Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years
Abstract
1. Introduction
2. Results
2.1. Meteorological Data for the Two Years of Study
2.2. Physicochemical Parameters of Savvatianno Grape Berries
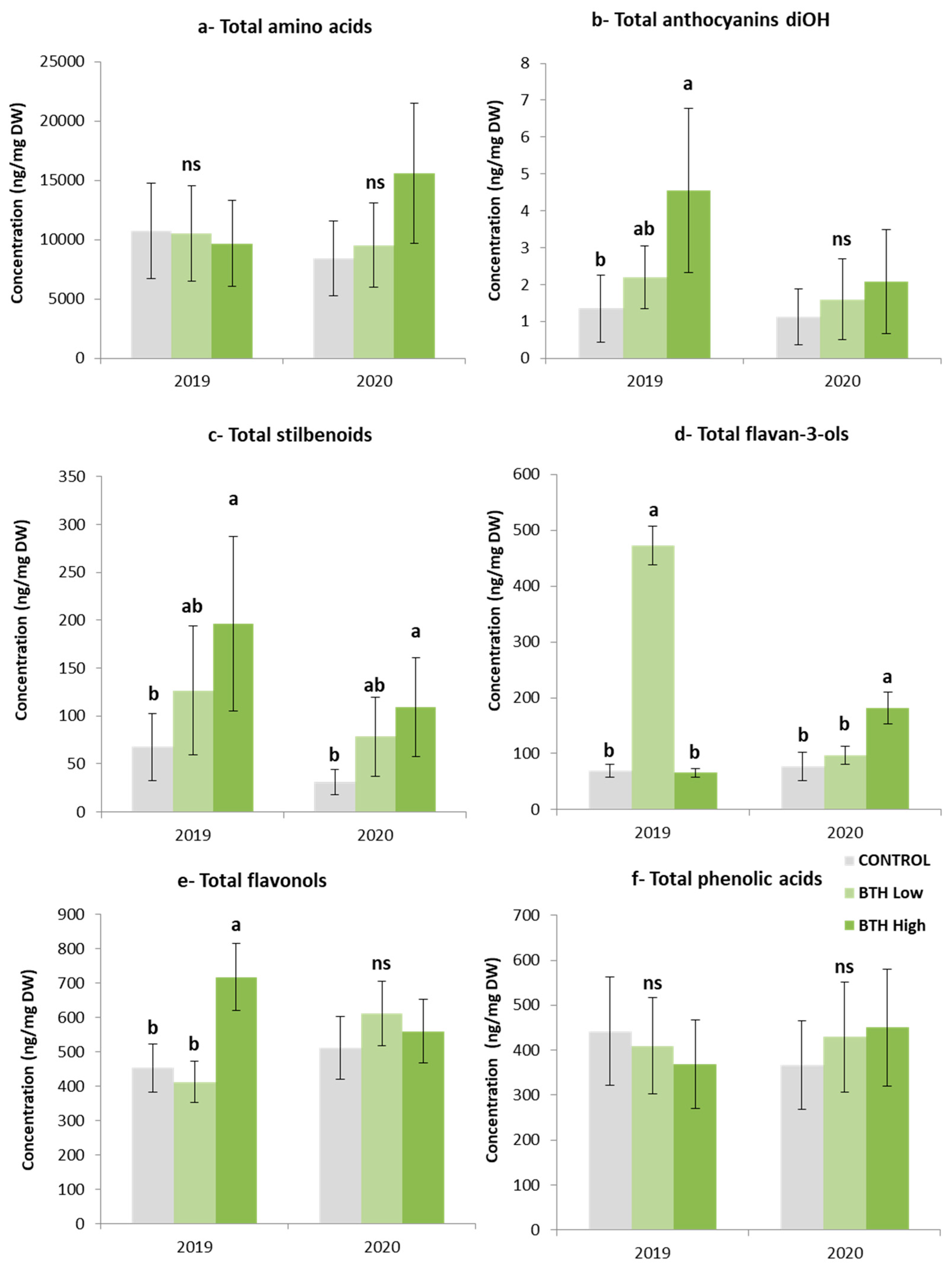
2.3. Metabolomic Analysis of Savvatiano Grape Berries in Response to BTH
2.4. Physicochemical Parameters of Savvatiano Experimental Wines
2.5. Color and Phenolic Parameters of the Three Experimental Savvatiano Wines in Response to Two Different Treatments and a Control
2.6. Physicochemical Parameters of Mouhtaro Grape Berries
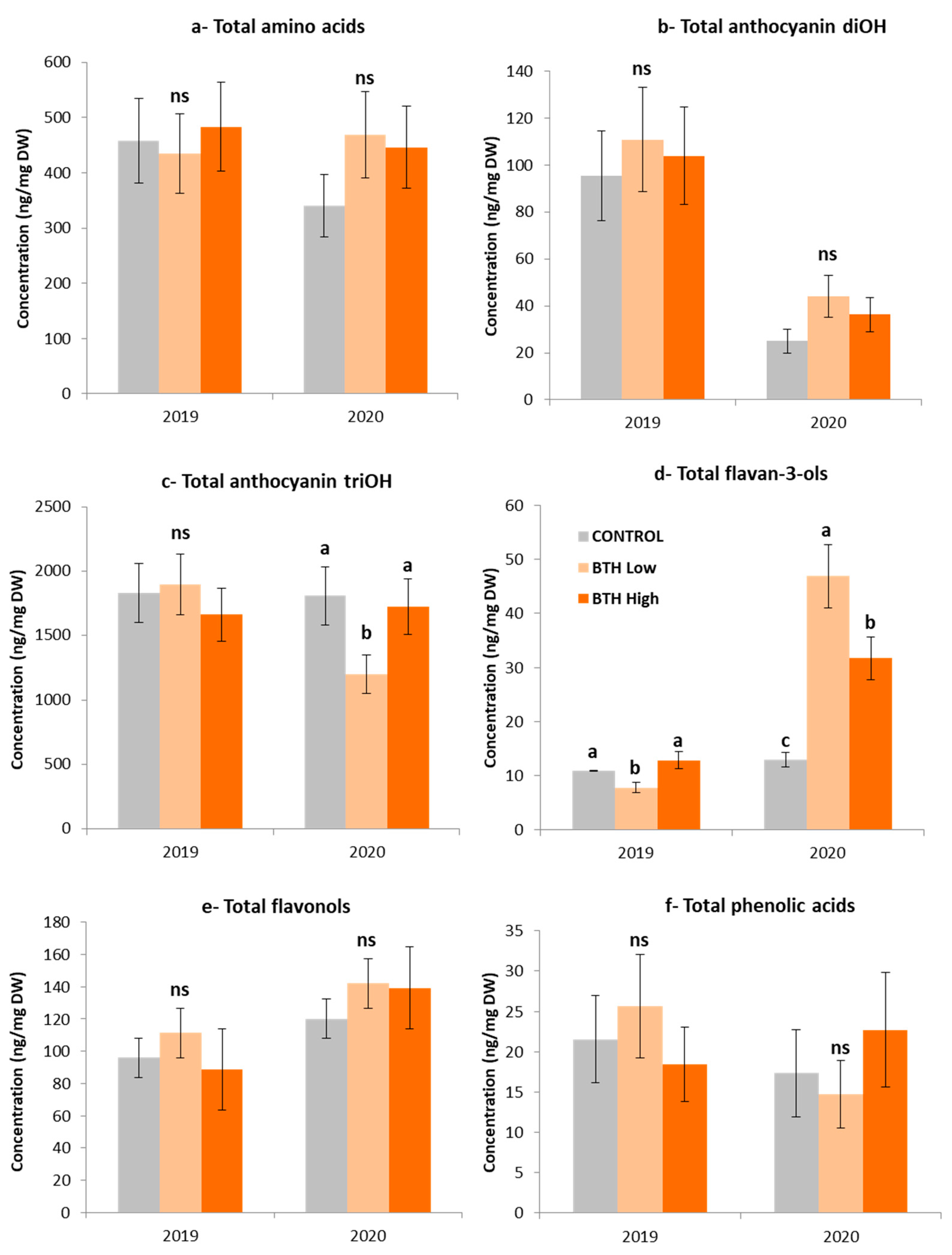
2.7. Metabolomic Analysis of Mouhtaro Grape Berries in Response to BTH Applications
2.8. Physicochemical Parameters of Mouhtaro Experimental Wines
2.9. Color and Phenolic Characteristics of the Mouhtaro Experimental Wines
2.10. Anthocyanins Content (mg/L) in Mouhtaro Experimental Wines
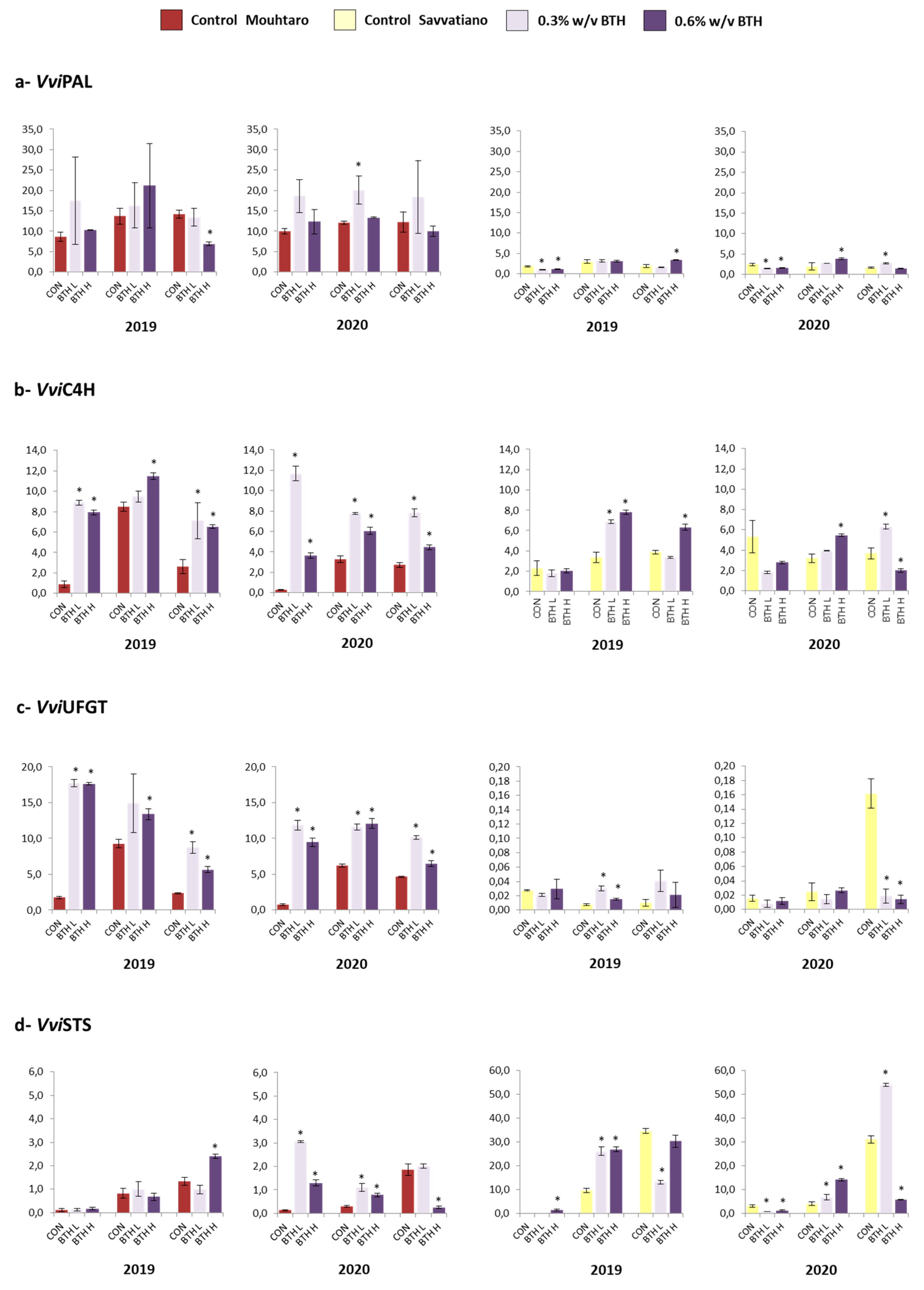
2.11. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Biostimulant Application
4.2. Physicochemical Results of the Must and Wine
4.3. Grape Metabolic Profile by UPLC-MS
4.4. RNA Extraction and Analysis of Gene Expression
4.5. Vinification Process
4.6. Wine Phenolics
4.6.1. Wine Color, Color Density
4.6.2. Total Anthocyanins
4.6.3. Total Phenolic Index, Follin–Ciocalteau, and Browning Test
4.6.4. Anthocyanins by HPLC
4.6.5. Tannin Determination with Methyl Cellulose Precipitation (MCP) Assay
4.6.6. Climate Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Gerós, H.; Moutinho-Pereira, J. Kaolin Foliar Application Has a Stimulatory Effect on Phenylpropanoid and Flavonoid Pathways in Grape Berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef]
- Grassi, F.; Labra, M.; Imazio, S.; Spada, A.; Sgorbati, S.; Scienza, A.; Sala, F. Evidence of a secondary grapevine domestication centre detected by SSR analysis. Theor. Appl. Genet. 2003, 107, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority. Research of Viticulture. Available online: https://www.statistics.gr/en/statistics/-/publication/SPG63 (accessed on 30 August 2022).
- Orsini, F.; Maggio, A.; Rouphael, Y.; De Pascale, S. “Physiological quality” of organically grown vegetables. Sci. Hortic. 2016, 208, 131–139. [Google Scholar] [CrossRef]
- Litskas, V.; Mandoulaki, A.; Vogiatzakis, I.N.; Tzortzakis, N.; Stavrinides, M. Sustainable Viticulture: First Determination of the Environmental Footprint of Grapes. Sustainability 2020, 12, 8812. [Google Scholar] [CrossRef]
- Mejía-Teniente, L.; Torres-Pacheco, I.; González-Chavira, M.M.; Ocampo-Velazquez, R.V.; Herrera-Ruiz, G.; Chapa-Oliver, A.M.; Guevara-González, R.G. Use of elicitors as an approach for sustainable agriculture. Afr. J. Biotechnol. 2010, 9, 9155–9162. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Mihai, R.; Cristina, S.; Helepciuc, F.; Brezeanu, A.; Stoian, G. Biotic and abiotic elicitors induce biosynthesis and accumulation of resveratrol with antitumoral activity in the long—Term Vitis vinifera L. callus cultures. Rom. Biotechnol. Lett. 2011, 16, 7. [Google Scholar]
