Adaptability and Stability of Faba Bean (Vicia faba L.) Accessions under Diverse Environments and Herbicide Treatments
Abstract
1. Introduction
2. Results
2.1. Phenological Traits
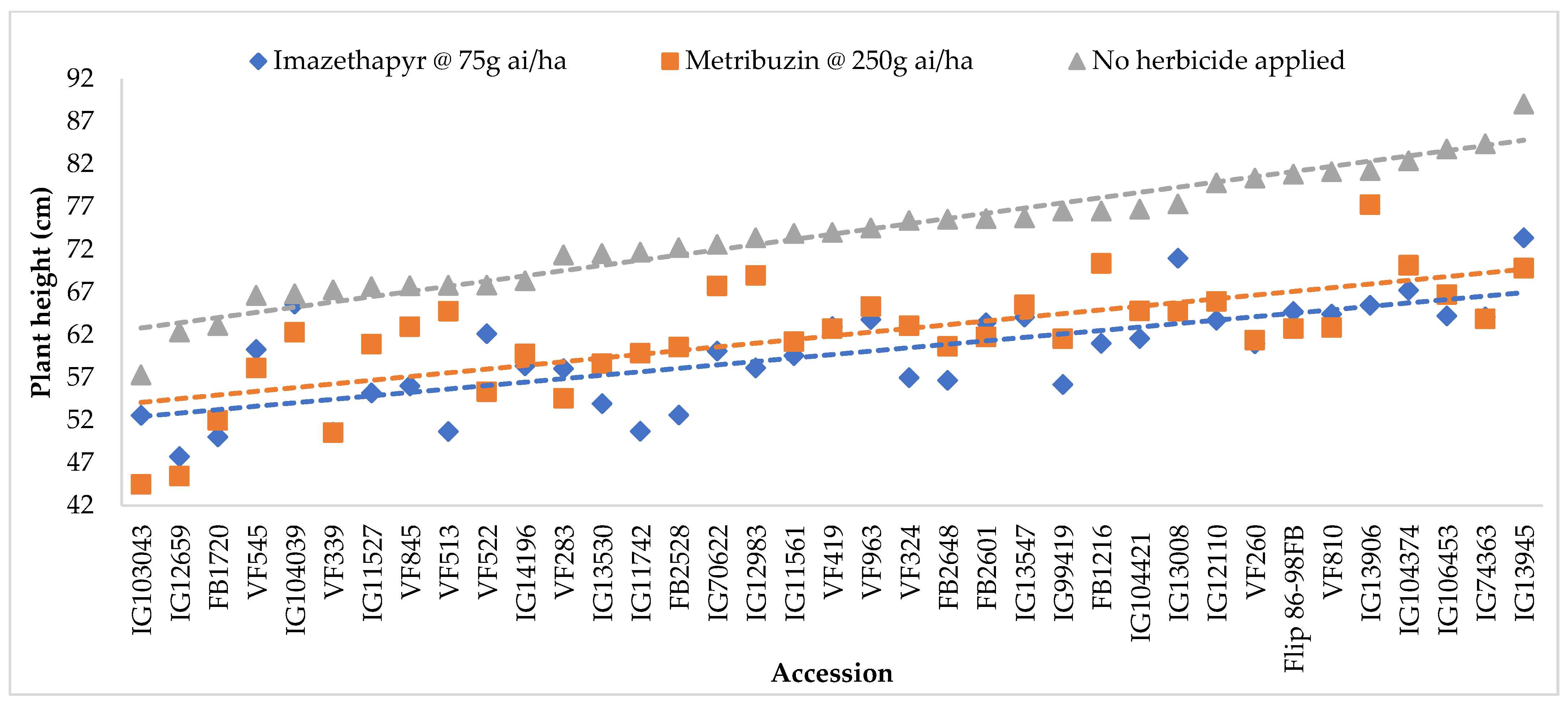
2.2. Plant Height
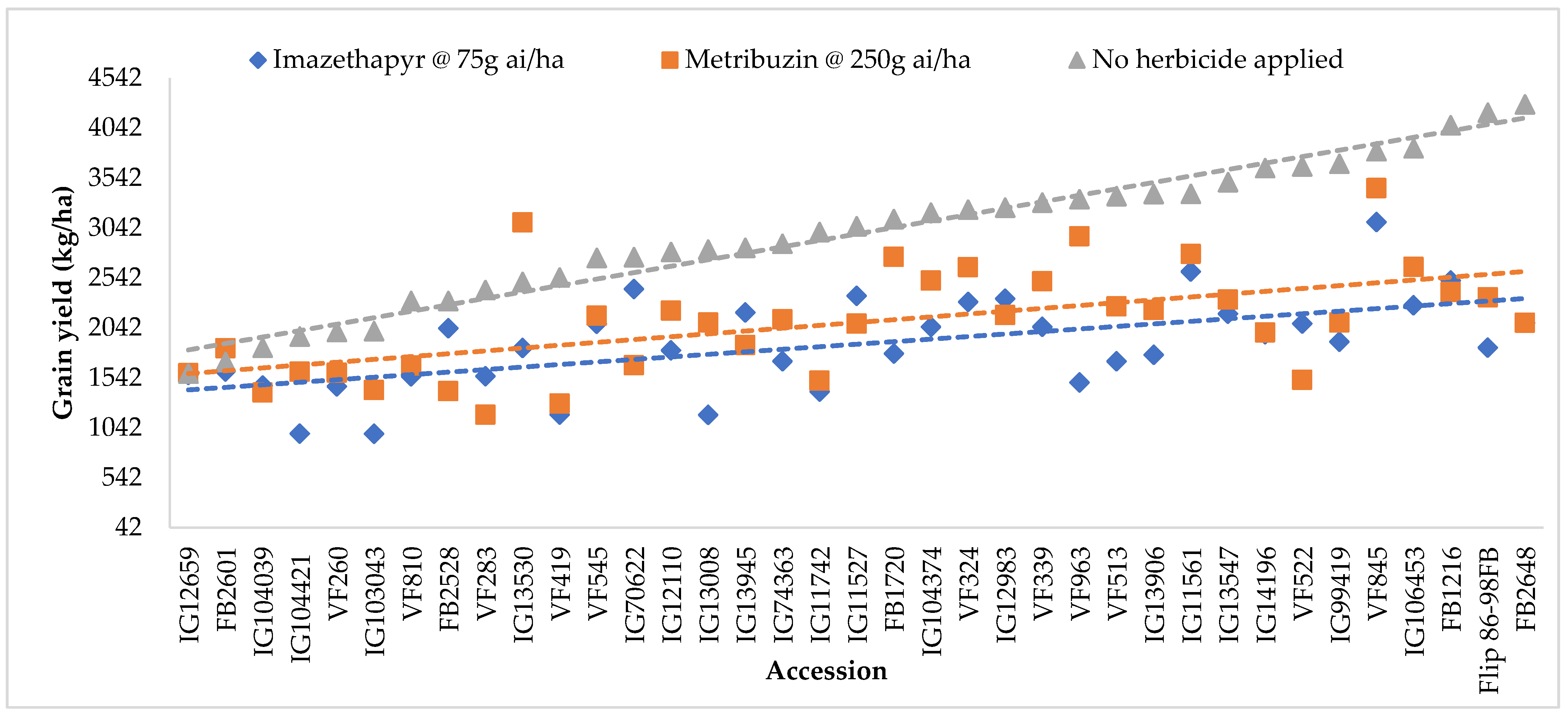
2.3. Grain Yield
2.4. GGE Analysis
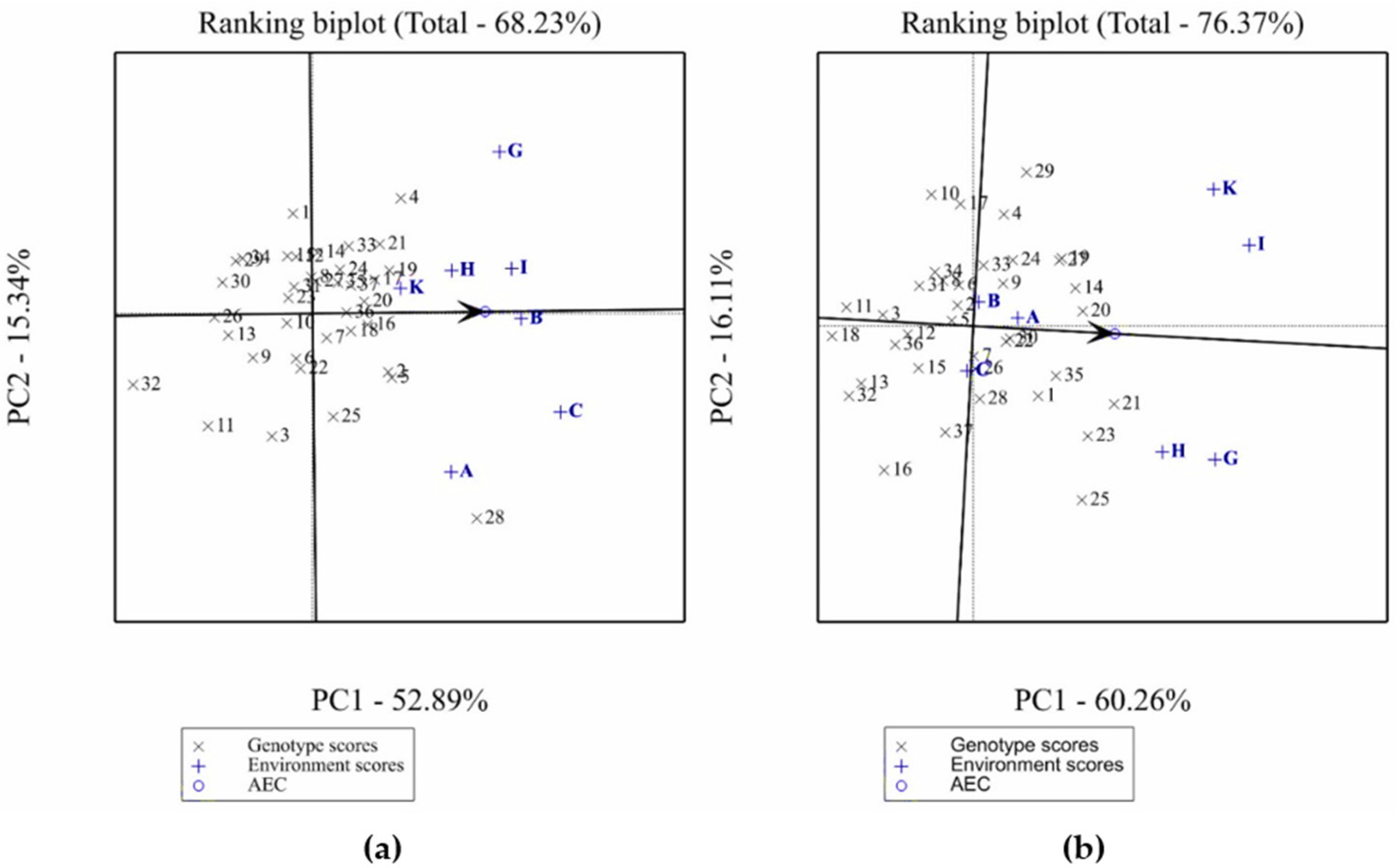
2.4.1. Evaluation of Test Environments
2.4.2. Identification of Mega-Environments and Specific Adapted Accessions
2.4.3. Performance of Tested Accessions
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experiments
4.3. Recorded Traits
4.4. Statistical Analysis
- The discriminating ability of the test environments was evaluated based on the length of the vector of each environment; the longer the environment vector, the more the discriminating ability of the environment.
- The mean performance of the genotype was graphically evaluated based on the line perpendicular to the average tester axis (ATA) that passes through the origin and separates entries with below-average means from those with above-average means; the genotypes located on the right side of this line are taller or have more yield than the ones located on the left side.
- The stability of the accessions was graphically represented by the projection from the genotype to the ATA; the longer the projection the greater is the GE interaction and therefore the lower the stability of the genotype across environments.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cubero, J.I. On the evolution of Vicia faba L. Theor. Appl. Genet. 1974, 45, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Caracuta, V.; Weinstein-Evron, M.; Kaufman, D.; Yeshurun, R.; Silvent, J.; Boaretto, E. 14,000-year-old seeds indicate the Levantine origin of the lost progenitor of faba bean. Sci. Rep. 2016, 6, 37399. [Google Scholar] [CrossRef]
- FAOSTAT Database. Available online: www.faostat.fao.org (accessed on 20 July 2021).
