Can Lunar and Martian Soils Support Food Plant Production? Effects of Horse/Swine Monogastric Manure Fertilisation on Regolith Simulants Enzymatic Activity, Nutrient Bioavailability, and Lettuce Growth
Abstract
1. Introduction
2. Results and Discussion
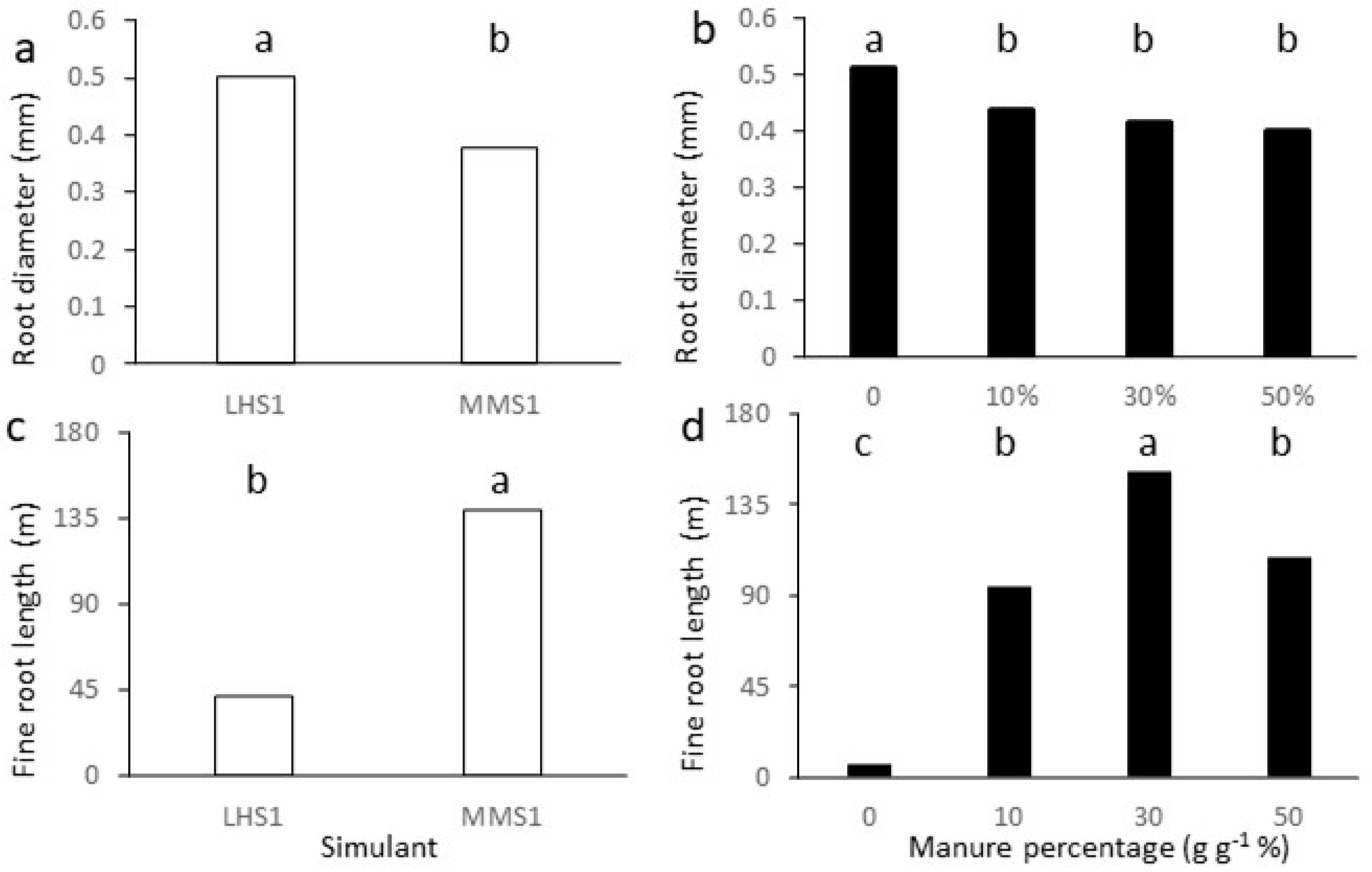
2.1. Biometric Parameters of Lettuce Plant
2.2. Physiological Parameters of Lettuce Plants
2.3. Extractable Nitrogen, Carbon, Microbial Biomass Nitrogen, and Carbon in Simulant/Manure Mixtures after Plant Growth
2.4. Enzymatic Activities in Simulant/Manure Mixtures after Plant Growth
2.5. Nutrient Bioavailability in Simulant/Manure Mixtures after Plant Growth
2.6. Leaf Mineral Content and Plant Nutrients Uptake
3. Materials and Methods
3.1. Main Mineralogical, Physico-Hydraulic, and Chemical Properties of MMS-1 and LHS-1 Simulants, Horse/Swine Monogastric Manure, and Related Simulant/Manure Mixtures
3.2. Plant Material, Growth Chamber Condition, and Experimental Treatments
3.3. Morpho and Physiological Measurements
3.4. Determination of Microbial Biomass Carbon and Nitrogen in Manure Amended Simulants
3.5. Enzymatic Activity Assay in Manure Amended Simulants
3.6. Nutrient Bioavailability in Simulant/Manure Mixtures after Plant Growth
3.7. Mineral Analysis and Calculation of Plant Nutrients Uptake
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fackrell, L.E. Plant growth experiments using martian simulants: Potential and limitations of agriculture on Mars. In Proceedings of the Geology Conference Paper; 2021; p. Abtract 1634. Available online: https://ui.adsabs.harvard.edu/abs/2021LPI....52.1684F/abstract (accessed on 25 October 2022).
- Gilrain, M.R.; Hogan, J.A.; Cowan, R.M.; Finstein, M.S.; Logendra, L.S. Preliminary Study of Greenhouse Grown Swiss Chard in Mixtures of Compost and Mars Regolith Simulant. In Proceedings of the SAE Technical Paper; 1999; p. No. 1999-01-2021. Available online: https://www.sae.org/publications/technical-papers/content/1999-01-2021/ (accessed on 25 October 2022).
- Mortley, D.G.; Aglan, H.A.; Bonsi, C.K.; Hill, W.A. Growth of Sweetpotato in Lunar and Mars Simulants. In Proceedings of the SAE Technical Paper; 2000; p. No. 2000-01-2289. Available online: https://www.sae.org/publications/technical-papers/content/2000-01-2289/ (accessed on 25 October 2022).
- Wamelink, G.W.W.; Frissel, J.Y.; Krijnen, W.H.J.; Verwoert, M.R.; Goedhart, P.W. Can Plants Grow on Mars and the Moon: A Growth Experiment on Mars and Moon Soil Simulants. PLoS ONE 2014, 9, e103138. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Vingiani, S.; Palladino, M.; El-Nakhel, C.; Duri, L.G.; Pannico, A.; Rouphael, Y.; De Pascale, S.; Adamo, P. Geo-mineralogical characterisation of Mars simulant MMS-1 and appraisal of substrate physico-chemical properties and crop performance obtained with variable green compost amendment rates. Sci. Total Environ. 2020, 720, 137543. [Google Scholar] [CrossRef] [PubMed]