- Murcia, G.; Fontana, A.; Pontin, M.; Baraldi, R.; Bertazza, G.; Piccoli, P.N. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases: Use of biostimulants in the vineyard for improved grape and wine quality. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Martins, V.; Billet, K.; Garcia, A.; Lanoue, A.; Gerós, H. Exogenous calcium deflects grape berry metabolism towards the production of more stilbenoids and less anthocyanins. Food Chem. 2019, 313, 126123. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, V.; Rath, S.K.; Prakash, R.; Saxena, S. Response surface statistical optimization of fermentation parameters for resveratrol production by the endophytic fungus Arcopilus aureus and its tyrosinase inhibitory activity. Biotechnol. Lett. 2020, 43, 627–644. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2020, 35, 2879–2889. [Google Scholar] [CrossRef]
- Ferrier, M.; Billet, K.; Drouet, S.; Tungmunnithum, D.; Malinowska, M.A.; Marchal, C.; Dedet, S.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Identifying Major Drivers of Antioxidant Activities in Complex Polyphenol Mixtures from Grape Canes. Molecules 2022, 27, 4029. [Google Scholar] [CrossRef]
- Anna Malinowska, M.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; LaNoue, A. Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2020, 12, 14–29. [Google Scholar] [CrossRef]
- Vos, I.A.; Emoritz, L.; Pieterse, C.; Van Wees, S.C.M. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front. Plant Sci. 2015, 6, 639. [Google Scholar] [CrossRef]
- Salifu, R.; Chen, C.; Sam, F.E.; Jiang, Y. Application of Elicitors in Grapevine Defense: Impact on Volatile Compounds. Horticulturae 2022, 8, 451. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, Q.; Li, C.; Duan, B.; Li, X.; Wei, M.; Li, J. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage. LWT 2019, 110, 48–53. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Friedrich, L.; Lawton, K.; Ruess, W.; Masner, P.; Specker, N.; Rella, M.G.; Meier, B.; Dincher, S.; Staub, T.; Uknes, S.; et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996, 10, 61–70. [Google Scholar] [CrossRef]
- Lawton, K.A.; Friedrich, L.; Hunt, M.; Weymann, K.; Delaney, T.; Kessmann, H.; Staub, T.; Ryals, J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abcisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of Benzothiadiazole and Methyl Jasmonate on the Volatile Compound Composition of Vitis vinifera L. Monastrell Grapes and Wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell. Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole. J. Int. Sci. Vigne Vin 2017, 51, 17–27. [Google Scholar]