- Karkanis, A.; Ntatsi, G.; Kontopoulou, C.K.; Pristeri, A.; Bilalis, D.; Savvas, D. Field pea in European cropping systems: Adaptability, biological nitrogen fixation and cultivation practices. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 325–336. [Google Scholar] [CrossRef]
- Kalburtji, K.L.; Mamolos, A.P. Competition between Canada thistle [Cirsium arvense (L.) Scop.] and faba bean (Vicia faba L.). J. Agron. Crop Sci. 2001, 186, 261–265. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Flores, F.; Rubiales, D. Differences in crenate broomrape parasitism dynamics on three legume crops using a thermal time model. Front. Plant Sci. 2016, 7, 1910. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M. Innovations in parasitic weeds management in legume crops. A review. Agron. Sustain. Dev. 2012, 32, 433–449. [Google Scholar] [CrossRef]
- Maalouf, F.; Khalil, S.; Ahmed, S.; Akintunde, A.N.; Kharrat, M.; El Shama’a, K.; Hajjar, S.; Malhotra, R.S. Yield stability of faba bean lines under diverse broomrape prone production environments. Field Crops Res. 2011, 124, 288–294. [Google Scholar] [CrossRef]
- Abou-Khater, L.; Maalouf, F.; Patil, B.S.; Balech, R.; Nacouzi, D.; Rubiales, D.; Kumar, S. Identification of Tolerance to Metribuzin and Imazethapyr Herbicides in Faba Bean (Vicia faba L.). Crop Sci. 2021, 61, 2593–2611. [Google Scholar] [CrossRef]
- Maalouf, F.; Naoum, A.A.; El Shamaa, K.; Patil, S.B. Wide range of genetic variability for herbicide tolerance in faba bean. In Proceedings of the International Conference on Pulses, Marrakesh, Morocco, 18–20 April 2016; pp. 108–109. [Google Scholar]
- Abbes, Z.; Kharrat, M.; Delavault, P.; Simier, P.; Chaïbi, W. Field evaluation of the resistance of some faba bean (Vicia faba L.) genotypes to the parasitic weed Orobanche foetida Poiret. Crop Prot. 2007, 26, 1777–1784. [Google Scholar] [CrossRef]
- Singh, N.P.; Yadav, I.S. Herbicide tolerant food legume crops: Possibilities and prospects. In Herbicides-Properties, Synthesis and Control of Weeds, in Agricultural and Biological Sciences; Hasaneen, M.N., Ed.; InTech: Shanghai, China, 2012; pp. 435–452. [Google Scholar]
- Burnside, O.C.; Wiens, M.J.; Holder, B.J.; Weisberg, S.; Ristau, E.A.; Johnson, M.M.; Cameron, J.H. Critical periods for weed control in dry beans (Phaseolus vulgaris). Weed Sci. 1998, 46, 301–306. [Google Scholar] [CrossRef]
- Maalouf, F.; Patil, S.B.; Rajendra, K.; Hamwieh, A.; Goyal, A.; Kumar, S. Breeding for post-emergence herbicide tolerance in cool-season food legumes. In Proceedings of the International Conference on Pulses, Marrakesh, Morocco, 18–20 April 2016; p. 65. [Google Scholar]
- Toker, C. Estimates of broad-sense heritability for seed yield and yield criteria in faba bean (Vicia faba L.). Hereditas 2004, 140, 222–225. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press LLC: Boca Raton, FL, USA, 2003; p. 271. [Google Scholar]
- Patel, C.M.; Patel, J.M.; Patel, C.J. Gene× Environment interaction and stability analysis for yield and yield determinant traits in castor (Ricinus communis L.). IOSR J. Agric. Vet. Sci. 2015, 8, 68–72. [Google Scholar]
- De Leon, N.; Jannink, J.L.; Edwards, J.W.; Kaeppler, S.M. Introduction to a special issue on genotype by environment interaction. Crop Sci. 2016, 56, 2081–2089. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, M. GE interaction and its breeding implications in pulses. Botanica 2006, 56, 31–36. [Google Scholar]
- Flores, F.; Hybl, M.; Knudsen, J.C.; Marget, P.; Muel, F.; Nadal, S.; Narits, L.; Raffiot, B.; Sass, O.; Solis, I.; et al. Adaptation of spring faba bean types across European climates. Field Crops Res. 2013, 145, 1–9. [Google Scholar] [CrossRef]
- Temesgen, T.; Keneni, G.; Sefera, T.; Jarso, M. Yield stability and relationships among stability parameters in faba bean (Vicia faba L.) genotypes. Crop J. 2015, 3, 258–268. [Google Scholar] [CrossRef]
- Dyke, G.V.; Lane, P.W.; Jenkyn, J.F. Sensitivity (stability) analysis of multiple variety trials, with special reference to data expressed as proportions or percentages. Exp. Agric. 1995, 31, 75–87. [Google Scholar] [CrossRef]
- Gedif, M.; Yigzaw, D. Genotype× environment interaction analysis for tuber yield of potato using a GGE biplot method in Amhara region, Ethiopia. Potato J. 2014, 41, 41–51. [Google Scholar]
- Wricke, G. Uber eine Methode zur Erfassung der okologischen Streubreite in Feldverzuchen. Z. Pflanzenzuchtg 1962, 47, 92–96. [Google Scholar]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, W. GGE biplot—A Windows application for graphical analysis of multi-environment trial data and other types of two-way data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Flores, F.; Nadal, S.; Solis, I.; Winkler, J.; Sass, O.; Stoddard, F.L.; Link, W.; Raffiot, B.; Muel, F.; Rubiales, D. Faba bean adaptation to autumn sowing under European climates. Agron. Sustain. Dev. 2012, 32, 727–734. [Google Scholar] [CrossRef]
- Sharma, S.R.; Singh, S.; Aggarwal, N.; Kaur, J.; Gill, R.K.; Kushwah, A.; Patil, S.B.; Kumar, S. Genetic variation for tolerance to post-emergence herbicide, imazethapyr in lentil (Lens culinaris Medik.). Arch. Agron. Soil Sci. 2018, 64, 1818–1830. [Google Scholar] [CrossRef]
