Advances in Plant Lipid Metabolism Responses to Phosphate Scarcity
Abstract
:1. Introduction
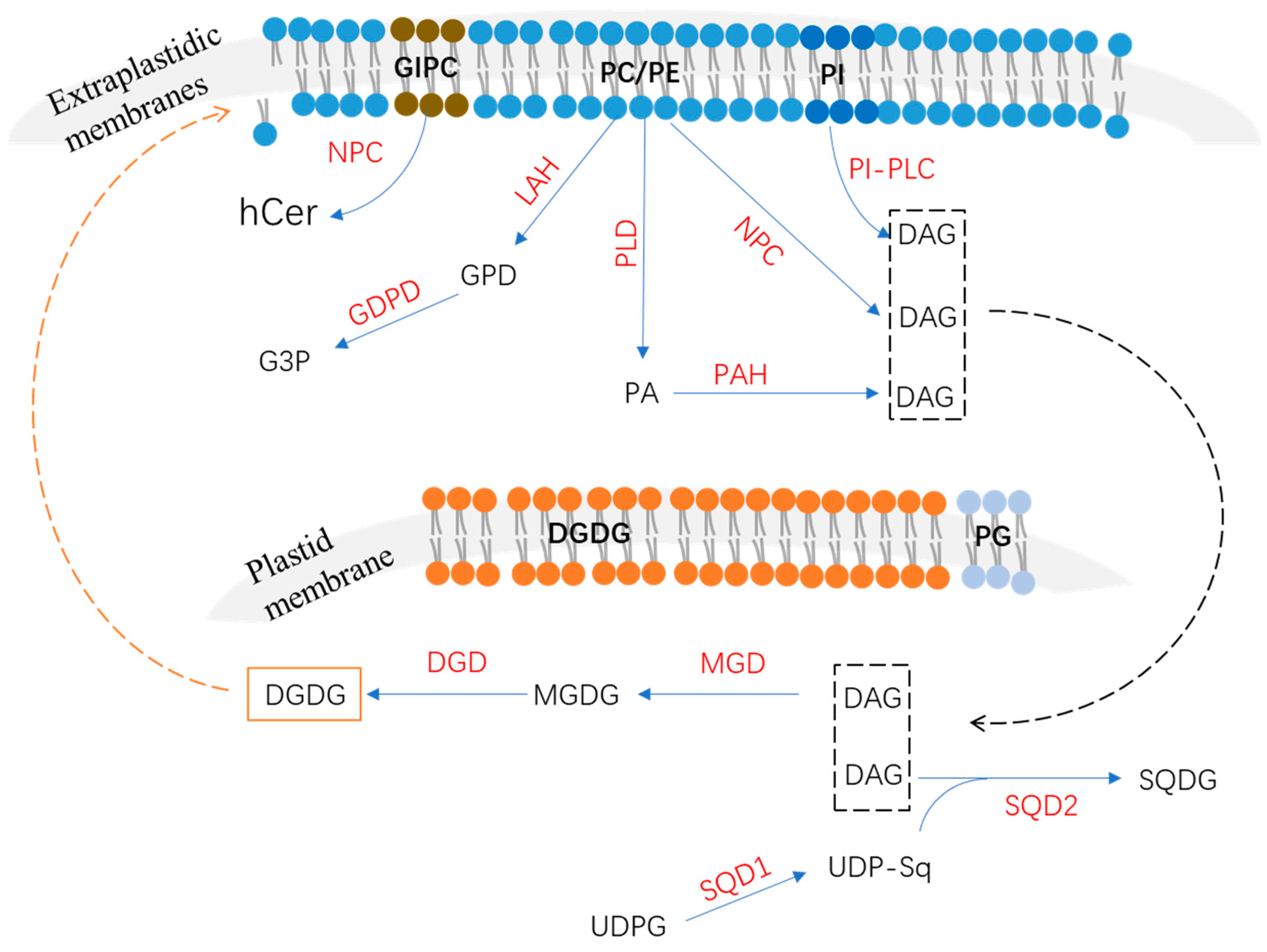
2. Phospholipids’ Degradation in Response to P Deficiency
2.1. Phospholipase C Pathway
2.2. Phospholipase D and Phosphatidic Acid Phosphatase Pathway
2.3. Lipid Acyl Hydrolase and Glycerophosphodiester Phosphodiesterase Pathway
3. Non-Phosphorus Lipid Biosynthesis
3.1. Biosynthesis of Monogalactosyldiacylglycerol and Digalactosyldiacylglycerol
3.2. Biosynthesis of Sulfoquinovosyldiacylglycerol
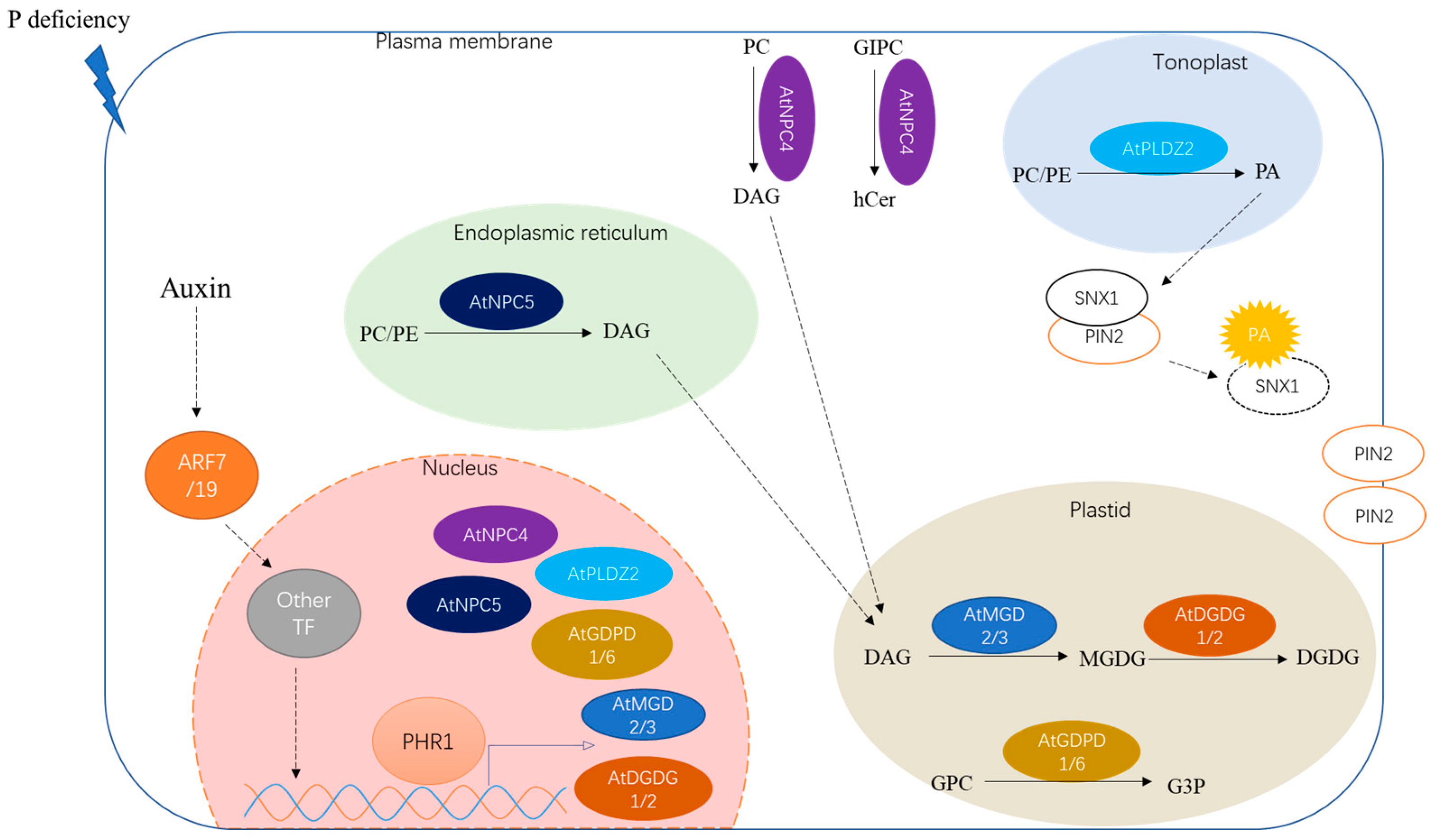
4. Other Aspects of Lipid Metabolism in Response to P Deficiency
4.1. Triacylglycerol Metabolism
4.2. Glucuronosyldiacylglycerol Metabolism
4.3. The De Novo Biosynthesis Pathway of Glycerolipid
5. Key Regulators in Lipid Metabolism
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liang, C.; Wang, J.; Zhao, J.; Tian, J.; Liao, H. Control of phosphate homeostasis through gene regulation in crops. Curr. Opin. Plant Biol. 2014, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners–the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. N. Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, D. Functions and regulation of phosphate starvationinduced secreted acid phosphatases in higher plants. Plant Sci. 2018, 271, 108–116. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Gutiérrez-Alanís, D.; Ojeda-Rivera, J.O.; Yong-Villalobos, L.; Cárdenas-Torres, L.; Herrera-Estrella, L. Adaptation to phosphate scarcity: Tips from Arabidopsis roots. Trends Plant Sci. 2018, 23, 721–730. [Google Scholar] [CrossRef]
- Nakamura, Y. Phosphate starvation and membrane lipid remodeling in seed plants. Prog. Lipid Res. 2013, 52, 43–50. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Zeng, L.; Lv, Y.; Ding, X.; Cheng, Y.; Zou, X. Plant lipid phosphate phosphatases: Current advances and future outlooks. Crit. Rev. Biotechnol. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Verma, L.; Kohli, P.S.; Maurya, K.; Abhijith, K.B.; Thakur, J.K.; Giri, J. Specific galactolipids species correlate with rice genotypic variability for phosphate utilization efficiency. Plant Physiol. Biochem. 2021, 168, 105–115. [Google Scholar] [CrossRef]
- Nakamura, Y.; Koizumi, R.; Shui, G.; Shimojima, M.; Wenk, M.R.; Ito, T.; Ohta, H. Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc. Natl. Acad. Sci. USA 2009, 106, 20978–20983. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, J.; Denton, A.K.; Usadel, B.; Pfaff, C. Phosphate starvation causes different stress responses in the lipid metabolism of tomato leaves and roots. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2020, 1865, 158763. [Google Scholar] [CrossRef] [PubMed]
