Effect of Temperature on the Germination of Five Coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America
Abstract
:1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Seed Collection and Preparation
4.2. Germination Experiments
4.3. Trial Design and Statistical Analysis
- Temperature: 18 °C, 22 °C, 26 °C, and 30 °C
- Provenance: Licantén, Las Cañas, Los Ruiles, Curanipe, and Quirihue
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez, R.; Quezada, M. Fagaceae. In Flora de Chile 2; Marticorena, C., Rodríguez, R., Eds.; Universidad de Concepción: Concepción, Chile, 2003; pp. 64–76. [Google Scholar]
- Barstow, M.; Rivers, M.C.; Baldwin, H. Nothofagus Glauca. IUCN Red List. Threat. Species 2017, eT32034A2809142. [Google Scholar] [CrossRef]
- Santelices-Moya, R.; Vergara, R.; Cabrera-Ariza, A.; Espinoza-Meza, S.; Silva-Flores, P. Variación intra-específica en Nothofagus glauca una especie endémica de los bosques mediterráneos de Chile. Bosque 2020, 41, 221–231. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Riveros, M.; Peñaloza, A.; Cavieres, L.; Faggi, A.M. Phytogeographic relationships and regional richness patterns of the cool temperate rainforest flora of southern South America. In High-Latitude Rainforests and Associated Ecosystems of the West Coasts of the Americas: Climate, Hydrology, Ecology and Conservation; Lawford, R.G., Alaback, P.B., Fuentes, E., Eds.; Springer: New York, NY, USA, 1996; pp. 134–172. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A. Cambio de Estructura en el Bosque de Nothofagus glauca (Phil.) Krasser. Ph.D. Thesis, Universidad de Chile, Santiago, Chile, 1975. Tesis Ingeniería Forestal. [Google Scholar]
- Valencia, D.; Saavedra, J.; Brull, J.; Santelices, R. Severidad del daño causado por los incendios forestales en los bosques remanentes de Nothofagus alessandrii Espinosa en la región del Maule de Chile. Gayana Botánica 2018, 75, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; p. 1600. [Google Scholar]
