Grape Berry Responses to Sequential Flooding and Heatwave Events: A Physiological, Transcriptional, and Metabolic Overview
Abstract
1. Introduction
2. Results and Discussion
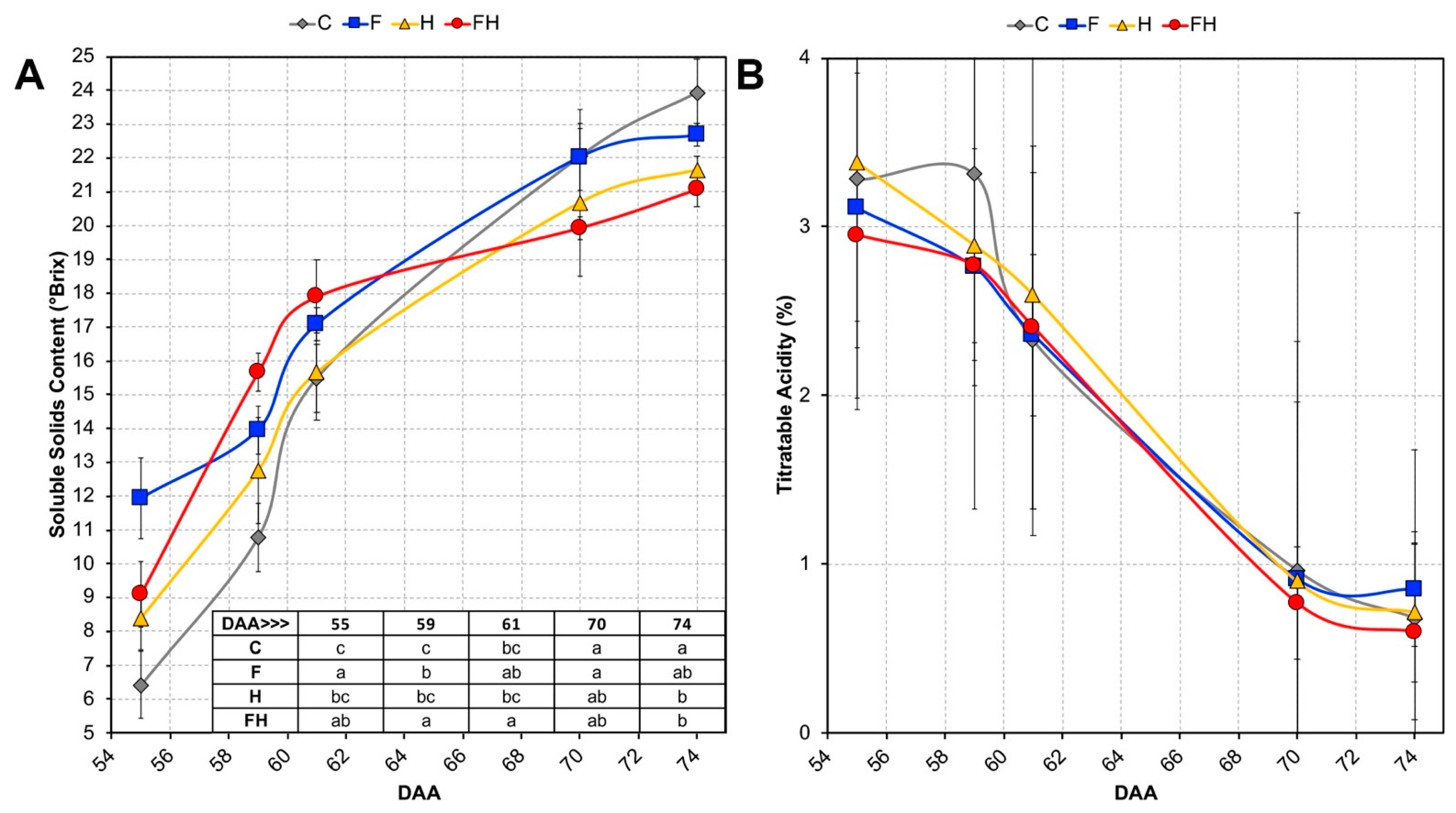
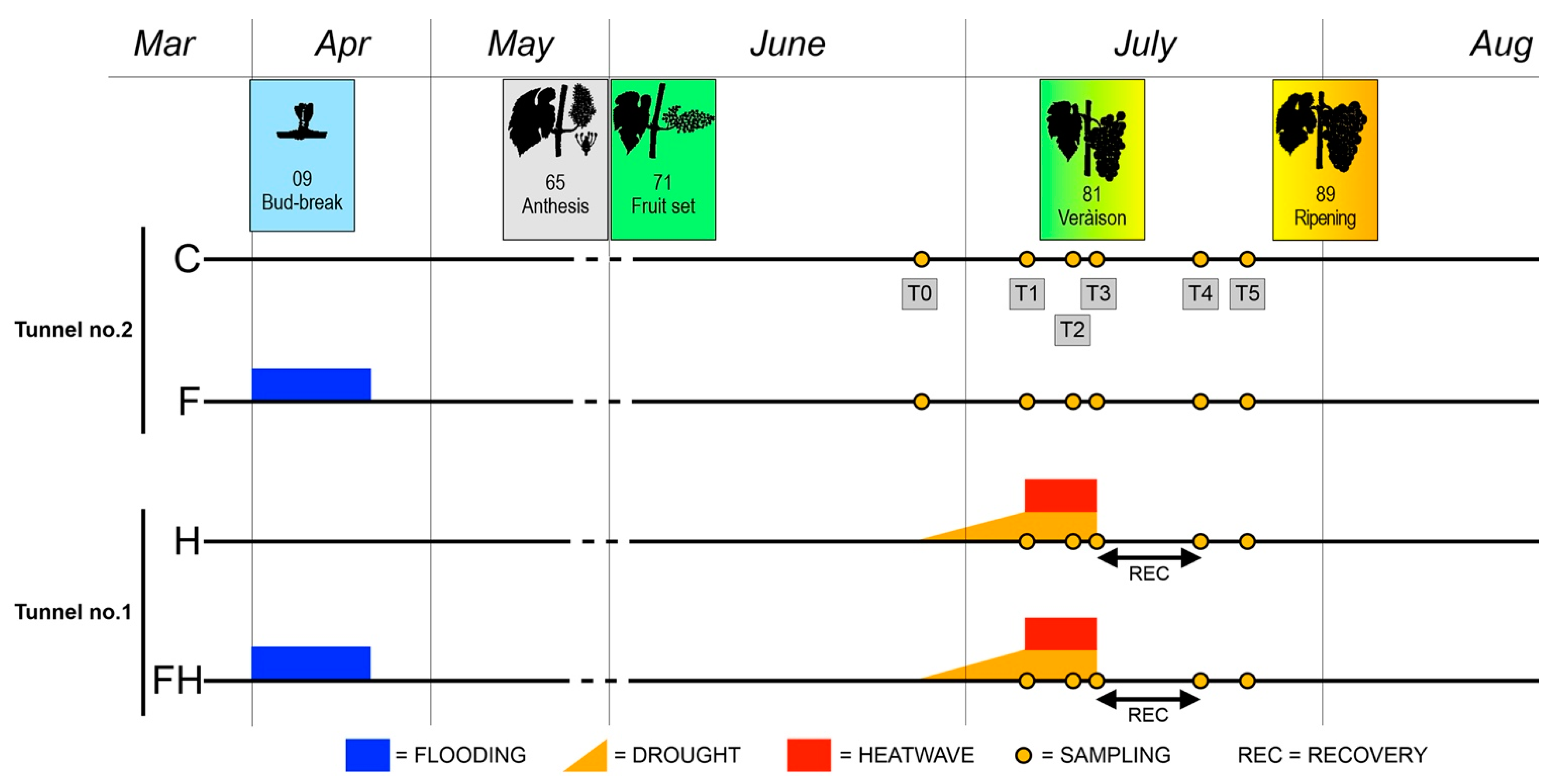
2.1. Experimental Conditions and Ripening Kinetics
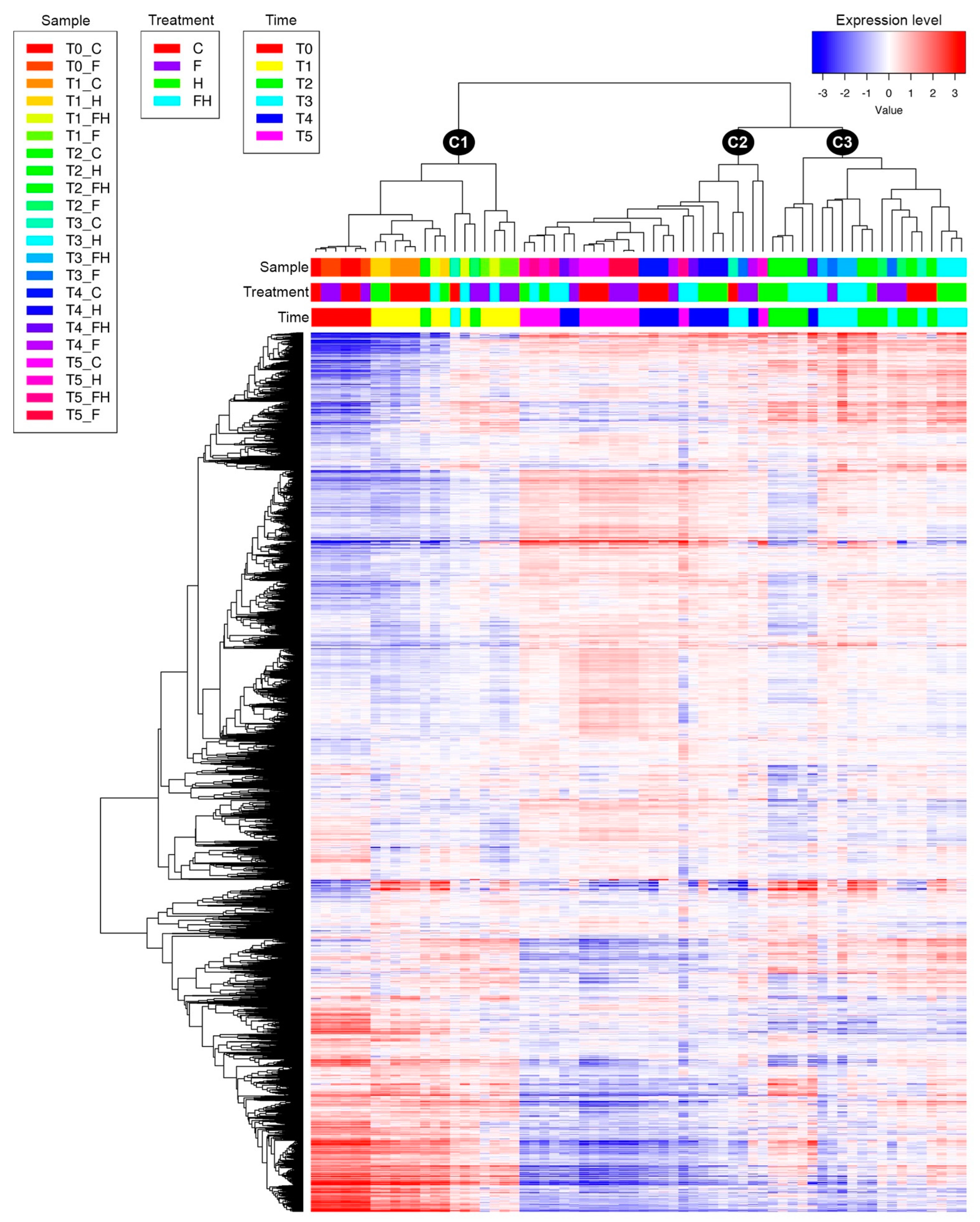
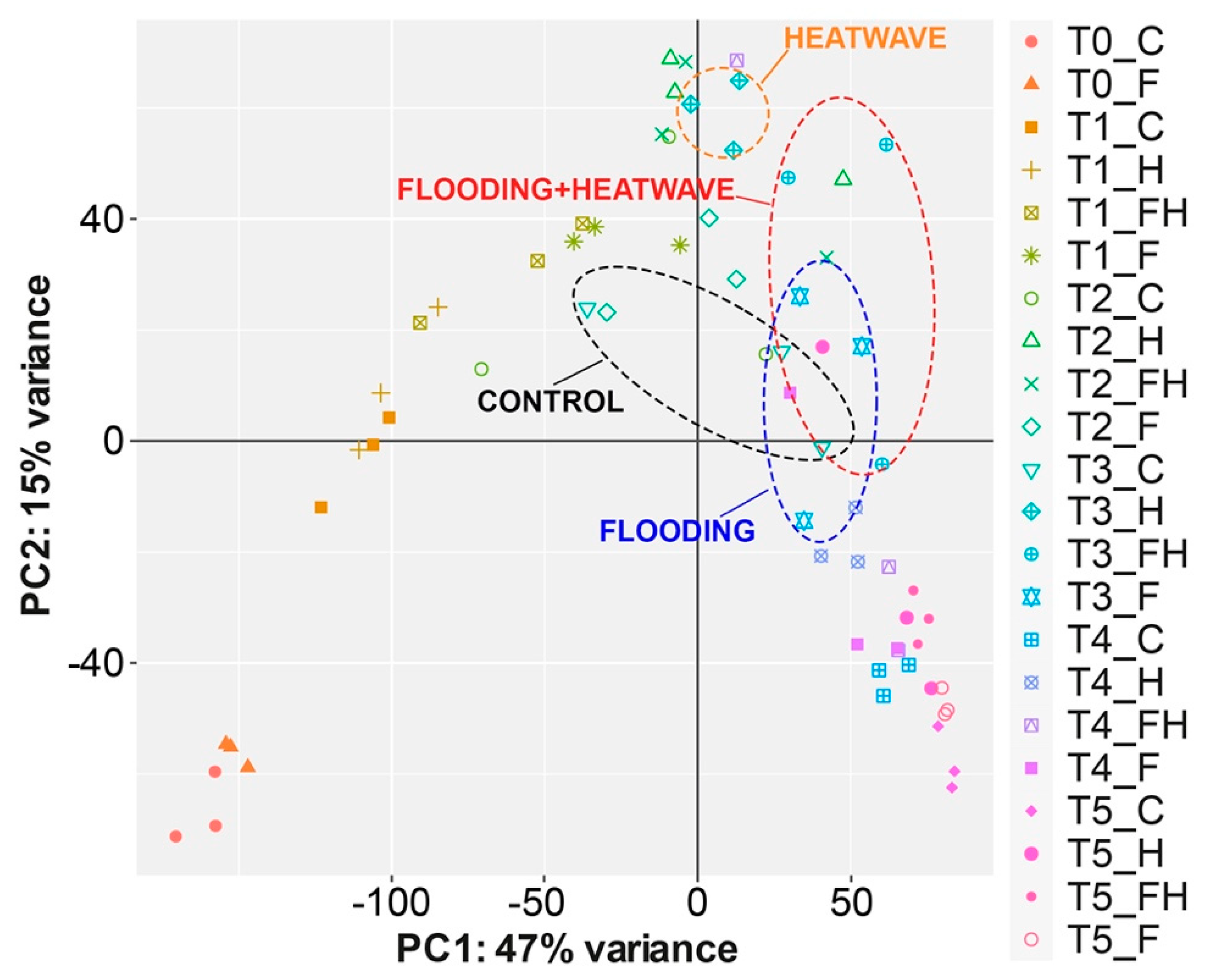
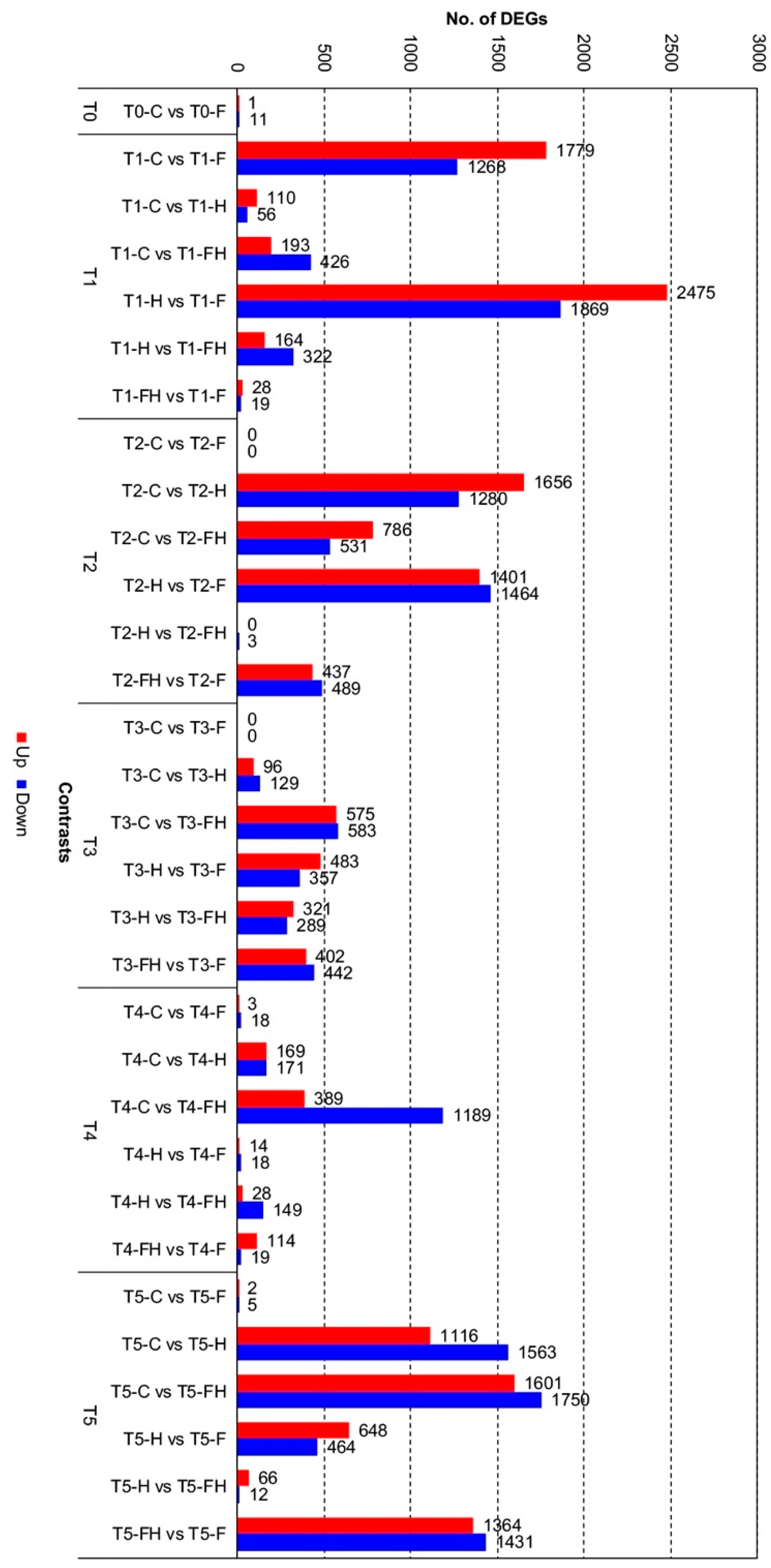
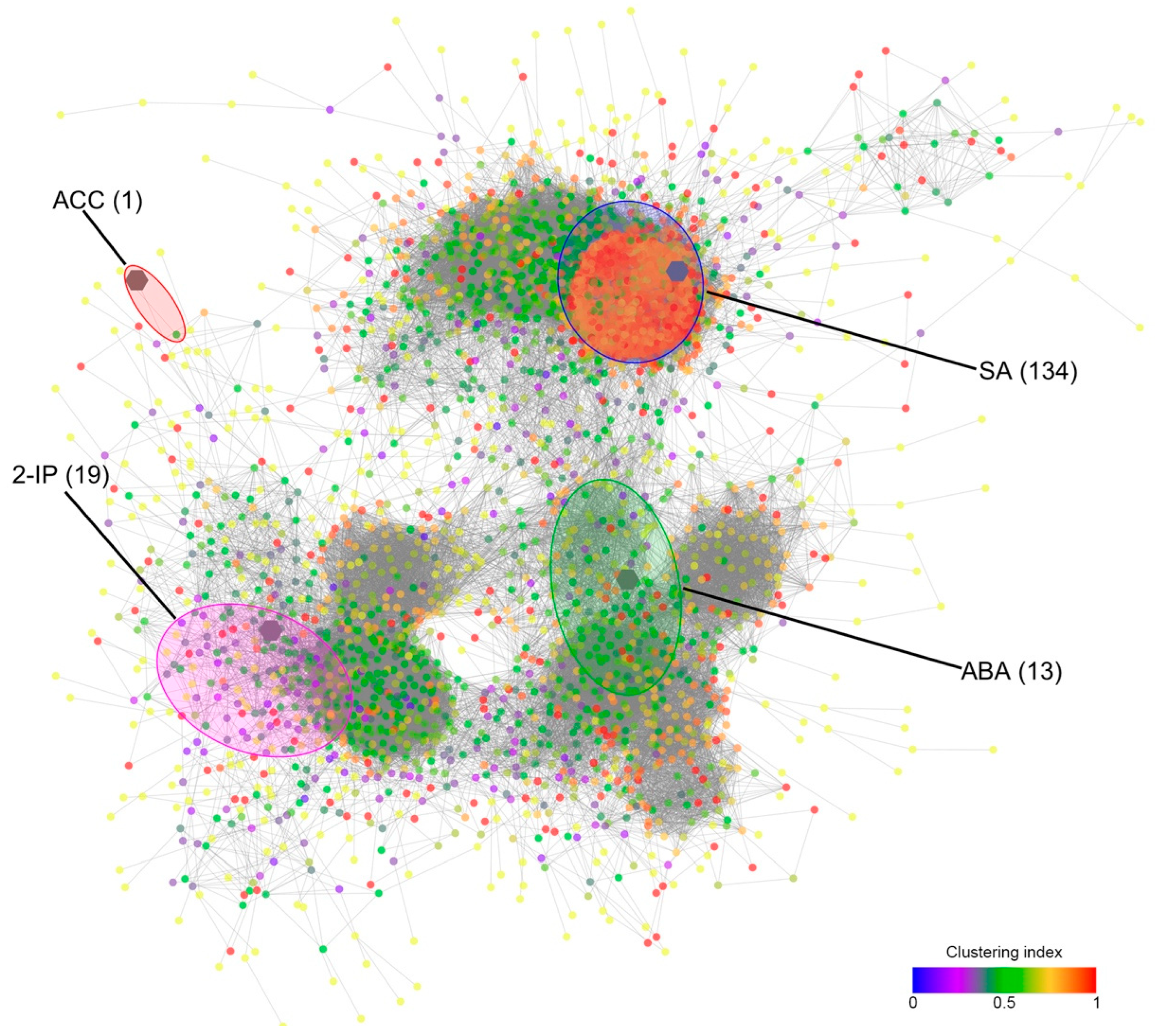
2.2. RNASeq Analyses
2.3. Hormones
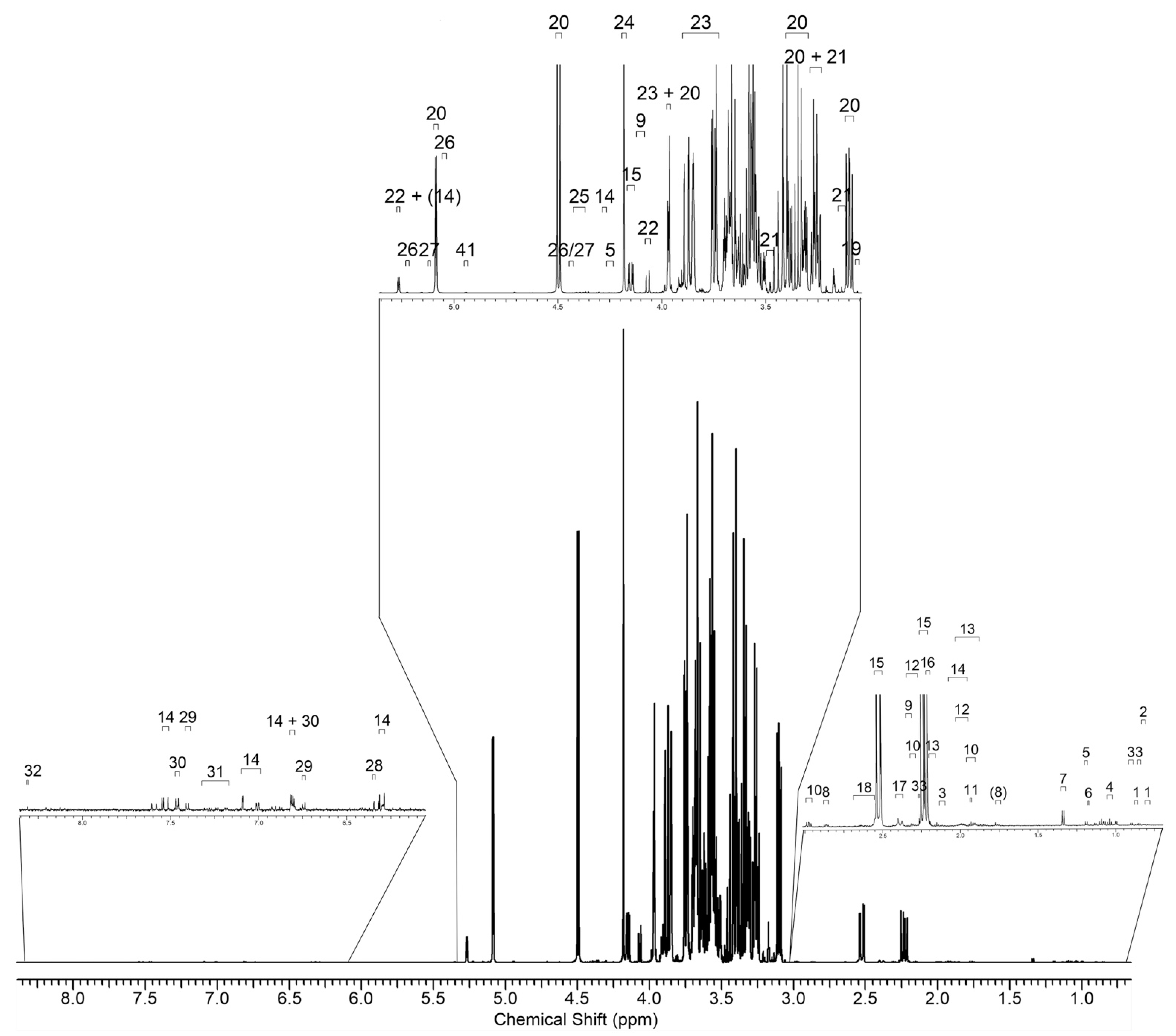
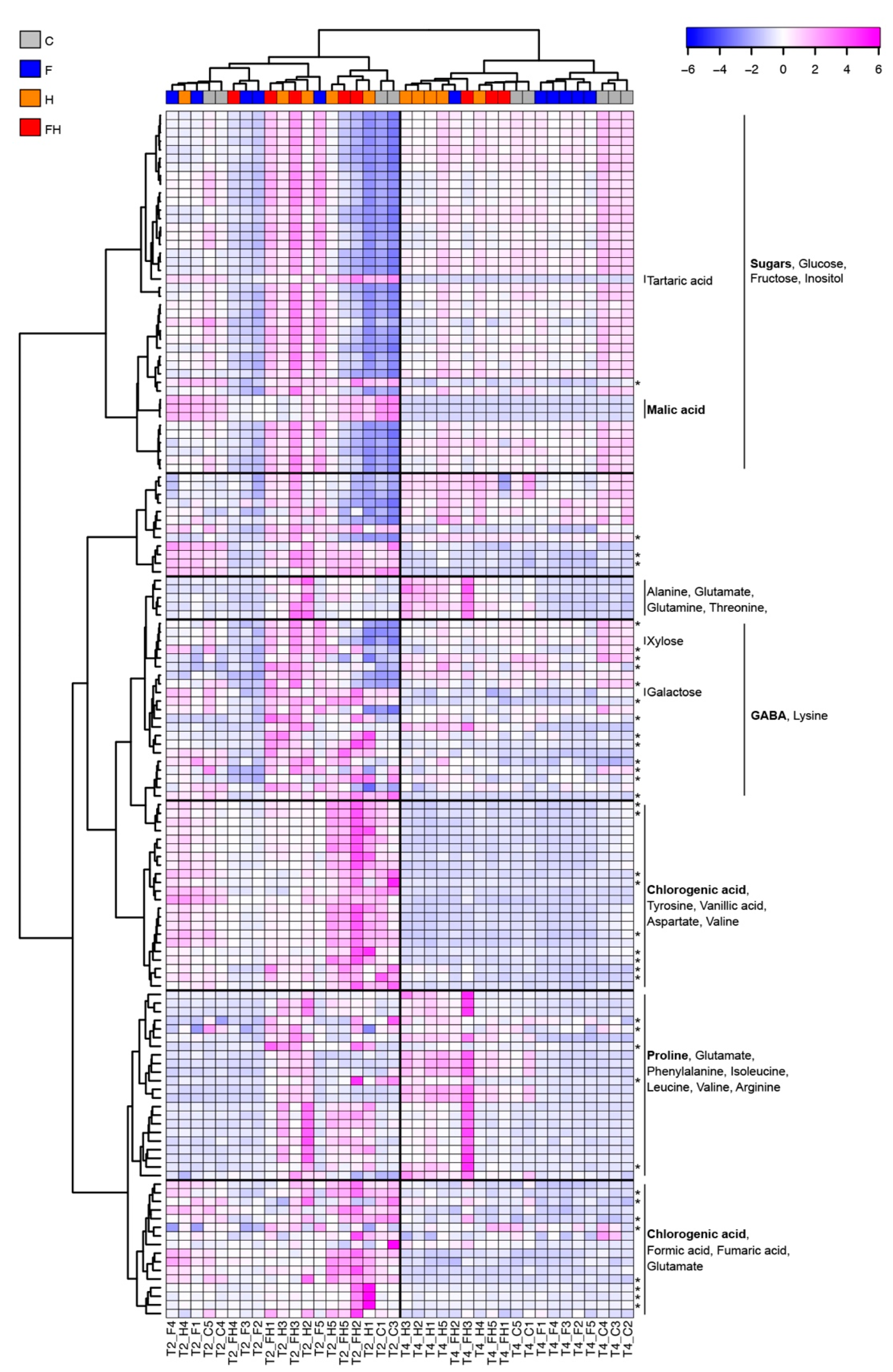
2.4. NMR Metabolic Profiling
2.5. Modelling the Berry Response to Stress
3. Materials and Methods
3.1. Plant Material, Experimental Setup, and Sampling
3.2. Meteorological and Biometrical Measurements