- Joyard, J.; Teyssier, E.; Miege, C.; Berny-Seigneurin, D.; Marechal, E.; Block, M.A.; Dorne, A.J.; Rolland, N.; Ajlani, G.; Douce, R. The biochemical machinery of plastid envelope membranes. Plant Physiol. 1998, 118, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Härtel, H.; Dormann, P.; Benning, C. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2000, 97, 10649–10654. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.X.; Stridh, M.H.; Larsson, K.E.; Liljenberg, C.; Sandelius, A.S. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 2003, 537, 128–132. [Google Scholar] [CrossRef]
- Okazaki, Y.; Takano, K.; Saito, K. Lipidomic analysis of soybean leaves revealed tissue-dependent difference in lipid remodeling under phosphorus-limited growth conditions. Plant Biotechnol. 2017, 34, 57–63. [Google Scholar] [CrossRef]
- Singh, A.; Bhatnagar, N.; Pandey, A.; Pandey, G.K. Plant phospholipase C family: Regulation and functional role in lipid signaling. Cell Calcium. 2015, 58, 139–146. [Google Scholar] [CrossRef]
- Sagar, S.; Singh, A. Emerging role of phospholipase C mediated lipid signaling in abiotic stress tolerance and development in plants. Plant Cell Rep. 2021, 40, 2123–2133. [Google Scholar] [CrossRef]
- Mueller-Roeber, B.; Pical, C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 2002, 130, 22–46. [Google Scholar] [CrossRef]
- Hunt, L.; Otterhag, L.; Lee, J.C.; Lasheen, T.; Hunt, J.; Seki, M.; Shinozaki, K.; Sommarin, M.; Gilmour, D.J.; Pical, C.; et al. Gene-specific expression and calcium activation of Arabidopsis thaliana phospholipase C isoforms. N. Phytol. 2004, 162, 643–654. [Google Scholar] [CrossRef]
- Tasma, I.M.; Brendel, V.; Whitham, S.A.; Bhattacharyya, M.K. Expression and evolution of the phosphoinositide-specific phospholipase C gene family in Arabidopsis thaliana. Plant Physiol. Biochem. 2008, 46, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kanehara, K.; Yu, C.Y.; Cho, Y.; Cheong, W.F.; Torta, F.; Shui, G.; Wenk, M.R.; Nakamura, Y. Arabidopsis AtPLC2 is a primary phosphoinositide-specific phospholipase c in phosphoinositide metabolism and the endoplasmic reticulum stress response. PLoS Genet. 2015, 11, e1005511. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Awai, K.; Masuda, T.; Yoshioka, Y.; Takamiya, K.; Ohta, H. A novel phosphatidylcholine-hydrolyzing phospholipase C induced by phosphate starvation in Arabidopsis. J. Biol. Chem. 2005, 280, 7469–7476. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, M.; Guo, L.; Wang, X. Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J. 2018, 94, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Li, M.; Narasimhan, R.; Roth, M.; Welti, R.; Wang, X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell. 2010, 22, 2642–2659. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Phillips, A.; Li, L.; Ali, U.; Li, Q.; Lu, S.; Hong, Y.; Wang, X.; Guo, L. Nonspecific phospholipase C4 hydrolyzes phosphosphingolipids and sustains plant root growth during phosphate deficiency. Plant Cell. 2021, 33, 766–780. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, K.; Jin, X.; Yan, J.; Lu, S.; Shen, Q.; Guo, L.; Hong, Y.; Wang, X.; Guo, L. Acylation of non-specific phospholipase C4 determines its function in plant response to phosphate deficiency. Plant J. 2021, 106, 1647–1659. [Google Scholar] [CrossRef]