- Paladines-Quezada, D.; Fernández-Fernández, J.; Moreno-Olivares, J.; Bleda-Sánchez, J.; Gómez-Martínez, J.; Martínez-Jiménez, J.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. cv Monastrell: Influence on Anthocyanin Concentration of Grapes and Wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Alatzas, A.; Kontoudakis, N.; Kouki, A.; Unlubayir, M.; Gémin, M.-P.; Tako, A.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Abscisic Acid and Chitosan Modulate Polyphenol Metabolism and Berry Qualities in the Domestic White-Colored Cultivar Savvatiano. Plants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Corio-Costet, M.; Dufour, M.; Cluzet, S.; Lambert, C.; Merdinoglu, D. “Biomolchem”: A tool to assess the defense status of grapevines after stimulations or not of cultivar or resistant genotypes, from genes to the field. Acta Hortic. 2013, 53–60. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, H.; Si, Z.; Xia, Y.; Chen, W.; Li, X. Benzothiadiazole-Mediated Induced Resistance to Colletotrichum musae and Delayed Ripening of Harvested Banana Fruit. J. Agric. Food Chem. 2016, 64, 1494–1502. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; López-Roca, J.M.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Effect of combined use of benzo-thiadiazole and methyl jasmonate on volatile compounds of Monastrell wine. Am. J. Enol. Vitic. 2014, 65, 238–243. [Google Scholar] [CrossRef]
- Andrea-Silva, J.; Cosme, F.; Ribeiro, L.F.; Moreira, A.S.P.; Malheiro, A.C.; Coimbra, M.A.; Domingues, M.R.M.; Nunes, F.M. Origin of the Pinking Phenomenon of White Wines. J. Agric. Food Chem. 2014, 62, 5651–5659. [Google Scholar] [CrossRef] [PubMed]
- Bellée, A.; Cluzet, S.; Dufour, M.-C.; Mérillon, J.-M.; Corio-Costet, M.-F. Comparison of the Impact of Two Molecules on Plant Defense and on Efficacy against Botrytis cinerea in the Vineyard: A Plant Defense Inducer (Benzothiadiazole) and a Fungicide (Pyrimethanil). J. Agric. Food Chem. 2018, 66, 3338–3350. [Google Scholar] [CrossRef]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; Adler, S.; Delanoue, G.; LaNoue, A. Semi-Targeted Metabolomics to Validate Biomarkers of Grape Downy Mildew Infection Under Field Conditions. Plants 2020, 9, 1008. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.J.; Degu, A.; Herrera, J.C.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Multi-Omics and Integrated Network Analyses Reveal New Insights into the Systems Relationships between Metabolites, Structural Genes, and Transcriptional Regulators in Developing Grape Berries (Vitis vinifera L.) Exposed to Water Deficit. Front. Plant Sci. 2017, 8, 1124. [Google Scholar] [CrossRef]
- González, R.; González, M.-R.; Martín, P. Abscisic acid and ethephon treatments applied to ‘Verdejo’ white grapes affect the quality of wine in different ways. Sci. Agric. 2018, 75, 381–386. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.I.F.; Guerrero, R.F.; Puertas, B.; Garcia-Parrilla, M.C.; Collado, I.G.; Cantos-Villar, E. Impact of preharvest and postharvest treatment combinations on increase of stilbene content in grape. OENO One 2013, 47, 203–212. [Google Scholar] [CrossRef]