3.3. RNA Isolation, RNAseq Analyses, and qPCR
3.4. Bioinformatic Analyses
3.5. Hormone Analysis
3.6. NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassi, F.; Labra, M.; Imazio, S.; Rubio, R.O.; Failla, O.; Scienza, A.; Sala, F. Phylogeographical Structure and Conservation Genetics of Wild Grapevine. Conserv. Genet. 2006, 7, 837–845. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kizildeniz, T.; Vucetic, V.; Dai, Z.; Luedeling, E.; van Leeuwen, C.; Gomès, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of Grapevine Phenology to Water Availability, Temperature and CO2 Concentration. Front. Environ. Sci. 2016, 4, 48. [Google Scholar] [CrossRef]
- Miras-Avalos, J.M.; Araujo, E.S. Optimization of Vineyard Water Management: Challenges, Strategies, and Perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Perria, R.; Ciofini, A.; Petrucci, W.A.; D’Arcangelo, M.E.M.; Valentini, P.; Storchi, P.; Carella, G.; Pacetti, A.; Mugnai, L. A Study on the Efficiency of Sustainable Wine Grape Vineyard Management Strategies. Agronomy 2022, 12, 392. [Google Scholar] [CrossRef]
- Bois, B.; Zito, S.; Calonnec, A. Climate vs Grapevine Pests and Diseases Worldwide: The First Results of a Global Survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Dinu, D.G.; Ricciardi, V.; Demarco, C.; Zingarofalo, G.; De Lorenzis, G.; Buccolieri, R.; Cola, G.; Rustioni, L. Climate Change Impacts on Plant Phenology: Grapevine (Vitis vinifera) Bud Break in Wintertime in Southern Italy. Foods 2021, 10, 2769. [Google Scholar] [CrossRef]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate Change Effects on Grapevine Physiology and Biochemistry: Benefits and Challenges of High Altitude as an Adaptation Strategy. Front. Plant Sci. 2022, 13, 1269. [Google Scholar] [CrossRef]
- Jones, G.V.; Edwards, E.J.; Bonada, M.; Sadras, V.O.; Krstic, M.P.; Herderich, M.J. Climate Change and Its Consequences for Viticulture. Manag. Wine Qual. Vol. One Vitic. Wine Qual. 2022, 1, 727–778. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. A Review on the Observed Climate Change in Europe and Its Impacts on Viticulture. Atmosphere 2022, 13, 837. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. Uniw. Śląski 2022, 7, 343–354. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Meyer, L.A. AR5 Synthesis Report: Climate Change 2014—IPCC. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 7 November 2022).
