.927* | −0.684 | 0.823* | 1 | |||||
| Cu | −0.663 | 0.970 * | 0.343 | 0.760 | −0.793 | −0.842 * | −0.870 * | 0.995 * | −0.965 * | −0.709 | 1 | ||||
| Zn | −0.803 | 0.467 | 0.739 | 0.143 | −0.208 | −0.178 | −0.267 | 0.690 | −0.460 | −0.014 | 0.635 | 1 | |||
| TAO | 0.634 | −0.979 * | −0.262 | −0.831 * | 0.933 * | 0.937 * | 0.946 * | −0.941 * | 0.983 * | 0.823 * | −0.953 * | −0.500 | 1 | ||
| TPP | 0.774 | −0.963 * | −0.338 | −0.853 * | 0.771 | 0.850 * | 0.810 | −0.956 * | 0.964 * | 0.705 | −0.965 * | −0.624 | 0.939 * | 1 | |
| TCh | −0.664 | 0.001 | 0.589 | 0.164 | −0.071 | −0.040 | 0.007 | 0.033 | −0.036 | −0.140 | −0.0133 | 0.253 | −0.106 | −0.167 | 1 |
| Compound | Calibration Curve | Linearity | Linear Range | Tr (min) |
|---|---|---|---|---|
| AOT | y= −0.1347x + 1.0678 | R2 = 0.9783 | 0.3–1.3 μM | - |
| TPP | y= 0.0018x + 0.0182 | R2 = 0.9898 | 0–400 ppm | - |
| Gallic acid | y = 25.36x + 21.562 | R2 = 0.9992 | 2–350 ppm | 6.39 |
| Chlorogenic acid | y = 25.567x + 32.541 | R2 = 0.9998 | 2–300 ppm | 16.63 |
| Caffeic acid | y = 45.356x + 37.156 | R2 = 0.9990 | 1.72–220 ppm | 17.74 |
| p-Coumaric acid | y = 60.223x + 228.38 | R2 = 0.9992 | 3–440 ppm | 21.26 |
| Myricetin | y = 41.599x + 10.346 | R2 = 0.9987 | 0.75–48 ppm | 6.26 |
| Ruine | y = 20.071x + 5.5868 | R2 = 0.9984 | 1.4–94 ppm | 7.02 |
| Quercetin | y = 44.696x + 26.656 | R2 = 0.9984 | 1.6–110 ppm | 8.16 |
| Luteolin | y = 21.996x – 10.039 | R2 = 0.9997 | 1.5–98 ppm | 8.59 |
| Kaempherol | y = 51.019x + 23.177 | R2 = 0.9986 | 1.5–97 ppm | 9.13 |
| Apigenin | y = 31.051x + 18.277 | R2 = 0.9985 | 1.5–95 ppm | 9.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukalova Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants 2022, 11, 377. https://doi.org/10.3390/plants11030377
Fukalova Fukalova T, García-Martínez MD, Raigón MD. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants. 2022; 11(3):377. https://doi.org/10.3390/plants11030377
Chicago/Turabian StyleFukalova Fukalova, Tamara, María Dolores García-Martínez, and María Dolores Raigón. 2022. "Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass" Plants 11, no. 3: 377. https://doi.org/10.3390/plants11030377
APA StyleFukalova Fukalova, T., García-Martínez, M. D., & Raigón, M. D. (2022). Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants, 11(3), 377. https://doi.org/10.3390/plants11030377