- Gupta, M.; Bindra, S.; Sood, A.; Singh, I.; Singh, G.; Gaur, P.M.; Chatuverdi, S.K.; Dixit, G.P.; Singh, S. Identifying new sources of tolerance to post emergence herbicides in chickpea (Cicer arietinum L.). J. Food Legumes 2017, 31, 5–9. [Google Scholar]
- Jefferies, M.L.; Willenborg, C.J.; Taran, B. Response of chickpea cultivars to imidazolinone herbicide applied at different growth stages. Weed Technol. 2016, 30, 664–676. [Google Scholar] [CrossRef]
- Sajja, S.; Samineni, S.; Gadekar, M.; Jayalakshmi, V.; Vijayakumar, A.; Yasin, M.; Varshney, R.K. Effect of post-emergence herbicide imazethapyr on phenological and agronomic traits in chickpea breeding lines. In Proceedings of the International Plant Breeding Congress (IPBC) and Eucarpia—Oil and Protein Crops Section Conference, Antalya, Turkey, 1–5 November 2015; p. 123. [Google Scholar]
- Taran, B.; Holm, F.; Banniza, S. Response of chickpea cultivars to pre-and post-emergence herbicide applications. Can. J. Plant Sci. 2013, 93, 279–286. [Google Scholar] [CrossRef]
- Gaur, P.; Jukanti, A.; Samineni, S.; Chaturvedi, S.; Singh, S.; Tripathi, S.; Singh, I.; Singh, G.; Das, T.; Aski, M.; et al. Large Genetic Variability in Chickpea for Tolerance to Herbicides Imazethapyr and Metribuzin. Agronomy 2013, 3, 524–536. [Google Scholar] [CrossRef]
- Pan, G.; Si, P.; Yu, Q.; Tu, J.; Powles, S. Non-target site mechanism of metribuzin tolerance in induced tolerant mutants of narrowleafed lupin (Lupinus angustifolius L.). Crop Pasture Sci. 2012, 63, 452–458. [Google Scholar] [CrossRef]
- Gaston, S.; Zabalza, A.; González, E.M.; Arrese-Igor, C.; Aparicio-Tejo, P.M.; Royuela, M. Imazethapyr, an inhibitor of the branched-chain amino acid biosynthesis, induces aerobic fermentation in pea plants. Physiol. Plant. 2002, 114, 524–532. [Google Scholar] [CrossRef]
- Maalouf, F.; Nachit, M.; Ghanem, M.E.; Singh, M. Evaluation of faba bean breeding lines for spectral indices, yield traits and yield stability under diverse environments. Crop Pasture Sci. 2015, 66, 1012–1023. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S.; Bukhari, N.A. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015, 16, 10214–10227. [Google Scholar] [CrossRef]
- Choukri, H.; Hejjaoui, K.; El-Baouchi, A. Heat and Drought Stress Impact on Phenology, Grain Yield, and Nutritional Quality of Lentil (Lens culinaris Medikus). Front. Nutr. 2020, 7, 596307. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Seidel, S.J.; Rachmilevitch, S.; Schütze, N.; Lazarovitch, N. Modelling the impact of drought and heat stress on common bean with two different photosynthesis model approaches. Environ. Model. Softw. 2016, 81, 111–121. [Google Scholar] [CrossRef]
- Taran, B.; Warkentin, T.D.; Vandenberg, A.; Holm, F.A. Variation in chickpea germplasm for tolerance to imazethapyr and imazamox herbicides. Can. J. Plant Sci. 2010, 90, 139–142. [Google Scholar] [CrossRef]
- Sharma, S.R.; Singh, S.; Aggarwal, N.; Kaur, J.; Gill, R.K.; Patil, S.B.; Kumar, S. Effect of post-emergence herbicide metribuzin application on morpho-physiological traits, yield and yield components in lentil (Lens culinaris Medik.). In Proceedings of the International Conference on Pulses, Marrakesh, Morocco, 18–20 April 2016; p. 100. [Google Scholar]
- Aboali, Z.; Saeedipour, S. Efficacy evaluation of some herbicides for weed management and yield attributes in broad bean (Vicia faba). Res. J. Environ. Sci. 2015, 9, 289. [Google Scholar]
- Schmidt, P. Estimating Heritability in Plant Breeding Programs. Ph.D. Thesis, University of Hohenheim, Rostock, Germany, 2019. [Google Scholar]
- Mohamed, K.M.M. The Relationship between Yield and Heritability in Ten Genotypes of Faba Bean (Vicia faba L.). Hered. Genet. 2019, 8, 197. [Google Scholar]
- Abdelmula, A.A.; Link, W.; Kittlitz, E.V.; Stelling, D. Heterosis and inheritance of drought tolerance in faba bean, Vicia faba L. Plant Breed. 1999, 118, 485–490. [Google Scholar] [CrossRef]
- Ceccarelli, S. Specific adaptation and breeding for marginal conditions. In Breeding Fodder Crops for Marginal Conditions; Rognli, O.A., Solberg, E., Schjelderup, I., Eds.; Springer: Dordrecht, The Netherlands, 1994; Volume 2, pp. 101–127. [Google Scholar]
- Atlin, G.N.; Frey, K.J. Selecting oat lines for yield in low-productivity environments. Crop Sci. 1990, 30, 556–561. [Google Scholar] [CrossRef]
- Wray, N.; Visscher, P. Estimating trait heritability. Nat. Educ. 2008, 1, 29. [Google Scholar]
- Schmidt, P.; Hartung, J.; Bennewitz, J.; Piepho, H.P. Heritability in plant breeding on a genotype-difference basis. Genetics 2019, 212, 991–1008. [Google Scholar] [CrossRef]
- Lubadde, G.; Tongoona, P.; Derera, J.; Sibiya, J. Analysis of genotype by environment interaction of improved pearl millet for grain yield and rust resistance. J. Agric. Sci. 2017, 9, 188–195. [Google Scholar] [CrossRef][Green Version]
- Alberts, M.J. A Comparison of Statistical Methods to Describe Genotype x Environment Interaction and Yield Stability in Multi-Location Maize Trials. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2004. [Google Scholar]
- Mustapha, M.; Bakari, H.R. Statistical evaluation of genotype by environment interactions for grain yield in millet (Penniisetum glaucum (L.) R. Br.). Int. J. Eng. Sci. 2014, 3, 7–16. [Google Scholar]