- Peters, C.; Kim, S.C.; Devaiah, S.; Li, M.; Wang, X. Non-specific phospholipase C5 and diacylglycerol promote lateral root development under mild salt stress in Arabidopsis. Plant Cell Environ. 2014, 37, 2002–2013. [Google Scholar] [CrossRef]
- Cruz-Ramírez, A.; Oropeza-Aburto, A.; Razo-Hernández, F.; Ramírez-Chávez, E.; Herrera-Estrella, L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2006, 103, 6765–6770. [Google Scholar] [CrossRef]
- Lin, D.L.; Yao, H.Y.; Jia, L.H.; Tan, J.F.; Xu, Z.H.; Zheng, W.M.; Xue, H.W. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. N. Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, W.; El Sheery, N.I.; Peters, C.; Li, M.; Wang, X.; Huang, J. Characterization of the Arabidopsis glycerophosphodiester phosphodiesterase (GDPD) family reveals a role of the plastid-localized AtGDPD1 in maintaining cellular phosphate homeostasis under phosphate starvation. Plant J. 2011, 66, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.H.; Nakamura, Y. Phosphate starvation-inducible GLYCEROPHOSPHODIESTER PHOSPHODIESTERASE6 is involved in Arabidopsis root growth. J. Exp. Bot. 2022, 73, 2995–3003. [Google Scholar] [CrossRef]
- Mehra, P.; Pandey, B.K.; Verma, L.; Giri, J. A novel glycerophosphodiester phosphodiesterase improves phosphate deficiency tolerance in rice. Plant Cell Environ. 2019, 42, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Giri, J. Rice and chickpea GDPDs are preferentially influenced by low phosphate and CaGDPD1 encodes an active glycerophosphodiester phosphodiesterase enzyme. Plant Cell Rep. 2016, 35, 1699–1717. [Google Scholar] [CrossRef]
- Awai, K.; Maréchal, E.; Block, M.A.; Brun, D.; Masuda, T.; Shimada, H.; Takamiya, K.; Ohta, H.; Joyard, J. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 10960–10965. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Awai, K.; Takamiya, K.; Ohta, H. Arabidopsis type B monogalactosyldiacylglycerol synthase genes are expressed during pollen tube growth and induced by phosphate starvation. Plant Physiol. 2004, 134, 640–648. [Google Scholar] [CrossRef]
- Kobayashi, K.; Awai, K.; Nakamura, M.; Nagatani, A.; Masuda, T.; Ohta, H. Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling, and are crucial for low-phosphate adaptation. Plant J. 2009, 57, 322–331. [Google Scholar] [CrossRef]
- Verma, L.; Bhadouria, J.; Rupam, B.K.; Singh, S.; Panchal, P.; Bhatia, C.; Eastmond, P.J.; Giri, J. Monogalactosyl Diacylglycerol Synthase 3 (OsMGD3) affects phosphate utilization and acquisition in rice. J. Exp. Bot. 2022, 73, 5033–5051. [Google Scholar] [CrossRef]
