Abstract
In agriculture, abiotic stress is one of the critical issues impacting the crop productivity and yield. Such stress factors lead to the generation of reactive oxygen species, membrane damage, and other plant metabolic activities. To neutralize the harmful effects of abiotic stress, several strategies have been employed that include the utilization of nanomaterials. Nanomaterials are now gaining attention worldwide to protect plant growth against abiotic stresses such as drought, salinity, heavy metals, extreme temperatures, flooding, etc. However, their behavior is significantly impacted by the dose in which they are being used in agriculture. Furthermore, the action of nanomaterials in plants under various stresses still require understanding. Hence, with this background, the present review envisages to highlight beneficial role of nanomaterials in plants, their mode of action, and their mechanism in overcoming various abiotic stresses. It also emphasizes upon antioxidant activities of different nanomaterials and their dose-dependent variability in plants’ growth under stress. Nevertheless, limitations of using nanomaterials in agriculture are also presented in this review.
1. Introduction
The upcoming challenges of rise in global population, decreasing arable lands, and escalating threats posed by climate change exert pressure on the need for developing new techniques and methods to increase yield potential during stressful conditions. Stressful conditions for plants arise from numerous biotic and abiotic factors, which impart stresses such as drought, salinity, temperature, and heavy metal leading to substantial modifications in plants. Thus, improving stress tolerance in crops is a major target of research to fulfill the food demand of growing populations. Over the last several decades, tremendous efforts are being taken to improve the agricultural yields through extensive application of chemicals that have long-lasting and profound effects on the environment and human health. Therefore, to feed the world population without damaging the environment, the application of novel technology is necessary.
Nanotechnology is a novel approach towards the improvement in the agricultural sector as it puts forth new ways to impart tolerance against various stresses and enhances the productivity [1]. Nanoparticles (NPs) are molecules with dimensions of 100 nm, diverse physicochemical properties, higher reactivity, and biochemical activity which depends on their high surface energy and the high surface-to-volume ratio [2]. Plants have the ability to synthesize NPs which are natural agents used for improving the morphology of the plants without imparting any negative effects [3]. In the current situation, NPs have the potentiality to boost plant morphogenesis, used as herbicides, nanopesticides, and nanofertilizers, etc., that can proficiently release their content in required amounts to target cellular organelles in plants. Still, certain potentials of NPs are not revealed due to a lack of mechanisms that are not cleared or nor yet studied.
Different types of NPs are developed such as those containing inorganic nonmetallic NPs, carbon-based NPs, metallic NPs, and organic polymeric materials based on the application and usage [4]. Effective nutrient supply requires specific nanofertilizers or nanoencapsulated nutrients that could act as an efficient tool towards sustainable mode of agricultural practices. These nanofertilizers would be an alternative to chemical fertilizers that, in turn, improve efficiency of resource utilization, reduce soil toxicity, and thus, usage of nanofertilizers will assist to diminish such problems [5]. Plants are sessile so they have to face extreme environmental stress conditions, such as salinity, drought, high and low temperatures, heavy metals, flooding, high and low light intensities, ultraviolet (UV), and others. The extreme environmental conditions induces bursts of reactive oxygen species (ROS) which causes macromolecules and membrane degradation, prompts cell toxicity, and diminishes the plant growth. Antioxidant machinery through enzymatic and non-enzymatic systems scavenges ROS to alleviate oxidative stress. Against various abiotic stress, NPs take part in the growth and development of plants followed by providing protection to plants [6]. NPs have the capability to modify those genes (and their expressions) that are involved in cell biosynthesis and organization, electron transport, and energy transport during stress responses [2]. From different experiments, it was concluded that NPs play a very important role in improvement of crop plants, but understanding of the appropriate mechanism [1,7,8,9,10] and the way of interaction of NPs with plants at different levels is still required at an early stage. Current review focuses on the concept, types, mode of metal/metalloid nanoparticles together with physiological impact of metalloid NPs on plants, their effect on growth and overcoming abiotic stress, and the underlying mechanisms.
2. Concepts and Types of Nanoparticles
The use of NPs has a novel approach, which allows a better understanding of interconnection of science and technology, and opens up new interventions in the field of biotechnology and agriculture [11]. Particles having dimensions between 1–100 nm are considered as NPs; they have high surface vitality and large surface to volume ratio that increases their reactivity [12]. Besides having small dimensions and high reactivity, each NP contains its unique physical and chemical properties. They are composed of three layers: the outer layer known as surface layer, middle layer known as shell layer, and the inner layer is called core layer. The shell layer is found chemically different from core layer [13]. In the present scenario, which depicts indulging of various materials and novel techniques to create a boom in agricultural crops and in improving crop quality, the application of NPs in the agriculture field shows potential results through increasing plant growth and production, as different NPs are applied through various methods, for instance, as herbicides, nanopesticides, nanofertilizers, etc. [14]. The major difference between mode of action of other elements and NPs in plants is that NPs are effectively released in required amounts and reach the targeted cellular organelles [12]. Although, despite having numerous initial studies on potential application of nanomaterials to attain the objective of flourishing agriculture, there is still a need to unfold their unique mode of action in plant system, which helps to boost the agriculture production one level up [15].
NPs have different sources of origin, namely natural, incidental, and engineered [16]. Natural occurrence of NPs is from volcanic eruptions, dust storms, mineral complexes, forest fire, photochemical reactions, etc. Incidental origin of NPs occurs through human interventional activities, such as exhaust from metallurgic activities, coal combustion, and industries [16]. Whereas, engineered NPs are generally classified into carbon-based NPs, metal-based NPs, metal magnetic NPs, dendrimers, and composite NPs. Metal and metal oxide-based NPs from the past several decades are comprehensively studied in agriculture field for the improvement of crop productivity, and increasing the plant resilience and tolerance under abiotic stress conditions [17]. Metal-based NPs include nanomaterials of gold (Au), silver (Ag), copper (Cu), aluminum (Al), and iron (Fe). Additionally, their oxides, such as titanium dioxide (TiO2), cerium oxide (CeO2), iron oxide (FeO), aluminum oxide (Al2O3), and zinc oxide (ZnO) are also gaining so much attention of scientists worldwide to tackle adverse environmental conditions [18,19,20]. The different types of nanoparticles are given in Table 1.

Table 1.
Categories and types of nanoparticles.
3. Synthesis of Metal and Metalloid Nanoparticles
The synthesis of metal and metalloid NPs is a promising part of nanotechnology, which offers solutions for wide areas including agriculture [33]. Engineered NPs have distinctive electrical, mechanical, physiochemical, optical, and imaging properties that can be controlled during synthesis process [34]. The difference between metal/metalloid NPs and their bulk material occurs on the basis of size, shape, and surface characteristics, such as presence of coatings, copious reactive sites, and mobility regulated by their aggregation state [35] that further depends on their pH, temperature, ionic strength, and concentration [36]. So far, a number of methods have been developed for controlled synthesis of NPs. Generally, there are two main approaches such as: (i) bottom-up approach and (ii) top-down approach [37]. These are further classified under many subclasses developed on the basis of operation, reaction condition, and adopted protocols.
Top-down pathway includes synthesis by gradual size reduction, which is achieved via various physical and chemical methods [38]. In general, it operates when particles are larger than nano-sized particles [34]. Whereas, in bottom-up means of synthesis, NPs are produced from atoms and molecules that include reduction/oxidation as core reaction [39]. This pathway is followed when metal particles are already smaller than nano-sized molecules. During synthesis, NPs aggregate through the action of reducing agents which also act as anti-agglomerating agents [34]. Plant extracts and chemicals act as reducing agents, as they contain alkaloids, terpenoids, flavonoids, phenols, carbohydrates, anthraquinones, and proteins, etc., which reduce the size of metal ions into NPs and stabilize the resultant NPs [40].
Moreover, bottom-up approach follows the involvement of biogenic substances. Biological agents required for the synthesis are bacteria, yeast, algae, cyanobacteria, fungi, flagella, viruses, plants, and even human cells [41]. For the reducing agent, microorganism and plant extracts are used [42]. Biological synthesis is more feasible, cost-effective, ecologically-friendly, and less toxic to the environment [41], due to their distinct optical, chemical, photoelectrochemical, and electronic properties [43]. A wide range of physical, chemical, and biological methods including environment-friendly green synthesis of NPs are developed and applied in various disciplines. The size of NPs can be manipulated by controlling various parameters such as pH, temperature, concentration, and exposure time to substrate [34]. For instance, a method was developed to manipulate the shape and size of AuNPs extracellularly produced by microorganisms through shifting the key growth parameters [43]. Some study shows that AuNPs’ synthesis occurs by using the plants rich in tannic acid, whereas to synthesize AgNPs, chemicals like trisodium citrate can be used as important catalysts [44,45]. The overview of nanoparticles’ synthesis is illustrated in Figure 1.
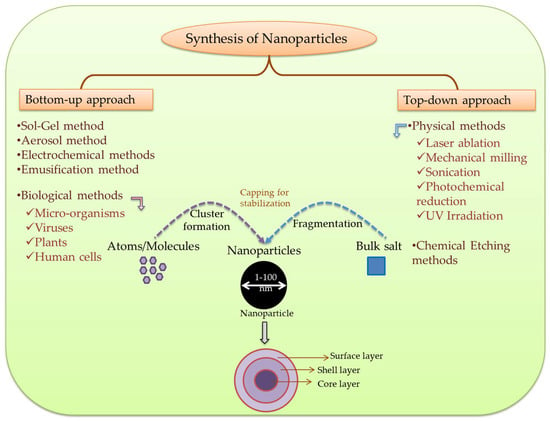
Figure 1.