- Dehghani, H.; Sabaghpour, S.H.; Sabaghnia, N. Genotype x environment interaction for grain yield of some lentil genotypes and relationship among univariate stability statistics. Span. J. Agric. Res. 2008, 3, 385–394. [Google Scholar] [CrossRef]
- Milioli, A.S.; Zdziarski, A.D.; Woyann, L.G.; Santos, R.D.; Rosa, A.C.; Madureira, A.; Benin, G. Yield stability and relationships among stability parameters in soybean genotypes across years. Chil. J. Agric. Res. 2018, 78, 299–309. [Google Scholar] [CrossRef]
- Westcott, B. Some methods of analysing genotype—Environment interaction. Heredity 1986, 56, 243–253. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A superiority measure of cultivar performance for cultivar× location data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- Fikere, M.; Tadesse, T.; Letta, T. Genotype-environment interactions and stability parameters for grain yield of faba bean (Vicia faba L.) genotypes grown in Southeastern Ethiopia. Int. J. Sustain. Crop Prod. 2008, 3, 80–87. [Google Scholar]
- Seife, A.; Tena, E. Genotype x environment interaction and yield stability analysis of sugarcane (Saccharum officinarum L.) genotypes. Int. J. Adv. Res. Biol. Sci. 2020, 7, 14–26. [Google Scholar]
- Stelling, D.; Ebmeyer, E.; Link, W. Yield stability in faba bean, Vicia faba L. Effects of heterozygosity and heterogeneity. Plant Breed. 1994, 112, 30–39. [Google Scholar] [CrossRef]
- Gharzeddin, K.; Maalouf, F.; Khoury, B.; Khater, L.A.; Christmann, S.; El Dine, N.A.J. Efficiency of different breeding strategies in improving the faba bean productivity for sustainable agriculture. Euphytica 2019, 215, 1–15. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr. Statistical Analysis of Regional Yield Trials: AMMI Analysis of Factorial Designs, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 278. [Google Scholar]
- Jursík, M.; Kočárek, M.; Kolářová, M.; Tichý, L. Effect of different soil and weather conditions on efficacy, selectivity and dissipation of herbicides in sunflower. Plant Soil Environ. 2020, 66, 468–476. [Google Scholar] [CrossRef]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar]
- Caseley, J. The Effect of Weather on Herbicide Performance. EPPO Bull. 1983, 13, 171–176. [Google Scholar] [CrossRef]
- Yang, R.C.; Crossa, J.; Cornelius, P.L.; Burgueño, J. Biplot analysis of genotype× environment interaction: Proceed with caution. Crop Sci. 2009, 49, 1564–1576. [Google Scholar] [CrossRef]
- Rakshit, S.; Ganapathy, K.N.; Gomashe, S.S.; Rathore, A.; Ghorade, R.B.; Kumar, M.N.; Patil, J.V. GGE biplot analysis to evaluate genotype, environment and their interactions in sorghum multi-location data. Euphytica 2012, 185, 465–479. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Gauch Jr, H.G. A simple protocol for AMMI analysis of yield trials. Crop Sci. 2013, 53, 1860–1869. [Google Scholar] [CrossRef]
- Tekalign, A.; Sibiya, J.; Derera, J.; Fikre, A. Analysis of genotype× environment interaction and stability for grain yield and chocolate spot (Botrytis fabae) disease resistance in faba bean (Vicia faba). Aust. J. Crop Sci. 2017, 11, 1228–1235. [Google Scholar] [CrossRef]
- Rubiales, D.; Ávila, C.M.; Sillero, J.C.; Hybl, M.; Narits, L.; Sass, O.; Flores, F. Identification and multi-environment validation of resistance to Ascochyta fabae in faba bean (Vicia faba). Field Crop. Res. 2012, 126, 165–170. [Google Scholar] [CrossRef]
- Navabi, A.; Yang, R.C.; Helm, J.; Spaner, D.M. Can spring wheat-growing megaenvironments in the northern Great Plains be dissected for representative locations or niche-adapted genotypes? Crop Sci. 2006, 46, 1107–1116. [Google Scholar] [CrossRef]
- Luo, J.; Pan, Y.B.; Que, Y.; Zhang, H.; Grisham, M.P.; Xu, L. Biplot evaluation of test environments and identification of mega-environment for sugarcane cultivars in China. Sci. Rep. 2015, 5, 15505. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Sharma, P.C.; Sharma, D.K.; Ravikiran, K.T.; Singh, Y.P.; Mishra, V.K.; Singh, R.K. Identification of mega-environments and rice genotypes for general and specific adaptation to saline and alkaline stresses in India. Sci. Rep. 2017, 7, 7968. [Google Scholar] [CrossRef]
- Kubure, T.E.; Raghavaiah, C.V.; Hamza, I. Production potential of faba bean (Vicia faba L.) genotypes in relation to plant densities and phosphorus nutrition on vertisols of central highlands of West Showa Zone, Ethiopia, east Africa. Adv. Crop Sci. Technol. 2016, 4, 2–9. [Google Scholar]
- Krisnawati, A.; Adie, M.M. Yield stability of soybean promising lines across environments. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 102, p. 012044. [Google Scholar]
- Gurmu, F.; Mohammed, H.; Alemaw, G. Genotype x environment interactions and stability of soybean for grain yield and nutrition quality. Afr. Crop Sci. J. 2009, 17, 87–99. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langeluddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Weber, E.; Bleiholder, H. Erläuterungen zu den bbch-dezimal-codes für die entwicklungsstadien von mais, raps, faba-bohne, sonnenblume und erbse-mit abbildungen. Gesunde Pflanzen 1990, 42, 308–321. [Google Scholar]
- Maalouf, F. Faba Bean Traits. 2018. Available online: http://www.cropontology.org/ontology/CO_665/Fababean (accessed on 15 June 2021).
