Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L.
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Design and Treatments
2.2. Soil Pot Experiment Design
2.3. Harvesting Plant
2.4. Assessment of Chlorophyll, Carotenoid Content and Photosynthetic Parameters
2.5. Assessment of Antioxidant Enzymes and Oxidative Stress Markers
2.6. Cadmium Assessment in Plants
2.7. Statistical Analysis
3. Results
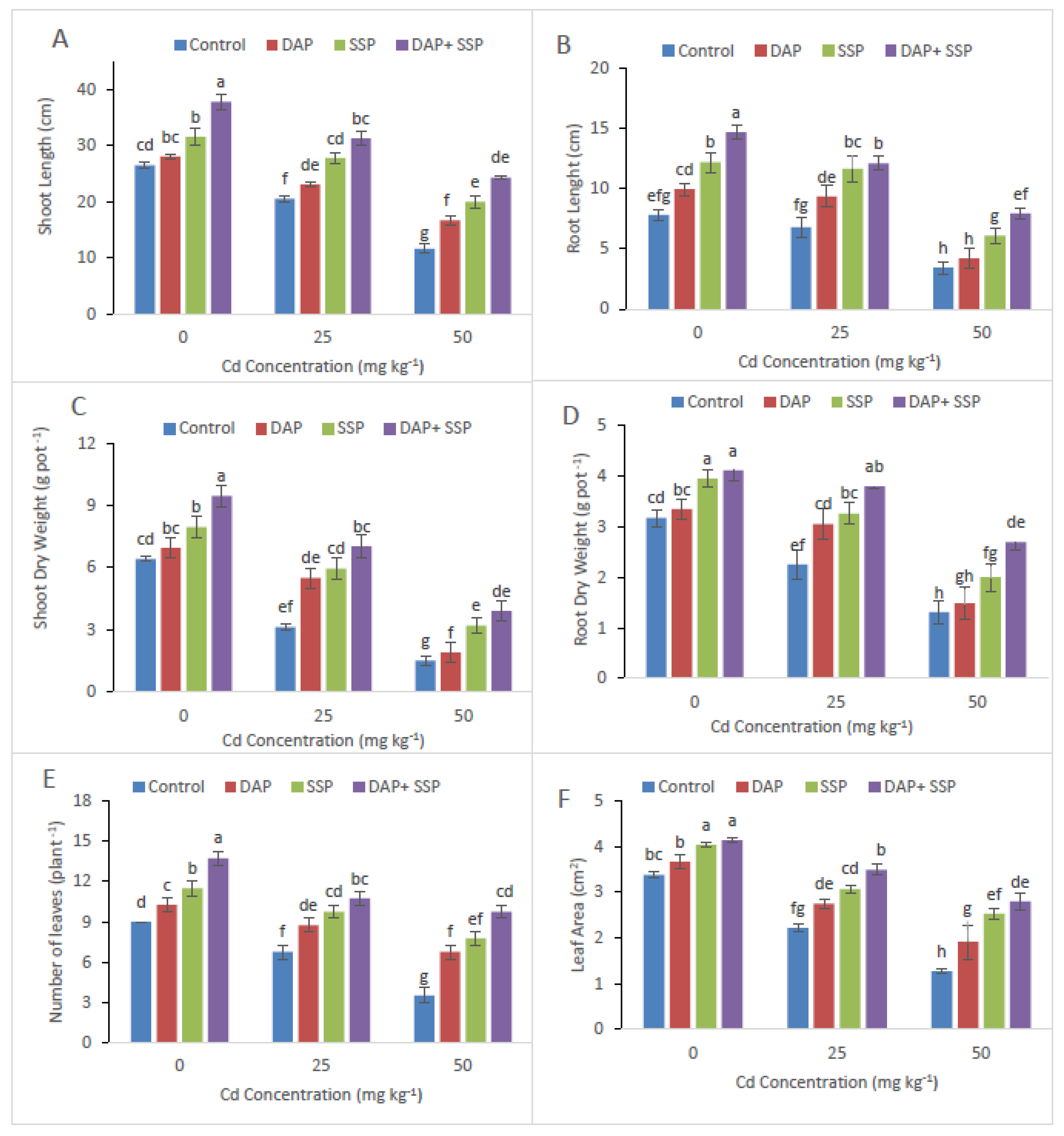
3.1. Calculation of P-Fertilizer Effects on Biomass and Growth
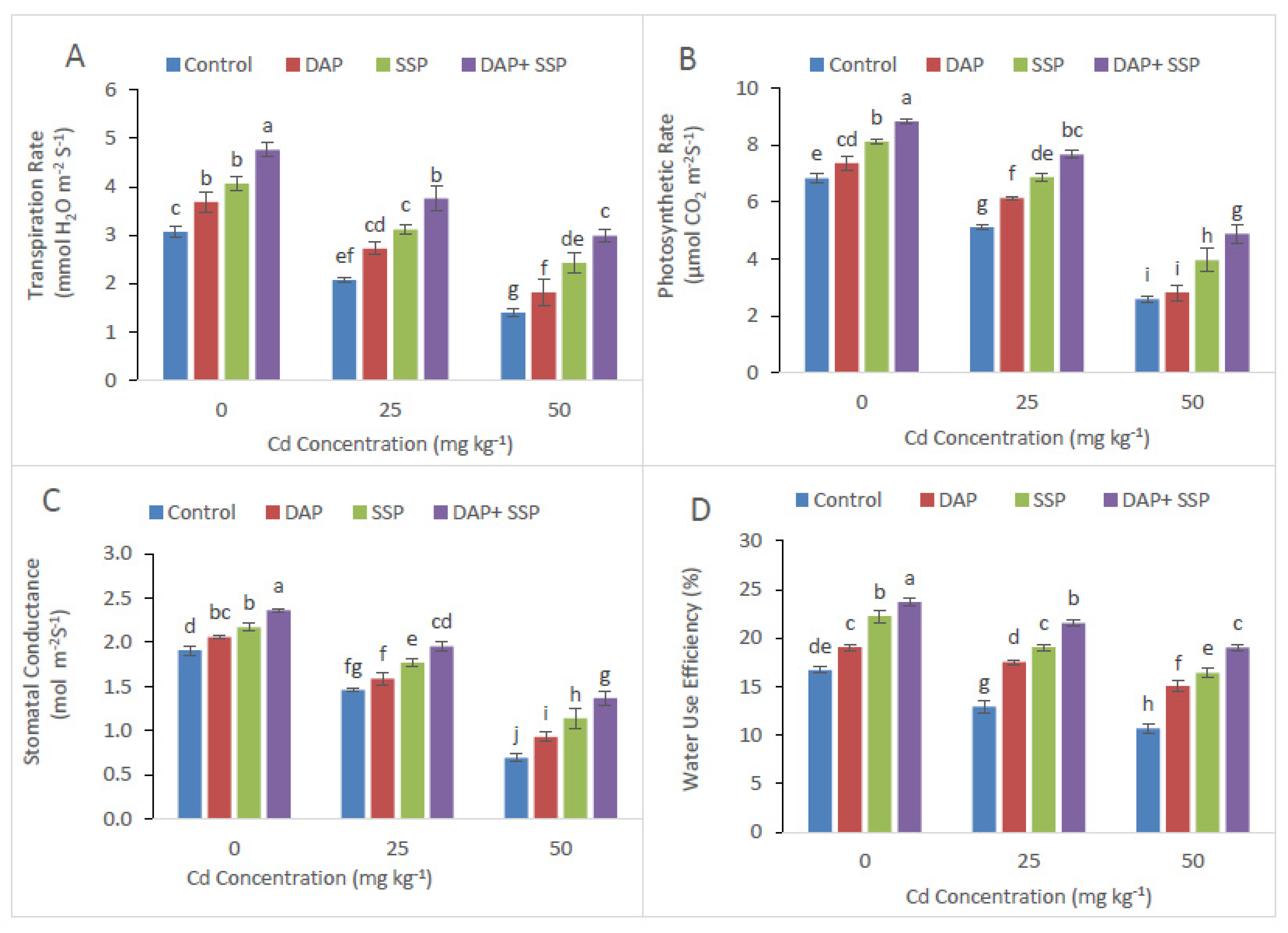
3.2. Calculation of P-Fertilizer Effects on Gas Exchange Traits and Chlorophyll Contents
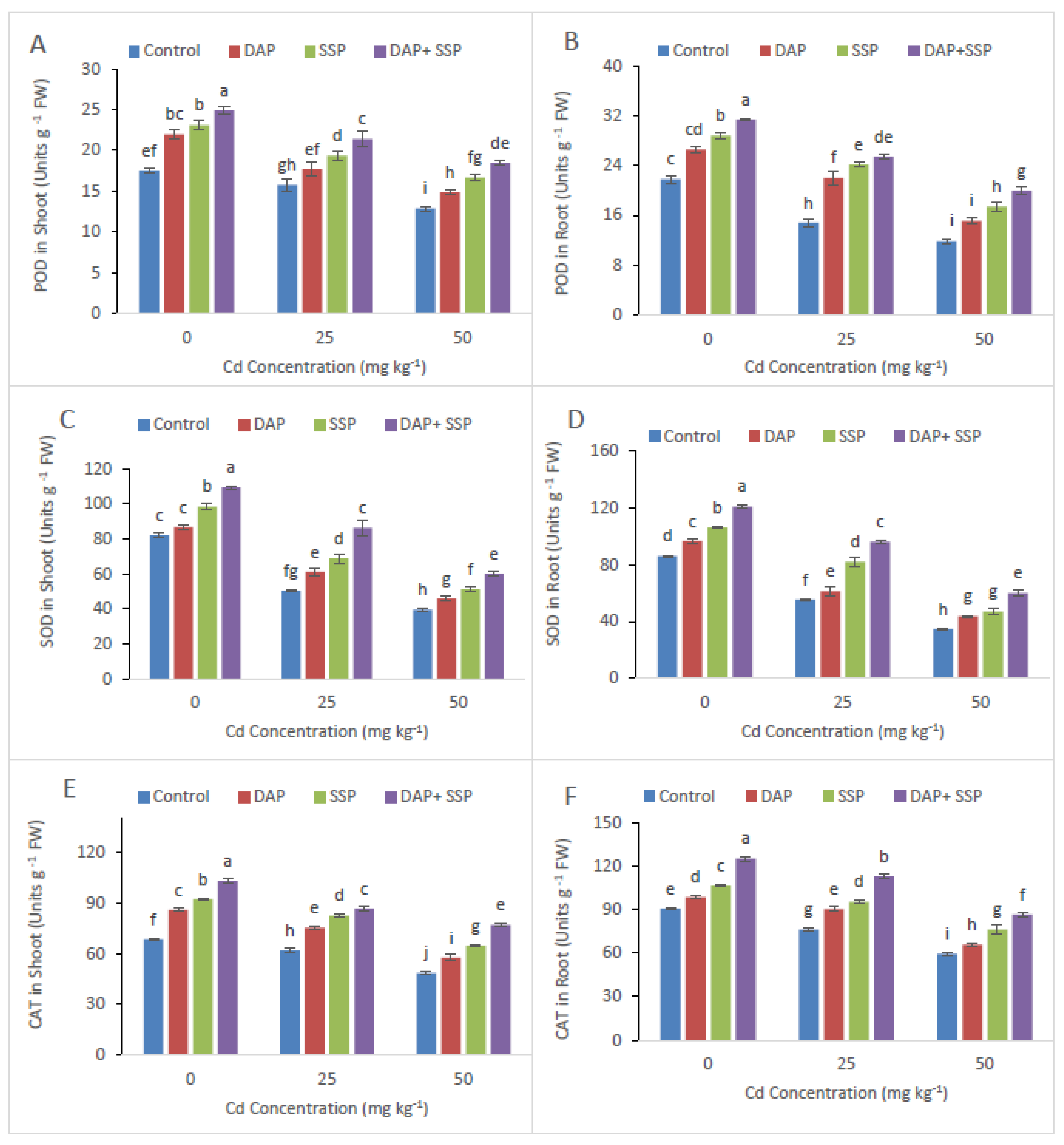
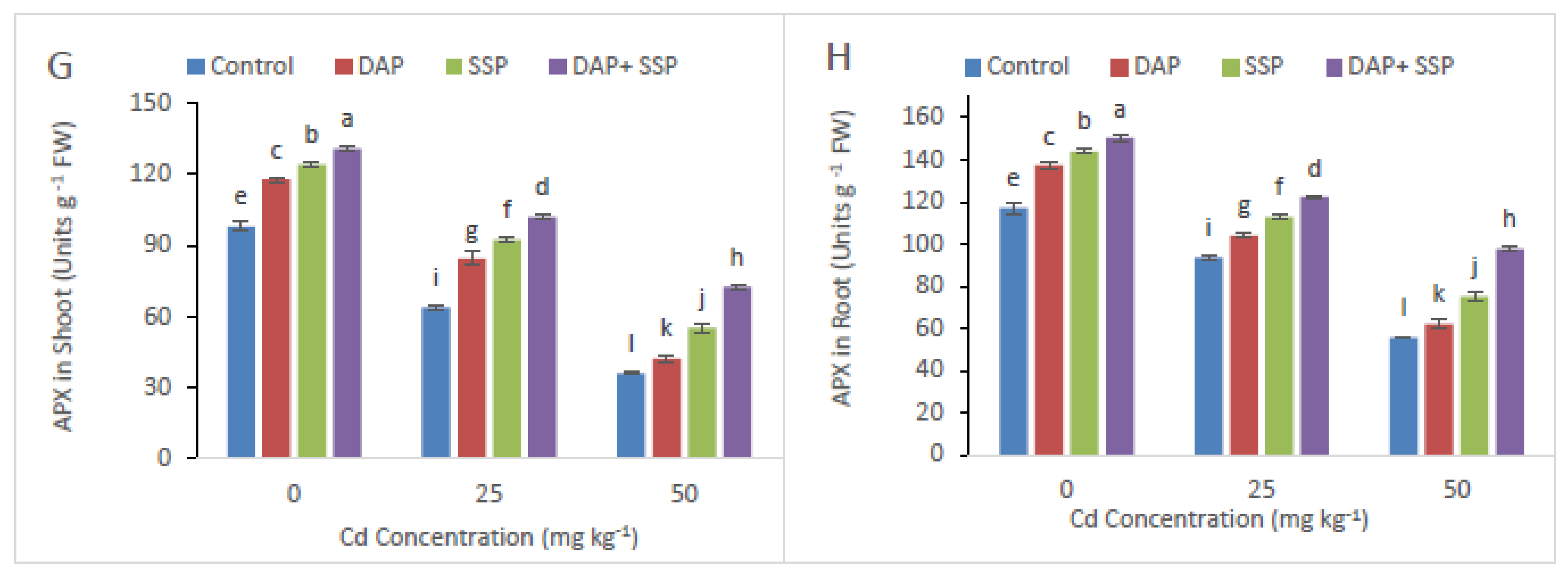
3.3. Calculation of P-Fertilizer Effects on EL, MDA and Antioxidant Enzyme Activities
3.4. Calculation of Accumulation of Cd in Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshmisha, A.; Agarwal, P.; Nikam, M. Assessing the Double Injustice of Climate Change and Urbanization on Water Security in Peri-urban Areas: Creating Citizen-Centric Scenarios. Water Insecurity Sanit. Asia 2019, 301, 1025–1033. [Google Scholar]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Naeem, A.; Zafar, M.; Khalid, H.; Zia-ur-Rehman, M.; Ahmad, Z.; Ayub, M.A.; Qayyum, M.F. Cadmium-Induced Imbalance in Nutrient and Water Uptake by Plants. In Cadmium Toxicity and Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 299–326. [Google Scholar]