An overview of nanoparticles’ synthesis.
4. Mode of Action of Nanoparticles in Plants
Several hypotheses have been made from the studies that were conducted to know the exact NPs’ mode of action (Figure 2). Certain studies showed that NPs which mediated growth of plants depends upon the concentration of NPs utilized; this can be toxic to plant growth at higher concentrations [46,47,48] or it can be beneficial when given in relevant concentrations [49,50]. Entry of NPs into the cells happens either by penetration or by transportation via particular channels located in the cellular membrane. NPs might function as stress signaling molecules which, in turn, cause induction in the expression of various genes involved in stressed condition. This includes the induction of expression of regulatory factors thus resulting in activation of defense system, and finally, exhibiting stress tolerance. Besides an acceptable level, NPs can maintain ROS at considerable level to induce ROS signaling network hence activating defense system of plant under stress conditions. Ruotolo et al. [51] performed meta-analysis of proteomics and transcriptomics studies where the response of different plant species to metal-based NPs was compared. It was found that common NPs which induced responses to stress include root architecture modification, antioxidant mechanism activation, and involvement of specific signaling pathway of phytohormones, although the effects were influenced by NPs’ nature and their duration of exposure [51,52]. For example, after exposure to NPs, the root architecture modification could be due to the downregulation of genes involved in trichoblast differentiation. This is the area from where the emergence of root hairs occurs hence trichoblasts come under specialized epidermal cells. Further, genes responsive to indole acetic acid (IAA) and ethylene (ET) were shown as the positive regulators of development of root hairs [51]. NPs’ treatment frequently alters biological pathways involved in defense mechanisms [51]. NPs’ treatment also upregulates genes that encode for proteins which play a primary role in ROS balance like NADPH oxidase, GST, superoxide dismutase (SOD), and peroxidases (POX) [51].
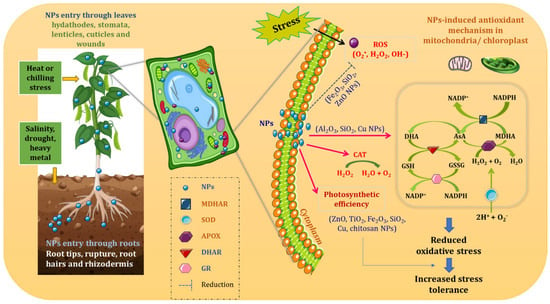
Figure 2.
Antioxidative mechanism of action of nanoparticles in plants under abiotic stress (NPs: nanoparticles; MDHAR: monodehydroascorbate reductase; SOD: superoxide dismutase; APOX: ascorbate peroxidase; DHAR: dehydroascorbate reductase; GR: glutathione reductase; ROS: reactive oxygen species).
The genes responsible for activation of antioxidant enzymes are upregulated by NPs [53]. Laware and Raskar [53] carried out an experiment to determine the effects of TiO2 NPs on onion seedlings, and from the results, they suggested that the activity of SOD enzyme was elevated by TiO2 NPs where the enzyme’s activity was further enhanced when the concentration of NPs was increased. However, only at low concentration of TiO2 NPs, there was an improvement in seedling growth and seed germination in onion which was suppressed at high concentration of TiO2 NPs [53]. One study showed an enhancement of seed germination and growth in Glycine max seeds when exposed to TiO2 and SiO2 NPs [54].
The studies also reported that NPs can be recognized by calcium-binding protein (CaBP) complex or as signaling molecules in the cytoplasm. Once NPs enter plant cells, NP-specific proteins are recognized which then triggers the downstream expression of stress-related genes [9,55]. As a result, a cascade of signaling pathways is induced intracellularly, and associated genes are upregulated whose expressions lead to plant’s increased tolerance responses to adverse environmental conditions. When Arabidopsis thaliana was exposed to salinity and drought conditions or treated with ABA, responsive to desiccation (RD20) gene expression was induced which harbors a specific conservative region for binding of calcium ion (EF-hand) [56]. In a study, increase in the expression of RD20A, particularly in Co and Fe NPs-supplemented plants, supported the hypothesis that NPs take part in induction of Ca2+- binding protein expression [55]. Besides that, NPs are also thought to impart a vital role in scavenging ROS by inducing the activities of antioxidant enzymes. Recently, very strong evidence was provided by Sun et al. [57] which shows that the expression of Cu/Zn SOD, Fe/Mn SOD, catalase (CAT), and ascorbate peroxidase (APX) was notably enhanced in plants that were treated with ZnO NPs under drought.
Various transcriptomics and proteomics studies have been carried out to assess plant and nanomaterial association [10]. Results from transcriptomics studies showed the effects of (≤50 nm size) Cu-based NPs which modulate the genes responsive to oxidative stress, brassinosteroid biosynthesis, and root formation [58]. Metabolomics studies on 40 nm sized Cu NPs in cucumber (Cucumis sativus) showed increase in secondary metabolite (such as acetyl glucosamine, phenyl lactate, 4-aminobutyrate) accumulation involved in cell signaling and defense responses, and decrease in metabolites of flavonoid and fatty acid synthesis, as well as riboflavin and amino acid metabolism [59]. Moreover, TiO2 NPs- treated tobacco plants had a significant elevation in transcript levels of miR399 and miR395 in transcriptome analysis, both of which are involved in regulation of adaptive responses of plant to nutrient stress, thus suggesting the fact that these miRNAs in tobacco plants have a significant role in responding to TiO2 NPs [60]. When the seedlings of A. thaliana were exposed to carbon nanodots of 3 nm, root elongation happened in a dose-dependent manner; transcriptomics analysis revealed that the genes involved in cellular response to phosphate starvation, UDP-glycosyltransferase activity, and stimulus response were upregulated whereas those which took part in chloroplast structure and function were downregulated [61]. Results from metabolomics study suggested the occurrence of defense response activation due to the augmentation of cell wall’s carbohydrate components.
Metal/Metalloid-Based Nanoparticles for Enhancing Plant Antioxidant Defense
Antioxidant defense system of plants comprise of various enzymes like CAT, APX, dehydroascorbate reductase (DHAR), guaiacol peroxidase (GPX), glutathione reductase (GR), and SOD and low molecular weight antioxidant compounds such as glutathione and ascorbate (Figure 2) [62,63]. It has been confirmed that enzyme-like activities are possessed by various NPs where nCeO2, nFe3O4, nCo3O4 NPs imitate CAT; nCeO2, nFe3O4, nCo3O4, nMnO2, nCuO, and nAu mimic peroxidase; nCeO2, nPt, and fullerene mimic SOD activity [62]. With all this information in hand, still, efficient techniques are required to detect enzymes mimicking activities of NPs when supplemented to the whole plant.
Maghemite γ-Fe2O3 nanomaterials (NMs) and magnetite Fe3O4 NMs are the most common forms among ferromagnetic FeO NMs [64,65,66]. It was first unveiled by Gao et al. [67] that Fe3O4 NPs have POD-like activity and the results showed that with decreasing Fe3O4 NPs particle size, the catalytic activity would be significantly increased [67,68]. In Fe3O4 NPs, the Fe is present in either ferrous (Fe2+) or in ferric (Fe3+) form where the POD-like activity is higher when NPs are in ferrous Fe2+ form [67]. Chen et al. [64] proved ferromagnetic FeO NPs can also act like CAT enzyme thus owning dual enzyme-like activity property. At an acidic pH of 4.8, hydrogen peroxide is catalyzed by ferromagnetic FeO NPs forming •OH thus exhibiting POD-like activity, whereas at neutral conditions ferromagnetic FeONPs exhibit CAT-like activity, decomposing hydrogen peroxide to H2O and O2. Side-by-side comparison of catalytic performance was done on two types of FeO ferromagnetic NPs on the basis of surface charge and similarity in sizes. From the results, it was known that POD-like activity was possessed by Fe3O4 NPs than γ-Fe2O3 NPs [64]. From all these, it can be concluded that ferromagnetic FeO NMs can perform multifunctional activities by combining enzyme-like and magnetic properties. In a study, doping γ-Fe2O3 NPs with yttrium has decreased the amount of H2O2 by 45% and peroxidation of membrane lipid by 28% in the leaves of B. napus, leading to alleviation of drought stress impacts on plant [69]. When maize grown in calcareous soil was foliar-sprayed with Fe3O4 NPs, scavenging of H2O2 was enhanced, and the rate of peroxidation of membrane lipid was brought down in comparison to the control [70]. Similarly, Fe3O4 NPs have been used to protect cadmium toxicity in tomato plants by reducing oxidative stress level [71]. Using all these results, it can be confirmed that γ-Fe2O3 and Fe3O4 NPs protect plants from environmental stresses. In addition to that, Li et al. [72] carried out an experiment in seedlings of Citrus maxima to compare γ-Fe2O3 and Fe3O4 NPs. It was found that Fe3O4 NPs have more antioxidant capacity than the γ-Fe2O3 NPs.
