Response of Transgenic Potato Plants Expressing Heterologous Genes of ∆9- or ∆12-Acyl-lipid Desaturases to Phytophthora infestans Infection
Abstract
:1. Introduction
2. Results
2.1. Morphophysiological Characteristics of Potato Plants
2.2. Inoculation of Potato Leaves with Phytophthora infestans
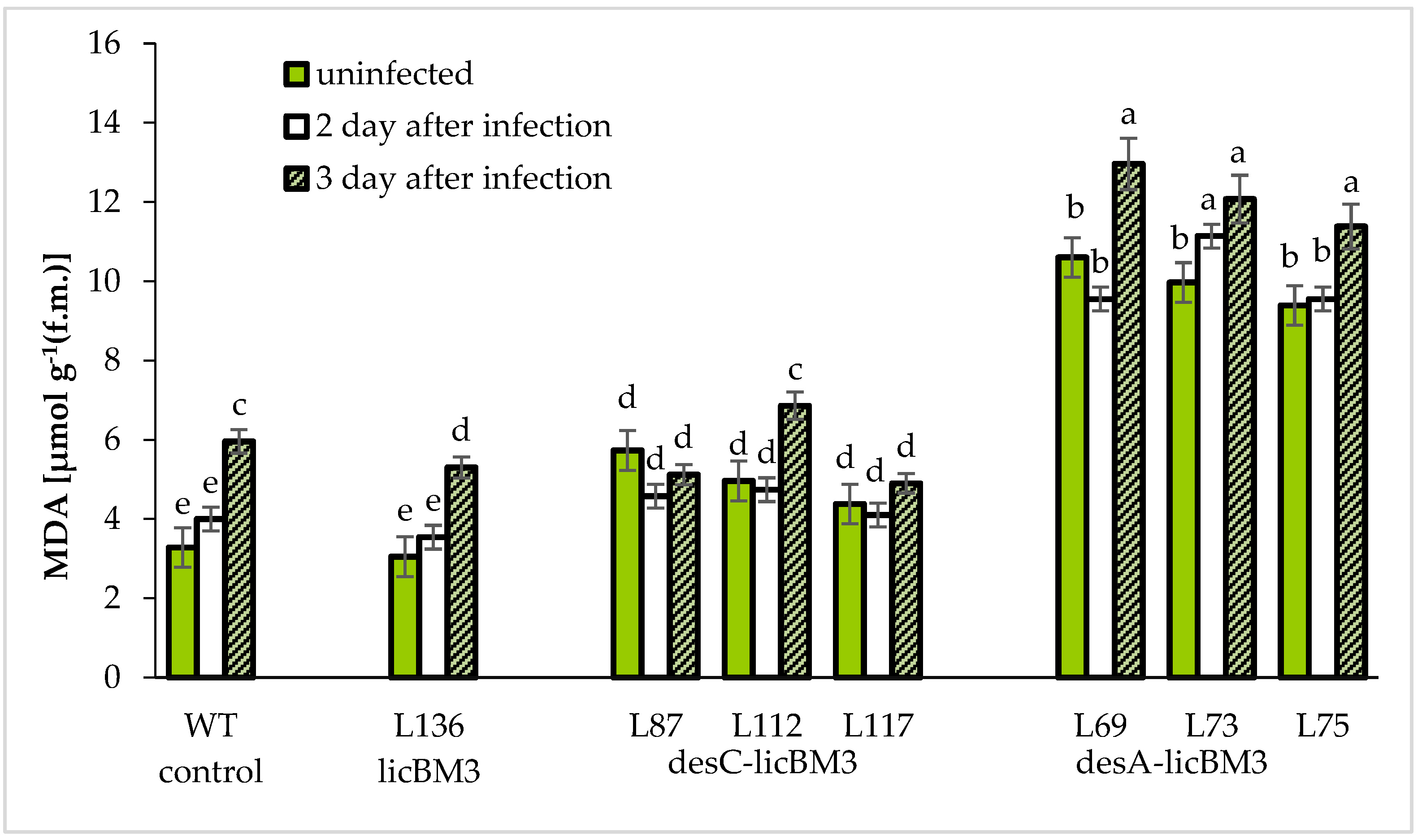
2.3. Lipid Peroxidation in Potato Plant Leaves
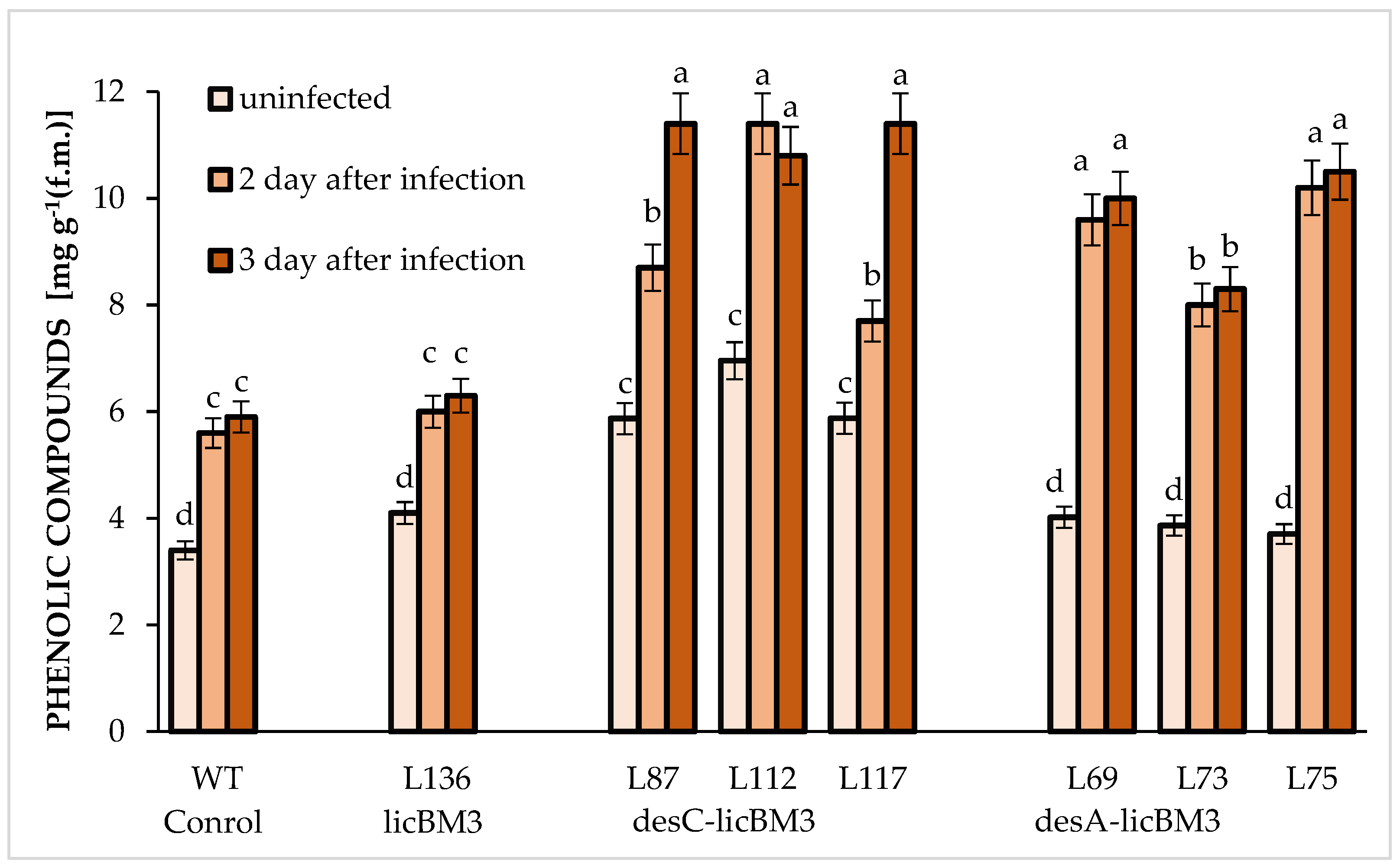
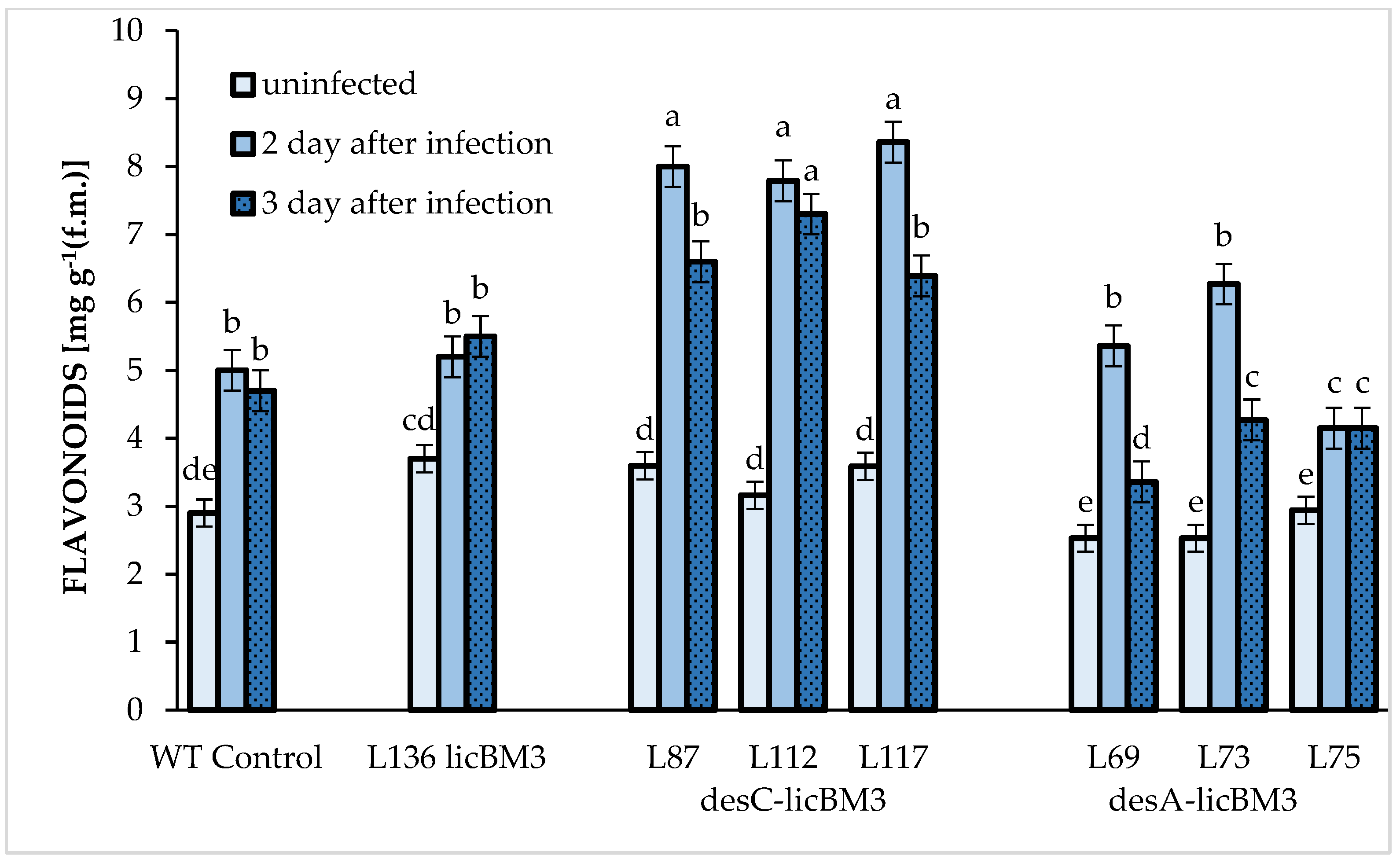
2.4. Content of Phenolic Compounds in Potato Leaves
3. Discussion
3.1. Morphophysiological Characteristics of Potato Plants
3.2. Leaf Resistance in Control and Transgenic Potato Plants to Phytophthora infestans
3.3. Lipid Peroxidation in Control and Transgenic Potato Plants under Normal Conditions and during Phytophthora infestation
3.4. Phenolic Compounds in Potato Leaf Resistance to Phytophthora infestans
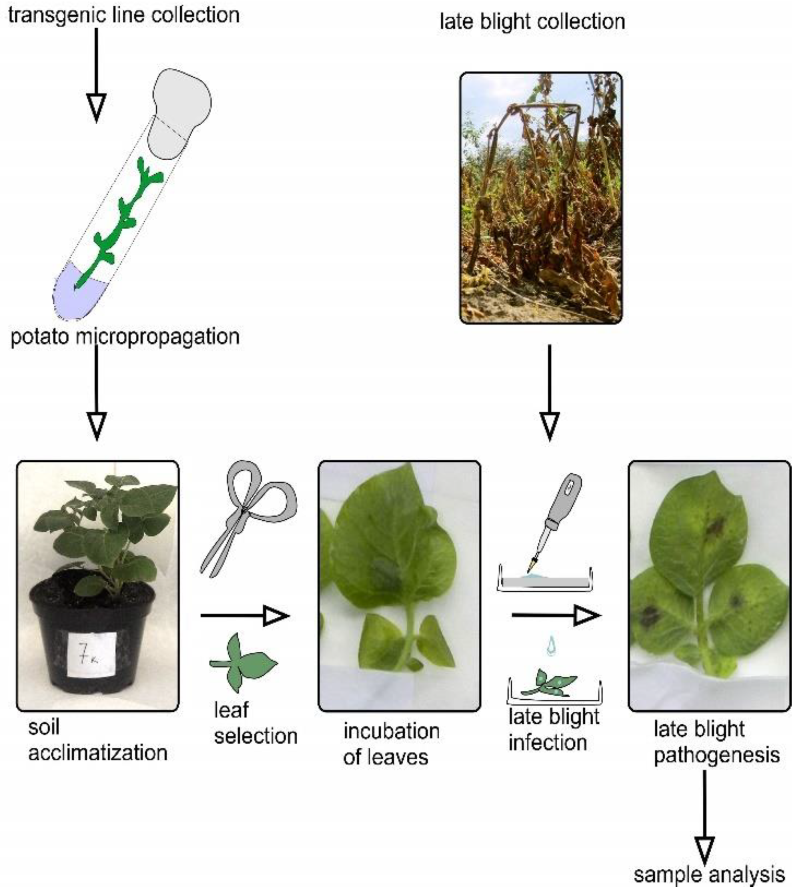
4. Materials and Methods
4.1. Generation of Transgenic Plants
4.2. Molecular Confirmation of Transgenic Plants
4.3. RNA Analysis
4.4. Experimental Conditions
4.5. Evaluation of Morphometric Parameters in Potato Plants
4.6. Stomata Distribution on the Leaf Surface
4.7. Inoculation of Potato Leaves with Phytophthora infestans and Assessment of Their Resistance
4.8. Determining the Content of Malondialdehyde (MDA)
4.9. Determining the Content of Total Phenolic Compounds and Flavonoids
4.10. Obtaining Protein Extracts from Plants
4.11. Determining the Molecular Weight of Fusion Proteins by Enzymogram Method