- Kirpichtchikova, T.A.; Manceau, A.; Spadini, L.; Panfili, F.; Marcus, M.A.; Jacquet, T. Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochim. Cosmochim. Acta 2006, 70, 2163–2190. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucera, T.; Horakova, H.; Sonska, A. Toxic metal ions in photoautotrophic organisms. Photosynthetica 2008, 46, 481–489. [Google Scholar] [CrossRef]
- Sarangthem, J.; Jain, M.; Gadre, R. Inhibition of δ-aminolevulinic acid dehydratase activity by cadmium in excised etiolated maize leaf segments during greening. Plant Soil Environ. 2011, 57, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Kanso, A.; Azoury, S.; Benizri, E.; Kobaissi, A.; Echevarria, G.; Sirguey, C. Improvement of Ni phytoextraction by Alyssum murale and its rhizosphere microbial activities by applying nitrogen fertilizer. Ecol. Res. 2018, 33, 811–821. [Google Scholar] [CrossRef]
- Hammami, H.; Parsa, M.; Mohassel, M.H.R.; Rahimi, S.; Mijani, S. Weeds ability to phytoremediate cadmium-contaminated soil. Int. J. Phytoremediat. 2016, 18, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Zheng, J.S.; Sharp, R.G. Phytoremediation in engineered wetlands: Mechanisms and applications. Procedia Environ. Sci. 2010, 2, 1315–1325. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhou, L. Application of green manure and pig manure to Cd-contaminated paddy soil increases the risk of Cd uptake by rice and Cd downward migration into groundwater: Field micro-plot trials. Water Air Soil Pollut. 2017, 228, 15–29. [Google Scholar]
- Jacobs, A.; Drouet, T.; Noret, N. Field evaluation of cultural cycles for improved cadmium and zinc phytoextraction with Noccaea caerulescens. Plant Soil 2018, 430, 381–394. [Google Scholar] [CrossRef]
- Jacobs, A.; Noret, N.; Baekel, A.V.; Lienard, A.; Colinet, G.; Drouet, T. Influence of edaphic conditions and nitrogen fertilizers on cadmium and zinc phytoextraction efficiency of 408 Noccaea caerulescens. Sci. Total Environ. 2019, 665, 649–659. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, Q. Growth responses and cadmium accumulation of Mirabilis jalapa L. under interaction between cadmium and phosphorus. J. Hazard. Mater. 2009, 167, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Afzal, J.; Wang, X.; Saleem, M.-H.; Sun, X.; Hussain, S.; Khan, I.; Rana, M.-S.; Ahmed, S.; Awan, S.-A.; Fiaz, S.; et al. Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa L.). Biocell 2021, 45, 1631–1649. [Google Scholar] [CrossRef]