CeO2 NMs are considered as the initial NMs, which have SOD-like activities exceeding the catalytic activity of native SOD [73]. The preliminary mechanism to possess enzyme-like activity is to have the ability to switch between two valence states (Ce3+ and Ce4+) with a significant level of oxygen vacancy on its surface [74]. CeO2 NMs retains longer when the cycling is between two oxidation states (Ce3+ and Ce4+) and remains uninterrupted with Ce3+ being continuously regenerated. Various studies have been carried out in the past to determine the multifunctional enzyme activity (SOD and CAT) of CeO2 nanozymes [73,75,76]. As a thumb rule, CeO2 NMs function as SOD-like when the ratio of Ce3+/Ce4+ is high and CAT-like when the ratio is low [77]. Under alkaline or neutral conditions, CeO2 NMs exhibit CAT-/SOD-like property whereas under acidic conditions OXD-/SOD-like property is exhibited by CeO2 NMs [76]. It is henceforth clear that O2•− and H2O2 can be scavenged by CeO2 NMs due to their ability to mimic ROS scavenging enzymes. Recently CeO2 NMs have attracted attention to scavenge ROS in plants under environmental stresses. The coating of anionic poly (acrylic acid) on CeO2 NPs (10nm) with low (35%) ratio of Ce3+/Ce4+ has been reported to scavenge ROS by 52% in the A. thaliana leaves subjected to abiotic stress [78]. Sorghum leaves under drought stress have been compared by spraying water (control) and CeO2 NPs to leaves, and it was observed that leaves sprayed with CeO2 NPs had decreased O2•− content by 41% and H2O2 content by 36% as compared to control [79]. In cotton roots, efficient reduction in accumulation of ROS by 46% has been observed when seeds were primed with poly (acrylic acid)-coated CeO2 NPs under salinity stress [80]. The results of transcriptomic analysis showed that tolerance to saline conditions had improved when seed priming with CeO2 NMs had been carried out which induced changes in expressions of gene family coding for antioxidant enzymes [80]. Thus, it is clear from previous studies that CeO2 NMs have dual roles of scavenging ROS and are an inducer of antioxidant enzymes.
Cobalt oxide (Co3O4) NPs have dual intrinsic POD-like and CAT-like enzyme activities [81]. Transfer of electrons between H2O2 and the substrates potentially offer Co3O4 NPs the ability to function like POD. Although Co3O4 NPs have dual intrinsic enzyme-like activities, its ability to function as CAT-like is weaker than that of its ability to function like POD. However, the CAT-like activity can be modified by changing the pH to neutral or to basic from acidic conditions [82]. Jahani et al. [83] did a field work of spraying Co3O4 NPs at different concentrations, where the foliar spray of these NPs at a concentration <100 mg L−1 induced growth of plant and did not cause production of ROS; however, at >250 mg L−1 concentration of Co3O4 NPs, ROS generation was induced and negatively affected growth and photosynthetic activity. It is still a mystery that the plant growth inducing effect of Co3O4 NMs is because of its ability to act enzyme-like or due to some other unknown function. Future research must be carried out to understand the association between Co3O4 NMs and plants under environmental stress.
Manganese NMs such as Mn3O4, MnO, and MnO2 have the ability to eliminate high amounts of ROS and also possess enzyme-like activities [84,85,86]. From the study of Ragg et al. [84], it is known that SOD-like activities are exhibited by MnO NPs where the enzyme-like activity is surprisingly greater as compared to native Mn-SOD. However, apart from SOD, multiple other enzyme activities have been mimicked by MnO2 such as OXD, POD, and CAT [85]. A very satisfying ROS scavenging efficacy was exhibited by Mn3O4 NPs where •OH was removed [86]. The fast redox exchange between two oxidative states of Mn (Mn2+ and Mn3+) is crucial for the intrinsic multifunctional enzyme-like activity of Mn3O4 NMs [87]. H2O2 and O2•− couple show a high degree of affinity for H2O2 and O2•− than any other transition metal couples. It was also found that Mn3O4 NPs’ ability to eliminate ROS was way superior to that of CeO2 NPs [86]. Hence manganese oxide-based NMs can be used as a promising therapeutic tool for treating ROS-mediated diseases [86,87,88]. Taking into account the abovementioned observations, more relevant studies regarding the catalytic and antioxidant activities of Mn3O4 NMs are needed in the coming future.
There are some other NPs that can be beneficial at low concentrations but toxic when supplied at higher concentrations. Zinc oxide (ZnO) NPs have been used in plants to overcome Zn deficiency and abiotic stresses. When ZnO NPs with the size of 90 ± 10 nm applied at varying concentration between 400–3200 mg Zn kg−1, levels of superoxide (O2−) radical were found to be elevated and a significant raise in SOD activity at a maximum dose was documented in maize [89]. On treating Gossypium hirsutum with ZnO NPs, enhanced POX and SOD activities with a subsequent drop in lipid peroxidation was reported [90]. ZnO NPs come in various shapes and sizes like spherical (38 nm), floral (59 nm), rod-like (>500 nm), and also Zn2+ ions; out of all these, the most protective form was found to be spherical ZnO NPs of size 38 nm which elicited the greatest oxidative stress responses (SOD, POX, MDA, CAT, H2O2 synthesis) in soybean [91].
The pretreatment by TiO2, ZnO NPs resulted in obvious increase in GPX and SOD activity which also improved the tolerance against heat stress, further lowering the levels of H2O2 and causing membrane stabilization (1.5 times) [92]. Gene expression analyses on A. thaliana exposed to ZnO NPs showed 660 up- and 826 downregulated genes [93]; further analyses on roots exposed to TiO2 NPs and fullerene soot (FS) NPs revealed 80 up- and 74 downregulated genes and 232 up- and 189 downregulated genes, respectively (expression difference > 2-fold).
Enhanced activities of APX, GPX, CAT, and GR were noticed when seedlings of Brassica juncea were treated with gold nanoparticles (GNPs) which also resulted in proline and H2O2 accumulation in an amount greater than usual in plants treated with GNPs which kept on increasing with increase in concentration of GNPs [94].
Extensive research is still being carried out to understand the interactions between plants and metallic oxide nanomaterials (NMs) [95,96]. Few metal-oxide NMs such as CeO2NMs, MnO2 NMs, cobalt oxide (Co3O4) NMs, and ferromagnetic FeO are available in mixed valence state and hence have the ability to function as nanozymes for scavenging free radicals [65,96,97].
5. Application of Metal and Metalloid Nanoparticles for Improving Abiotic Stress Tolerance
Abiotic stresses are major problems for agriculture productivity and extension. They include drought, salinity, alkalinity, submergence, mineral and metal toxicity/deficiencies, and many others that reduce crop growth and productivity. Plants adapt and mitigate abiotic stresses by alterations in morphological, physiological, biochemical, and molecular levels to combat various stresses. Researchers have revealed that NPs help plants to overcome abiotic stresses by their concentration-dependent impact on plant growth and development [98]. The effect of abiotic stresses and the ways by which NPs combat abiotic stress and impart tolerance is depicted in Table 2. Recapitulation of the possible interaction between NPs and plant metabolisms is essential to explore the novel insights in the field of plants’ stress tolerance.

Table 2.
Positive effect of various types of nanoparticles on some plant species under different abiotic stress conditions.
5.1. Drought
Among different stresses, drought is a frequently occurring stress, causing scarcity of water followed by high temperature and loss of water uptake by the plants. It is mainly found in the dry and semiarid regions thereby affects plant growth at early stage, i.e., starting from seed germination to seed setting [116]. Drought stress can be transformed by different NPs’ application such as studies reported that drought stress tolerance in plants imparted by silica NPs. According to Ashkavand et al. [117], application of silica NPs in hawthorns improved seedling growth and physiological parameters under drought stress. Similar results were observed in Triticum aestivum, which improved starch and gluten content thereby improving growth and yield under drought condition [107]. This amendment is due to the ability of TiO2 to facilitate germination of seeds and growth of seedlings. TiO2 also helps to increase biomass, keep relative water content (RWC), and boost antioxidative enzymes in plants under drought stress [6]. Jute seeds treated with CaNP (hydroxyapatite nanoparticle) showed improved tolerance against drought stress via biosynthesis of proline and thus controlling the level of proline [118]. Although drought stress severely hampers the corn seedlings and decelerates its growth, whereas treatment with yttrium-doped Fe2O3 NPs improved photosynthetic machinery with increased chlorophyll, carotenoid content, and also ameliorated the negative impacts of drought on B. napus [69].
According to Sedghi et al. [119], ZnO in G. max improved seed germination percentage and dry weight, by utilizing seed reserves at faster rate due to the increased activity of gibberellins. Similarly, Fe2O3 enhanced tolerance against drought stress by modifying carbohydrate metabolism and stomatal movements. Studies conducted in maize proved that nano ZnO downregulate photosynthetic pigment degradation and thus enhance the rate of photosynthesis and stomatal movements. Starch and sucrose synthesis were also enhanced by manipulating key enzymes such as UDP glucose pyrophosphorylase, phosphoglucoisomerase, and cytoplasmic invertase leading to better performance under drought stress [57]. This makes ZnO a potential nano agent to mitigate the negative effects of drought stress. Van Nguyen et al. [103] reported that in maize, CuO NPs positively regulate pigment system and ROS scavenging mechanism to cope with drought stress. Application of the same NP at low concentration via roots and leaves has been found to improve crop performance by enhancing the performance of chlorophyll and photosynthetic enzymes such as RubisCO and thereby photosynthesis. It also helps in supplement uptake, fortifying stress resilience, and positively impacts on yield.
5.2. Salinity
Salt stress is the most noteworthy universal concern that influences crop growth and productivity. Unusual increase in sodium (Na+) and chloride (Cl−) generates cytotoxicity and imbalance in nutrition further coupled with oxidative stress due to ROS production followed by implementing a strategy of osmoregulation. During osmoregulation, the plant will accumulate the organic compounds such as amino acids, polyols, sugars, glycine betaine, and quaternary ammonium compounds which further results in decreased osmotic potential. Another key solution is ion homeostasis where the concentration of Na+ is reduced and K+ concentration will be increased in the cell to overcome the ROS affect and to start the activity of enzymatic machinery [120,121].