- Héberger, K.; Csomós, E.; Simon-Sarkadi, L. Principal Component and Linear Discriminant Analyses of Free Amino Acids and Biogenic Amines in Hungarian Wines. J. Agric. Food Chem. 2003, 51, 8055–8060. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Formation of amino acid derivatives in white and red wines during fermentation: Effects of non-Saccharomyces yeasts and Oenococcus oeni. Food Chem. 2020, 343, 128415. [Google Scholar] [CrossRef] [PubMed]
- Vivas de Gaulejac, N.; Vivas, N.; Guerra, C.; Nonier, M. Anthocyanin in grape skins during the maturation of Vitis vinifera L. cv. Cabernet Sauvignon and Merlot Noir from different Bordeaux terroirs. J. Int. Sci. Vigne Vin 2001, 35, 149–156. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole Enhances Resveratrol and Anthocyanin Biosynthesis in Grapevine, Meanwhile Improving Resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and Stability of Pigments Derived from the Acetaldehyde-Mediated Condensation between Malvidin 3-O-Glucoside and (+)-Catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef]
- González-Muñoz, B.; Garrido-Vargas, F.; Pavez, C.; Osorio, F.; Chen, J.; Bordeu, E.; O’Brien, J.A.; Brossard, N. Wine astringency: More than just tannin–protein interactions. J. Sci. Food Agric. 2021, 102, 1771–1781. [Google Scholar] [CrossRef]
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Koyama, R.; Roberto, S.R.; de Souza, R.T.; Borges, W.F.S.; Anderson, M.; Waterhouse, A.L.; Cantu, D.; Fidelibus, M.W.; Blanco-Ulate, B. Exogenous Abscisic Acid Promotes Anthocyanin Biosynthesis and Increased Expression of Flavonoid Synthesis Genes in Vitis vinifera × Vitis labrusca Table Grapes in a Subtropical Region. Front. Plant Sci. 2018, 9, 323. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; de Urturi, I.S.; Rubio-Bretón, P.; Román, S.M.-S.; Murillo-Peña, R.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Garde-Cerdán, T. Application of Elicitors, as Conventional and Nano Forms, in Viticulture: Effects on Phenolic, Aromatic and Nitrogen Composition of Tempranillo Wines. Beverages 2022, 8, 56. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Revilla, E.; García-Beneytez, E.; Cabello, F.; Martín-Ortega, G.; Ryan, J.-M. Value of high-performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J. Chromatogr. A 2001, 915, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.C.; Lambert, C.; Bouscaut, J.; Mérillon, J.M.; Corio-Costet, M.F. Benzothiadiazole-primed defence responses and enhanced differential expression of defence genes in Vitis vinifera infected with biotrophic pathogens Erysiphe necator and Plasmopara viticola. Plant Pathol. 2012, 62, 370–382. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Effects of benzothiadiazole on disease resistance and soluble sugar accumulation in grape berries and its possible cellular mechanisms involved. Postharvest Biol. Technol. 2015, 102, 51–60. [Google Scholar] [CrossRef]