4.12. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 117–145. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gechev, T.; Petrov, V. Reactive oxygen species and abiotic stress in plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier (Woodhead Publishing): Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Bidel, L.P.R.; Coumans, M.; Baissac, Y.; Doumans, P.; Jay-Allemand, C. Recent advances in polyphenol research. In Biological Activity in Plant Cells; Santos-Buelga, C., Escribano-Bailon, M., Lattanzio, V., Eds.; Academic Press: Oxford, UK, 2010; Volume 2, pp. 163–205. [Google Scholar]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Siberian raspberries: LC-MS profile, seasonal variation, antioxidant activity and, thermal stability of Rubus matsumuranus phenolome. Plants 2021, 10, 2317. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal–plant interactions. Genetics 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Parvez, M.M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar] [CrossRef]
- Vyacheslavova, A.O.; Berdichevets, I.N.; Tyurin, A.A.; Shimshilashvili, K.R.; Mustafaev, O.N.; Goldenkova-Pavlova, I.V. Expression of heterologous genes in plant systems: New possibilities. Russ. J. Genet. 2012, 48, 1067–1079. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, H.R.; Gao, J.; Huang, Y.J.; Zhang, B.; Chen, K.S. Two ω-3 FADs are associated with peach fruit volatile formation. Int. J. Mol. Sci. 2016, 17, 464. [Google Scholar] [CrossRef] [Green Version]
- Berestovoy, M.A.; Pavlenko, O.S.; Goldenkova-Pavlova, I.V. Plant fatty acid desaturases: Role in the life of plants and biotechnological potential. Biol. Bull. Rev. 2020, 10, 127–139. [Google Scholar] [CrossRef]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 gene in plants: Occurrence, regulation, and role. Front. Plant. Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasymenko, I.M.; Sakhno, L.A.; Kyrpa, T.N.; Ostapchuk, A.M.; Hadjiev, T.A.; Goldenkova-Pavlova, I.V.; Sheludko, Y.V. Characterization of Nicotiana tabacum plants expressing hybrid genes of cyanobacterial Δ9 or Δ12 acyl-lipid desaturases and thermostable lichenase. Russ. J. Plant Physiol. 2015, 62, 283–291. [Google Scholar] [CrossRef]
- Orlova, I.V.; Serebriiskaya, T.S.; Popov, V.; Merkulova, N.; Nosov, A.M.; Trunova, T.I.; Tsydendambaev, V.D.; Los, D.A. Transformation of tobacco with a gene for the thermophilic acyl-lipid desaturase enhances the chilling tolerance of plants. Plant Cell Physiol. 2003, 44, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Madi, L.; Wang, X.; Kobiler, I.; Lichter, A.; Prusky, D. Stress on avocado fruits regulates Δ9-stearoyl ACP desaturase expression, fatty acid composition, antifungal diene level and resistance to Colletotrichum gloeosporioides attack. Physiol. Mol. Plant Pathol. 2003, 62, 277–283. [Google Scholar] [CrossRef]
- Yur’eva, N.O.; Kirsanova, S.N.; Kukushkina, L.N.; Pchelkin, V.P.; Sobol’kova, G.I.; Nikiforova, K.R.; Tsydendambaev, V.D. Expression of the gene encoding Δ12 acyl-lipid desaturase from Synechocystis sp. PCC 6803 improves potato plant resistance to late blight infection. Russ. J. Plant Physiol. 2014, 61, 672–678. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched led lights in a plant factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Tian, F.; Wigneron, J.P.; Ciais, P.; Chave, J.; Ogée, J.; Peñuelas, J.; Rebild, A.; Domec, J.-C.; Tong, X.; Brandt, M.; et al. Coupling of ecosystem-scale plant water storage and leaf phenology observed by satellite. Nat. Ecol. Evol. 2018, 2, 1428–1435. [Google Scholar] [CrossRef] [Green Version]