NPs help in mitigating such stresses by activating specific genes, accumulating osmolytes, and providing free nutrients and amino acids. In Cucurbita pepo, treatment with SiO2 NPs improved the plant transpiration rate, water use efficiency (WUE), enzyme carbonic anhydrase activity, and defense response against salinity stress [122]. Correspondingly, TiO2 (anatase) alters the photoreduction activity and hinders linolenic acid in the electron transport chain (ETC) [123]. A study carried out in Abelmoschus esculentus revealed that foliar application of ZnO improves photosynthetic functionality and enzymatic machinery to reduce negative impacts of salinity stress. It positively impacted on plant growth and resulted in enhanced photosynthesis by improving the efficiency of photosystem II. It also helps to maintain RWC thus decreasing membrane damage [124]. Similarly, combined application of ZnO and Si as foliar spray in mango seedlings augmented the carbon assimilation and nutrient uptake further leading to improved growth conditions [125]. Various reports on SiO2 application confirmed improved vegetative growth, increased epicuticular wax layer, accumulation of proline, and salt stress genes were up- or downregulated to mitigate salinity impact in different plants such as Solanum lycopersicum, strawberry, and Ocimum basilicum [126,127,128].
AgNPs is a well-known nanomaterial; it has been reported that AgNPs act as potential nano agents to mitigate salinity stress. AgNPS in T. aestivum increased the accumulation of POD, proline, and sugar, further followed by enhanced germination [129]. Similarly, treatment with CeO, CNTs, and graphene NPs improved the assimilation of photosynthetic carbon, increased the proteins and amino acids at reproductive stage, and thus imparted tolerance against salinity stress in cotton and Catharanthus roseus [80,130]. ZnO enhanced salt tolerance by lowering the contents of malondialdehyde (MDA) and Na+ in lupine plants, and improved germination in cumin seeds. Application of n-ZnO diminished the negative effects of NaCl through enhancing photosynthetic system, proper osmoregulation, and bringing down the levels of MDA and Na+ [19].
5.3. Extreme Temperature
Temperatures above maximum threshold level are called heat stress and below a minimum threshold level are known as cold stress. These stresses can create an imbalance of cell homeostasis and promote serious hindrance which may even lead to the death of the plants. Extreme temperature directly imparts a combination of heat, and as a consequence, oxidative stress leading to the excessive production of reactive species and further alterations in physiological and biochemical activity such as production of various osmolytes and heat shock proteins (HSPs) that can protect proteins and cell structures, and enhance antioxidant mechanism to restore the redox potential and homeostasis [131].
NPs such as selenium were found to be effective in combating high temperature stress. Djanaguiraman et al. [79] reported that application of selenium NPs in sorghum improved their antioxidant machinery to scavenge ROS produced as a result of heat, thus alleviating heat stress. Similar results of SeNPs were observed in L. esculentum that imparted tolerance against both high and low temperature stresses [108]. Photosynthetic apparatus of wheat plants was highly affected by heat, however, use of AgNPs imparted tolerance against heat stress and improved the morphological features such as root shoot length, root number, fresh and dry weight, leaf area, and number [132]. Furthermore, application of NPs such as ZnO regulated the antioxidative system and chilling response transcription factors under chilling stress in Oryza sativa L. [133].
5.4. Metal/Metalloid Toxicity
Application of NPs are arising as a competent technique in the field of phytoremediation due to the effective interaction of the NPs with plants’ metabolism and metal ions. Phytoremediation is a sustainable technique for the removal of hazardous wastes from environment using potent plant candidates [134]. The NPs promoted growth of different plant species exposed to heavy metal toxicity by mitigating the oxidative stress elicited by heavy metals [111,135]. Application of 100 μM silicon dioxide improved the Cd, Cu, and Mn stress tolerance potential of A. pygmaea by augmenting biomass accumulation and increasing the activities of different biocatalysts in the plant [111]. Moreover, the silicon dioxide increased the absorption and accumulation of heavy metals in roots and thus prevented the translocation of the toxic compounds to the leaves [111]. NPs have the ability to immobilize the toxic metal ions and nanofibrous composite membranes using polyvinyl alcohol, and polyacrylonitrile have the metal chelation efficiency that aids in the removal of Cr and Cd [136]. This study also validated the metal chelation efficiency of NPs depends on the positive or negative charge it possesses on the surface [136]. The NPs have the potential to protect the membrane of the plant exposed to stress by preventing the membrane degradation through low MDA accumulation of NPs- treated plants exposed to metal stress [90]. In Leucaena leucocephala, ZnO NPs induced elevation of SOD, CAT, and APX activity that contributes to the reduction of MDA content under Cd and Pb stresses [90]. Addition of magnetic nano-Fe3O4 into the growing media of wheat seedlings contaminated with Pb, Zn, Cd, and Cu (10 mM) increased the activity of SOD and POD, and thus alleviated the MDA accumulation [135]. Fe NPs which upregulate the activity of antioxidant enzymes and glyoxalase through the accumulation of phytochelatins and glutathione simultaneously resulted in the boosting up of the tolerance to arsenic in rice [110]. Exposure to NPs recovered the mineral acquisition and thus maintained the biosynthesis of photosynthetic pigments in finger millet [137]. Parallel responses were observed in G. hirsutum when it was treated with ZnO NPs for tolerating Cd and Pb stresses [138]. The potential of ZnO NPs in the clearing of HM- contaminated media was established in a study performed in rice [109].
5.5. Flooding
The plants exposed to prolonged anaerobic condition as a result of flooding stress exhibit growth retardation and severe loss in crop productivity. Protein metabolism plays a significant role in the flooding stress tolerance of plants. Application of Ag NPs augmented the stress tolerance potential of soybean seedling by downregulation of protein mis-folding induced by flooding stress [112]. During flooding stress, augmentation of glyoxalase II 3, alcohol dehydrogenase 1, and pyruvate decarboxylase 2 genes was noticed, whereas upon the exposure of Ag NPs, the flood-induced metabolic changes were regulated and it reflected on the downregulation of all these enzymes [112]. Influence of Ag NPs in the production of the glyoxalase II 3 was one of the prominent outcomes of proteomics and this enzyme is considered as an indicator of cytotoxicity. When nicotinic acid and potassium nitrate (KNO3) were incorporated with Ag NPs, it further boosts up the flood tolerance in plants [114]. Another metal NP of Al2O3 also showed significant contribution in flood stress tolerance of soybean [115]. Moreover, NPs aid to fasten the recovery kinetics of flooding stress; soybean exposed to aluminum oxide nanoparticles (Al2O3 NPs) has the potential to recovery by the involvement of S-adenosyl-l-methionine-dependent methyltransferases and enolase [139]. The findings from the study conducted by Mustafa and Komatsu [115] give clear indication on the influence of size of NPs in flood tolerance, rather than the quantity and types. Three different sizes of Al2O3 NPs triggered different metabolic responses in plants under flood. The catalytic activity of isocitrate dehydrogenase was increased with the application of 5 nm Al2O3 NPs, but 30–60 nm Al2O3 NPs induced ribosomal protein production under flood. Whereas by the high concentration, 135 nm Al2O3 NPs, improved permeability of the mitochondrial membrane [115]. The differential imprints of 2, 15, and 50–80 nm Ag NPs on the tolerance mechanisms of the soybean under flood stress was reported by Mustafa et al. [140]. Of the three sizes, 15 nm Ag NPs was more effective due to the increase in ribosomal proteins, and amino acid metabolism-related proteins with a reduction in protein synthesis-related proteins.
5.6. Other Abiotic Stresses
Apart from salinity, drought, temperature, and heavy metal stresses, other stresses such as high light, UV, and nutrient stresses can cause oxidative stress in plants, altering their growth and development. NPs such as TiO2 play a significant role in mitigating light stress by catalyzing the redox reaction, which leads to the generation of superoxide and hydroxide radicals. UV imparts negative consequences on growth as it induces oxidative stress. Photosynthetic apparatus would be highly damaged leading to ROS production and change in leaf structure following exposure to UV-B whereas application of SiNPs enhanced the antioxidant machinery to regulate oxidative stress resulting from UV-B exposure [8]. Thus, NPs modulate abiotic stress-induced responses at different levels in plants, and may be considered as potential tools for abiotic stress management in crops.
6. Dose-Dependent Variability of the Nanoparticle Action
Entry of NPs into the plant cells occurs via roots and leaves, and cause differential morphological and physiological changes, which either become inhibitory or stimulatory, depending upon the NPs’ properties, such as: chemical nature, size, reactivity, and the concentration of NPs. The inhibitory impacts of metallic NPs are apparent through its toxicity in plants. A number of research studies on plant–NPs interaction shows that NPs have both negative and positive effects, depending on the specific properties of NPs, their concentrations, reactivity, and plant species [141,142,143,144,145]. For instance, Lin and Xing [146] showed that seed supplemented with ZnO NPs at high concentration of 2000 mg L−1 negatively affected the germination of corn and ryegrass. Similarly, Ma et al. [147] observed the impacts of gadolinium (III) oxide (Gd2O3), cerium (IV) oxide (CeO2), ytterbium oxide (Yb2O3), and lanthanum (III) oxide (La2O3) at high concentration on the growth of cabbage, lettuce, radish, rape, cucumber, tomato, and wheat, and propounded that CeO2 inhibited the root elongation of lettuce at the concentration of 2000 mg L−1, while La2O3, Gd2O3, and Yb2O3 at 2000 mg L−1 suppressed the root elongation of all these seven plant species. Likewise, seed treated with TiO2 and aluminum oxide (Al2O3) affected seed germination, growth, and development of tobacco plants. A study of other researchers also showed the reduced growth of C. annum seedlings supplemented with 1 mg L−1 Ag NPs [148]. Inhibition of Lemna minor growth and the decreased activity of POX, CAT, and SOD activity were reported under CuO NPs (200 mg L−1) [149]. Moreover, ZnO NPs significantly declined the biomass of rye seedlings as well as affected the root anatomy by shrinking root tip, epidermal, and cortex cell deformation [146].