- Duri, L.G.; El-Nakhel, C.; Caporale, A.G.; Ciriello, M.; Graziani, G.; Pannico, A.; Palladino, M.; Ritieni, A.; De Pascale, S.; Vingiani, S.; et al. Mars Regolith Simulant Ameliorated by Compost as in situ Cultivation Substrate Improves Lettuce Growth and Nutritional Aspects. Plants 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Aronne, G. Biometric anatomy of seedlings developed onboard of Foton-M2 in an automatic system supporting growth. Acta Astronaut. 2008, 62, 505–513. [Google Scholar] [CrossRef]
- Wheeler, R.M. Agriculture for Space: People and Places Paving the Way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Duri, L.G.; Caporale, A.G.; Rouphael, Y.; Vingiani, S.; Palladino, M.; De Pascale, S.; Adamo, P. The Potential for Lunar and Martian Regolith Simulants to Sustain Plant Growth: A Multidisciplinary Overview. Front. Astron. Space Sci. 2022, 8, 747821. [Google Scholar] [CrossRef]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef]
- Tibbitts, T.W.; Alford, D.K. Controlled Ecological Life Support System—Use of Higher Plants. In Proceedings of the NASA Conference Publication; NASA: O’Hare: Chicago, IL, USA, 1980; p. 2231. [Google Scholar]
- Wheeler, R.M. Plants for human life support in space: From Myers to Mars. Gravit. Space Res. 2010, 23, 25–35. [Google Scholar]
- Wheeler, R.M.; Stutte, G.W.; Subbarao, G.V.; Yorio, N.C. Plant growth and human life support for space travel. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; CRC Press-Taylor & Francis Group: Abingdon, UK, 2001; p. 18. [Google Scholar]
- Khodadad, C.L.M.; Hummerick, M.E.; Spencer, L.E.; Dixit, A.R.; Richards, J.T.; Romeyn, M.W.; Smith, T.M.; Wheeler, R.M.; Massa, G.D. Microbiological and Nutritional Analysis of Lettuce Crops Grown on the International Space Station. Front. Plant Sci. 2020, 11, 199. [Google Scholar] [CrossRef]
- Morrow, R.C.; Remiker, R.W.; Mischnick, M.J.; Tuominen, L.K.; Lee, M.C.; Crabb, T.M. A Low Equivalent System Mass Plant Growth Unit for Space Exploration; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Benaroya, H.; Metzger, P.; Muscatello, A. Special Issue on In Situ Resource Utilization. J. Aerosp. Eng. 2013, 26, 1–4. [Google Scholar] [CrossRef]
- Fackrell, L.E.; Schroeder, P.A. Growing Plants on Mars: Potential and Limitations of Martian Soil As a Plant Growth Medium Based on Results from Analogue Studies. In Proceedings of the ASA, CSSA and SSSA International Annual Meetings, Virtual, 9–13 November 2020. [Google Scholar]
- Yu, Q.; Hu, X.; Ma, J.; Ye, J.; Sun, W.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G.; Colla, G.; Fortezza, R.; De Pascale, S. Agro-biology for bioregenerative Life Support Systems in long-term Space missions: General constraints and the Italian efforts. J. Plant Interact. 2009, 4, 241–252. [Google Scholar] [CrossRef]
- Stoklosa, A.M.; Weiss, I.; Bugbee, B.; Perchonok, M.H.; Mauer, L.J. Composition and Functional Properties of Apogee and Perigee Compared to Common Terrestrial Wheat Cultivars. Int. J. Food Prop. 2011, 14, 996–1006. [Google Scholar] [CrossRef]
- Gregory, J.; Kirkegaard, J.A. Growth and Function of Root Systems. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murray, B.G., Murray, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 230–237. [Google Scholar]
