MdWRKY120 Enhance Apple Susceptibility to Alternaria alternata
Abstract
1. Introduction
2. Results
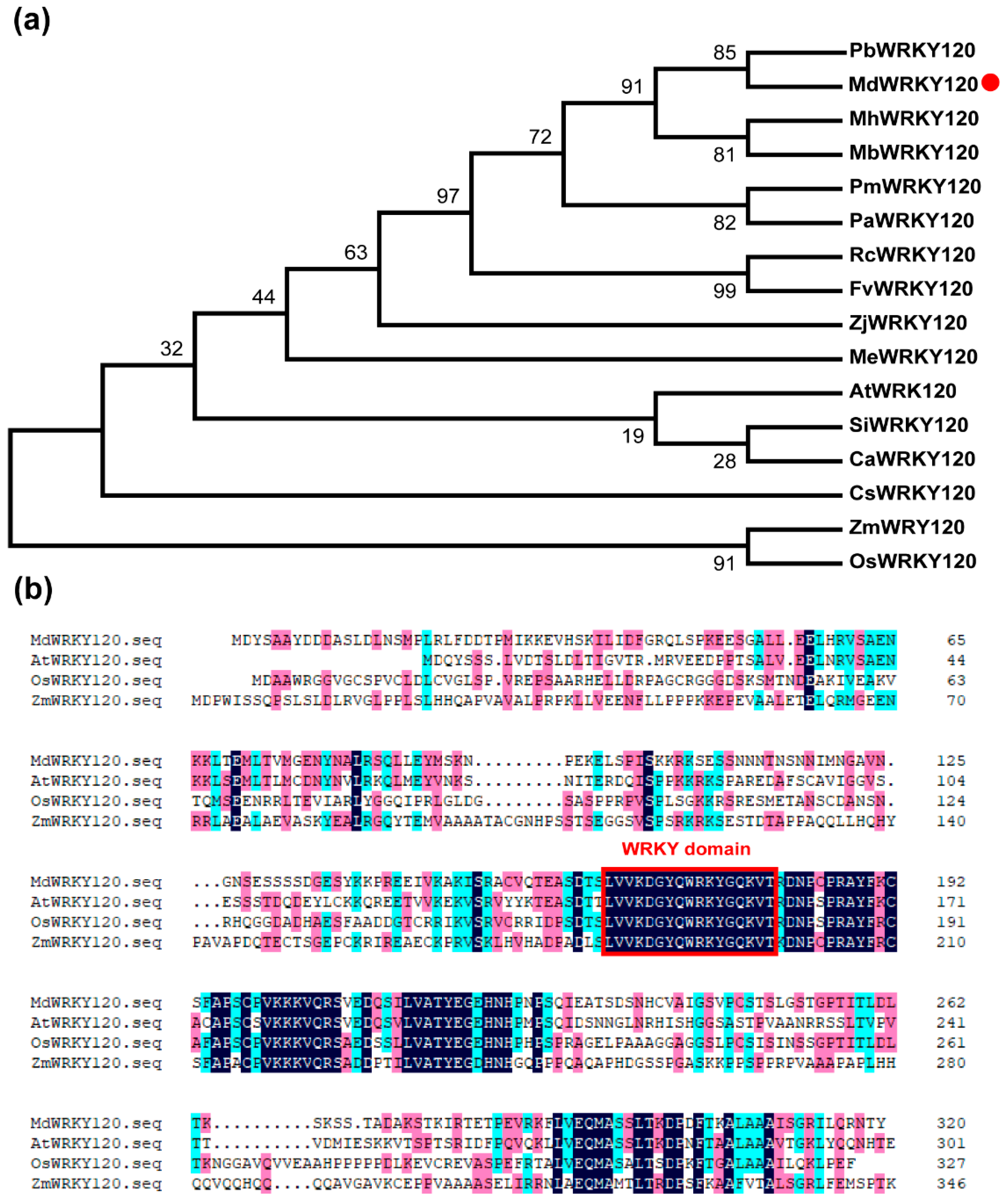
2.1. Identification of MdWRKY120 in Apple
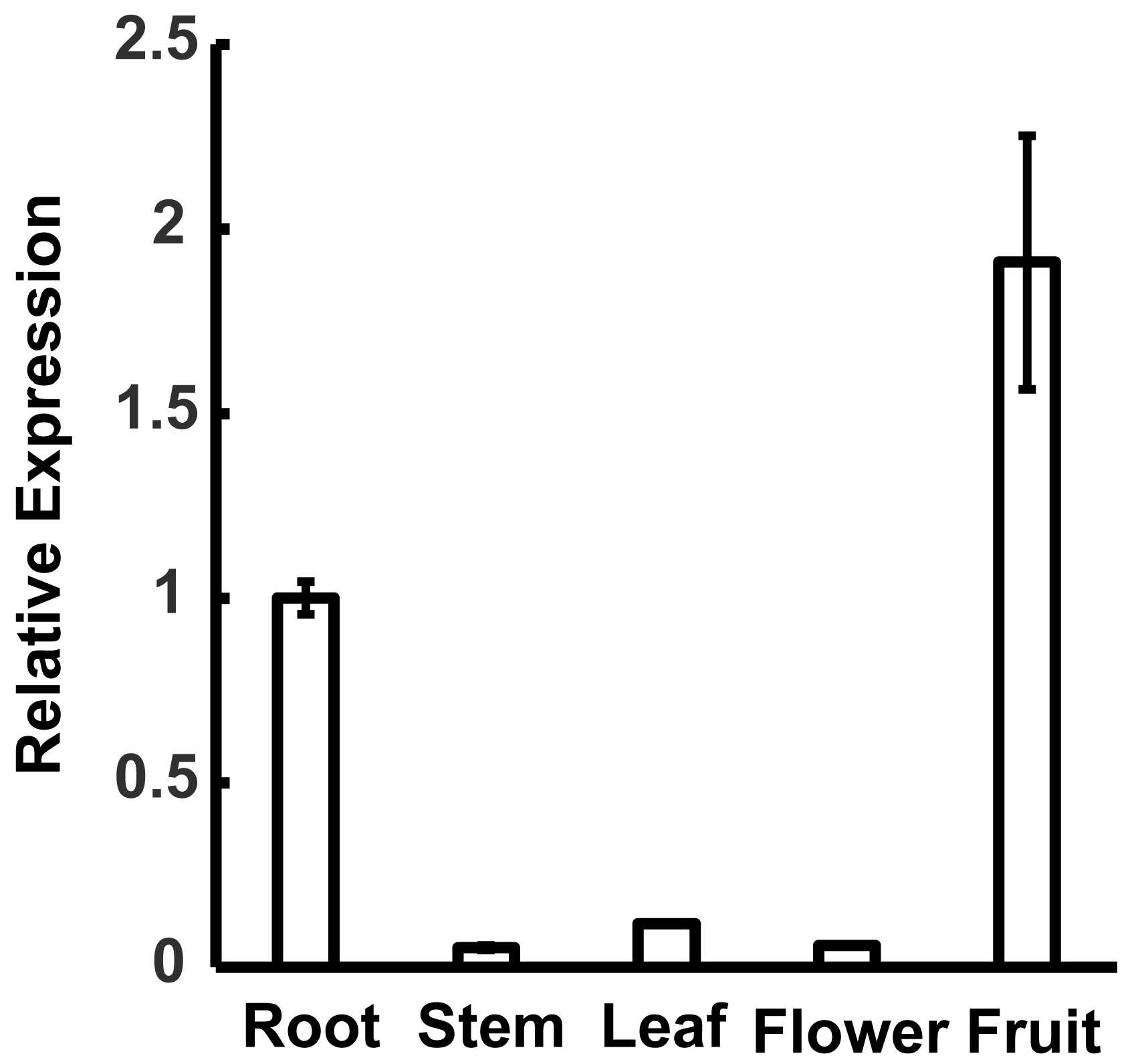
2.2. Expression of MdWRKY120 in Multiple Organs
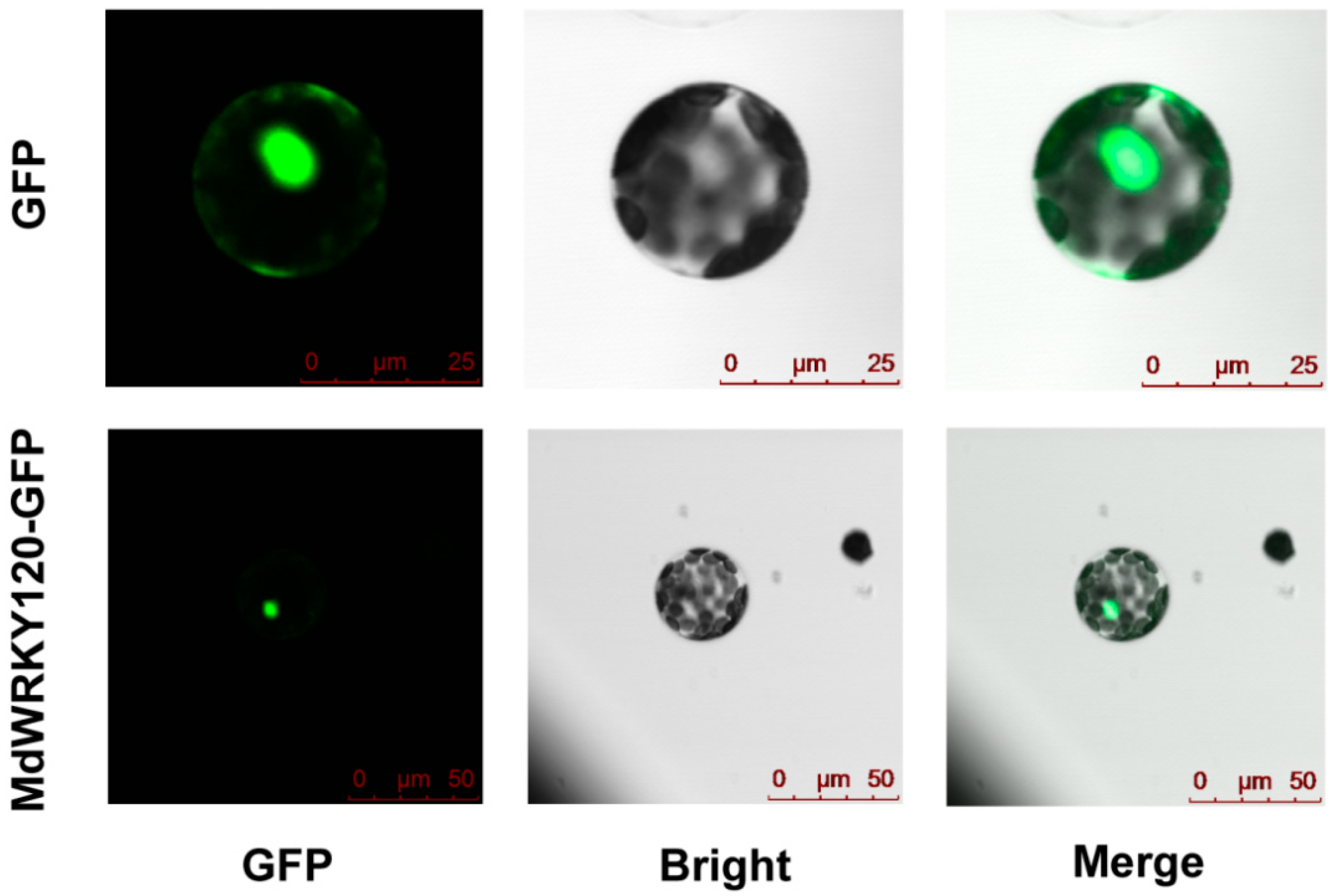
2.3. Subcellular Localization of MdWRKY120
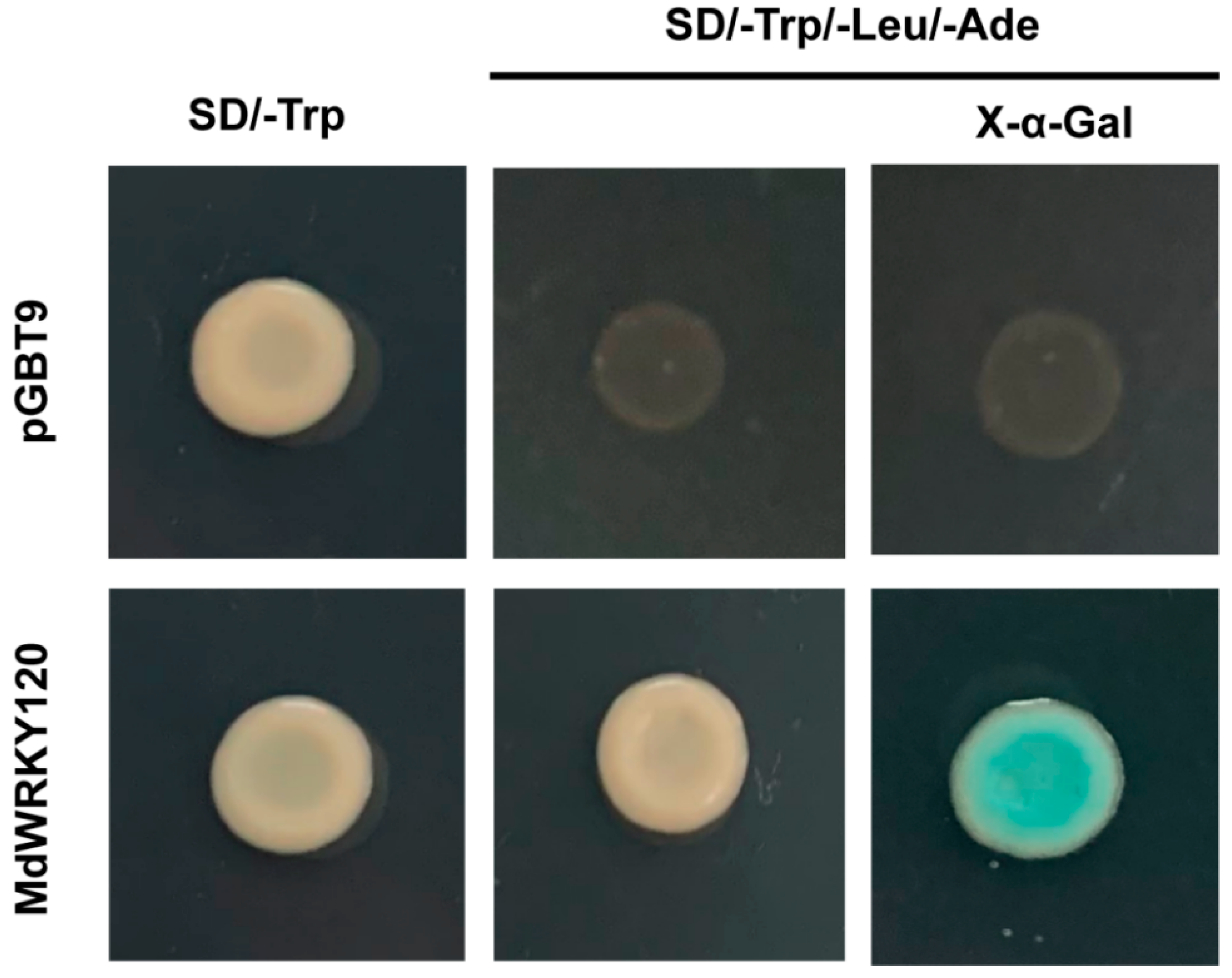
2.4. Transcription Activation Analysis of MdWRKY120
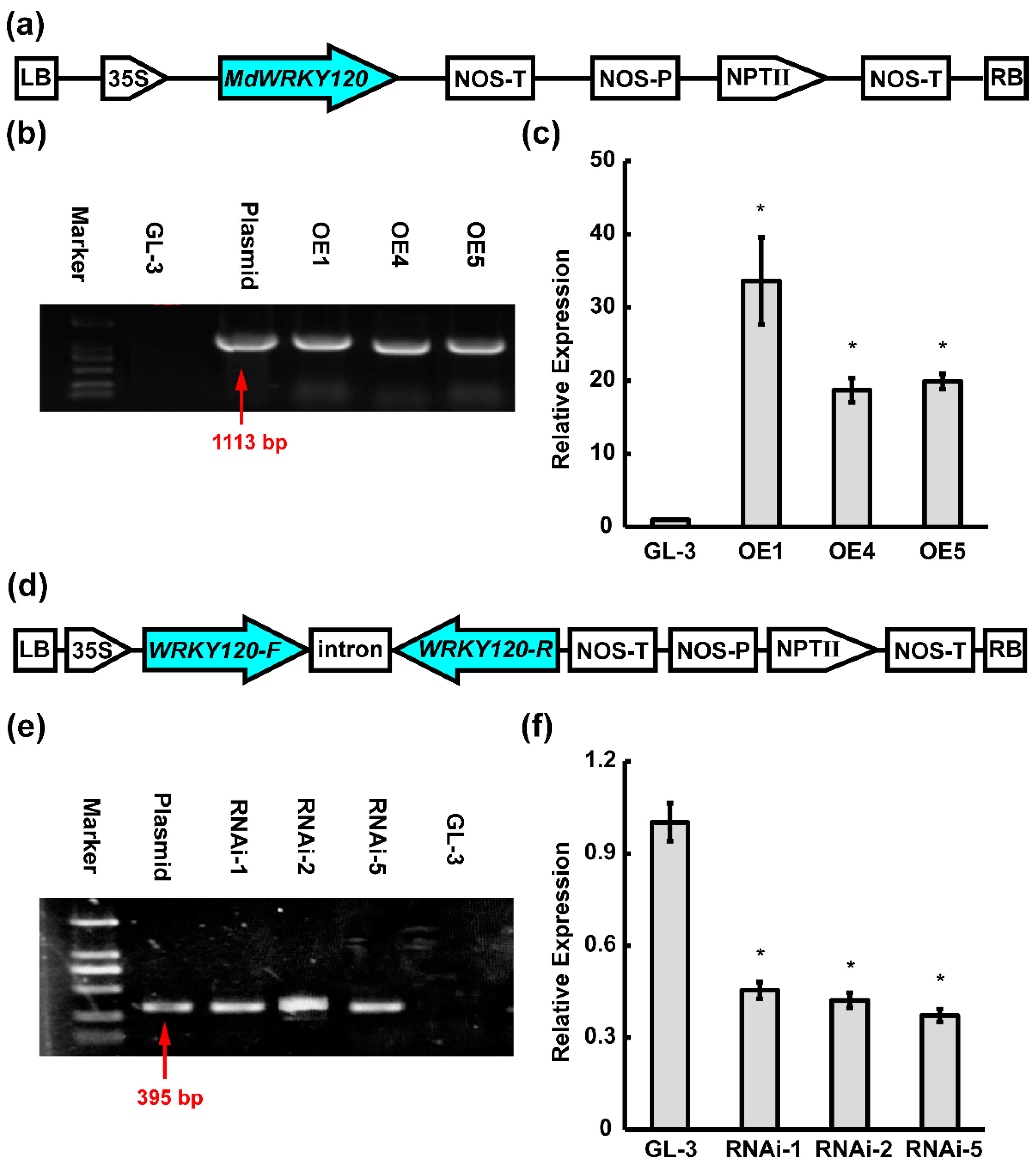
2.5. Overexpression and RNAi Knockdown of MdWRKY120 in ‘GL-3′ Apple
2.6. MdWRKY120 Confers A. Alternata Resistance to Apple
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cloning MdWRKY120 Gene
4.3. Sequence Alignment and Phylogenetic Analysis
4.4. Subcellular Localization
4.5. Transcriptional Activation Analysis in Yeast Cells
4.6. Vector Construction and Apple ‘GL-3′ Transformation
4.7. PCR Confirmation of Transgenic Plants
4.8. Gene Expression Analysis
4.9. Alternaria Mali Culture and Infection
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, S.; Peduto Hand, F. Determining the sources of primary and secondary inoculum and seasonal inoculum dynamics of fungal pathogens causing fruit rot of deciduous holly. Plant Dis. 2019, 103, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Ishii, Y.; Honda, A.