- Kelly, A.A.; Froehlich, J.E.; Dörmann, P. Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell. 2003, 15, 2694–7206. [Google Scholar] [CrossRef]
- Kelly, A.A.; Dörmann, P. DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J. Biol. Chem. 2002, 277, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Brown, C.; Mäler, L. Expression and purification of DGD2, a chloroplast outer membrane-associated glycosyltransferase for galactolipid synthesis. Biochemistry 2020, 59, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Sanda, S.; Leustek, T.; Theisen, M.J.; Garavito, R.M.; Benning, C. Recombinant Arabidopsis SQD1 converts UDP-glucose and sulfite to the sulfolipid head group precursor UDP-sulfoquinovose in vitro. J. Biol. Chem. 2001, 276, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jain, A.; Xue, Y.; Wang, X.; Zhao, G.; Liu, L.; Hu, Z.; Hu, S.; Shen, X.; Liu, X.; et al. OsSQD1 at the crossroads of phosphate and sulfur metabolism affects plant morphology and lipid composition in response to phosphate deprivation. Plant Cell Environ. 2020, 43, 1669–1690. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Xu, C.; Benning, C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc. Natl. Acad. Sci. USA 2002, 99, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Angkawijaya, A.E.; Nguyen, V.C.; Nakamura, Y. Enhanced root growth in phosphate-starved Arabidopsis by stimulating de novo phospholipid biosynthesis through the overexpression of LYSOPHOSPHATIDIC ACID ACYLTRANSFERASE 2 (LPAT2). Plant Cell Environ. 2017, 40, 1807–1818. [Google Scholar] [CrossRef]
- Wang, X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005, 139, 566–573. [Google Scholar] [CrossRef]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef]
- Yamaryo, Y.; Dubots, E.; Albrieux, C.; Baldan, B.; Block, M.A. Phosphate availability affects the tonoplast localization of PLDzeta2, an Arabidopsis thaliana phospholipase D. FEBS Lett. 2008, 582, 685–690. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem. Sci. 2006, 31, 694–699. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Quettier, A.L.; Kroon, J.T.; Craddock, C.; Adams, N.; Slabas, A.R. Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell. 2010, 22, 2796–2811. [Google Scholar] [CrossRef] [Green Version]
- Brindley, D.N.; Pilquil, C. Lipid phosphate phosphatases and signaling. J. Lipid Res. 2009, 50, S225–S230. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tsuchiya, M.; Ohta, H. Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin. J. Biol. Chem. 2007, 282, 29013–29021. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Bläsing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M. Biosynthesis and functions of the plant sulfolipid. Prog. Lipid Res. 2011, 50, 234–239. [Google Scholar] [CrossRef]