- Burdziej, A.; Bellée, A.; Bodin, E.; Fonayet, J.V.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.-F. Three Types of Elicitors Induce Grapevine Resistance against Downy Mildew via Common and Specific Immune Responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a Grapevine R2R3-MYB Transcription Factor That Regulates the Phenylpropanoid Pathway. Plant Physiol. 2005, 140, 499–511. [Google Scholar] [CrossRef]
- Boss, P.; Davies, C.; Robinson, S. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 1996, 32, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.J.; Thomas, M.R.; Robinson, S. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. Cell Mol. Biol. 2007, 49, 772–785. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 46. [Google Scholar] [CrossRef]
- Ferreira, V.; Matus, J.T.; Pinto-Carnide, O.; Carrasco, D.; Arroyo-García, R.; Castro, I. Genetic analysis of a white-to-red berry skin color reversion and its transcriptomic and metabolic consequences in grapevine (Vitis vinifera cv. ‘Moscatel Galego’). BMC Genom. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The Grapevine R2R3-MYB Transcription Factor VvMYBF1 Regulates Flavonol Synthesis in Developing Grape Berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef]
- Bogs, J.; Jaffé, F.W.; Takos, A.M.; Walker, A.; Robinson, S.P. The Grapevine Transcription Factor VvMYBPA1 Regulates Proanthocyanidin Synthesis during Fruit Development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) Tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis vinifera L. cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative Insight upon Chitosan Solution and Chitosan Nanoparticles Application on the Phenolic Content, Antioxidant and Antimicrobial Activities of Individual Grape Components of Sousão Variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; de Urturi, I.S.; Rubio-Bretón, P.; Román, S.M.-S.; Baroja, E.; Ramírez-Rodríguez, G.; Delgado-López, J.; Pérez-Álvarez, E. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2023, 402, 134244. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kumar, V.; Sikron-Persi, N.; Dynkin, I.; Weiss, D.; Perl, A.; Fait, A.; Oren-Shamir, M. Over 1000-Fold Synergistic Boost in Viniferin Levels by Elicitation of Vitis vinifera cv. Gamay Red Cell Cultures over Accumulating Phenylalanine. J. Agric. Food Chem. 2022, 70, 5049–5056. [Google Scholar] [CrossRef]
- Ju, Y.-L.; Liu, B.-C.; Xu, X.-L.; Wu, J.-R.; Sun, W.; Fang, Y.-L. Targeted metabolomic and transcript level analysis reveals the effects of exogenous strigolactone and methyl jasmonate on grape quality. Sci. Hortic. 2022, 299, 111009. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wines and Musts; Methods: OIVMA–AS313–01; OIV–MA–AS2–10; International Organisation of Vine and Wine: Paris, France, 2018; Volume 2, Available online: https://www.oiv.int/public/medias/3731/oiv-ma-as313-01.pdf (accessed on 30 August 2022).