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; et al. Function of genes encoding Acyl-CoA synthetase and Enoyl-CoA hydratase for host-selective act-toxin biosynthesis in the tangerine pathotype of Alternaria alternata. Phytopathology 2009, 99, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Terakami, S.; Okada, K.; Shimizu, T.; Adachi, Y.; Katayose, Y.; Fujisawa, H.; Wu, J.; Kanamori, H.; Yamamoto, T.; et al. Identification of candidate genes responsible for the susceptibility of apple (Malus × domestica Borkh.) to Alternaria blotch. BMC Plant Biol. 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Meng, D.; Li, Y.; Bai, Y.; Li, M.; Cheng, L. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress. Plant Physiol. Biochem. 2016, 103, 71–83. [Google Scholar] [CrossRef]
- Wei, K.F.; Chen, J.; Chen, Y.F.; Wu, L.J.; Xie, D.X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012, 19, 153–164. [Google Scholar] [CrossRef]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Xiong, C.; Zhao, S.; Yu, X.; Sun, Y.; Li, H.; Ruan, C.; Li, J. Yellowhorn drought-induced transcription factor XsWRKY20 acts as a positive regulator in drought stress through ROS homeostasis and ABA signaling pathway. Plant Physiol. Biochem. 2020, 155, 187–195. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Wang, H.; Zhang, L.; Wang, F.; Yu, D. Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J. 2013, 74, 730–745. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, C.; Liu, W.; Wang, Y. The WRKY53 transcription factor enhances stilbene synthesis and disease resistance by interacting with MYB14 and MYB15 in Chinese wild grape. J. Exp. Bot. 2020, 71, 3211–3226. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, Q.; Lan, J.; Zhang, T.; Liu, X.; Miao, R.; Mou, C.; Nguyen, T.; Wang, J.; Zhang, X.; et al. WRKY transcription factor OsWRKY29 represses seed dormancy in rice by weakening abscisic acid response. Front. Plant Sci. 2020, 11, 691. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Li, M.; Sui, S. CpWRKY71, a WRKY transcription factor gene of wintersweet (Chimonanthus praecox), promotes flowering and leaf senescence in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 45–47. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, L.; Weng, Y.; Zou, H.; Li, X.; Yang, S.; Qiu, S.; Huang, X.; Huang, J.; Hussain, A.; et al. ChiIV3 Acts as a novel target of WRKY40 to mediate pepper immunity against Ralstonia solanacearum infection. Mol. Plant Microbe Interact. 2019, 32, 1121–1133. [Google Scholar] [CrossRef]
- Chakraborty, J.; Ghosh, P.; Sen, S.; Das, S. Epigenetic and transcriptional control of chickpea WRKY40 promoter activity under Fusarium stress and its heterologous expression in Arabidopsis leads to enhanced resistance against bacterial pathogen. Plant Sci. 2018, 276, 250–267. [Google Scholar] [CrossRef]
- Schön, M.; Töller, A.; Diezel, C.; Roth, C.; Westphal, L.; Wiermer, M.; Somssich, I.E. Analyses of wrky18 wrky40 plants reveal critical roles of SA/EDS1 signaling and indole-glucosinolate biosynthesis for Golovinomyces orontii resistance and a loss-of resistance towards Pseudomonas syringae pv. tomato AvrRPS4. Mol. Plant Microbe Interact. 2013, 26, 758–767. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Li, Y.; Chu, X.; Wu, C.; Guo, X. GhWRKY40, a multiple stress-responsive cotton WRKY gene, plays an important role in the wounding response and enhances susceptibility to Ralstonia solanacearum infection in transgenic Nicotiana benthamiana. PLoS ONE 2014, 9, e93577. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants. Int. J. Mol. Sci. 2018, 19, 3046–3061. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhang, Y.; Wu, S.; Wang, S.; Hao, L.; Wang, S.; Li, T. Md-miR156ab and Md-miR395 target WRKY transcription factors to influence apple resistance to leaf spot disease. Front. Plant Sci. 2017, 8, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; He, M.H.; Ouyang, H.B.; Zhu, W.; Pan, Z.C.; Sui, Q.J.; Shang, L.P.; Zhan, J. Cross-resistance of the pathogenic fungus Alternaria alternata to fungicides with different modes of action. BMC Microbiol. 2019, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, F.; Su, Y.; Jiang, Q.; Yuan, Y.; Nie, X.; Zhou, Y.; Zhang, X.; Wang, Z.; Wang, F.; et al. MdIPT8, an isopentenyl transferase enzyme, enhances the resistance of apple to Colletotrichum gloeosporioides infection. Sci. Hortic. 2022, 303, 111245. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Nejat, N.; Mantri, N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Dang, F.; Lin, J.; Xue, B.; Chen, Y.; Guan, D.; Wang, Y.; He, S. CaWRKY27 negatively regulates H2O2-mediated thermotolerance in pepper (Capsicum annuum). Front. Plant Sci. 2018, 9, 1633. [Google Scholar] [CrossRef]
- Wang, C.; He, X.; Li, Y.; Wang, L.; Guo, X.; Guo, X. The cotton MAPK kinase GhMPK20 negatively regulates resistance to Fusarium oxysporum by mediating the MKK4-MPK20-WRKY40 cascade. Mol. Plant Pathol. 2018, 19, 1624–1638. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Wang, Z.; Lin, L.; Cui, M.; Long, Y.; Xing, Z. Genome-wide identification of WRKY transcription factors in the Asteranae. Plants 2019, 8, 393. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genomics. 