- Basnet, R.; Zhang, J.; Hussain, N.; Shu, Q. Characterization and mutational analysis of a monogalactosyldiacylglycerol synthase gene OsMGD2 in rice. Front. Plant Sci. 2019, 10, 992. [Google Scholar] [CrossRef]
- Okazaki, Y.; Shimojima, M.; Sawada, Y.; Toyooka, K.; Narisawa, T.; Mochida, K.; Tanaka, H.; Matsuda, F.; Hirai, A.; Hirai, M.Y.; et al. A chloroplastic UDP-glucose pyrophosphorylase from Arabidopsis is the committed enzyme for the first step of sulfolipid biosynthesis. Plant Cell 2009, 21, 892–909. [Google Scholar] [CrossRef]
- Pant, B.D.; Burgos, A.; Pant, P.; Cuadros-Inostroza, A.; Willmitzer, L.; Scheible, W.R. The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation. J. Exp. Bot. 2015, 66, 1907–1918. [Google Scholar] [CrossRef]
- Kaup, M.T.; Froese, C.D.; Thompson, J.E. A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol. 2002, 129, 1616–1626. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Song, L.; An, C. ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol. 2011, 156, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Pourtau, N.; Marès, M.; Purdy, S.; Quentin, N.; Ruël, A.; Wingler, A. Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 2004, 219, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 2013, 4, 1510. [Google Scholar] [CrossRef] [PubMed]
- Angkawijaya, A.E.; Nguyen, V.C.; Nakamura, Y. LYSOPHOSPHATIDIC ACID ACYLTRANSFERASES 4 and 5 are involved in glycerolipid metabolism and nitrogen starvation response in Arabidopsis. N. Phytol. 2019, 224, 336–351. [Google Scholar] [CrossRef]
- Li, M.; Welti, R.; Wang, X. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases D zeta1 and D zeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol. 2006, 142, 750–761. [Google Scholar] [CrossRef]
- Gaude, N.; Nakamura, Y.; Scheible, W.R.; Ohta, H.; Dörmann, P. Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J. 2008, 56, 28–39. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T.; Takamiya, K.; Ohta, H. Membrane lipid alteration during phosphate starvation is regulated by phosphate signaling and auxin/cytokinin cross-talk. Plant J. 2006, 47, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Narise, T.; Kobayashi, K.; Baba, S.; Shimojima, M.; Masuda, S.; Fukaki, H.; Ohta, H. Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol. Biol. 2010, 72, 533–544. [Google Scholar] [CrossRef]
- Pérez-Torres, C.A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef]
- Chevalier, F.; Cuyas, L.; Jouhet, J.; Gros, V.R.; Chiarenza, S.; Secco, D.; Whelan, J.; Seddiki, K.; Block, M.A.; Nussaume, L.; et al. Interplay between jasmonic acid, phosphate signaling and the regulation of glycerolipid homeostasis in Arabidopsis. Plant Cell Physiol. 2019, 60, 1260–1273. [Google Scholar] [CrossRef]