- Martins, V.; Unlubayir, M.; Teixeira, A.; Gerós, H.; Lanoue, A. Calcium and methyl jasmonate cross-talk in the secondary metabolism of grape cells. Plant Physiol. Biochem. 2021, 165, 228–238. [Google Scholar] [CrossRef]
- Billet, K.; Delanoue, G.; Arnault, I.; Besseau, S.; Oudin, A.; Courdavault, V.; Marchand, P.A.; Giglioli-Guivarc’h, N.; Guérin, L.; LaNoue, A. Vineyard evaluation of stilbenoid-rich grape cane extracts against downy mildew: A large-scale study. Pest Manag. Sci. 2018, 75, 1252–1257. [Google Scholar] [CrossRef]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, D.; Merkouropoulos, G.; Kogkou, C.; Arseniou, S.; Alatzas, A.; Proxenia, N.; Hatzopoulos, P.; Kotseridis, Y. Explore the Rare—Molecular Identification and Wine Evaluation of Two Autochthonous Greek Varieties: “Karnachalades” and “Bogialamades”. Plants 2021, 10, 1556. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Kanapitsas, A.; Lola, D.; Goulioti, E.; Kontoudakis, N.; Leventis, G.; Tsiknia, M.; Kotseridis, Y. Effect of Nitrogen Fertilization on Savvatiano (Vitis vinifera L.) Grape and Wine Composition. Beverages 2022, 8, 29. [Google Scholar] [CrossRef]
- Sudraud, P. Interpretation of Red Wine Absorption Curves. Annu. Technol. Agric. 1958, 7, 203–208. [Google Scholar]
- Ribèreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. The Chemistry of Wine; Wiley: West Sessex, UK, 2006; Volume 2, pp. 1–441. [Google Scholar]
- Ribereau-Gayon, P.; Stonestreet, E. Le dosage des anthocyannes’ dans Je vin rouge. Bull. De La Soc. Chim. De Fr. 1965, 9, 2649–2652. [Google Scholar]
- Ribéreau–Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, the Chemistry of Wine Stabilization and Treatments; John Wiley and Sons Ltd.: West Sussex, UK, 2000; Volume 2, pp. 157–162. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of Principal Polyphenolic Components in Relation to Antioxidant Characteristics of Aged Red Wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Kramlinga, T.E. Browning of White Wines and an Accelerated Test for Browning Capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar] [CrossRef]
- Sarneckis, C.; Dambergs, R.; Jones, P.; Mercurio, M.; Herderich, M.; Smith, P. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Lagouvardos, K.; Kotroni, V.; Bezes, A.; Koletsis, I.; Kopania, T.; Lykoudis, S.; Mazarakis, N.; Papagiannaki, K.; Vougioukas, S. The automatic weather stations NOANN network of the National Observatory of Athens: Operation and database. Geosci. Data J. 2017, 4, 4–16. [Google Scholar] [CrossRef]
| Meteorological Data | |||||
|---|---|---|---|---|---|
| Savvatiano | Mouhtaro | ||||
| 2019 | 2020 | 2019 | 2020 | ||
| Mean Temperature (°C) | Pre-veraison 1 | 22.8 | 22.2 | 19.62 | 19.42 |
| Post-veraison 2 | 22.6 | 23.8 | 24.7 | 26 | |
| Growing season 3 | 22.8 | 22.5 | 21.11 | 22.32 | |
| Rainfall (mm) | Pre-veraison | 363.8 | 266.5 | 146.2 | 207.4 |
| Post-veraison | 16.8 | 5.2 | 0.0 | 84.0 | |
| Growing season | 112.0 | 299.2 | 146.2 | 291.4 | |
| Annual 4 | 492.6 | 570.9 | 505.4 | 587.5 | |
| 2019 | |||||
| Phenolic Stage | Treatment | Berry Volume (mg/berry) | Total Soluble Solids (oBrix) | Total Acidity (Tartaric Acid g/L) | pH |
| V | Control | 2.21 ± 0.29 ab | 14.26 ± 0.75 b | 5.42 ± 0.24 b | 2.89 ± 0.12 a |
| BTH Low | 2.23 ± 0.16 a | 14.95 ± 0.30 ab | 5.70 ± 0.32 ab | 2.77 ± 0.02 a | |
| BTH High | 1.83 ± 0.05 b | 15.3 ± 0.17 a | 6.15 ± 0.22 a | 2.87 ± 0.15 a | |
| MV | Control | 2.70 ± 0.16 a | 17.13 ± 0.55 b | 4.75 ± 0.11 a | 3.14 ± 0.07 b |
| BTH Low | 2.39 ± 0.12 b | 18.13 ± 0.28 a | 4.45 ± 0.35 a | 3.32 ± 0.04 a | |
| BTH High | 2.07 ± 0.06 c | 18.66 ± 0.11 a | 4.72 ± 0.19 a | 3.35 ± 0.01 a | |
| H | Control | 2.30 ± 0.06 b | 19.53 ± 0.11 a | 4.40 ± 0.67 a | 3.35 ± 0.09 a |