- Kane, C.N.; Jordan, G.J.; Jansen, S.; McAdam, S.A. A permeable cuticle, not open stomata, is the primary source of water loss from expanding leaves. Front. Plant Sci. 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Noguchi, K.O.; Terashima, I. Distinct light responses of the adaxial and abaxial stomata in intact leaves of Helianthus annuus L. Plant Cell Environ. 2008, 31, 1307–1316. [Google Scholar] [CrossRef]
- Wang, W.; Jiao, F. Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity. Planta 2019, 250, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. J. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Shi, S.-L.; Li, X.-L.; Li, C.-N.; Zhang, C.-M.; Kang, W.-J.; Yin, G.-L. Effects of autotoxicity on alfalfa (Medicago sativa): Seed germination, oxidative damage and lipid peroxidation of seedlings. Agronomy 2021, 11, 1027. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Allakhverdiev, S.I.; Los, D.A.; Kuznetsov, V.V. Signaling role of reactive oxygen species in plants under stress. Russ. J. Plant Physiol. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Chng, C.P.; Sadovsky, Y.; Hsia, K.J.; Huang, C. Site-specific peroxidation modulates lipid bilayer mechanics. Extrem. Mech. Lett. 2021, 42, 101148. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Windisch, S.; Walter, A.; Moradtalab, N.; Walker, F.; Höglinger, B.; El-Hasan, A.; Ludewig, U.; Neumann, G.; Grosch, R. Role of benzoic acid and lettucenin A in the defense response of lettuce against soil-borne pathogens. Plants 2021, 10, 2336. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Garcia, G.P.; Santos, F.N.; Zanotta, S.; Eberlin, M.N.; Carazzone, C. Metabolomics of Solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef] [Green Version]