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic Stress Effects on Grapevine (Vitis Vinifera L.): Focus on Abscisic Acid-Mediated Consequences on Secondary Metabolism and Berry Quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Feller, U. Stomatal Opening at Elevated Temperature: An Underestimated Regulatory Mechanism. Gen. Appl. Plant Physiol. Spec. Issue 2006, 32, 19–31. [Google Scholar]
- Gamon, J.A.; Pearcy, R.W. Leaf Movement, Stress Avoidance and Photosynthesis in Vitis Californica. Oecologia 1989, 79, 475–481. [Google Scholar] [CrossRef]
- Carbonneau, A. Recherche Sur Les Systèmes de Conduite de La Vigne. Essai de Maîtrise Du Micro-Climat et de La Plante Entière Pour Produire Économiquement Du Raisin de Qualité; INRAE: Paris, France, 1980. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate Change, Wine, and Conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Jones, P.R.; Gawel, R.; Francis, I.L.; Waters, E.J. The Influence of Interactions between Major White Wine Components on the Aroma, Flavour and Texture of Model White Wine. Food Qual. Prefer. 2008, 19, 596–607. [Google Scholar] [CrossRef]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic Effects of Elevated Temperature on Organic Acid Degradation in Ripening Vitis Vinifera Fruit. J. Exp. Bot. 2014, 65, 5975. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Duchêne, E.; Schneider, C. Grapevine and Climatic Changes: A Glance at the Situation in Alsace. Agronomie 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Vierra, G. Pretenders at the Table: Are Table Wines No Longer Food Friendly? Wine Bus. Mon. 2004, 11, 17–21. [Google Scholar]
- Pillet, J.; Egert, A.; Pieri, P.; Lecourieux, F.; Kappel, C.; Charon, J.; Gomès, E.; Keller, F.; Delrot, S.; Lecourieux, D. VvGOLS1 and VvHsfA2 Are Involved in the Heat Stress Responses in Grapevine Berries. Plant Cell Physiol. 2012, 53, 1776–1792. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Santa María, E.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance Responses in Ripening Berries of Vitis Vinifera L. Cv Muscat Hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and Night Heat Stress Trigger Different Transcriptomic Responses in Green and Ripening Grapevine (Vitis Vinifera) Fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Lecourieux, D. Dissecting the Biochemical and Transcriptomic Effects of a Locally Applied Heat Treatment on Developing Cabernet Sauvignon Grape Berries. Front. Plant Sci. 2017, 8, 53. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, J.; Zhang, Z.; Hou, L.; Liu, X. VvBAP1, a Grape C2 Domain Protein, Plays a Positive Regulatory Role Under Heat Stress. Front. Plant Sci. 2020, 11, 1497. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, A.K. Global Transcriptome Analysis of Heat Stress Response of Grape Variety. Vitis J. Grapevine Res. 2021, 60, 143–151. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The Physiology of Drought Stress in Grapevine: Towards an Integrative Definition of Drought Tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional Regulation of Anthocyanin Biosynthesis in Ripening Fruits of Grapevine under Seasonal Water Deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Cardone, M.F.; Perniola, R.; Catacchio, C.R.; Alagna, F.; Rotunno, S.; Crupi, P.; Antonacci, D.; Velasco, R.; Ventura, M.; Bergamini, C. Grapevine Adaptation to Drought: New Candidate Genes for the Genotype-Dependent Response. BIO Web Conf. 2019, 15, 01016. [Google Scholar] [CrossRef][Green Version]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock-Scion Interactions through Gene Expression Regulation. Agronomy 2020, 10, 680. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.E.; Leontaridou, K.; Kanellis, A.K.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The Effect of Water Deficit on Two Greek Vitis Vinifera l. Cultivars: Physiology, Grape Composition and Gene Expression during Berry Development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Perdomo, P.; Murphy, J.A.; Berkowitz, G.A. Physiological Changes Associated with Performance of Kentucky Bluegrass Cultivars during Summer Stress. HortScience 1996, 31, 1182–1186. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and Heat Stress Injury to Two Cool-Season Turfgrasses in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The Combined Effect of Drought Stress and Heat Shock on Gene Expression in Tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Murata, N. Revised Scheme for the Mechanism of Photoinhibition and Its Application to Enhance the Abiotic Stress Tolerance of the Photosynthetic Machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Lehr, P.P.; Hernández-Montes, E.; Ludwig-Müller, J.; Keller, M.; Zörb, C. Abscisic Acid and Proline Are Not Equivalent Markers for Heat, Drought and Combined Stress in Grapevines. Aust. J. Grape Wine Res. 2022, 28, 119–130. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis Vinifera L. ssp. Vinifera)—Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Coetzee, C.; du Toit, W.J. A Comprehensive Review on Sauvignon Blanc Aroma with a Focus on Certain Positive Volatile Thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Marais, J. Sauvignon Blanc Cultivar Aroma—A Review. South Afr. J. Enol. Vitic. 2017, 15, 41–45. [Google Scholar] [CrossRef][Green Version]
- Cataldo, E.; Salvi, L.; Paoli, F.; Sbraci, S.; Battista Mattii, G. Influence of Water Stress on Grape Quality and Aroma Precursors in Sauvignon Blanc Grown in Bolgheri Area (Tuscany). BIO Web Conf. 2019, 13, 03001. [Google Scholar] [CrossRef]
- Ben Salem-Fnayou, A.; Bouamama, B.; Ghorbel, A.; Mliki, A. Investigations on the Leaf Anatomy and Ultrastructure of Grapevine (Vitis Vinifera) under Heat Stress. Microsc. Res. Tech. 2011, 74, 756–762. [Google Scholar] [CrossRef]
- Chapman, D.J.; De-Felice, J.; Barber, J. Growth Temperature Effects on Thylakoid Membrane Lipid and Protein Content of Pea Chloroplasts. Plant Physiol. 1983, 72, 225–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Keller, M. Grape Berry Transpiration Is Determined by Vapor Pressure Deficit, Cuticular Conductance, and Berry Size. Am. J. Enol. Vitic. 2015, 66, 454–462. [Google Scholar] [CrossRef]
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, Anatomy and Development of the Pericarp after Anthesis in Grape, Vitis Vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Pilati, S.; Perazzolli, M.; Malossini, A.; Cestaro, A.; Demattè, L.; Fontana, P.; Dal Ri, A.; Viola, R.; Velasco, R.; Moser, C. Genome-Wide Transcriptional Analysis of Grapevine Berry Ripening Reveals a Set of Genes Similarly Modulated during Three Seasons and the Occurrence of an Oxidative Burst at Vèraison. BMC Genom. 2007, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on Steroids. Brassinosteroids Are Involved in Grape Berry Ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, N.; Tindjau, R.; Wong, D.C.J.; Matzat, T.; Haslam, T.; Song, C.; Gambetta, G.A.; Kunst, L.; Castellarin, S.D. Drought Stress Modulates Cuticular Wax Composition of the Grape Berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.S.; Kim, H.; Kim, H.J.; Suh, M.C. Arabidopsis Cuticular Wax Biosynthesis Is Negatively Regulated by the DEWAX Gene Encoding an AP2/ERF-Type Transcription Factor. Plant Cell 2014, 26, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G.; Hale, C.R. The Hormone Content of Ripening Grape Berries and the Effects of Growth Substance Treatments. Plant Physiol. 1973, 51, 629–634. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Chernys, J.T.; Zeevaart, J.A.D. Characterization of the 9-Cis-Epoxycarotenoid Dioxygenase Gene Family and the Regulation of Abscisic Acid Biosynthesis in Avocado. Plant Physiol. 2000, 124, 343–353. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquezar, B.; Zacarías, L. Cloning and Characterization of Two 9-Cis-Epoxycarotenoid Dioxygenase Genes, Differentially Regulated during Fruit Maturation and under Stress Conditions, from Orange (Citrus Sinensis L. Osbeck). J. Exp. Bot. 2006, 57, 633–643. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of Drought Tolerance by Gene Manipulation of 9-Cis-Epoxycarotenoid Dioxygenase, a Key Enzyme in Abscisic Acid Biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid Cleavage by NCED5 Fine-Tunes ABA Accumulation and Affects Seed Dormancy and Drought Tolerance with Other NCED Family Members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA Is Required for the Accumulation of APX1 and MBF1c during a Combination of Water Deficit and Heat Stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef]