| BTH Low | 2.45 ± 0.07 a | 19.73 ± 0.49 a | 4.35 ± 0.30 a | 3.37 ± 0.03 a | |
| BTH High | 2.12 ± 0.03 c | 19.96 ± 0.35 a | 3.90 ± 0.25 a | 3.39 ± 0.07 a | |
| 2020 | |||||
| Phenolic Stage | Treatment | Berry volume (mg/berry) | Total Soluble Solids (oBrix) | Total Acidity (Tartaric Acid g/L) | pH |
| V | Control | 2.64 ± 0.05 a | 15.66± 0.73 a | 6.2 ± 0.17 a | 3.25 ± 0.03 a |
| BTH Low | 2.63 ± 0.06 a | 14.83 ± 0.64 a | 6.4 ± 0.44 a | 3.19 ± 0.09 a | |
| BTH High | 2.56 ± 0.10 a | 15.00 ± 0.17 a | 6.5 ± 0.43 a | 3.20 ± 0.04 a | |
| MV | Control | 2.85 ± 0.11 a | 17.76 ± 0.28 a | 5.3 ± 0.34 a | 3.32 ± 0.09 a |
| BTH Low | 2.58 ± 0.07 b | 16.53 ± 0.89 a | 5.45 ± 0.22 a | 3.22 ± 0.02 a | |
| BTH High | 2.59 ± 0.08 b | 16.50 ± 0.70 a | 5.6 ± 0.31 a | 3.24 ± 0.04 a | |
| H | Control | 2.56 ± 0.23 a | 20.13 ± 0.40 a | 4.49 ± 0.26 a | 3.36 ± 0.03 a |
| BTH Low | 2.82 ± 0.19 a | 19.53 ± 0.25 ab | 4.6 ± 0.17 a | 3.37 ± 0.07 a | |
| BTH High | 2.55 ± 0.14 a | 19.10 ± 0.25 b | 4.63 ± 0.20 a | 3.39 ± 0.13 a | |
| Savvatiano | |||||
|---|---|---|---|---|---|
| Vintage | Treatment | Ethanol (v/v %) | Total Acidity (Tart. Acid g/L) | Volatile Acidity (Ac. Ac. g/L) | pH |
| 2019 | Control | 10.8 ± 0.2 a | 6.0 ± 0.3 a | 0.2 ± 0.04 a | 3.10 ± 0.04 a |
| BTH Low | 10.8 ± 0.3 a | 6.0 ± 0.1 a | 0.2 ± 0.03 a | 3.20 ± 0.12 a | |
| BTH High | 11.5 ± 0.5 a | 6.2 ± 0.3 a | 0.2 ± 0.01 a | 3.17 ± 0.07 a | |
| 2020 | Control | 11.8 ± 0.2 a | 4.1 ± 0.1 a | 0.10 ± 0.01 a | 3.47 ± 0.02 a |
| BTH Low | 10.8 ± 0.1 b | 4.3 ± 0.1 a | 0.10 ± 0.02 a | 3.35 ± 0.01 a | |
| BTH High | 10.7 ± 0.2 b | 4.6 ± 0.5 a | 0.10 a ± 0.06 a | 3.33 ± 0.11 a | |
| Vintage | Treatment | TPI | 420 nm | Total Polyphenol Concentration (Gal. Ac. mg/L) | k Factor |
|---|---|---|---|---|---|
| Savvatiano 2019 | Control | 5.37 ± 0.38 b | 0.047 ± 0.003 b | 23.2 ± 1.0 b | 0.0031 ± 0.0005 a |
| BTH Low | 5.72 ± 0.46 ab | 0.0513 ± 0.007 ab | 25.0 ± 2.0 b | 0.0035 ± 0.0002 a | |
| BTH High | 6.42 ± 0.47 a | 0.058 ± 0.002 a | 31.1 ± 1.5 a | 0.0036 ± 0.0001 a | |
| Savvatiano 2020 | Control | 5.23 ± 0.06 a | 0.05 ± 0.005 a | 25.2 ± 1.6 a | 0.060 ± 0.0004 b |
| BTH Low | 4.46 ± 0.31 b | 0.04 ± 0.005 b | 20.6 ± 1.9 b | 0.0641 ± 0.005 a | |
| BTH High | 4.45 ± 0.37 b | 0.04 ± 0.001 b | 21.5 ± 1.4 b | 0.067 ± 0.002 a |
| 2019 | |||||
| Phenologic Stage | Treatment | Berry Volume (mg/berry) | Total Soluble Solids (oBrix) | Total Acidity (Tartaric Acid g/L) | pH |
| V | Control | 1.51 ± 0.15 a | 14.1 ± 0.8 b | 12.3 ± 0.4 a | 2.86 ± 0.03 a |
| BTH Low | 1.39 ± 0.10 ab | 15.9 ± 0.1 a | 13.0 ± 0.6 a | 2.88 ± 0.13 a | |
| BTH High | 1.22 ± 0.02 b | 16.2 ± 1.1 a | 12.1 ± 0.2 a | 2.87 ± 0.15 a | |
| MV | Control | 2.02 ± 0.10 a | 18.9 ± 1.6 a | 9.5 ± 1.1 a | 3.50 ± 0.02 a |
| BTH Low | 1.77 ± 0.05 b | 19.1 ± 0.3 a | 9.2 ± 0.2 a | 3.41 ± 0.16 a | |
| BTH High | 1.59 ± 0.02 b | 18.0 ± 0.8 a | 9.4 ± 0.6 a | 3.50 ± 0.05 a | |
| H | Control | 2.11 ± 0.01 a | 23.7 ± 0.4 a | 8.1 ± 0.2 a | 3.31 ± 0.01 a |
| BTH Low | 2.10 ± 0.07 ab | 23.4 ± 0.3 a | 8.1 ± 0.1 a | 3.69 ± 0.07 b | |
| BTH High | 2.01 ± 0.02 b | 23.2 ± 0.5 a | 7.4 ± 0.2 b | 3.76 ± 0.02 b | |
| 2020 | |||||
| Phenolic Stage | Treatment | Berry volume (mg/berry) | Total Soluble Solids (oBrix) | Total Acidity (Tartaric Acid g/L) | pH |
| V | Control | 1.74 ± 0.12 a | 14.7 ± 0.2 a | 17.9 ± 0.1 a | 2.97 ± 0.06 a |
| BTH Low | 1.88 ± 0.10 a | 15.0 ± 0.8 a | 16.8 ± 0.4 b | 3.05 ± 0.05 a | |
| BTH High | 1.90 ± 0.11 a | 14.3 ± 0.1 a | 17.4 ± 0.2 ab | 3.08 ± 0.02 a | |
| MV | Control | 2.38 ± 0.06 a | 17.7 ± 0.3 a | 10.3 ± 0.4 a | 3.21 ± 0.09 a |
| BTH Low | 2.16 ± 0.19 ab | 18.3 ± 0.5 a | 10.3 ± 0.4 a | 3.23 ± 0.03 a | |