- Pauliuc, D.; Florina, D.; Mircea, O. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, D.; Musmeci, S.; Pulgar, J.; Onofri, C.; Parisi, B.; Sasso, R.; Mandolino, G.; Lombardi-Boccia, G. Caffeic acid and α-chaconine influence the resistance of potato tuber to Phthorimaea operculella (Lepidoptera: Gelechiidae). Am. J. Potato Res. 2019, 96, 403–413. [Google Scholar] [CrossRef]
- Koltunov, Y.V. The effect of the stem rot at composition and content of phenolic compounds in leaves of birch (Betula pendula Roth). Chem. Plant Raw Mater. 2019, 3, 169–176. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Williamson, G.; Plumb, G.W.; Garcia-Conesa, M.T. Glycosylation, esterification, and polymerization of flavonoids and hydroxycinnamates: Effects on antioxidant properties. In Plant Polyphenols 2; Springer: Boston, MA, USA, 1999; pp. 483–494. [Google Scholar]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Demin, I.N.; Deryabin, A.N.; Sinkevich, M.S.; Trunova, T.I. Insertion of cyanobacterial desA gene coding for Δ12-acyl-lipid desaturase increases potato plant resistance to oxidative stress induced by hypothermia. Russ. J. Plant Physiol. 2008, 55, 639–648. [Google Scholar] [CrossRef]
- Wada, H.; Combos, Z.; Murata, N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 1990, 347, 200–203. [Google Scholar] [CrossRef]
- Kiseleva, L.L.; Serebriiskaya, T.S.; Horvath, I.; Vigh, L.; Lyukevich, A.A.; Los, D.A. Expression of the gene for the delta 9 acyl-lipid desaturase in the thermophilic cyanobacterium. J. Mol. Microbiol. Biotechnol. 2000, 2, 331–338. [Google Scholar] [PubMed]