- Agren, G.I.; Franklin, O. Root: Shoot Ratios, Optimization and Nitrogen Productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc. R. Soc. B Biol. Sci. 2012, 279, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root Architecture Responses: In Search of Phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Seiferlin, K.; Ehrenfreund, P.; Garry, J.; Gunderson, K.; Hütter, E.; Kargl, G.; Maturilli, A.; Merrison, J.P. Simulating Martian regolith in the laboratory. Planet. Space Sci. 2008, 56, 2009–2025. [Google Scholar] [CrossRef]
- Sajjad, N. Effect of organic fertilizers on physical attribute and organoleptic properties of Lettuce varieties. Pure Appl. Biol. 2020, 9, 1637–1645. [Google Scholar] [CrossRef]
- Caporale, A.G.; Palladino, M.; De Pascale, S.; Duri, L.G.; Rouphael, Y.; Adamo, P. How to make the Lunar and Martian soils suitable for food production—Assessing the changes after manure addition and implications for plant growth. J. Environ. Manag. 2023, 325, 116455. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of biochar and biochar particle size on plant-available water of sand, silt loam, and clay soil. Soil Tillage Res. 2021, 212, 104992. [Google Scholar] [CrossRef]
- Duggan, T.; Jones, P. Lettuce (Lactuca sativa ‘Webb’s Wonderful’) shoot and root growth in different grades of compost and vermicomposted compost. Acta Hortic. 2016, 1146, 33–40. [Google Scholar] [CrossRef]
- Roitsch, T. Source-sink regulation by sugar and stress. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef][Green Version]
- Murakami, T.; Yamada, K.; Yoshida, S. Root distribution of field-grown crisphead lettuce (Lactuca sativa L.) under different fertilizer and mulch treatment. Soil Sci. Plant Nutr. 2002, 48, 347–355. [Google Scholar] [CrossRef]
- Rowse, H.R. The effect of irrigation on the length, weight, and diameter of lettuce roots. Plant Soil 1974, 40, 381–391. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of Internal and External Factors on Root Growth and Development. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 331–346. [Google Scholar]
- Jackson, L.E.; Stivers, L.J. Root Distribution of Lettuce under Commercial Production: Implications for Crop Uptake of Nitrogen. Biol. Agric. Hortic. 1993, 9, 273–293. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Sarker, B.C.; Karmoker, J. Effects of phosphorus deficiency on the root growth of lentil seedlings grown in rhizobox. Bangladesh J. Bot. 2009, 38, 215–218. [Google Scholar] [CrossRef]
- Beroueg, A.; Lecompte, F.; Mollier, A.; Pagès, L. Genetic Variation in Root Architectural Traits in Lactuca and Their Roles in Increasing Phosphorus-Use-Efficiency in Response to Low Phosphorus Availability. Front. Plant Sci. 2021, 12, 658321. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rojas-Pierce, M.; Yan, X.; Blair, M.W.; Pedraza, F.; Muñoz, F.; Tohme, J.; Lynch, J.P. Quantitative Trait Loci for Root Architecture Traits Correlated with Phosphorus Acquisition in Common Bean. Crop Sci. 2006, 46, 413–423. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Root strategies for phosphorus acquisition. In Plant Ecophysiology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 83–116. [Google Scholar]