- Pandey, B.K.; Verma, L.; Prusty, A.; Singh, A.P.; Bennett, M.J.; Tyagi, A.K.; Giri, J.; Mehra, P. OsJAZ11 regulates phosphate starvation responses in rice. Planta 2021, 254, 8. [Google Scholar] [CrossRef]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, H.; Wan, R.; Liu, Y.; Xu, Z.; Tian, W.; Ruan, W.; Wang, F.; Deng, M.; Wang, J.; et al. Identification of vacuolar phosphate efflux transporters in land plants. Nat. Plants. 2019, 5, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.S.; Maurya, K.; Thakur, J.K.; Bhosale, R.; Giri, J. Significance of root hairs in developing stress-resilient plants for sustainable crop production. Plant Cell Environ. 2022, 45, 677–694. [Google Scholar] [CrossRef]
- Mo, X.; Liu, G.; Zhang, Z.; Lu, X.; Liang, C.; Tian, J. Mechanisms underlying soybean response to phosphorus deficiency through integration of omics analysis. Int. J. Mol. Sci. 2022, 23, 4592. [Google Scholar]
- Zhou, M.; Zhu, S.; Mo, X.; Guo, Q.; Li, Y.; Tian, J.; Liang, C. Proteomic analysis dissects molecular mechanisms underlying plant responses to phosphorus deficiency. Cells 2022, 11, 651. [Google Scholar] [PubMed]
| Gene | Species | Pi Starvation | Tissues | Subcellular Localization | Pathway | Functions in Adaption to Pi Starvation | References |
|---|---|---|---|---|---|---|---|
| AtNPC4 | Arabidopsis thaliana | Up-regulated | Shoot and root | Plasma membrane | Hydrolyzing PC to generate DAG. Hydrolyzing GIPC to generate hCer. | Knockout of AtNPC4 decreases the loss in GIPC and impedes root growth under Pi starvation. | [23,24,26,27] |
| AtNPC5 | Arabidopsis thaliana | Up-regulated | Leaf | Cytosol | Hydrolyzing PC and PE to generate DAG. | Knockout of AtNPC5 decreases the accumulation of DGDG in both leaf and root under Pi starvation. | [28] |
| AtPLDX2 | Arabidopsis thaliana | Up-regulated | Shoot and root | Tonoplasts | Hydrolyzing PC and PE to generate PA. | Knockout of AtPLDZ2 decreases the amount of DGDG and increases the amounts of PC and PE in root, decreases primary root length, and increases root hair density and root hair length under Pi starvation. | [24,29,30] |
| AtGDPD1 | Arabidopsis thaliana | Up-regulated | Shoot and root | Plastids | Hydrolyzing glycerophosphodiesters into G3P and the corresponding alcohols. | Knockout of AtGDPD1 decreases G3P content, Pi content, and seedling growth rate under Pi starvation. | [31] |
| AtGDPD6 | Arabidopsis thaliana | Up-regulated | Flower and primary root | Nd | Hydrolyzing GPC to generate G3P. | Overexpression of AtGDPD6 increases root length and knockout of AtGDPD6 decreases root length under Pi starvation. | [32] |
| OsGDPD2 | Oryza sativa | Up-regulated | Shoot and root | Nd | Hydrolyzing glycerophosphodiesters into G3P and the corresponding alcohols. | Overexpression of OsGDPD2 increases Pi content, root growth, and biomass accumulation under Pi starvation. | [33] |
| CaGDPD1 | Cicer arietinum | Up-regulated | Root | Endoplasmic reticulum | Having enzyme activities on GPC and GPE. | Nd. | [34] |