- Li, Y.; Luo, T.; Lu, Q. Plant height as a simple predictor of the root to shoot ratio: Evidence from alpine grasslands on the Tibetan Plateau. J. Veg. Sci. 2008, 19, 245–252. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Naeem, M.; Hussain, A.; Azmi, U.R.; Maqsood, S.; Imtiaz, U.; Ali, H.; Ghani, U. Comparative anatomical studies of epidermis with different stomatal patterns in some selected plants using compound light microscopy. Intern. J. Sci. Res. Publ. 2019, 9, 375–380. [Google Scholar] [CrossRef]
- Coopoosamy, M.R.; Naidoo, K.K. Anatomical features of medicinal importance of Aloe excelsa (Berger). Afr. J. Biotechnol. 2011, 10, 7622–7632. [Google Scholar] [CrossRef]
- Maricle, B.R.; Koteyeva, N.K.; Voznesenskaya, E.V.; Thomasson, J.R.; Edwards, G.E. Diversity in leaf anatomy, and stomatal distribution and conductance, between salt marsh and freshwater species in the C4 genus Spartina (Poaceae). New Phytol. 2009, 184, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Horodecka, E. Laboratory methods of evaluating tomato resistance to late blight (Phytophthora infestans (Mont.) de Bary). Acta Agrobot. 1989, 42, 133. [Google Scholar] [CrossRef] [Green Version]
- Nechaeva, T.L.; Nikolaeva, T.N.; Zagoskina, N.V. Salicylic and hydroxybenzoic acids affect the accumulation of phenolic compounds in tea-plant cultures in vitro. Biol. Bull. 2020, 47, 374–380. [Google Scholar] [CrossRef]
- Goncharuck, E.A.; Kazantseva, V.V.; Zagoskina, N.V. Effect of hypothermia on the composition of phenolics in buckwheat plants with different ploidy. Russ. J. Plant Physiol. 2021, 68, 1227–1235. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]


| Variants | WT Plants (Control) | licBM3 Plants, L136 | desC Plants | desA Plants | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | L87 | L112 | L117 | L69 | L73 | L75 | |||