- Goedhart, P.W.; Thissen, J.T.N.M. Biometris Genstat Procedure Library Manual, 19th ed.; Wageningen: Wageningen, The Netherlands, 2018. [Google Scholar]
- Becker, H.C.; Leon, J. Stability analysis in plant breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Yan, W.; Cornelius, P.L.; Crossa, J.; Hunt, L.A. Two types of GGE biplots for analyzing multi-environment trial data. Crop Sci. 2001, 41, 656–663. [Google Scholar] [CrossRef]
- Papastylianou, P.; Vlachostergios, D.N.; Dordas, C.; Tigka, E.; Papakaloudis, P.; Kargiotidou, A.; Pratsinakis, E.; Koskosidis, A.; Pankou, C.; Kousta, A.; et al. Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability 2021, 13, 2586. [Google Scholar] [CrossRef]

| df | DFLR | DMAT | PLHT | GY | |
|---|---|---|---|---|---|
| Genotype × Environment | 396 | 949.1 *** | 206 | 728.8 *** | 800.4 *** |
| Herbicide treatments (T) | 2 | 2.52 | 4.8 | 36.56 *** | 33.5 *** |
| Genotypes (G) | 36 | 1859.3 *** | 199.2 *** | 278.1 *** | 268.1 *** |
| G × T | 72 | 156.5 *** | 97.1 * | 70.7 | 125.5 *** |
| h2 | - | 0.97 | 0.99 | 0.60 | 0.40 |
| Environment | Environment Details | Means | DFLR | DMAT | PLHT (cm) | GY (Kg/ha) |
|---|---|---|---|---|---|---|
| A | Marchouch 2014/2015 treated by metribuzin 250 g ai/ha | Range | ND | ND | 28–78 | 440–5995 |
| Mean ± SE | ND | ND | 55.8 ± 1.5 | 2344 ± 620 | ||
| B | Marchouch 2014/2015 treated by imazethapyr 75 g ai/ha | Range | ND | ND | 37–82 | 220–6380 |
| Mean ± SE | ND | ND | 55.1 ± 8.9 | 1450 ± 1158 | ||
| C | Marchouch 2014/2015 with no herbicide treatment | Range | 78–97 | 131–147 | 50–112 | 333–7333 |
| Mean ± SE | 84.9 ± 3.81 | 137.9 ± 3.6 | 72.5 ± 7.5 | 3545 ± 1360 | ||
| D | Marchouch-2016/2017 treated by metribuzin 250 g ai/ha | Range | 37–47 | 100–109 | 32–73 | 363–3091 |
| Mean ± SE | 41.1 ± 1.6 | 103.8 ± 1.9 | 52.1 ± 8.2 | 1857 ± 392.2 | ||
| E | Marchouch-2016/2017 treated by imazethapyr 75 g ai/ha | Range | 45–53 | 101–111 | 28–75 | 330–2486 |
| Mean ± SE | 47.9 ± 1.6 | 107 ± 2.26 | 48.2 ± 9.9 | 1385 ± 392 | ||
| F | Marchouch-2016/2017 with no herbicide treatment | Range | 34–44 | 96–106 | 41–82 | 1089–3729 |
| Mean ± SE | 39.3 ± 2.0 | 99.91 ± 1.8 | 65.4 ± 5.7 | 2470 ± 499.5 | ||
| G | Terbol-2015/2016 treated by metribuzin 250 g ai/ha | Range | 93–130 | 165–173 | 18–77 | 0–4190 |
| Mean ± SE | 103.6 ± 4.4 | 168.8 ± 2.3 | 51.8 ± 8.2 | 1720 ± 665 | ||
| H | Terbol-2015/2016 treated by imazethapyr 75 g ai/ha | Range | 93–131 | 165–173 | 33.0–76.7 | 57–3689 |
| Mean ± SE | 102.8 ± 3.0 | 170.1 ± 2.75 | 57.1 ± 7.3 | 1439 ± 739.8 | ||
| I | Terbol-2015/16 with no herbicide treatment | Range | 93–122 | 165–171 | 39.3–93 | 352–4184 |
| Mean ± SE | 98.8 ± 1.6 | 166.1 ± 1.97 | 68.5 ± 7.3 | 2313 ± 474 | ||
| J | Terbol-2018/2019 treated metribuzin 250 g ai/ha | Range | 99–130 | 175–183 | 49–105 | 321.2–4782 |
| Mean ± SE | 107.1 ± 2.1 | 178.7 ± 1.53 | 79.7 ± 7.98 | 2423 ± 745.3 | ||
| K | Terbol-2018/2019 with Imazethapyr 75 g ai/ha | Range | 99–130 | 175–185 | 48–103 | 781–5369 |
| Mean ± SE | 106.8 ± 2.3 | 175 ± 1.33 | 72.3 ± 8.3 | 2757 ± 679 | ||
| L | Terbol-2018/2019 with no herbicide treatment | Range | 93–130 | 175–183 | 52–113 | 912–7788 |
| Mean ± SE | 106 ± 2.1 | 176.8 ± 0.8 | 83.5 ± 7.6 | 3424 ± 1173 |
| Environment | Environment Characteristics | df | PLHT | h2_PLHT | GY | h2_GY |
|---|---|---|---|---|---|---|
| A | Marchouch 2014/2015 treated by metribuzin 250 g ai/ha | 36 | 3755.1 *** | 0.95 | 385.6 *** | 0.50 |
| B | Marchouch 2014/2015 treated by imazethapyr 75 g ai/ha | 36 | 99.1 *** | 0.50 | 107.1 *** | 0.50 |
| C | Marchouch 2014/2015 with no herbicide treatment | 36 | 203.4 *** | 0.50 | 62.7 ** | 0.50 |
| D | Marchouch-2016/2017 treated by metribuzin 250 g ai/ha | 36 | 64.9 ** | 0.03 | 69.8 * | 0.00 |
| E | Marchouch-2016/2017 treated by imazethapyr 75 g ai/ha | 36 | 46.3 | 0.05 | 82.1 * | 0.00 |
| F | Marchouch-2016/2017 with no herbicide treatment | 36 | 82.1 * | 0.32 | 83.5 * | 0.00 |
| G | Terbol-2015/2016 treated by metribuzin 250 g ai/ha | 36 | 149.7 *** | 0.18 | 170.5 *** | 0.01 |