- Gao, X.P.; Flaten, D.N.; Tenuta, M.; Grimmett, M.G.; Gawalko, E.J.; Grant, C.A. Soil solution dynamics and plant uptake of cadmium and zinc by durum wheat following phosphorus fertilization. Plant. Soil 2011, 338, 423–434. [Google Scholar] [CrossRef]
- Afzal, J.; Saleem, M.H.; Batool, F.; Elyamine, A.M.; Rana, M.S.; Shaheen, A.; El-Esawi, M.A.; Tariq Javed, M.; Ali, Q.; Arslan Ashraf, M.; et al. Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes. Biomolecules 2020, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; El-Esawi, M.A.; Rana, M.S.; Saleem, M.H.; Riaz, M.; Ashraf, U.; Potcho, M.P.; Duan, M.; Rajput, I.A. Molybdenum Supply Alleviates the Cadmium Toxicity in Fragrant Rice by Modulating Oxidative Stress and Antioxidant Gene Expression. Biomolecules 2020, 10, 1582. [Google Scholar] [CrossRef]
- Maqbool, A.; Ali, S.; Rizwan, M.; Arif, M.S.; Yasmeen, T.; Riaz, M.; Alkahtani, S. N-Fertilizer (Urea) Enhances the Phytoextraction of Cadmium through Solanum nigrum L. Int. J. Environ. Res. Public Health 2020, 17, 3850. [Google Scholar] [CrossRef]
- Yang, W.; Dai, H.; Skuza, L.; Wei, S. Strengthening role and the mechanism of optimum nitrogen addition in relation to Solanum nigrum L. Cd hyperaccumulation in soil. Ecotoxicol. Environ. Saf. 2019, 182, 109444. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids pigments of photosynthetic biomembranes. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Ecosyst. Environ. Res. 1998, 253, 122–130. [Google Scholar] [CrossRef]