- Belmehdi, O.; El Harsal, A.; Benmoussi, M.; Laghmouchi, Y.; Senhaji, N.S.; Abrini, J. Effect of light, temperature, salt stress and pH on seed germination of medicinal plant Origanum elongatum (Bonnet) Emb. & Maire. Biocatal. Agric. Biotechnol. 2018, 16, 126–131. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; p. 392. [Google Scholar]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Cabello, A.; Espinoza, N.; Espinoza, S.; Cabrera, A.; Santelices, R. Effect of pre-germinative treatments on Nothofagus glauca seed germination and seedling growth. N. Z. J. For. Sci. 2019, 49, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moya, R.S.; Meza, S.E.; Díaz, C.M.; Ariza, A.C.; Calderón, S.D.; Peña-Rojas, K. Variability in seed germination and seedling growth at the intra- and inter-provenance levels of Nothofagus glauca (Lophozonia glauca), an endemic species of Central Chile. N. Z. J. For. Sci. 2017, 47, 10. [Google Scholar] [CrossRef] [Green Version]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High Temperature-Induced Abscisic Acid Biosynthesis and Its Role in the Inhibition of Gibberellin Action in Arabidopsis Seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izydorczyk, C.; Nguyen, T.N.; Jo, S.; Son, S.; Tuan, P.A.; Ayele, B.T. Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signalling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.). Plant Cell Environ. 2018, 41, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Donoso, C. Variación Natural en Especies de Nothofagus en Chile. Bosque 1987, 8, 85–97. [Google Scholar] [CrossRef]
- Buamscha, M.G.; Contardi, L.T.; Dumroese, R.K.; Enricci, J.A.; Escobar, R.; Gonda, H.E.; Jacobs, D.F.; Landis, T.D.; Luna, T.; Mexal, J.G.; et al. Producción de Plantas en Viveros Forestales; Contardi, L.T., Gonda, H.E., Tolone, G., Salimbeni, J., Eds.; Consejo Federal de Inversiones; Universidad Nacional de la Patagonia San Juan Bosco; Centro de Investigación y Extensión Forestal Andino: Patagonia, Argentina, 2012; p. 220. [Google Scholar]
- DMC. Reporte Anual de la Evolución del Clima en Chile; Dirección Meteorológica de Chile: Santiago, Chile, 2020; p. 44. [Google Scholar]
- ISTA. International Rules for Seed Testing; ISTA (International Seed Testing Association): Zurich, Switzerland, 2006. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Kamran, M.; Wang, D.; Xie, K.; Lu, Y.; Shi, C.; El Sabagh, A.; Gu, W.; Xu, P. Pre-sowing seed treatment with kinetin and calcium mitigates salt induced inhibition of seed germination and seedling growth of choysum (Brassica rapa var. parachinensis). Ecotoxicol. Environ. Saf. 2021, 227, 112921. [Google Scholar] [CrossRef] [PubMed]