Several studies have shown that NPs at concentrations below certain limits stimulates seed germination [150,151], and plant growth and development [152,153]. For developing the better understanding of NPs’ influence on plant growth, further studies could be done based on the types and concentration of NPs.
Experimental findings of Suriyaprabha et al. [154] show that SiO2 promoted seed sprouting of maize seedlings by increasing the nutrient uptake. A study related to TiO2 NPs’ impacts on soybean plant resulted in increased germination by enhancing the activity of nitrate reductase. Moreover, the NP-treated seed has the capability of increased water uptake, better water utilization, and increased nutrient uptake from the soil [155]. ZnO NPs at low concentration (10–20 μg mL−1) reportedly enhanced the seed germination as well as stimulated the plant growth of soybean [119], onion [23], peanut [156], wheat [157], and in cluster bean, Cyamopsis tetragonoloba [158]. Furthermore, Kumar et al. [159] also stated that Au NP at 10 and 80 μg mL−1 increased the plant growth and yield as well as enhanced the number and leaf area along with chlorophyll and sugar content in A. thaliana. Reportedly, the addition of Ag NPs at 20–60 ppm stimulated the plant length of mustard, beans, and corn, and also increased carbohydrate, chlorophyll, and protein content in B. juncea [160,161]. In Table 3, we tried to show the positive and negative impacts of various nanoparticles on plants.

Table 3.
Dose-dependent impacts of nanoparticles on different plant species.
7. Priming with Nanoparticles: An Emerging Stress Elicitor
Seed priming is the most effective method for mitigation of stress tolerance and enhancement of crop production in plants [171]. Priming approaches are established to augment germination and seedling growth by changing seed vigor or physiological status of the seed [172,173]. In the recent few years, nanopriming method of seed priming with synthetic NPs gained significance in crop advancement owing to their small size and distinctive physicochemical properties of nanomaterials [174]. NPs, besides improving plant growth, also safeguard from various kinds of stresses. Heavy metals (HMs) are bound to the NPs’ surface due to its great surface area and lesser size, therefore decreasing its accessibility [2]. NPs can simulate the antioxidant enzyme activity in nano-enzymes, which can scavenge from oxidative stress [175]. Photosynthesis is a key metabolic process in plants and a highly vulnerable approach, which alleviates oxidative and osmotic stress, and its usual working can be sustained. In photosynthesis apparatus, photosystem II, RubisCo, and ATP are the chief goals under stress conditions [176,177]. The SiO2 NPs enhanced chlorophyll, transpiration rate, WUE, and carbonic anhydrase activity in Cucurbita pepo under salinity conditions [122]. Likewise, TiO2 alters the photoreduction activity and prevents linolenic acid in the electron transport chain. It also reduces the oxygen evolution rate of chloroplast [123]. Numerous stress responses are exhibited by plants like changes in molecular machineries, stress response gene expression, and generation of antioxidative enzymes, which helps to exhibit significant function in scavenging the plants in severe environmental conditions [178]. Plants guard themselves from osmotic stress by generating different organic osmolytes like polyols and trehalose, and diverse amino acids like glycine and proline. NPs provide sustenance to plants in mitigating this defense mechanism [179]. In stress situations, ROS are generated by cell organelles, and this is the sign of abiotic stress conditions. Plants are furnished with enzymatic apparatus to cope with oxidative stress levied by the environment [2].
Priming induces enhancement in amylases, lipases, and proteases enzyme activities that degrade macromolecules for growth and development of embryos. It also mitigates stress at the germination stage and eventually results in greater rates of seedling appearance and efficacious seedling formation [180]. These biological impacts provide assistance to farmers in that they decrease the time, fertilization, and expenditure of re-seeding. Nanopriming increases α-amylase activity in rice plants and ensuing greater soluble sugar concentration for supportive seedling growth. However, more ROS generation was found in germinating seeds of nanopriming treatment in contrast to control rice plants, indicating that both ROS and aquaporins exhibit significant function in increasing the seed germination [181,182]. Diverse approaches for nanopriming mediating seed germination were suggested, comprising formation of nanopores for augmented water uptake, restarting antioxidant systems, formation of hydroxyl radicals for cell wall relaxing, and nanocatalysts for rapid starch hydrolysis [181].
8. Biochemical Mechanism of Metal/Metalloid-Based Nanoparticles to Mitigate Abiotic Stresses
NPs are essential implements which act as nanofertilizers, pesticides, herbicides, etc., for the proper growth and development of plants under various environmental stresses, though the exact mechanisms in particular are still undiscovered [15]. It is believed that there are some biochemical mechanisms such as detoxification pathway, especially based on the activities of enzymatic antioxidants behind the mitigation process of stress-induced damage using NPs. The reactivity of NPs is dependent upon some essential factors like shape, size, composition, surface properties, stability, chemical properties, purity and production process, and most importantly, dose applied [183,184,185,186]. Additionally, the susceptibility of NPs to different environments are mainly due to the transformation of their configuration phase and oxidation process [187]. The core conformation of NPs may vary plant species to species and are dependent upon the changes of environments leading to alter their chemical and physical properties that eventually exert different responses [188]. Khan et al. [9] reported that metal/metalloid NPs can combat the adverse effects of abiotic stresses in crops. Generally, NPs’ uptake take place via plasmodesmata, and the translocation of NPs occurs via apoplast and symplast [189]. They also demonstrated that application of NPs enhanced biomass levels, chlorophyll contents as well as photosynthetic processes, antioxidant machineries, synthesis of osmolytes, and carbohydrate contents in plant cells. Beside these, when NPs enter into the plant cells, it not only promotes N2 levels and protein contents but also regulate the gene expression during both biotic and abiotic stresses [189,190]. According to Sharifi et al. [175], NPs can simulate the antioxidant defense system as nano-enzymes which restrict the production of ROS under stress environments. NP supplementation increased the activities of some enzymatic antioxidants viz., SOD, CAT, APX, POX, etc., and also boost up the levels of glutathione levels, proline levels, and the phytochelatin synthesis in plants [190]. Mahato et al. [191] also reported that NPs restrict the generation of oxidative stress by upregulating the antioxidant defense system under different stressed conditions viz., salt stress, temperature stress, drought stress, UV stress, etc. Thus, in this viewpoint, the enhancement of mentioned parameters due to NP supplementation are responsible for the increase in tolerability in plants under environmental stresses.
According to Liu and Lal [192] and Ranjan et al. [193], there are various kinds of NPs (viz., Mg NPs, TiO2 NPs, ZnO NPs, Cu NPs as CuO, Ag NPs as AgNO3, SiO4, Mn NPs as MnSO4, Ca NPs as CaCO3, Mo NPs, phosphorous NPs as [Ca5(PO4)3OH], AlO4 carbon nanotubes, Fe2O3 NPs, and chitosan complex of Cu or Zn) have been used in field conditions for proper growth and yield of agricultural crops. At first, NPs choose lateral root synapse to enter into the plant rhizosphere and outreach towards xylem via cortex and then pericycle [194]. However, their association with plants takes place on the basis of some biochemical activities which may activate not only the transport of ions into the cell but also reacts with -SH and -COOH groups, and modifies protein levels in the plant cells [195]. Additionally, NPs are able to form a network with the transporters present in the membrane of plant root cells to fetch inside the plants [196,197]. Thus, the transport of NPs into the cytoplasm occurs from roots to shoots, stem, leaves via cuticle, and ultimately in the grain but the main entrance is xylem [198,199]. Upon entry into the cell cytoplasm NPs form complexes with diverse cell organelles and consistently begin the metabolic pathways required for growth and yield of the plants [200]. In Figure 3, we have illustrated the effect of nanoparticles on abiotic stresses schematically, also, Table 4 lists the biochemical activities of some of the most common metal/metalloid-based NPs to combat the effects of abiotic stress.
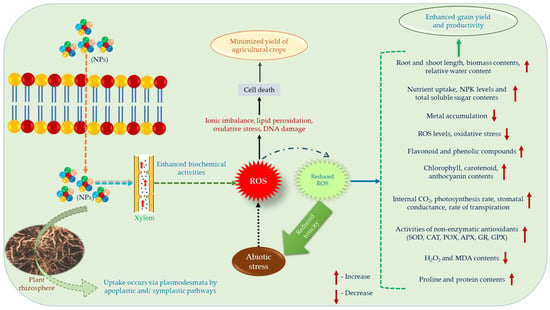
Figure 3.
Schematic representation of uptake and impact of NPs during abiotic stress.

Table 4.
Biochemical activities of some metal/metalloid-based NPs to combat abiotic stress effects.