- Umezawa, T.; Okamoto, M.; Kushiro, T.; Nambara, E.; Oono, Y.; Seki, M.; Kobayashi, M.; Koshiba, T.; Kamiya, Y.; Shinozaki, K. CYP707A3, a Major ABA 8′-Hydroxylase Involved in Dehydration and Rehydration Response in Arabidopsis Thaliana. Plant J. 2006, 46, 171–182. [Google Scholar] [CrossRef]
- Coombe, B.G. Research on Development and Ripening of the Grape Berry. Am. J. Enol. Vitic. 1992, 43, 101–110. [Google Scholar]
- Chervin, C.; El-Kereamy, A.; Roustan, J.P.; Latché, A.; Lamon, J.; Bouzayen, M. Ethylene Seems Required for the Berry Development and Ripening in Grape, a Non-Climacteric Fruit. Plant Sci. 2004, 167, 1301–1305. [Google Scholar] [CrossRef]
- Kim, C.Y.; Liu, Y.; Thorne, E.T.; Yang, H.; Fukushige, H.; Gassmann, W.; Hildebrand, D.; Sharp, R.E.; Zhang, S. Activation of a Stress-Responsive Mitogen-Activated Protein Kinase Cascade Induces the Biosynthesis of Ethylene in Plants. Plant Cell 2003, 15, 2707–2718. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S. Phosphorylation of 1-Aminocyclopropane-1-Carboxylic Acid Synthase by MPK6, a Stress-Responsive Mitogen-Activated Protein Kinase, Induces Ethylene Biosynthesis in Arabidopsis. Plant Cell 2004, 16, 3386–3399. [Google Scholar] [CrossRef]
- Yoon, G.M.; Kieber, J.J. 1-Aminocyclopropane-1-Carboxylic Acid as a Signaling Molecule in Plants. AoB Plants 2013, 5, plt017. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Gudenschwager, O.; Chervin, C.; Campos-Vargas, R.; González-Agüero, M.; Defilippi, B.G. Study on Differential Expression of 1-Aminocyclopropane-1-Carboxylic Acid Oxidase Genes in Table Grape Cv. Thompson Seedless. Postharvest Biol. Technol. 2013, 76, 163–169. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Erban, A.; Sousa, L.; Pais, M.S.; Kopka, J.; Fortes, A.M. Search for Transcriptional and Metabolic Markers of Grape Pre-Ripening and Ripening and Insights into Specific Aroma Development in Three Portuguese Cultivars. PLoS ONE 2013, 8, e60422. [Google Scholar] [CrossRef]
- Fonseca, S.; Chico, J.M.; Solano, R. The Jasmonate Pathway: The Ligand, the Receptor and the Core Signalling Module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Changes in Transcription of Cytokinin Metabolism and Signalling Genes in Grape (Vitis Vinifera L.) Berries Are Associated with the Ripening-Related Increase in Isopentenyladenine. BMC Plant Biol. 2015, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Action of Jasmonates in Plant Stress Responses and Development—Applied Aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef]
- Kazan, K. Diverse Roles of Jasmonates and Ethylene in Abiotic Stress Tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A Dynamic Interplay between Phytohormones Is Required for Fruit Development, Maturation, and Ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Montefiori, M.; Galer, A.L.; Neil Emery, R.J.; Allan, A.C.; Jameson, P.E. Endogenous Cytokinin in Developing Kiwifruit Is Implicated in Maintaining Fruit Flesh Chlorophyll Levels. Ann. Bot. 2013, 112, 57–68. [Google Scholar] [CrossRef]
- Böttcher, C.; Boss, P.K.; Davies, C. Increase in Cytokinin Levels during Ripening in Developing Vitis Vinifera Cv. Shiraz Berries. Am. J. Enol. Vitic. 2013, 64, 527–531. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The Involvement of Cytokinins in Plant Responses to Environmental Stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Argueso, C.T.; Ferreira, F.J.; Kieber, J.J. Environmental Perception Avenues: The Interaction of Cytokinin and Environmental Response Pathways. Plant. Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and Proteomic Changes in the Xylem Sap of Maize under Drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Thomas, S.G.; Phillips, A.L.; Hedden, P. Molecular Cloning and Functional Expression of Gibberellin 2-Oxidases, Multifunctional Enzymes Involved in Gibberellin Deactivation. Proc. Natl. Acad. Sci. USA 1999, 96, 4698–4703. [Google Scholar] [CrossRef]
- Fortes, A.M.; Teixeira, R.T.; Agudelo-Romero, P. Complex Interplay of Hormonal Signals during Grape Berry Ripening. Molecules 2015, 20, 9326–9343. [Google Scholar] [CrossRef] [PubMed]
- Oraei, M.; Panahirad, S.; Zaare-Nahandi, F.; Gohari, G. Pre-Véraison Treatment of Salicylic Acid to Enhance Anthocyanin Content of Grape (Vitis Vinifera L.) Berries. J. Sci. Food Agric. 2019, 99, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, T.K.; Marak, T. Salicylic Acid and Oxalic Acid in Enhancing the Quality and Extending the Shelf Life of Grape Cv. Thompson Seedless. Food Sci. Technol. Int. 2022, 28, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Dwivedi, U.N. Delayed Ripening of Banana Fruit by Salicylic Acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Raskin, I. Role of Salicylic Acid in Plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Morris, K.; Mackerness, S.A.H.; Page, T.; Fred John, C.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic Acid Has a Role in Regulating Gene Expression during Leaf Senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef]
- Martínez, C.; Pons, E.; Prats, G.; León, J. Salicylic Acid Regulates Flowering Time and Links Defence Responses and Reproductive Development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against Heat Stress-Induced Oxidative Damage in Arabidopsis Involves Calcium, Abscisic Acid, Ethylene, and Salicylic Acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Nunan, K.J.; Davies, C.; Robinson, S.P.; Fincher, G.B. Expression Patterns of Cell Wall-Modifying Enzymes during Grape Berry Development. Planta 2001, 214, 257–264. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Deng, H.; Ma, W.; Zhou, Q.; Liu, Z. Genome-Wide Identification of the MADS-Box Transcription Factor Family in Autotetraploid Cultivated Alfalfa (Medicago Sativa L.) and Expression Analysis under Abiotic Stress. BMC Genom. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, T.; Cheng, Z.; Zhao, D.; Tao, J. R2R3-MYB Transcription Factor PlMYB108 Confers Drought Tolerance in Herbaceous Peony (Paeonia Lactiflora Pall.). Int. J. Mol. Sci. 2021, 22, 11884. [Google Scholar] [CrossRef]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The Botrytis Susceptible1 Gene Encodes an R2R3MYB Transcription Factor Protein That Is Required for Biotic and Abiotic Stress Responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef]
- Marino, G.; Funk, C. Matrix Metalloproteinases in Plants: A Brief Overview. Physiol. Plant. 2012, 145, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gupta, S.; Gupta, P.; Dhawan, S.S.; Lal, R.K. In Silico Identification of MiRNA and Targets from Chrysopogon Zizanioides (L.) Roberty with Functional Validation from Leaf and Root Tissues. Appl. Biochem. Biotechnol. 2020, 192, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Biswas, R.; Anjum, N.; Das, A.K.; Maiti, M.K. Rice Matrix Metalloproteinase OsMMP1 Plays Pleiotropic Roles in Plant Development and Symplastic-Apoplastic Transport by Modulating Cellulose and Callose Depositions. Sci. Rep. 2018, 8, 2783. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, X.L.; Wang, X.F.; Xia, G.H.; Pan, Q.H.; Fan, R.C.; Wu, F.Q.; Yu, X.C.; Zhang, D.P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef]