| H | Terbol-2015/2016 treated by imazethapyr 75 g ai/ha | 36 | 95.0 *** | 0.14 | 89.7 *** | 0.06 |
| I | Terbol-2015/2016 with no herbicide treatment | 36 | 135.0 *** | 0.01 | 352.5 *** | 0.12 |
| J | Terbol-2018/2019 treated metribuzin 250 g ai/ha | 36 | 74.9 * | 0.01 | 139.2 *** | 0.00 |
| K | Terbol-2018/2019 with Imazethapyr 75 g ai/ha | 36 | 97.9 *** | 0.01 | 130.7 *** | 0.24 |
| L | Terbol-2018/2019 with no herbicide treatment | 36 | 160.1 *** | 0.02 | 74.4 * | 0.00 |
| Accession | Accession Number | Cultivar Superiority | Static Stability | Wricke’s Eco-valence | Finlay- Wilkinson |
|---|---|---|---|---|---|
| IG11561 | 1 | 331.9(23) | 189.4(19) | 519.8(14) | 0.9074(16) |
| IG12110 | 2 | 170.6(4) | 189.4(18) | 515.7(13) | 0.9941(18) |
| VF283 | 3 | 351.7(26) | 218.2(23) | 750.1(24) | 1.1284(27) |
| IG13906 | 4 | 199.2(6) | 155.3(12) | 764.4(25) | 0.8296(12) |
| IG74363 | 5 | 157.7(3) | 289.9(31) | 834.9(27) | 1.2719(32) |
| IG13530 | 6 | 342.3(25) | 207.5(21) | 201.2(2) | 1.2292(30) |
| IG13547 | 7 | 216.6(10) | 238.5(28) | 620.5(18) | 1.0971(24) |
| VF513 | 8 | 399.6(28) | 143(10) | 664.3(20) | 0.9026(15) |
| VF522 | 9 | 429.5(31) | 206.1(20) | 912.3(30) | 1.0141(19) |
| IG11742 | 10 | 381.7(27) | 231.9(26) | 498.4(11) | 1.142(28) |
| IG12659 | 11 | 646.1(36) | 120.5(6) | 1312.2(33) | 0.5057(1) |
| VF419 | 12 | 266.6(17) | 161.4(14) | 360.3(5) | 0.9258(17) |
| IG104039 | 13 | 401.7(29) | 563.2(36) | 2596.2(37) | 1.7049(37) |
| FB2648 | 14 | 327.4(21) | 270.4(30) | 557.2(16) | 1.2729(33) |
| FB2528 | 15 | 402.1(30) | 157.6(13) | 514.3(12) | 0.8565(14) |
| FB2601 | 16 | 212.1(8) | 104.1(1) | 540.6(15) | 0.6932(6) |
| IG104374 | 17 | 155.1(2) | 207.8(22) | 368.3(6) | 1.1143(25) |
| IG104421 | 18 | 216.4(9) | 174.5(16) | 271.8(3) | 1.0751(22) |
| IG106453 | 19 | 177(5) | 162.2(15) | 641.8(19) | 0.8476(13) |
| Flip 86–98FB | 20 | 199.6(7) | 129.9(8) | 298.8(4) | 1.0777(23) |
| IFB1216 | 21 | 220.4(11) | 227.5(25) | 672.2(21) | 1.1187(26) |
| IG11527 | 22 | 328.2(22) | 118.3(5) | 742.4(23) | 0.7(7) |
| VF845 | 23 | 339.8(24) | 139(9) | 181.3(1) | 1.0289(20) |
| IG99419 | 24 | 326.1(20) | 178.8(17) | 831.6(26) | 0.7987(11) |
| VF324 | 25 | 284.5(19) | 225.1(24) | 905.8(29) | 1.0561(21) |
| VF339 | 26 | 557.5(34) | 125.1(7) | 471.9(9) | 0.6844(5) |
| VF963 | 27 | 227.3(13) | - | 477.7(10) | 0.7321(8) |
| IG13945 | 28 | 84.7(1) | 390.2(35) | 2204(36) | 1.249(31) |
| IG14196 | 29 | 447.2(33) | 357.2(33) | 1382.4(34) | 1.3134(34) |
| FB1720 | 30 | 565.3(35) | 107.3(2) | 680.9(22) | 0.641(3) |
| IG13008 | 31 | 263.8(16) | 379.8(34) | 1229.9(31) | 1.5122(36) |
| IG103043 | 32 | 717.7(37) | 263.8(29) | 2035.4(35) | 0.7726(10) |
| IG70622 | 33 | 272.5(18) | 114(4) | 437.8(8) | 0.7637(9) |
| VF545 | 34 | 441.5(32) | 143.4(11) | 1299.7(32) | 0.5994(2) |
| IG12983 | 35 | 263.7(15) | 112.7(3) | 599.3(17) | 0.6791(4) |
| VF810 | 36 | 222.1(12) | 233.7(27) | 413.1(7) | 1.1556(29) |
| VF260 | 37 | 231.4(14) | 351.6(32) | 839.4(28) | 1.4862(35) |
| Trait | Method | Cultivar Superiority | Finlay and Wilkinson | Static Stability |
|---|---|---|---|---|
| PLHT | Finlay and Wilkinson | −0.4 * | - | - |
| Static stability | −0.1 | 0.9 *** | - | |
| Wricke’s eco-valence | 0.3 | 0.3 | 0.7 *** | |
| GY | Finlay and Wilkinson | −0.3 | - | - |
| Static stability | −0.6 *** | 0.2 | - | |
| Wricke’s eco-valence | −0.3 | 0.0 | 0.6 *** |
| Accession | Accession Number | Cultivar Superiority | Static Stability | Wricke’s Eco-valence | Finlay- Wilkinson |
|---|---|---|---|---|---|
| IG11561 | 1 | 22,485(9) | 8927(13) | 29,797(3) | 0.3013(17) |
| IG12110 | 2 | 28,697(15) | 12,597(19) | 38,689(6) | 0.2918(23) |
| VF283 | 3 | 48,317(33) | 3891(5) | 36,277(5) | 0.2909(28) |
| IG13906 | 4 | 26,485(14) | 13,087(22) | 73,390(22) | 0.2973(30) |
| IG74363 | 5 | 38,191(26) | 13,269(23) | 70,087(19) | 0.2864(5) |
| IG13530 | 6 | 31,653(20) | 13,365(24) | 69,391(18) | 0.2972(27) |
| IG13547 | 7 | 14,591(2) | 16,564(28) | 88,280(27) | 0.3218(21) |
| VF513 | 8 | 22,008(8) | 22,278(31) | 126,327(33) | 0.2951(29) |
| VF522 | 9 | 33,940(23) | 9839(16) | 80,135(24) | 0.2838(22) |