- Czabator, F.J. Germination value: An index combining speed and completeness of pine seed germination. For. Sci. 1962, 8, 386–396. [Google Scholar]
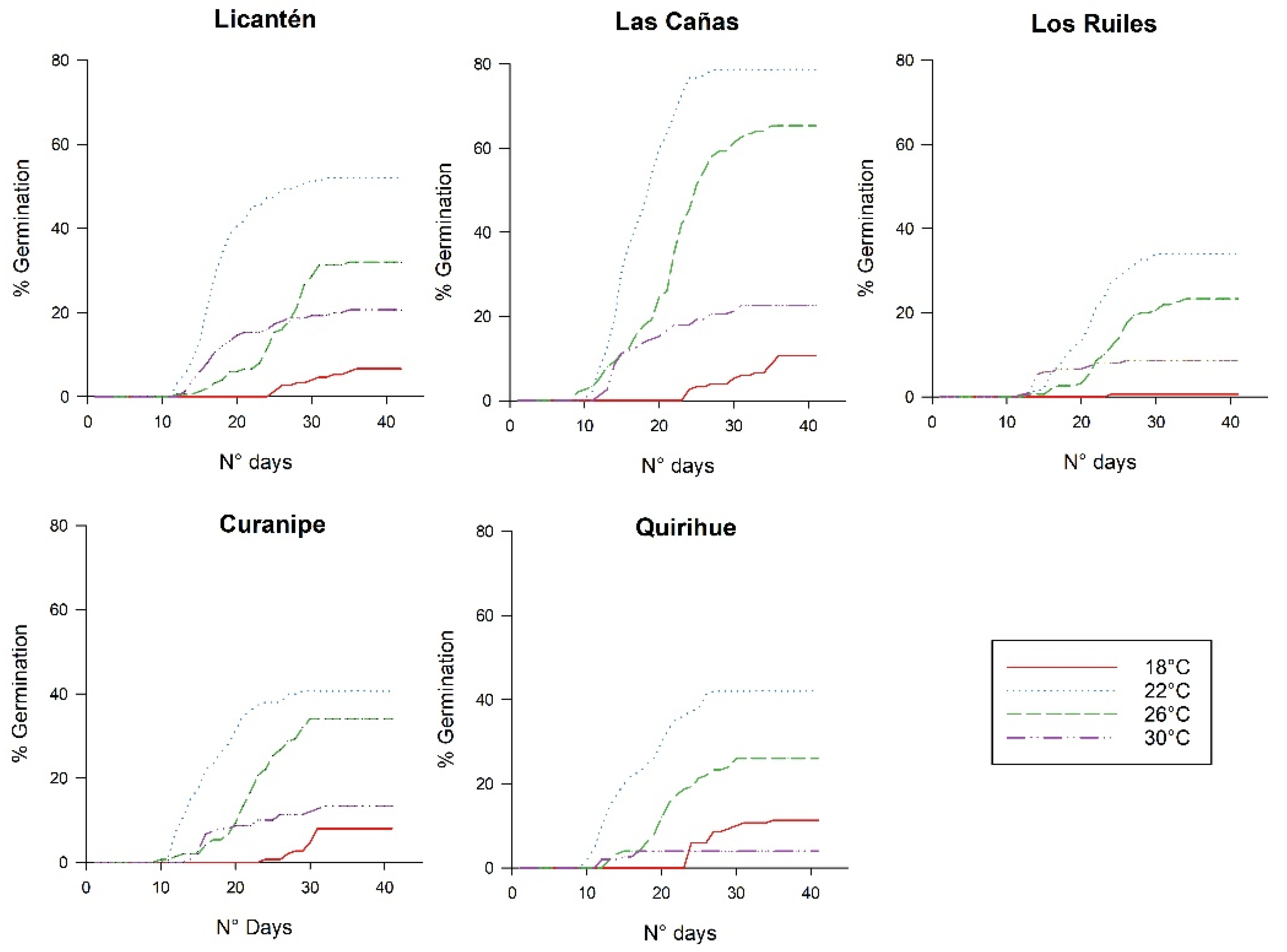


| Provenance | Temperature (°C) | Germination Ratio (%) | Germination Energy (%) | Germination Start Day | Average Germination Speed (Seed/Day) | Germination Vigor |
|---|---|---|---|---|---|---|
| Licantén | 18 | 6.7 ± 0.67 d | 6.8 ± 0.33 d | 25.0 ± 0.0 c | 0.1 ± 0.22 c | 0.0 ± 0.0 d |
| 22 | 52.0 ± 0.01 a | 45.0 ± 2.26 a | 11.0 ± 0.94 a | 1.5 ± 0.07 a | 2.4 ± 0.11 a | |
| 26 | 32.0 ± 0.01 b | 31.4 ± 1.44 b | 16.0 ± 0.94 b | 0.7 ± 0.03 b | 0.9 ± 0.04 b | |
| 30 | 20.7 ± 0.67 c | 14.6 ± 0.71 c | 13.0 ± 0.47 ab | 0.6 ± 0.03 b | 0.4 ± 0.02 c | |
| Las Cañas | 18 | 10.7 ± 0.54 c | 6.2 ± 0.11 c | 24.3 ± 0.27 b | 0.2 ± 0.01 d | 0.1 ± 0.0 d |
| 22 | 78.7 ± 0.54 a | 77.1 ± 3.42 a | 12.0 ± 0.94 a | 2.4 ± 0.11 a | 9.1 ± 0.41 a | |
| 26 | 65.3 ± 0.54 a | 57.5 ± 2.97 a | 10.7 ± 0.72 a | 1.7 ± 0.08 b | 4.0 ± 0.2 b | |
| 30 | 22.7 ± 0.54 b | 14.0 ± 0.65 b | 12.0 ± 0.47 a | 0.7 ± 0.04 c | 0.6 ± 0.03 c | |
| Los Ruiles | 18 | 0.7 ± 0.54 d | 0.7 ± 0.54 d | 0.0 ± 0.0 c | 0.0 ± 0.0 d | 0.0 ± 0.0 d |
| 22 | 34.0 ± 0.0 a | 31.2 ± 0.0 a | 16.0 ± 0.94 ab | 0.8 ± 0.04 a | 1.3 ± 0.05 a | |
| 26 | 23.3 ± 0.54 b | 19.3 ± 0.9 b | 21.0 ± 0.94 b | 0.5 ± 0.02 b | 0.5 ± 0.03 b | |
| 30 | 8.7 ± 0.54 c | 6.7 ± 0.49 c | 14.0 ± 0.47 a | 0.3 ± 0.01 c | 0.2 ± 0.02 c | |
| Curanipe | 18 | 8.0 ± 0.94 d | 8.0 ± 0.42 b | 24.3 ± 1.19 b | 0.1 ± 0.01 d | 0.0 ± 0.0 d |
| 22 | 40.7 ± 0.54 a | 34.6 ± 1.62 a | 12.0 ± 0.47 a | 1.3 ± 0.06 a | 2.3 ± 0.11 a | |
| 26 | 34.0 ± 0.0 b | 34.0 ± 0.0 a | 13.0 ± 0.47 a | 0.8 ± 0.03 b | 1.3 ± 0.06 b | |
| 30 | 13.3 ± 0.54 c | 8.1 ± 0.38 b | 14.0 ± 0.47 a | 0.4 ± 0.02 c | 0.2 ± 0.01 c | |
| Quirihue | 18 | 11.3 ± 0.54 c | 10.8 ± 0.47 c | 24.0 ± 0.94 b | 0.2 ± 0.02 c | 0.3 ± 0.01 c |
| 22 | 42.0 ± 0.0 a | 35.4 ± 1.72 a | 10.0 ± 0.47 a | 1.3 ± 0.08 a | 5.2 ± 0.25 a | |
| 26 | 26.0 ± 0.0 b | 23.3 ± 1.1 b | 13.0 ± 0.47 a | 0.6 ± 0.03 b | 1.7 ± 0.08 b | |
| 30 | 4.0 ± 0.0 d | 3.8 ± 0.14 d | 12.0 ± 0.47 a | 0.1 ± 0.01 d | 0.1 ± 0.01 d |
| Provenance | Latitude | Longitude | Elevation (m a.s.l.) | M.A.T 1 (°C) | M.A.R 2 (mm Year−1) |