- Zhang, X.Z. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhang, R.R.; Liu, Y.; Xue, W.L.; Chen, R.X.; Du, S.T.; Jin, C.W. Slow-release nitrogen fertilizers can improve yield and reduce Cd concentration in pakchoi (Brassica chinensis L.) grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 25074–25083. [Google Scholar] [CrossRef] [PubMed]
- Al Khateeb, W.; Al-Qwasemeh, H. Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L., a crop wild relative for tomato; comparative study. Physiol. Mol. Biol. 2014, 20, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Wang, S.; Li, Y.; Zhu, J. Root system responses of hyperaccumulator Solanum nigrum L. to Cd. J. Soils Sedim. 2013, 13, 1069–1074. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Wu, J.F.; Shang, D.R.; Ning, J.S.; Zhai, Y.X.; Shend, X.F.; Ding, H.Y. Subcellular distribution and chemical forms of cadmium in the edible seaweed, Porphyra yezoensis. Food Chem. 2015, 168, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Ullah, I.; Khan, A.L.; Hong, S.J.; Waqas, M.; Park, G.S.; Kwak, Y.; Choi, J.; Jung, B.K.; Park, M.; et al. Phytostabilization and physicochemical responses of Korean ecotype Solanum nigrum L. to cadmium contamination. Water Air Soil Pollut. 2014, 225, 2147. [Google Scholar] [CrossRef]