2018, 19, 490. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Rafii, M.Y.; Mahmud, T.M.M.; Azizi, P.; Osman, M.; Abiri, R.; Taheri, S.; Kalhori, N.; Shabanimofrad, M.; et al. Improvement of drought tolerance in rice (Oryza sativa L.): Genetics, genomic tools, and the WRKY gene family. BioMed Res. Int. 2018, 2018, 3158474. [Google Scholar] [CrossRef]
- Shi, W.Y.; Du, Y.T.; Ma, J.; Min, D.H.; Jin, L.G.; Chen, J.; Chen, M.; Zhou, Y.B.; Ma, Y.Z.; Xu, Z.S.; et al. The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int. J. Mol. Sci. 2018, 19, 4087. [Google Scholar] [CrossRef]
- Guo, W.; Chen, W.; Zhang, Z.; Guo, N.; Liu, L.; Ma, Y.; Dai, H. The hawthorn CpLRR-RLK1 gene targeted by ACLSV-derived vsiRNA positively regulate resistance to bacteria disease. Plant Sci. 2020, 300, 110641. [Google Scholar] [CrossRef]
- Gu, L.; Li, L.; Wei, H.; Wang, H.; Su, J.; Guo, Y.; Yu, S. Identification of the group IIa WRKY subfamily and the functional analysis of GhWRKY17 in upland cotton (Gossypium hirsutum L.). PLoS ONE 2018, 13, e0191681. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Abeysinghe, J.K.; Lam, K.M.; Ng, D.W. Differential regulation and interaction of homoeologous WRKY18 and WRKY40 in Arabidopsis allotetraploids and biotic stress responses. Plant J. 2019, 97, 352–367. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef]