9. Limitations of Using Nanoparticles for Crop Production
Though the supplementation with NPs caused positive impact on agricultural crops to mitigate various kinds of environmental stresses, all NPs cannot possess proper defense as it varies from species to species differentially [246]. There are several reports based on the NPs’ phytotoxicity that induced the synthesis of ROS and oxidative damage [198,247,248,249,250,251]. According to Gottschalk et al. [252] and Navarro et al. [253], the application of NPs in high dose caused toxicity whereas in low dose, NPs contributed a positive effect in combating abiotic stress-induced oxidative damage through antioxidant defense system [254,255]. NPs executed harmful effects by producing genotoxicity and oxidative stress in plants [146,247,256,257,258,259] that also affected the physicochemical metabolic pathways [94] by hampering the mineral uptake in agricultural crops [260]. The toxicity of NPs is dependent on not only the dose applied but also on the application process and its shape and size [251,261,262]. According to Manke et al. [263], the conformational alteration in shape and size of the NPs can lead to ROS production by affecting biochemical metabolism. They also demonstrated that the phytotoxicity of NPs is responsible for severe physiological deterioration by inducing inflammation, cell signaling, and genotoxicity. Ebbs et al. [251] reported that in plants, the toxicity levels of NPs regarding uptake, accumulation, and transportation also rely on the composition and surface area. Metal/metalloid-based NPs trigger Fenton reactions to generate free radicals that eventually produce ROS in plants [264]. There are some factors that are responsible for an imbalance of redox status of NPs, as a result, the antioxidant defense system would be downregulated and the generation of free radicals would be enhanced [265]. Priester et al. [266] stated that further investigation on the degree of NPs’ toxicity is vital for NPs’ supplementation in crops. Their uptake and accumulation should also be examined for better understanding. Therefore, keeping in mind these limitations, all the factors viz., size, shape, composition, surface area, application procedures, redox state, applied dose etc., should be investigated properly before application of NPs in agricultural fields to avoid ecotoxicological risks for both plants and humans.
10. Conclusions
Crop production globally has undergone several challenges in terms of climate and stresses. To overcome such challenges, nanotechnology has come up as a key component for sustainable development. Nanomaterials have the properties to nullify the harmful effects of abiotic stresses in plants by activating the antioxidant defense system of plants. Due to their property of being able to penetrate in plants and large surface area, they have more effective adsorption and targeted delivery, can be responsible in regulating photosynthetic efficiency and water uptake, and detoxifying reactive oxygen species, thereby enhancing seed germination, growth, and yield of crops. By careful analysis of dosage to be used for different nanomaterials, they can be sustainably utilized in the agriculture for better productivity. However, there is still a need for the risk assessment and fate of nanomaterials in plants and soil as well as their interaction with the ecosystem.
Author Contributions
Conceptualization, M.S. and M.H.; writing—original draft preparation, M.S., K.V., V.K., N.A., S.D., R.J., E.J., S.A. and M.H., writing—review and editing, J.T.P., D.K.C., M.F. and M.H.; visualization, M.H. and M.F.; supervision, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All information is presented in this article.
Acknowledgments
The authors thank Farzana Nowroz, Department of Agronomy, Sher-e-Bangla Agricultural University, for her critical reading of the manuscript. Mirza Hasanuzzaman acknowledge International Union of Biological Sciences (IUBS) New Initiative Grant for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 10. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Du, W.; Barrios, A.C.; Armendariz, R., Jr.; Zuverza-Mena, N.; Ji, Z.; Chang, C.H.; Zink, J.I.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; et al. Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ. Pollut. 2017, 222, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.; Zhang, Y.; Cai, J.; Zhang, W.; Pan, B. Arsenate adsorption by hydrous ferric oxide nanoparticles embedded in cross-linked anion exchanger: Effect of the host pore structure. ACS Appl. Mater. Interfaces 2016, 8, 3012–3020. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil. Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Tripathi, D.K.; Chauhan, D.K.; Sharma, S.; Sahi, S. Potential applications and avenues of nanotechnology in sustainable agriculture. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 473–500. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Vishwakarma, K.; Singh, S.; Sharma, S.; Dubey, N.K.; Singh, V.K.; Liu, S.; Tripathi, D.K.; Chauhan, D.K. Understanding the plant and nanoparticle interface at transcriptomic and proteomic level: A concentric overview. Plant Gene 2017, 11, 265–272. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.K.; Cho, J.; Kannan, A.G.; Lee, Y.S.; Kim, D.W. Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Manjunatha, R.L.; Naik, D.; Usharani, K.V. Nanotechnology application in agriculture: A review. J. Pharm. Phytochem. 2019, 8, 1073–1083. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of Nanoparticles in Plants. In Nanotechnology and Plant Science, 1st ed.; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Taran, N.; Storozhenko, V.; Svietlova, N.; Batsmanova, L.; Shvartau, V.; Kovalenko, M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- Skiba, E.; Adamczyk-Szabela, D.; Wolf, W.M. Metal based nanoparticles interactions with plants. In Plant Responses to Nanomaterials. Recent Interventions and Physiological and Biochemical Responses; Singh, V.P., Singh, S., Prasad, S.M., Chauhan, D.K., Tripathi, D.K., Eds.; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Sepehrizadeh, Z.; Mahdavi, M.; Shahverdi, A.R.; Faramarzi, M.A. Metal, metalloid, and oxide nanoparticles for therapeutic and diagnostic oncology. Nano Biomed. Eng. 2016, 8, 246–267. [Google Scholar] [CrossRef][Green Version]
- Kalisz, A.; Húska, D.; Jurkow, R.; Dvořák, M.; Klejdus, B.; Caruso, G.; Sękara, A. Nanoparticles of cerium, iron, and silicon oxides change the metabolism of phenols and flavonoids in butterhead lettuce and sweet pepper seedlings. Environ. Sci. Nano 2021, 8, 1945–1959. [Google Scholar] [CrossRef]
- Alonso, J.; Barandiarán, J.M.; Fernández Barquín, L.; García-Arribas, A. Magnetic nanoparticles, synthesis, properties, and applications. In Magnetic Nanostructured Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Jain, K.; Jain, N.K.; Kesharwani, P. Types of dendrimers. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 95–123. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Shojaei, T.R.; Salleh, M.A.M.; Tabatabaei, M.; Mobli, H.; Aghbashlo, M.; Rashid, S.A.; Tan, T. Applications of nanotechnology and carbon nanoparticles in agriculture. In Synthesis, Technology and Applications of Carbon Nanomaterials; Suraya, A.R., Raja, N.I.R.O., Mohd, Z.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, characterization, and applications of metal nanoparticles. In Advances in Pharmaceutical Product Development and Research, Biomaterials and Bionanotechnology, 1st ed.; Tekade, R.K., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Wiesner, M.R.; Lowry, G.V.; Alvarez, P.; Dionysiou, D.; Biswas, P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef]
- Fernandez, Y.A.D.; Gschneidtner, T.A.; Wadell, C.; Fornander, L.H.; Avila, S.L.; Langhammer, C.; Westerlund, F.; Moth-Poulsen, K. The conquest of middle-earth: Combining top-down and bottom-up nanofabrication for constructing nanoparticle based devices. Nanoscale 2014, 6, 14605–14616. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S.; Yudha, S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Tocopherol-mediated synthesis of silver nanoparticles and preparation of antimicrobial PBAT/silver nanoparticles composite films. LWT 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Jadhav, K.; Deore, S.; Dhamecha, D.; Hr, R.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of silver nanoparticles: Characterization, biocompatibility studies, and anticancer activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Silicon and plant growth promoting rhizobacteria differentially regulate AgNP-induced toxicity in Brassica juncea: Implication of nitric oxide. J. Hazard. Mater. 2020, 390, 121806. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R.G.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Mishra, R.K.; Singh, S.; Singh, S.; Vishwakarma, K.; Sharma, S.; Singh, V.P.; Singh, P.K.; Prasad, S.M.; Dubey, N.K.; et al. Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: Implication of the ascorbate-glutathione cycle. Front. Plant Sci. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Maestri, E.; Pagano, L.; Marmiroli, M.; White, J.C.; Marmiroli, N. Plant response to metal-containing engineered nanomaterials: An omics-based perspective. Environ. Sci. Technol. 2018, 52, 2451–2467. [Google Scholar] [CrossRef]
- Fiol, D.F.; Terrile, M.C.; Frik, J.; Mesas, F.A.; Álvarez, V.A.; Casalongué, C.A. Nanotechnology in plants: Recent advances and challenges. J. Chem. Technol. Biotechnol. 2021, 96, 2095–2108. [Google Scholar] [CrossRef]
- Laware, S.L.; Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 749–760. Available online: https://www.ijcmas.com/vol-3-7/S.L.Laware%20and%20Shilpa%20Raskar.pdf (accessed on 5 November 2021).