- Höfer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis Cytochrome P450 CYP86A1 Encodes a Fatty Acid ω-Hydroxylase Involved in Suberin Monomer Biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef]
- Laffaru Singpho, N.; Sharma, J.G. Importance of Cytochrome P450 Gene Family from Metabolite Biosynthesis to Stress Tolerance: A Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 775, p. 012012. [Google Scholar]
- Serra, O.; Soler, M.; Hohn, C.; Sauveplane, V.; Pinot, F.; Franke, R.; Schreiber, L.; Prat, S.; Molinas, M.; Figueras, M. CYP86A33-Targeted Gene Silencing in Potato Tuber Alters Suberin Composition, Distorts Suberin Lamellae, and Impairs the Periderm’s Water Barrier Function. Plant Physiol. 2009, 149, 1050–1060. [Google Scholar] [CrossRef]
- Xiao, F.; Goodwin, S.M.; Xiao, Y.; Sun, Z.; Baker, D.; Tang, X.; Jenks, M.A.; Zhou, J.M. Arabidopsis CYP86A2 Represses Pseudomonas Syringae Type III Genes and Is Required for Cuticle Development. EMBO J. 2004, 23, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Liu, J.; Bennett, M.H.; Mansfield, J.W.; Desikan, R. Arabidopsis Histidine Kinase 5 Regulates Salt Sensitivity and Resistance against Bacterial and Fungal Infection. New Phytol. 2012, 194, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Horák, J.; Chaban, C.; Mira-Rodado, V.; Witthöft, J.; Elgass, K.; Grefen, C.; Cheung, M.K.; Meixner, A.J.; Hooley, R.; et al. The Histidine Kinase AHK5 Integrates Endogenous and Environmental Signals in Arabidopsis Guard Cells. PLoS ONE 2008, 3, e2491. [Google Scholar] [CrossRef] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (Pyridoxine) and Its Derivatives Are Efficient Singlet Oxygen Quenchers and Potential Fungal Antioxidants. Photochem. Photobiol. 2007, 71, 129–134. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, N.; Wang, F.; Song, S.; Guo, J.; Pang, Z.; Zhang, J. Effect of Components in Homogeneous Extraction Suspensions of Pea and Sweet Buckwheat on γ-Aminobutyric Acid Synthesis. Czech J. Food Sci. 2019, 37, 292–300. [Google Scholar] [CrossRef]
- Huang, S.H.; Zhang, J.Y.; Wang, L.H.; Huang, L.Q. Effect of Abiotic Stress on the Abundance of Different Vitamin B6 Vitamers in Tobacco Plants. Plant Physiol. Biochem. 2013, 66, 63–67. [Google Scholar] [CrossRef]
- Kaur, C.; Singla-Pareek, S.L.; Sopory, S.K. Glyoxalase and Methylglyoxal as Biomarkers for Plant Stress Tolerance. CRC. Crit. Rev. Plant Sci. 2014, 33, 429–456. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ghosh, A.; Kaur, C.; Pareek, A.; Singla-Pareek, S.L. Glyoxalase Pathway and Drought Stress Tolerance in Plants. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 379–399. ISBN 9783319288994. [Google Scholar]
- Gupta, B.K.; Sahoo, K.K.; Ghosh, A.; Tripathi, A.K.; Anwar, K.; Das, P.; Singh, A.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Manipulation of Glyoxalase Pathway Confers Tolerance to Multiple Stresses in Rice. Plant Cell Environ. 2018, 41, 1186–1200. [Google Scholar] [CrossRef]
- Manoj, V.M.; Anunanthini, P.; Swathik, P.C.; Dharshini, S.; Ashwin Narayan, J.; Manickavasagam, M.; Sathishkumar, R.; Suresha, G.S.; Hemaprabha, G.; Ram, B.; et al. Comparative Analysis of Glyoxalase Pathway Genes in Erianthus Arundinaceus and Commercial Sugarcane Hybrid under Salinity and Drought Conditions. BMC Genom. 2019, 19, 986. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous Silicon Attenuates Cadmium-Induced Oxidative Stress in Brassica Napus L. by Modulating Asa-Gsh Pathway and Glyoxalase System. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef]
- Guillaumie, S.; Fouquet, R.; Kappel, C.; Camps, C.; Terrier, N.; Moncomble, D.; Dunlevy, J.D.; Davies, C.; Boss, P.K.; Delrot, S. Transcriptional Analysis of Late Ripening Stages of Grapevine Berry. BMC Plant Biol. 2011, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry Ripening: Recently Heard through the Grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- H.P., R. Metabolism of Tartaric and Malic Acids in Vitis: A Review—Part B. Vitis 1982, 21, 346–358. [Google Scholar]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine Abiotic Stress Assessment and Search for Sustainable Adaptation Strategies in Mediterranean-like Climates. A Review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Meggio, F. The Interplay between Grape Ripening and Weather Anomalies in Northern Italy—A Modelling Exercise. Oeno One 2022, 56, 353–373. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The Influence of Alternative Pathways of Respiration That Utilize Branched-Chain Amino Acids Following Water Shortage in Arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the Vascular Brassinosteroid Receptor BRL3 Confers Drought Resistance without Penalizing Plant Growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef]
- Xu, J.; Cai, M.; Li, J.; Chen, B.; Chen, Z.; Jia, W.; Xu, Z. Physiological, Biochemical and Metabolomic Mechanisms of Mitigation of Drought Stress-Induced Tobacco Growth Inhibition by Spermidine. Ind. Crops Prod. 2022, 181, 114844. [Google Scholar] [CrossRef]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.R.; Marriott, A.S.; Bergström, E.; et al. Molecular Mechanisms of Desiccation Tolerance in the Resurrection Glacial Relic Haberlea Rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. In Proceedings of the Molecular Plant; Cell Press: Cambridge, MA, USA, 2012; Volume 5, pp. 418–429. [Google Scholar]
- Sicher, R.C.; Barnaby, J.Y. Impact of Carbon Dioxide Enrichment on the Responses of Maize Leaf Transcripts and Metabolites to Water Stress. Physiol. Plant. 2012, 144, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Hasnain, G.; Logue, T.; Lynch, M.; Wu, S.; Guan, J.C.; Alseekh, S.; Fernie, A.R.; Hanson, A.D.; McCarty, D.R. A Core Metabolome Response of Maize Leaves Subjected to Long-Duration Abiotic Stresses. Metabolites 2021, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Li, M.Q.; Gao, X.X.; Liu, L.N.; Wu, X.F.; Zhou, J.H. Metabolic Response of Maize Plants to Multi-Factorial Abiotic Stresses. Plant Biol. 2016, 18, 120–129. [Google Scholar] [CrossRef]
- Semel, Y.; Schauer, N.; Roessner, U.; Zamir, D.; Fernie, A.R. Metabolite Analysis for the Comparison of Irrigated and Non-Irrigated Field Grown Tomato of Varying Genotype. Metabolomics 2007, 3, 289–295. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from Less: Plant Growth under Limited Water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Erxleben, A.; Gessler, A.; Vervliet-Scheebaum, M.; Reski, R. Metabolite Profiling of the Moss Physcomitrella Patens Reveals Evolutionary Conservation of Osmoprotective Substances. Plant Cell Rep. 2012, 31, 427–436. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite Adjustments in Drought Tolerant and Sensitive Soybean Genotypes in Response to Water Stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. In Proceedings of the Molecular Plant; Cell Press: Cambridge, MA, USA, 2012; Volume 5, pp. 401–417. [Google Scholar]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting Rice Polyamine Metabolism under Controlled Long-Term Drought Stress. PLoS ONE 2013, 8, e60325. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Suravajhala, P.; Rathnagiri, P.; Sreenivasulu, N. Intriguing Role of Proline in Redox Potential Conferring High Temperature Stress Tolerance. Front. Plant Sci. 2022, 13, 867531. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological Characteristics and Metabolomics of Transgenic Wheat Containing the Maize C4 Phosphoenolpyruvate Carboxylase (PEPC) Gene under High Temperature Stress. Protoplasma 2017, 254, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.D.; El-gawad, H.G.A.; El-yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. Gaba: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive Oxygen Species and Temperature Stresses: A Delicate Balance between Signaling and Destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in Plants: Just a Metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Zhang, L.; Becker, D.F. Connecting Proline Metabolism and Signaling Pathways in Plant Senescence. Front. Plant Sci. 2015, 6, 552. [Google Scholar] [CrossRef]