- Carr, M.K.V.; Dodds, S.M. Some Effects of Soil Compaction on Root Growth and Water Use of Lettuce. Exp. Agric. 1983, 19, 117. [Google Scholar] [CrossRef]
- Taylor, S.A.; Ashcroft, G.L. Physical Edaphology: The Physics of Irrigated and Nonirrigated Soils; Freeman, W.H., Ed.; W.H. Freeman Co.: San Francisco, CA, USA, 1972; ISBN 0716708183. [Google Scholar]
- Mortimer, S.R. Root length/leaf area ratios of chalk grassland perennials and their importance for competitive interactions. J. Veg. Sci. 1992, 3, 665–673. [Google Scholar] [CrossRef]
- Klepper, B. Root-shoot relationships. In Plant Roots: The Hidden Half; Waisel, Y., Kafkafi, U., Eshel, A., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 265–286. [Google Scholar]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef]
- Butler, A.; Marimon-Junior, B.; Maracahipes, L.; Marimon, B.S.; Silvério, D.V.; Oliveira, E.A.; Lenza, E.; Feldpausch, T.R.; Meir, P.; Grace, J. Absorbing Roots Areas and Transpiring Leaf Areas at the Tropical Forest and Savanna Boundary in Brazil. In Savannas: Climate, Biodiversity and Ecological Significance; Perrault, C., Bellamy, L., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 107–126. [Google Scholar]
- Atkin, R.K.; Barton, G.E.; Robinson, D.K. Effect of Root-growing Temperature on Growth Substances in Xylem Exudate of Zea mays. J. Exp. Bot. 1973, 24, 475–487. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J.J.W.A. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef]
- Hasenstein, K.H.; van Loon, J.J.W.A. Clinostats and other rotating systems—Design, function, and limitations. In Generation and Applications of Extra-Terrestrial Environments on Earth; Beysens, D.A., van Loon, J.J.W.A., Eds.; River Publishers: Delft, The Netherlands, 2015; pp. 147–156. ISBN 978-879323754-4/978-879323753-7. [Google Scholar]
- Kiss, J.Z.; Millar, K.D.L.; Edelmann, R.E. Phototropism of Arabidopsis thaliana in microgravity and fractional gravity on the International Space Station. Planta 2012, 236, 635–645. [Google Scholar] [CrossRef]
- Rivera, C.M.; Battistelli, A.; Moscatello, S.; Proietti, S.; Rouphael, Y.; Cardarelli, M.; Colla, G. Influence of simulated microgravity on growth, yield, and quality of leafy vegetables: Lettuce and rocket. Eur. J. Hortic. Sci. 2006, 71, 45–51. [Google Scholar]
- Manzano, A.; Herranz, R.; den Toom, L.A.; te Slaa, S.; Borst, G.; Visser, M.; Medina, F.J.; van Loon, J.J.W.A. Novel, Moon and Mars, partial gravity simulation paradigms and their effects on the balance between cell growth and cell proliferation during early plant development. NPJ Microgravity 2018, 4, 9. [Google Scholar] [CrossRef]