| Plant height *, cm | 17.3 ± 1.8 C | 20.3 ± 1.3 B | 19.4 ± 1.1 B | 17.1 ± 0.9 C | 21.4 ± 1.4 B | 25.7 ± 2.1 A | 24.3 ± 2.3 A | 25.2 ± 2.14 A | |
| Number of internodes *, pcs | 9 ± 1 B | 10 ± 1 B | 10 ± 1 B | 10 ± 1 B | 10 ± 1 B | 15 ± 1 A | 15 ± 1 A | 15 ± 1 A | |
| Plant weight *, g | 3.21 ± 0.21 B | 3.69 ± 0.21 B | 3.56 ± 0.2 B | 3.89 ± 0.23 B | 3.82 ± 0.26 B | 6.42 ± 0.39 A | 6.35 ± 0.42 A | 6.45 ± 0.38 A | |
| Leaf weight *, g | 2.56 ± 0.15 B | 3.02 ± 0.16 A | 3.05 ± 0.1 A | 3.11 ± 0.15 A | 3.01 ± 0.17 A | 3.46 ± 0.13 A | 3.31 ± 0.12 A | 3.23 ± 1.11 A | |
| Water content in leaves, % | 75.3 ± 2.1 A | 81.6 ± 2.3 A | 85.0 ± 2.2 A | 83.4 ± 1.9 A | 87.0 ± 2.1 A | 90.9 ± 2.4 A | 92.1 ± 2.4 A | 92.6 ± 2.5 A | |
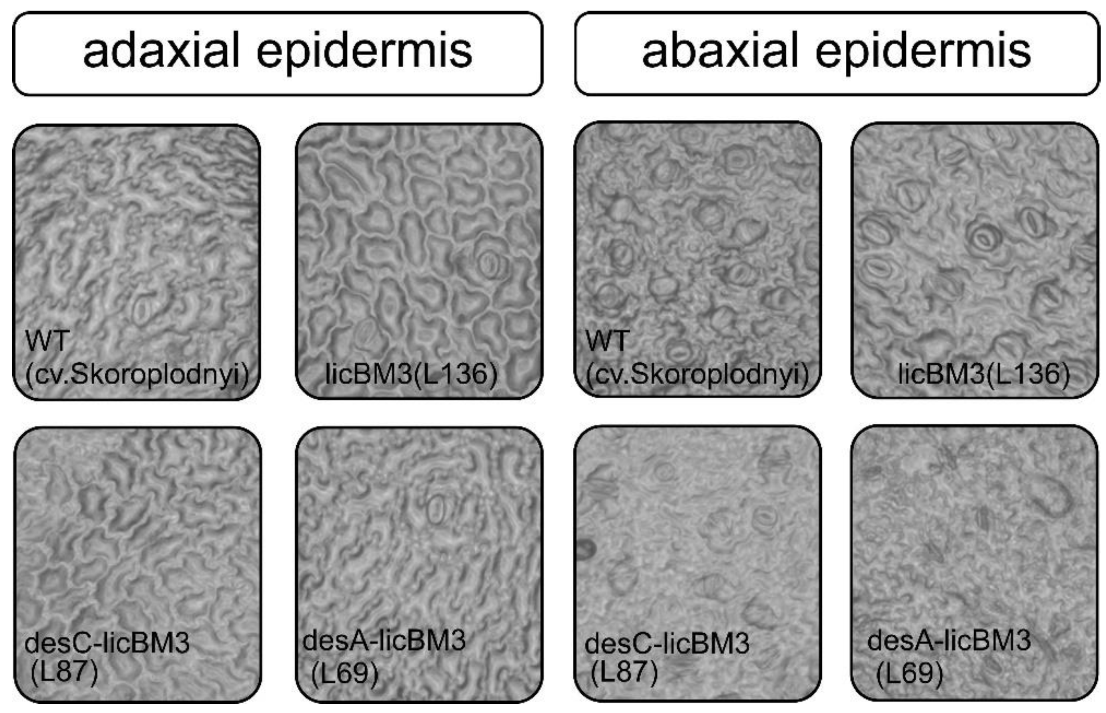
| Variants | Number of Stomata | Area Stomata, mm | Area Epidermis Cells | Stomatal Index | ||||
|---|---|---|---|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | Adaxial | Abaxial | Adaxial | Abaxial | |
| WT (control) | 1 | 23 | 39 | 40.2 | 79.4 | 140.8 | 2.07 | 47.8 |
| licBM3 plants (L136) | 4 | 16 | 52.8 | 47.9 | 84.4 | 88.2 | 8.3 | 33.3 |
| desC plants (L87) | 1 | 10 | 43.1 | 31.3 | 127.3 | 118.9 | 2.07 | 20.7 |
| desA plants (L69) | 2 | 9 | 44.5 | 48.1 | 160.6 | 93.3 | 4.15 | 18.7 |
| Transgene | Line | Resistance Indicator, Points | |
|---|---|---|---|
| Second Day after Infection | Third Day after Infection | ||
| WT (control) | 5.5 ± 0.7 E | 4.2 ± 0.41 D | |
| licBM3 | L136 | 6.2 ± 0.2 D | 4.5 ± 0.6 D |
| desC-licBM3 | L87 | 7.1 ± 0.38 C | 6.8 ± 0.88 B |
| L112 | 6.9 ± 0.27 C | 6.7 ± 0.2 B | |
| L117 | 7.3 ± 0.1 C | 6.2 ± 0.54 C | |
| desA-licBM3 | L69 | 7.8 ± 0.68 B | 7 ± 0.82 B |