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan Delays Ripening and ROS Production in Guava (Psidium Guajava L.) Fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Daniels, D.G.H.; King, H.G.C.; Martin, H.F. Antioxidants in Oats: Esters of Phenolic Acids. J. Sci. Food Agric. 1963, 14, 385–390. [Google Scholar] [CrossRef]
- Daniels, D.G.H.; Martin, H.F. Antioxidants in Oats: Mono-esters of Caffeic and Ferulic Acids. J. Sci. Food Agric. 1967, 18, 589–595. [Google Scholar] [CrossRef]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.A.; Roberts, K.; Martin, C. The AmMYB308 and AmMYB330 Transcription Factors from Antirrhinum Regulate Phenylpropanoid and Lignin Biosynthesis in Transgenic Tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X.; Li, H.; Dong, J.; Li, J.; Gong, Z.; et al. Cold-Induced CBF–PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the PhyB Thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water Stress Induces Changes in Polyphenol Profile and Antioxidant Capacity in Poplar Plants (Populus Spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. In Proceedings of the Journal of Experimental Botany; Oxford Academic: Oxford, UK, 2002; Volume 53, pp. 979–987. [Google Scholar]
- Iwasaki, M.; Paszkowski, J. Epigenetic Memory in Plants. EMBO J. 2014, 33, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Ruperti, B.; Botton, A.; Populin, F.; Eccher, G.; Brilli, M.; Quaggiotti, S.; Trevisan, S.; Cainelli, N.; Guarracino, P.; Schievano, E.; et al. Flooding Responses on Grapevine: A Physiological, Transcriptional, and Metabolic Perspective. Front. Plant Sci. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, F.M.; Bonghi, C.; Tonutti, P. Postharvest Water Loss Induces Marked Changes in Transcript Profiling in Skins of Wine Grape Berries. Postharvest Biol. Technol. 2009, 52, 247–253. [Google Scholar] [CrossRef]
- Klepikova, A.V.; Kasianov, A.S.; Chesnokov, M.S.; Lazarevich, N.L.; Penin, A.A.; Logacheva, M. Effect of Method of Deduplication on Estimation of Differential Gene Expression Using RNA-Seq. PeerJ 2017, 2017, e3091. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of RNA-Seq Experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The Grapevine Genome Sequence Suggests Ancestral Hexaploidization in Major Angiosperm Phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Vitulo, N.; Forcato, C.; Carpinelli, E.C.; Telatin, A.; Campagna, D.; D’Angelo, M.; Zimbello, R.; Corso, M.; Vannozzi, A.; Bonghi, C.; et al. A Deep Survey of Alternative Splicing in Grape Reveals Changes in the Splicing Machinery Related to Tissue, Stress Condition and Genotype. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef]
- Nonis, A.; Ruperti, B.; Pierasco, A.; Canaguier, A.; Adam-Blondon, A.F.; Di Gaspero, G.; Vizzotto, G. Neutral Invertases in Grapevine and Comparative Analysis with Arabidopsis, Poplar and Rice. Planta 2008, 229, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of Ethylene Production and Ethylene Receptor Expression in Regulating Apple Fruitlet Abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Simon, P. Q-Gene: Processing Quantitative Real-Time RT-PCR Data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, E45. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Rapid and Sensitive Hormonal Profiling of Complex Plant Samples by Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Plant Methods 2011, 7, 37. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Picone, G.; Trimigno, A.; Tessarin, P.; Donnini, S.; Rombolà, A.D.; Capozzi, F. 1H NMR Foodomics Reveals That the Biodynamic and the Organic Cultivation Managements Produce Different Grape Berries (Vitis Vinifera L. Cv. Sangiovese). Food Chem. 2016, 213, 187–195. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.P.; Van Leeuwen, C.; Hilbert, G.; Lavialle, O.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR and Chemometrics to Characterize Mature Grape Berries in Four Wine-Growing Areas in Bordeaux, France. J. Agric. Food Chem. 2005, 53, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.; Federica, M.; Eva, Z.; Martina, R.; Young, H.C.; Robert, V. NMR Metabolic Fingerprinting Based Identification of Grapevine Metabolites Associated with Downy Mildew Resistance. J. Agric. Food Chem. 2009, 57, 9599–9606. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic Characterization of Palatinate German White Wines According to Sensory Attributes, Varieties, and Vintages Using NMR Spectroscopy and Multivariate Data Analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mulas, G.; Galaffu, M.G.; Pretti, L.; Nieddu, G.; Mercenaro, L.; Tonelli, R.; Anedda, R. NMR Analysis of Seven Selections of Vermentino Grape Berry: Metabolites Composition and Development. J. Agric. Food Chem. 2011, 59, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2008, 36, D402–D408. [Google Scholar] [CrossRef]
- Kuhn, S.; Schlörer, N.E. Facilitating Quality Control for Spectra Assignments of Small Organic Molecules: Nmrshiftdb2—A Free in-House NMR Database with Integrated LIMS for Academic Service Laboratories. Magn. Reson. Chem. 2015, 53, 582–589. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Charng, Y.; Mitra, S.; Yu, S.-J. Maintenance of Abiotic Stress Memory in Plants: Lessons Learned from Heat Acclimation. Plant Cell 2022. [Google Scholar] [CrossRef]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of Amino Acid, Nucleotide, and Phosphate Metabolism in Saccharomyces Cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of Amino Acids in Wine after the End of Fermentation by Saccharomyces Cerevisiae Strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botton, A.; Girardi, F.; Ruperti, B.; Brilli, M.; Tijero, V.; Eccher, G.; Populin, F.; Schievano, E.; Riello, T.; Munné-Bosch, S.; et al. Grape Berry Responses to Sequential Flooding and Heatwave Events: A Physiological, Transcriptional, and Metabolic Overview. Plants 2022, 11, 3574. https://doi.org/10.3390/plants11243574
Botton A, Girardi F, Ruperti B, Brilli M, Tijero V, Eccher G, Populin F, Schievano E, Riello T, Munné-Bosch S, et al. Grape Berry Responses to Sequential Flooding and Heatwave Events: A Physiological, Transcriptional, and Metabolic Overview. Plants. 2022; 11(24):3574. https://doi.org/10.3390/plants11243574
Chicago/Turabian StyleBotton, Alessandro, Francesco Girardi, Benedetto Ruperti, Matteo Brilli, Veronica Tijero, Giulia Eccher, Francesca Populin, Elisabetta Schievano, Tobia Riello, Sergi Munné-Bosch, and et al. 2022. "Grape Berry Responses to Sequential Flooding and Heatwave Events: A Physiological, Transcriptional, and Metabolic Overview" Plants 11, no. 24: 3574. https://doi.org/10.3390/plants11243574
APA StyleBotton, A., Girardi, F., Ruperti, B., Brilli, M., Tijero, V., Eccher, G., Populin, F., Schievano, E., Riello, T., Munné-Bosch, S., Canton, M., Rasori, A., Cardillo, V., & Meggio, F. (2022). Grape Berry Responses to Sequential Flooding and Heatwave Events: A Physiological, Transcriptional, and Metabolic Overview. Plants, 11(24), 3574. https://doi.org/10.3390/plants11243574