- Pandey, S.P.; Roccaro, M.; Schön, M.; Logemann, E.; Somssich, I.E. Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J. 2010, 64, 912–923. [Google Scholar] [CrossRef]
- Karim, A.; Jiang, Y.; Guo, L.; Ling, Z.; Ye, S.; Duan, Y.; Li, C.; Luo, K. Isolation and characterization of a subgroup IIa WRKY transcription factor PtrWRKY40 from Populus trichocarpa. Tree Physiol. 2015, 35, 1129–1139. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple slat stress tolerance by activing MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–321. [Google Scholar] [CrossRef]
- Dai, H.; Li, W.; Han, G.; Yang, Y.; Ma, Y.; Li, H.; Zhang, Z. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic. 2013, 164, 202–208. [Google Scholar] [CrossRef]
- Chen, K.; Guo, Y.; Song, M.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. Dual Role of MdSND1 in the Biosynthesis of Lignin and in Signal Transduction in Response to Salt and Osmotic Stress in Apple. Hortic. Res. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tang, X.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z.; Chen, K.; Tang, X.; et al. Functional Identification of MdMYB5 Involved in Secondary Cell Wall Formation in Apple. Fruit Res. 2021, 1, 1–10. [Google Scholar] [CrossRef]

| (X) Value of Grade | Percentage of the Disease Spots in Total Leaves | Severity |
|---|---|---|
| 0 | X = 0 | No disease spot |
| 1 | 0 < X ≤ 10 | The disease spot area < 1/10 |
| 3 | 10 < X ≤ 25 | 1/10 < The disease spot area < 1/4 |
| 5 | 25 < X ≤ 50 | 1/4 < The disease spot area < 2/5 |
| 7 | 50 < X ≤ 75 | 2/5 < The disease spot area < 3/4 |
| 9 | X > 75.0 | The disease spot area > 3/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, X.; Guo, W.; Shi, J.; Chen, W.; Lei, Y.; Ma, Y.; Dai, H. MdWRKY120 Enhance Apple Susceptibility to Alternaria alternata. Plants 2022, 11, 3389. https://doi.org/10.3390/plants11233389
Liu L, Li X, Guo W, Shi J, Chen W, Lei Y, Ma Y, Dai H. MdWRKY120 Enhance Apple Susceptibility to Alternaria alternata. Plants. 2022; 11(23):3389. https://doi.org/10.3390/plants11233389
Chicago/Turabian StyleLiu, Lifu, Xiaoming Li, Wei Guo, Jiajun Shi, Wenjun Chen, Yingying Lei, Yue Ma, and Hongyan Dai. 2022. "MdWRKY120 Enhance Apple Susceptibility to Alternaria alternata" Plants 11, no. 23: 3389. https://doi.org/10.3390/plants11233389
APA StyleLiu, L., Li, X., Guo, W., Shi, J., Chen, W., Lei, Y., Ma, Y., & Dai, H. (2022). MdWRKY120 Enhance Apple Susceptibility to Alternaria alternata. Plants, 11(23), 3389. https://doi.org/10.3390/plants11233389