| IG11742 | 10 | 48,025(32) | 12,793(20) | 62,918(14) | 0.3075(11) |
| IG12659 | 11 | 41,996(29) | 8638(12) | 128,754(34) | 0.2864(34) |
| VF419 | 12 | 50,741(34) | 2913(2) | 28,269(2) | 0.2863(16) |
| IG104039 | 13 | 43,884(30) | 9326(14) | 135,959(35) | 0.3008(24) |
| FB2648 | 14 | 24,007(11) | 24,953(34) | 119,856(31) | 0.2838(35) |
| FB2528 | 15 | 40,875(28) | 2728(1) | 57,220(12) | 0.3219(3) |
| FB2601 | 16 | 56,023(36) | 3523(3) | 106,197(30) | 0.2833(10) |
| IG104374 | 17 | 31,852(21) | 15,414(27) | 90,573(28) | 0.3029(2) |
| IG104421 | 18 | 60,697(37) | 5081(6) | 63,444(15) | 0.3014(32) |
| IG106453 | 19 | 20,382(5) | 12,874(21) | 72,541(21) | 0.2953(7) |
| Flip 86-98FB | 20 | 20,704(6) | 13,909(25) | 49,157(9) | 0.2921(20) |
| IFB1216 | 21 | 17,744(3) | 16,801(29) | 91,972(29) | 0.2975(31) |
| IG11527 | 22 | 25,508(13) | 6357(9) | 34,188(4) | 0.2908(9) |
| VF845 | 23 | 4164(1) | 23,917(33) | 255,879(37) | 0.2882(26) |
| IG99419 | 24 | 30,080(18) | 19,235(30) | 82,221(25) | 0.2918(13) |
| VF324 | 25 | 24,172(12) | 5996(8) | 70,922(20) | 0.3094(14) |
| VF339 | 26 | 28,852(17) | 8030(11) | 46,802(7) | 0.2951(15) |
| VF963 | 27 | 20,374(4) | ND | 73,431(23) | 0.284(12) |
| IG13945 | 28 | 28,731(16) | 7384(10) | 58,930(13) | 0.2695(25) |
| IG14196 | 29 | 31,459(19) | 15,412(26) | 66,326(17) | 0.2951(33) |
| FB1720 | 30 | 40,017(27) | 10,146(17) | 123,054(32) | 0.3219(8) |
| IG13008 | 31 | 31,970(22) | 23,600(32) | 144,645(36) | 0.2974(4) |
| IG103043 | 32 | 44,948(31) | ND | 49,900(10) | 0.2833(1) |
| IG70622 | 33 | 37,920(25) | 9628(15) | 55,610(11) | 0.286(37) |
| VF545 | 34 | 23,971(10) | ND | 64,208(16) | 0.2917(19) |
| IG12983 | 35 | 21,023(7) | 10,532(18) | 46,866(8) | 0.2908(18) |
| VF810 | 36 | 36,277(24) | 5569(7) | 15,481(1) | 0.2953(6) |
| VF260 | 37 | 54,013(35) | 3550(4) | 85,871(26) | 0.1424(36) |
| Environment Symbol | Environment (Site-Season-Treatment Details) | Rainfall (mm) | Supplemental Irrigation (mm) | Air Temperature (°C) | ||
|---|---|---|---|---|---|---|
| Average | Average Min | Average Max | ||||
| A | Marchouch-2014/2015 treated by metribuzin 250 g ai/ha | 291.4 | 0 | 13.12 | 5.61 | 23.64 |
| B | Marchouch-2014/2015 treated by imazethapyr 75 g ai/ha | 0 | ||||
| C | Marchouch-2014/2015 with no herbicide treatment | 0 | ||||
| D | Marchouch-2016/2017 treated by metribuzin 250 g ai/ha | 211 | 0 | 14.05 | −2.4 | 42.99 |
| E | Marchouch-2016/2017 treated by imazethapyr 75 g ai/ha | 0 | ||||
| F | Marchouch-2016/2017 with no herbicide treatment | 0 | ||||
| G | Terbol-2015/2016 treated by metribuzin 250 g ai/ha | 343 | 30 | 11.5 | −0.44 | 24.62 |
| H | Terbol-2015/2016 treated by imazethapyr 75 g ai/ha | 30 | ||||
| I | Terbol-2015/2016 with no herbicide treatment | 30 | ||||
| J | Terbol-2018/2019 treated metribuzin 250 g ai/ha | 810.2 | 0 | 11.7 | −0.28 | 32.3 |
| K | Terbol-2018/2019 with Imazethapyr 75 g ai/ha | 0 | ||||
| L | Terbol-2018/2019 with no herbicide treatment | 0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Khater, L.; Maalouf, F.; Jighly, A.; Rubiales, D.; Kumar, S. Adaptability and Stability of Faba Bean (Vicia faba L.) Accessions under Diverse Environments and Herbicide Treatments. Plants 2022, 11, 251. https://doi.org/10.3390/plants11030251
Abou-Khater L, Maalouf F, Jighly A, Rubiales D, Kumar S. Adaptability and Stability of Faba Bean (Vicia faba L.) Accessions under Diverse Environments and Herbicide Treatments. Plants. 2022; 11(3):251. https://doi.org/10.3390/plants11030251
Chicago/Turabian StyleAbou-Khater, Lynn, Fouad Maalouf, Abdulqader Jighly, Diego Rubiales, and Shiv Kumar. 2022. "Adaptability and Stability of Faba Bean (Vicia faba L.) Accessions under Diverse Environments and Herbicide Treatments" Plants 11, no. 3: 251. https://doi.org/10.3390/plants11030251
APA StyleAbou-Khater, L., Maalouf, F., Jighly, A., Rubiales, D., & Kumar, S. (2022). Adaptability and Stability of Faba Bean (Vicia faba L.) Accessions under Diverse Environments and Herbicide Treatments. Plants, 11(3), 251. https://doi.org/10.3390/plants11030251