- Jiang, H.M.; Yang, J.C.; Zhang, J.F. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut. 2007, 3, 750–756. [Google Scholar] [CrossRef]
- Bao, T.; Sun, T.; Sun, L. Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and nonhyperaccumulator Solanum lycopersicum L. Afr. J. Biotechnol. 2011, 10, 17180–17185. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqbool, A.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Hussain, A.; Mansha, A.; Ali, S.; Alshaya, H.; Okla, M.K. Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L. Plants 2022, 11, 236. https://doi.org/10.3390/plants11030236
Maqbool A, Rizwan M, Yasmeen T, Arif MS, Hussain A, Mansha A, Ali S, Alshaya H, Okla MK. Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L. Plants. 2022; 11(3):236. https://doi.org/10.3390/plants11030236
Chicago/Turabian StyleMaqbool, Arosha, Muhammad Rizwan, Tahira Yasmeen, Muhammad Saleem Arif, Afzal Hussain, Asim Mansha, Shafaqat Ali, Huda Alshaya, and Mohammad K. Okla. 2022. "Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L." Plants 11, no. 3: 236. https://doi.org/10.3390/plants11030236
APA StyleMaqbool, A., Rizwan, M., Yasmeen, T., Arif, M. S., Hussain, A., Mansha, A., Ali, S., Alshaya, H., & Okla, M. K. (2022). Phosphorus Fertilizers Enhance the Phytoextraction of Cadmium through Solanum nigrum L. Plants, 11(3), 236. https://doi.org/10.3390/plants11030236