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Wu, G.R.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–172. [Google Scholar]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Takahashi, S.; Katagiri, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. An Arabidopsis Gene Encoding a Ca2+-Binding Protein is induced by Abscisic Acid during Dehydration. Plant Cell Physiol. 2000, 41, 898–903. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-Induced Drought Tolerance is Associated with Melatonin Synthesis and Metabolism in Maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; ur Rehman, M.Z.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ. Sci. Technol 2016, 50, 2000–2010. [Google Scholar] [CrossRef]
- Frazier, T.P.; Burklew, C.E.; Zhang, B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Func. Integr. Genom. 2014, 14, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Yang, Z.; Qu, J.; Xun, H.; Dou, R.; Gao, X.; Wang, L. Phenotypic, transcriptional, physiological and metabolic responses to carbon nanodot exposure in Arabidopsis thaliana (L.). Environ. Sci. Nano 2018, 5, 2672–2685. [Google Scholar] [CrossRef]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. In Nanotechnology and Plant Science; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–17. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Chen, F.; Yue, L.; Zou, H.; Lyu, J.; Wang, Z. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: Trends, meta-analysis, and prospect. Sci. Total Environ. 2021, 780, 146578. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Roy, A.; Sahoo, R.; Ray, C.; Dutta, S.; Pal, T. Soft template induced phase selective synthesis of Fe2O3 nanomagnets: One step towards peroxidase-mimic activity allowing colorimetric sensing of thioglycolic acid. RSC Adv. 2016, 6, 32308–32318. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Peng, F.F.; Zhang, Y.; Gu, N. Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chin. Chem. Lett. 2008, 19, 730–733. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Ghanati, F.; Modarres-Sanavi, A.M.; Khoshgoftarmanesh, A.H. Physiological effects of repeated foliar application of magnetite nanoparticles on maize plants. J. Agron. Crop Sci. 2017, 203, 593–602. [Google Scholar] [CrossRef]
- Rahmatizadeh, R.; Arvin, S.M.J.; Jamei, R.; Mozaffari, H.; Reza Nejhad, F. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact. 2019, 14, 474–481. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Xiao, L.; Wang, Y.; Wang, X. Interaction mechanisms between α-Fe2O3, γ-Fe2O3 and Fe3O4 nanoparticles and Citrus maxima seedlings. Sci. Total Environ. 2018, 625, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutasemimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host-guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (nanozymes): Current advances and future perspectives. Front Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Co3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 378, 30–37. [Google Scholar] [CrossRef]
- Jahani, M.; Khavari-Nejad, R.A.; Mahmoodzadeh, H.; Saadatmand, S. Effects of foliar application of cobalt oxide nanoparticles on growth, photosynthetic pigments, oxidative indicators, non-enzymatic antioxidants and compatible osmolytes in canola (Brassica napus L.). Acta Biol. Cracov. Bot. 2019, 61, 29–42. [Google Scholar] [CrossRef]
- Ragg, R.; Schilmann, A.M.; Korschelt, K.; Wieseotte, C.; Kluenker, M.; Viel, M.; Volker, L.; Preiss, S.; Herzberger, J.; Frey, H.; et al. Intrinsic superoxide dismutase activity of MnO nanoparticles enhances the magnetic resonance imaging contrast. J. Mater. Chem. B 2016, 4, 7423–7428. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Liu, C.; Guan, Y.; Ren, J.; Qu, X. Manganese dioxide nanozymes as responsive cytoprotective shells for individual living cell encapsulation. Angew. Chem. Int. Ed. Engl. 2017, 56, 13661–13665. [Google Scholar] [CrossRef]
- Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S.; Wang, X.; Wu, J.; Li, S.; Wei, H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018, 9, 2927–2933. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. Engl. 2017, 56, 14267–14271. [Google Scholar] [CrossRef]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants-a soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Asthir, B.; Kaur, G.; Kalia, A.; Sharma, A. Zinc oxide and titanium dioxide nanoparticles influence heat stress tolerance mediated by antioxidant defense system in wheat. Cereal Res. Commun. 2021, 49, 1–2. [Google Scholar] [CrossRef]
- Landa, P.; Vankova, R.; Andrlova, J.; Hodek, J.; Marsik, P.; Storchova, H.; White, J.C.; Vanek, T. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J. Hazard. Mater. 2012, 241–242, 55–62. [Google Scholar] [CrossRef]
- Gunjan, B.; Zaidi, M.G.H.; Sandeep, A. Impact of gold nanoparticles on physiological and biochemical characteristics of Brassica juncea. J. Plant Biochem. Physiol. 2014, 2, 133. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.F.; Tan, Z.Q.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nanobiotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- An, Z.; Yan, J.; Zhang, Y.; Pei, R. Applications of nanomaterials for scavenging reactive oxygen species in the treatment of central nervous system diseases. J. Mater. Chem. B 2020, 8, 8748–8767. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, A.D. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 2017, 63, 65–75. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: A sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of chitosan—PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef]
- Abou-Zeid, H.; Ismail, G. The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. to salt stress. Egypt. J. Bot. 2018, 58, 73–85. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2021, 40, 1–12. [Google Scholar] [CrossRef]
- Mozafari, A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria× ananassa Duch.) to cope with drought stress. Plant. Cell. Tiss.Organ. Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Pandey, K.; Anas, M.; Hicks, V.K.; Green, M.J.; Khodakovskaya, M.V. Improvement of commercially Valuable traits of industrial crops by application of carbon-based nanomaterials. Sci. Rep. 2019, 9, 19358. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Moghadam, H.R.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef]
- Haghighi, M.; Abolghasemi, R.; da Silva, J.A.T. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 2014, 178, 231–240. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic effects of zinc oxide nanoparticles and bacteria reduce heavy metals toxicity in rice (Oryza sativa L.) plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z.; Xie, Y. Determination of heavy metal tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob. Ecol. Cons. 2020, 24, e01306. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Hossain, Z.; Komatsu, S. Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J. Proteom. 2015, 122, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of poly (epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Hashimoto, T.; Mustafa, G.; Nishiuchi, T.; Komatsu, S. Comparative analysis of the effect of inorganic and organic chemicals with silver nanoparticles on soybean under flooding stress. Int. J. Mol. Sci. 2020, 21, 1300. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteom. Res. 2016, 15, 4464–4475. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomášková, I.; Struve, D. Effect of SiO2 Nanoparticles on Drought Resistance in Hawthorn Seedlings. For. Res. Pap. 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Das, A.; Ray, R.; Mandal, N.; Chakrabarti, K. An analysis of transcripts and enzyme profiles in drought stressed jute (Corchorus capsularis) and rice (Oryza sativa) seedlings treated with CaCl2, hydroxyapatite nano-particle and β-amino butyric acid. Plant Growth Regul. 2016, 79, 401–412. [Google Scholar] [CrossRef]
- Sedghi, M.; Hadi, M.; Toluie, S.G. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. WUT-Ser. Biol. 2013, 16, 73–78. [Google Scholar]
- Isayenkov, S.V. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Su, M.; Liu, C.; Qu, C.; Zheng, L.; Chen, L.; Huang, H.; Liu, X.; Wu, X.; Hong, F. Nano-anatase relieves the inhibition of electron transport caused by linolenic acid in chloroplasts of spinach. Biol. Trace Elem. Res. 2009, 131, 99. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Oprica, L.; Grigore, M.N.; Bara, I.; Vochita, G. Salinity and SiO2 Impact on Growth and Biochemical Responses of Basil (Ocimum Basilicum L.) Seedlings. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Mohamed, A.K.S.H.; Qayyum, M.F.; Abdel-Hadi, A.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- McGehee, D.L.; Alimohammadi, M.; Khodakovskaya, M.V. Carbonbased nanomaterials as stimulators of production of pharmaceutically active alkaloids in cell culture of Catharanthus roseus. Nanotechnology 2019, 30, 275102. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Raja, N.I.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran. J. Sci. Technol. Transac. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza Sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Yin, X.; Ma, H.; Hsiao, B.S. Highly permeable nanofibrous composite microfiltration membranes for removal of nanoparticles and heavy metal ions. Sep. Purif. Technol. 2020, 233, 115976. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Foliar application of chitosan nanoparticle improves yield, mineral content and boost innate immunity in finger millet plants. Carbohydr. Polym. 2021, 258, 117691. [Google Scholar] [CrossRef]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar] [CrossRef]
- Yasmeen, F.; Raja, N.I.; Mustafa, G.; Sakata, K.; Komatsu, S. Quantitative proteomic analysis of post-flooding recovery in soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2016, 143, 136–150. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of soybean root exposed to varying sizes of silver nanoparticles under flooding stress. J. Proteom. 2016, 148, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Arshad, M.; Khokhar, M.F.; Qazi, I.A.; Hamza, A.; Virk, N. Growth response of wheat to titania nanoparticles application. NUST J. Engin. Sci. 2014, 7, 42–46. [Google Scholar]
- Van Nhan, L.; Ma, C.; Rui, Y.; Cao, W.; Deng, Y.; Liu, L.; Xing, B. The effects of Fe2O3 nanoparticles on physiology and insecticide activity in non-transgenic and Bt-transgenic cotton. Front. Plant Sci. 2016, 6, 1263. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Ihtisham, M.; Noori, A.; Yadav, S.; Sarraf, M.; Kumari, P.; Brestic, M.; Imran, M.; Jiang, F.; Yan, X.; Rastogi, A. Silver nanoparticle’s toxicological effects and phytoremediation. Nanomaterials 2021, 11, 2164. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef]
- Song, G.; Hou, W.; Gao, Y.; Wang, Y.; Lin, L.; Zhang, Z.; Niu, Q.; Ma, R.; Mu, L.; Wang, H. Effects of CuO nanoparticles on Lemna minor. Bot. Stud. 2016, 57, 1–8. [Google Scholar] [CrossRef]
- Hatami, M. Stimulatory and inhibitory effects of nanoparticulates on seed germination and seedling vigor indices. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 48, pp. 357–385. [Google Scholar] [CrossRef]
- Klanjšček, T.; Muller, E.B.; Holden, P.A.; Nisbet, R.M. Host–symbiont interaction model explains non-monotonic response of soybean growth and seed production to nano-CeO2 exposure. Environ. Sci. Technol. 2017, 51, 4944–4950. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, M.C.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Silica nanoparticles for increased silica availability in maize (Zea mays. L) Seeds under hydroponic conditions. Curr. Nanosci. 2012, 8, 902–908. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanisamy, K.; Babu, K.; Sharma, N.K. Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J. Glob. Biosci. 2014, 3, 415–422. Available online: http://www.mutagens.co.in/jgb/vol.03/2/04.pdf (accessed on 12 August 2021).