| L73 | 8.1 ± 0.55 AB | 7.5 ± 0.43 A | |
| L75 | 8.4 ± 0.61 A | 7.2 ± 0.17 AB | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsypurskaya, E.V.; Nikolaeva, T.N.; Lapshin, P.V.; Nechaeva, T.L.; Yuorieva, N.O.; Baranova, E.N.; Derevyagina, M.K.; Nazarenko, L.V.; Goldenkova-Pavlova, I.V.; Zagoskina, N.V. Response of Transgenic Potato Plants Expressing Heterologous Genes of ∆9- or ∆12-Acyl-lipid Desaturases to Phytophthora infestans Infection. Plants 2022, 11, 288. https://doi.org/10.3390/plants11030288
Tsypurskaya EV, Nikolaeva TN, Lapshin PV, Nechaeva TL, Yuorieva NO, Baranova EN, Derevyagina MK, Nazarenko LV, Goldenkova-Pavlova IV, Zagoskina NV. Response of Transgenic Potato Plants Expressing Heterologous Genes of ∆9- or ∆12-Acyl-lipid Desaturases to Phytophthora infestans Infection. Plants. 2022; 11(3):288. https://doi.org/10.3390/plants11030288
Chicago/Turabian StyleTsypurskaya, Elena V., Tatiana N. Nikolaeva, Petr V. Lapshin, Tatiana L. Nechaeva, Natalya O. Yuorieva, Ekaterina N. Baranova, Marina K. Derevyagina, Lyudmila V. Nazarenko, Irina V. Goldenkova-Pavlova, and Natalia V. Zagoskina. 2022. "Response of Transgenic Potato Plants Expressing Heterologous Genes of ∆9- or ∆12-Acyl-lipid Desaturases to Phytophthora infestans Infection" Plants 11, no. 3: 288. https://doi.org/10.3390/plants11030288
APA StyleTsypurskaya, E. V., Nikolaeva, T. N., Lapshin, P. V., Nechaeva, T. L., Yuorieva, N. O., Baranova, E. N., Derevyagina, M. K., Nazarenko, L. V., Goldenkova-Pavlova, I. V., & Zagoskina, N. V. (2022). Response of Transgenic Potato Plants Expressing Heterologous Genes of ∆9- or ∆12-Acyl-lipid Desaturases to Phytophthora infestans Infection. Plants, 11(3), 288. https://doi.org/10.3390/plants11030288