|---|---|---|---|---|---|
| Licantén | 34°55′49″ S | 72° 5′11″ W | 403 | 12.5 | 829 |
| Las Cañas | 35°27′27″ S | 72°28′11″ W | 165 | 12.9 | 831 |
| Los Ruiles | 35°50′2″ S | 72°30′36″ W | 202 | 12.4 | 851 |
| Curanipe | 35°51′24″ S | 72°37′13″ W | 129 | 13.1 | 817 |
| Quirihue | 36°2′18″ S | 72°36′35″ W | 342 | 12.0 | 898 |
| Provenance | Weight of 1000 Seeds (g) | Number of Seeds per Kilogram | Dimerous Seeds | Trimerous Seeds | |||
|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Lenght (mm) | Width (mm) | Thickness (mm) | |||
| Licantén | 468.3 ± 0.3 | 2136 ± 10.4 | 17.1 ± 1.8 | 14.2 ± 1.8 | 17.2 ± 0.1 | 10.4 ± 0.1 | 9.7 ± 0.1 |
| Las Cañas | 537.5 ± 0.4 | 1861 ± 14.0 | 19.5 ± 0.1 | 12.1 ± 0.2 | 19.0 ± 0.1 | 10.8 ± 0.1 | 9.9 ± 0.1 |
| Los Ruiles | 683.7 ± 0.5 | 1463 ± 10.3 | 19.7 ± 1.6 | 13.2 ± 1.8 | 19.6 ± 0.2 | 11.3 ± 0.2 | 11.2 ± 0.1 |
| Curanipe | 395.5 ± 0.3 | 2530 ± 9.9 | 19.5 ± 0.3 | 11.3 ± 0.2 | 17.5 ± 0.3 | 10.3 ± 0.1 | 8.2 ± 0.2 |
| Quirihue | 379.0 ± 0.4 | 2641 ± 14.9 | 19.3 ± 0.1 | 12.1 ± 0.1 | 18.5 ± 0.2 | 9.7 ± 0.1 | 9.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santelices-Moya, R.E.; González Ortega, M.; Acevedo Tapia, M.; Cartes Rodríguez, E.; Cabrera-Ariza, A.M. Effect of Temperature on the Germination of Five Coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America. Plants 2022, 11, 297. https://doi.org/10.3390/plants11030297
Santelices-Moya RE, González Ortega M, Acevedo Tapia M, Cartes Rodríguez E, Cabrera-Ariza AM. Effect of Temperature on the Germination of Five Coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America. Plants. 2022; 11(3):297. https://doi.org/10.3390/plants11030297
Chicago/Turabian StyleSantelices-Moya, Rómulo E., Marta González Ortega, Manuel Acevedo Tapia, Eduardo Cartes Rodríguez, and Antonio M. Cabrera-Ariza. 2022. "Effect of Temperature on the Germination of Five Coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America" Plants 11, no. 3: 297. https://doi.org/10.3390/plants11030297
APA StyleSantelices-Moya, R. E., González Ortega, M., Acevedo Tapia, M., Cartes Rodríguez, E., & Cabrera-Ariza, A. M. (2022). Effect of Temperature on the Germination of Five Coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America. Plants, 11(3), 297. https://doi.org/10.3390/plants11030297