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. 2013. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013, 461, 462–468. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud. Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef] [PubMed]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Labeeb, M.; Badr, A.; Haroun, S.A.; Mattar, M.Z.; El-Kholy, A.S.; El-Mehasseb, I.M. Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanz. 2020, 72, 113–127. [Google Scholar] [CrossRef]
- de Almeida, G.H.G.; Siqueira-Soares, R.C.; Mota, T.R.; de Oliveira, D.M.; Abrahão, J.; Foletto-Felipe, M.P.; dos Santos, W.D.; Ferrarese-Filho, O.; Marchiosi, R. Aluminum oxide nanoparticles affect the cell wall structure and lignin composition slightly altering the soybean growth. Plant Physiol. Biochem. 2021, 159, 335–346. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, C.; He, X.; Zhang, B.; Yang, J.; Sun, M.; Luo, W.; Feng, S.; Zhang, J.; Wang, G.; et al. Effects of ceria nanoparticles and CeCl3 on plant growth, biological and physiological parameters, and nutritional value of soil grown common bean (Phaseolus vulgaris). Small 2020, 16, 1907435. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense-linked hormones in watermelon seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO nanoparticle- based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Faryabi, K.; Talaei, A.J.; Shekha, M.S.; Ale-Ebrahim, M.; Salihi, A.; Nanakali, N.M.Q.; Aziz, F.M.; Rasti, B.; Hasan, A.; et al. Antioxidant properties of gold nanozyme: A review. J. Mol. Liq. 2020, 297, 112004. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.D.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Shomali, A.; Aliniaeifard, S.; Didaran, F.; Lotfi, M.; Mohammadian, M.; Seif, M.; Strobel, W.R.; Sierka, E.; Kalaji, H.M. Synergistic effects of melatonin and gamma-aminobutyric acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells 2021, 10, 1631. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Ardebili, Z.O.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.B.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed priming with sorghum water extract improves the performance of camelina (camelina sativa (L.) crantz.) under salt stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Teske, S.S.; Detweiler, C.S. The biomechanisms of metal and metaloxide nanoparticles’ interactions with cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Shchyogolev, S.Y. Interactions of plants with noble metal nanoparticles (review). Seľskokhozyaistvennaya Biol. 2017, 52, 13–24. [Google Scholar] [CrossRef]
- Burman, U.; Kumar, P. Plant response to engineered nanoparticles. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Elsevier Academic Press: West Bengal, India, 2018; Volume 1. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Singh, D.; Sillu, D.; Kumar, A.; Agnihotri, S. Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environ. Sci. Nano 2021, 8, 1308–1325. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Mahato, D.K.; Mishra, A.K.; Kumar, P. Nanoencapsulation for agri-food applications and associated health and environmental concerns. Front. Nutr. 2021, 8, 663229. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, B. Nanotechnology a potential tool to mitigate abiotic stress in crop plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Watanabe, T.; Misawa, S.; Hiradate, S.; Osaki, M. Root mucilage enhances aluminum accumulation in Melastoma malabathricum, an aluminum accumulator. Plant Signal. Behav. 2008, 3, 603–605. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2009, 10, 2296–2302. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Sharif, F.; Westerhoff, P.; Herckes, P. Sorption of trace organics and engineered nanomaterials on to wet land plant material. Environ. Sci. Process. Impacts 2013, 15, 267–274. [Google Scholar] [CrossRef]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci. 2009, 110, 138–155. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Bai, X.; Zheng, L.; Zhao, J.; Li, Y.-F.; Zhang, Z.; Gao, Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg(II) in soybean (Glycine max). Environ. Sci. Nano 2020, 7, 1807–1817. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Qados, A.M.S.A. Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean (Vicia faba L.) plants. Am. J. Exp. Agric. 2015, 7, 78–95. [Google Scholar] [CrossRef]
- Qados, A.M.S.A.; Moftah, A.E. Influence of silicon and nano-silicon on germination, growth and yield of faba bean (Vicia faba L.) under salt stress conditions. Am. J. Exp. Agric. 2015, 5, 509–524. [Google Scholar] [CrossRef]
- Azimi, R.; Borzelabad, M.J.; Feizi, H.; Azimi, A. Interaction of SiO2 nanoparticles with seed prechilling on germination and early seedling growth of tall wheatgrass (Agropyron elongatum L.). Pol. J. Chem. Tech. 2014, 16, 25–29. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Aliabadi, M.M.; Nosratabadi, A.F. Effect of silica nanoparticles on Basil (Ocimum basilicum) Under Salinity Stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Janmohammadi, M. Effect of nanosilicon particles application on salinity tolerance in early growth of some lentil genotypes. Ann. UMCS Biol. 2014, 69, 39–55. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Horticult. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Haghighi, M.; Afifipour, Z.; Mozafarian, M. The effect of N-Si on tomato seed germination under salinity levels. J. Biol. Environ. Sci. 2012, 6, 87–90. Available online: https://uludag.edu.tr/dosyalar/jbes/16/mak12.pdf (accessed on 25 November 2021).
- Katiyar, P.; Yadu, B.; Korram, J.; Satnami, M.L.; Kumar, M.; Keshavkant, S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 2020, 92, 18–27. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Aghdam, M.T.B.; Mohammadi, H.; Ghorbanpour, M. Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 2016, 39, 139–146. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, H.; Maali-Amiri, R.; Zeinali, H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 2015, 62, 779–787. [Google Scholar] [CrossRef]
- Kiapour, H.; Moaveni, P.; Habibi, D.; Sani, B. Evaluation of the application of gibbrellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.). Int. J. Agron. Agric. Res. 2015, 6, 138–150. Available online: https://www.innspub.net/wp-content/uploads/2015/04/IJAAR-V6No4-p138-150.pdf (accessed on 20 November 2021).
- Mohammadi, R.; Maali-Amiri, R.; Abbasi, A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013, 152, 403–410. [Google Scholar] [CrossRef]
- Wahid, I.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. silver nanoparticle regulates salt tolerance in wheat through changes in aba concentration, ion homeostasis, and defense systems. Biomolecules 2020, 10, 1506. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on fenugreek seed germination under salinity levels. Russ. Agricult. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 2015, 5, 436–467. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Hossain, Z.; Komatsu, S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015, 128, 280–297. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nano silver application and dark storage. Turk. J. Biol. 2014, 38, 130–139. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hatami, M. Spray treatment with silver nanoparticles plus thidiazuron increases anti-oxidant enzyme activities and reduces petal and leaf abscission in four cultivars of geranium (Pelargonium zonale) during storage in the dark. J. Hort. Sci. Biotech. 2014, 89, 712–718. [Google Scholar] [CrossRef]
- Kazemipour, S.; Hashemabadi, D.; Kaviani, B. Effect of silver nanoparticles on the vase life and quality of cut chrysanthemum (Chrysanthemum morifolium L.) flower. Eur. J. Exp. Biol. 2013, 3, 298–302. [Google Scholar]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (Zno-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Noohpisheh, Z.; Amiri, H.; Mohammadi, A.; Farhadi, S. Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2021, 155, 267–280. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease them accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia Ur Rehman, M.Z.U.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.U.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.; Sahi, S. Zinc oxide nanoparticles (ZnO NPS) alleviate heavy metal-induced toxicity in Leucaena leucocephala Seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Hussain, S.; Niazi, M.B.K.; Shahid, M.; Song, F. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri Snt22 reduced the translocation of cadmium from soil to wheat plants. J. Hazard. Mater. 2020, 398, 123175. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. green copper nanoparticles drom a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Rehman, M.Z.U.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.-S. Iron nanoparticle-induced activation of plasma membrane Hþ-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- Madhavi, V.; Prasad, T.; Reddy, A.V.B.; Madhavi, G. Plant growth promoting potential of nano-bioremediation under Cr (VI) stress. Int. J. Nanotechnol. Appl. 2013, 3, 1–10. Available online: http://www.tjprc.org/publishpapers/2-6-1372741377-1.%20Plant%20growth%20-full.pdf (accessed on 1 November 2021).
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of nano-particles of FeSO4 on the growth and ion content of sunflower under saline condition. J. Plant. Nutr. 2017, 40, 615–623. [Google Scholar] [CrossRef]
- Sicard, C.; Perullini, M.; Spedalieri, C.; Coradin, T.; Brayner, R.; Livage, J.; Jobbáagy, M.; Bilmes, S.A. CeO2 nanoparticles for the protection of photosynthetic organisms immobilized in silica gels. Chem. Mater. 2011, 23, 1374–1378. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 2018, 3, 22–39. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Su, Y.B.; Takahiro, M.; Fugetsu, B. Multi-Walled carbon nanotubes induce oxidative stress and vacuolar structure changes to Arabidopsis T87 suspension cells. Nano Biomed. 2010, 2, 170–181. [Google Scholar]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Bradfield, S.J.; Kumar, P.; White, J.C.; Musante, C.; Ma, X. Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ. Sci. Nano 2016, 3, 114–126. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowak, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef]
- Solaiman, A.S.; El-feky, S.A.; Darwish, E. Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J. Hortic. For. 2015, 7, 36–47. [Google Scholar] [CrossRef]
- Shen, C.X.; Zhang, Q.F.; Li, J.; Bi, F.C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Yadav, T.; Mungray, A.A.; Mungray, A.K. Fabricated nanoparticles: Current status and potential phytotoxic threats. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 230, pp. 83–110. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophies levels: Plant and human lymphocytes. Chemosphere 2015, 81, 1253–1262. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jósko, I.; Xing, B. The toxicity to plants of the sewage sludges containing multiwalled carbon nanotubes. J. Hazard. Mater. 2011, 186, 436–442. [Google Scholar] [CrossRef]
- Syu, Y.Y.; Hung, J.H.; Chen, J.C.; Chuang, H.W. Impact of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Aronstam, R. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Aronstam, R.S.; Chen, D.; Huang, Y. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. Vitr. 2010, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).