| AtMGD2 | Arabidopsis thaliana | Up-regulated | Root | Plastid | Catalyzing DAG and UDP-Gal into MGDG. | Knockout of AtMGD2 decreases root length under Pi starvation. | [35,36,37] |
| AtMGD3 | Arabidopsis thaliana | Up-regulated | Root | Plastid | Catalyzing DAG and UDP-Gal into MGDG. | Knockout of AtMGD3 decreases root length and shoot and root fresh weight under Pi starvation. | [35,36,37] |
| OsMGD3 | Oryza sativa | Up-regulated | Root | Plastid | Catalyzing DAG and UDP-Gal into MGDG. | Knockout of OsMGD3 decreases shoot dry weight and Pi use efficiency; overexpression of OsMGD3 increases shoot dry weight, lateral root number, root Pi acquisition efficiency, and total P content per plant shoot under Pi starvation. | [38] |
| AtDGD1 | Arabidopsis thaliana | Up-regulated | Leaf | Chloroplast | Catalyzing MGDG and UDP-Gal into DGDG. | Knockout of AtDGD1 decreases the amount of DGDG under Pi starvation. | [14,39] |
| AtDGD2 | Arabidopsis thaliana | Up-regulated | Leaf | Chloroplast | Catalyzing MGDG and UDP-Gal into DGDG. | Knockout of AtDGD2 decreases the amount of DGDG under Pi starvation. | [14,39,40,41] |
| AtSQD1 | Arabidopsis thaliana | Up-regulated | Leaf | Chloroplast | Catalyzing UDPG and sulfite into UDP-sulfoquinovose. | Nd. | [42] |
| OsSQD1 | Oryza sativa | Up-regulated | Shoot and root | Chloroplast | Catalyzing UDPG and sulfite into UDP-sulfoquinovose. | Knockout of OsSQD1 decreases root growth and increases the Pi and total P concentration of shoot and root under Pi starvation. | [43] |
| AtSQD2 | Arabidopsis thaliana | Up-regulated | Leaf | Chloroplast | Catalyzing UDP-sulfoquinovose and DAG into SQDG; catalyzing UDP-GlcA and DAG into GlcADG. | Knockout of AtSQD2 decreases the amount of SQDG and reduces plant fresh weight under Pi starvation. | [44] |
| SlDGAT2 | Solanum lycopersicum | Up-regulated | Leaf and root | Nd | Catalyzing DAG and acyl-CoA into TAG. | Nd. | [12] |
| AtLPAT2 | Arabidopsis thaliana | Up-regulated | Root | Endoplasmic reticulum | Catalyzing LPA into PA. | Overexpression of AtLPAT2 increases the length of primary root and the amount of PC in root under Pi starvation. | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liang, C.; Tian, J.; Xue, Y. Advances in Plant Lipid Metabolism Responses to Phosphate Scarcity. Plants 2022, 11, 2238. https://doi.org/10.3390/plants11172238
Zhu S, Liang C, Tian J, Xue Y. Advances in Plant Lipid Metabolism Responses to Phosphate Scarcity. Plants. 2022; 11(17):2238. https://doi.org/10.3390/plants11172238
Chicago/Turabian StyleZhu, Shengnan, Cuiyue Liang, Jiang Tian, and Yingbin Xue. 2022. "Advances in Plant Lipid Metabolism Responses to Phosphate Scarcity" Plants 11, no. 17: 2238. https://doi.org/10.3390/plants11172238
APA StyleZhu, S., Liang, C., Tian, J., & Xue, Y. (2022). Advances in Plant Lipid Metabolism Responses to Phosphate Scarcity. Plants, 11(17), 2238. https://doi.org/10.3390/plants11172238