| BTH High | 2.06 ± 0.10 b | 17.6 ± 0.3 a | 10.8 ± 0.2 a | 3.16 ± 0.03 a | |
| H | Control | 2.05 ± 0.09 a | 24.6 ± 0.5 a | 7.6 ± 0.3 a | 3.37 ± 0.12 b |
| BTH Low | 1.90 ± 0.23 a | 23.6 ± 0.3 b | 8.7 ± 0.9 a | 3.51 ± 0.09 ab | |
| BTH High | 1.96 ± 0.80 a | 23.6 ± 0.2 b | 8.6 ± 0.2 a | 3.62 ± 0.08 a | |
| Vintage | Treatment | Ethanol (v/v %) | Total Acidity (Tart. Acid g/L) | pH | Volatile Acidity (Ac. Ac. g/L) |
|---|---|---|---|---|---|
| Mouhtaro 2019 | Control | 13.7 ± 0.4 a | 6.2 ± 0.3 a | 3.43 ± 0.04 a | 0.43 ± 0.07 a |
| BTH Low | 13.4 ± 0.3 a | 5.7 ± 0.4 ab | 3.66 ± 0.24 a | 0.53 ± 0.15 a | |
| BTH High | 13.3 ± 0.2 a | 5.4 ± 0.2 b | 3.82 ± 0.31 a | 0.57 ± 0.02 a | |
| Mouhtaro 2020 | Control | 14.2 ± 0.4 a | 6.8 ± 0.5 a | 3.8 ± 0.21 a | 0.27 ± 0.03 a |
| BTH Low | 13.2 ± 0.3 b | 6.1 ± 0.6 ab | 3.8 ± 0.22 a | 0.33 ± 0.03 a | |
| BTH High | 13.4 ± 0.2 b | 5.6 ± 0.1 b | 3.9 ± 0.06 a | 0.29 ± 0.02 a |
| Stage | Treatment | Total Phenolic Index | Color Intensity | Total Polyphenol Concentration (Gal. Ac. mg/L) | Total Anthocyanins (mg/L) | MCP |
|---|---|---|---|---|---|---|
| Mouhtaro 2019 | Control | 42.5 ± 2.5 b | 13.0 ± 0.3 b | 1506.5 ± 81.6 b | 355.5 ± 4.5 b | 232.3 ± 14.6 b |
| BTH Low | 46.0 ± 1.7 b | 14.0 ± 0.5 a | 1675.4 ± 60.2 ab | 385.1 ± 12.7 a | 234.5 ± 12.5 b | |
| BTH High | 51.4 ± 2.2 a | 13.3 ± 0.2 ab | 1847.4 ± 133.1 a | 363.9 ± 9.9 ab | 339.7 ± 13.6 a | |
| Mouhtaro 2020 | Control | 36.9 ± 3.7 b | 12.5 ± 0.2 ab | 1273.1 ± 49.3 c | 325.5 ± 2.0 b | 222.3 ± 6.6 c |
| BTH Low | 45.7 ± 3.7 a | 13.0 ± 0.1 a | 1442.1 ± 32.4 b | 344.4 ± 8.1 a | 237.8 ± 6.4 b | |
| BTH High | 47.7 ± 3.4 a | 12.2 ± 0.4 b | 1610.7 ± 51.8 a | 321.9 ± 3.8 b | 323.1 ± 7.1 a |
| Vintage | Treatment | Dp3G | Cy3G | Pt3G | Pn3G | Mlv3G | MlvAc | MlvCm |
Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|
| Mouhtaro 2019 | Control | 32.0 ± 2.5 a | 2.2 ± 0.1 b | 41.1 ± 1.9 b | 5.7 ± 0.5 b | 260.0 ± 8.0 b | 10.2 ± 1.0 a | 4.7 ± 0.4 a | 348.1 ± 7.9 b |
| BTH Low | 38.2 ± 4.1 a | 2.6 ± 0.1 a | 50.1 ± 2.7 a | 7.8 ± 1.1 a | 265.1 ± 1.9 a | 8.4 ± 0.4 b | 4.3 ± 0.6 ab | 372.6 ± 4.8 a | |
| BTH High | 38.7 ± 4.5 a | 2.4 ± 0.1 a | 49.5 ± 3.6 a | 7.8 ± 1.1 a | 251.1 ± 6.2 c | 8.1 ± 0.4 b | 3.5 ± 0.5 b | 354.6 ± 8.6 b | |
| Mouhtaro 2020 | Control | 27.0 ± 2.5 b | 2.3 ± 0.1 ab | 26.5 ± 1.6 b | 2.6 ± 0.1 a | 248.4 ± 3.1 a | 12.6 ± 1.3 a | 6.4 ± 0.8 a | 319.6 ± 3.4 ab |
| BTH Low | 33.7 ± 1.2 a | 2.4 ± 0.1 a | 29.4 ± 0.6 a | 3.1 ± 0.4 a | 246.1 ± 1.4 ab | 10.6 ± 1.2 a | 5.5 ± 0.3 a | 325.6 ± 0.9 a | |
| BTH High | 34.2 ± 0.3 a | 2.1 ± 0.1 b | 25.8 ± 0.6 b | 2.7 ± 0.1 a | 241.0 ± 4.9 b | 10.9 ± 0.1 a | 5.4 ± 0.1 a | 317.1 ± 3.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliordos, D.-E.; Alatzas, A.; Kontoudakis, N.; Unlubayir, M.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years. Plants 2023, 12, 1179. https://doi.org/10.3390/plants12051179
Miliordos D-E, Alatzas A, Kontoudakis N, Unlubayir M, Hatzopoulos P, Lanoue A, Kotseridis Y. Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years. Plants. 2023; 12(5):1179. https://doi.org/10.3390/plants12051179
Chicago/Turabian StyleMiliordos, Dimitrios-Evangelos, Anastasios Alatzas, Nikolaos Kontoudakis, Marianne Unlubayir, Polydefkis Hatzopoulos, Arnaud Lanoue, and Yorgos Kotseridis. 2023. "Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years" Plants 12, no. 5: 1179. https://doi.org/10.3390/plants12051179
APA StyleMiliordos, D.-E., Alatzas, A., Kontoudakis, N., Unlubayir, M., Hatzopoulos, P., Lanoue, A., & Kotseridis, Y. (2023). Benzothiadiazole Affects Grape Polyphenol Metabolism and Wine Quality in Two Greek Cultivars: Effects during Ripening Period over Two Years. Plants, 12(5), 1179. https://doi.org/10.3390/plants12051179