- Paul, A.-L.; Amalfitano, C.E.; Ferl, R.J. Plant growth strategies are remodeled by spaceflight. BMC Plant Biol. 2012, 12, 232. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative Study on Several Determination Methods of Chlorophyll Content in Plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Giordano, M.; Pannico, A.; Carillo, P.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Rouphael Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space. Life 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Maggiore, T. Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharvest Biol. Technol. 2007, 45, 73–80. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Moran, M.S.; Papuga, S.A.; Thorp, K.R.; Alonso, L.; Moreno, J.; Ponce-Campos, G.; Rascher, U.; Wang, G. Plant chlorophyll fluorescence: Active and passive measurements at canopy and leaf scales with different nitrogen treatments. J. Exp. Bot. 2016, 67, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Burger, M.; Yang, L.; Gong, P.; Wu, Z. Changes in Soil Carbon and Enzyme Activity As a Result of Different Long-Term Fertilization Regimes in a Greenhouse Field. PLoS ONE 2015, 10, e0118371. [Google Scholar] [CrossRef]
- Sax, M.S.; Scharenbroch, B.C. Assessing Alternative Organic Amendments as Horticultural Substrates for Growing Trees in Containers1. J. Environ. Hortic. 2017, 35, 66–78. [Google Scholar] [CrossRef]
- Werheni Ammeri, R.; Di Rauso Simeone, G.; Hidri, Y.; Abassi, M.S.; Mehri, I.; Costa, S.; Hassen, A.; Rao, M.A. Combined bioaugmentation and biostimulation techniques in bioremediation of pentachlorophenol contaminated forest soil. Chemosphere 2022, 290, 133359. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.P. (Ed.) Methods of Soil Enzymology; SSSA Book Series; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2011; ISBN 9780891188582. [Google Scholar]
- Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Martínez, F.; Castillo, S.; Pérez, S.; Carmona, E.; Ordovás, J.; Avilés, M. Effect of different soilless growing systems on biological properties of growth media in strawberry. Acta Hortic. 2005, 697, 417–423. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, P.K.; Niranjan, A.; Tewari, S.K. Use of a Bioaugmented Organic Soil Amendment in Combination with Gypsum for Withania somnifera Growth on Sodic Soil. Pedosphere 2016, 26, 299–309. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Soil zymography as a powerful tool for exploring hotspots and substrate limitation in undisturbed subsoil. Soil Biol. Biochem. 2018, 124, 210–217. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Zarebanadkouki, M.; Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Spatiotemporal patterns of enzyme activities in the rhizosphere: Effects of plant growth and root morphology. Biol. Fertil. Soils 2018, 54, 819–828. [Google Scholar] [CrossRef]
- Hummel, C.; Boitt, G.; Santner, J.; Lehto, N.J.; Condron, L.; Wenzel, W.W. Co-occurring increased phosphatase activity and labile P depletion in the rhizosphere of Lupinus angustifolius assessed with a novel, combined 2D-imaging approach. Soil Biol. Biochem. 2021, 153, 107963. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation—Coupling soil zymography with 14C imaging. Soil Biol. Biochem. 2013, 67, 106–113. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Bacon, J.; Gomez, A.; Muntau, H.; Queaviller, P. Certification of the Content (Mass Fraction) of Cd, Cr, Cu, Ni, Pb and Zn in an Organic-Rich Soil Following Harmonised EDTA and Acetic acid Extraction Procedures; BCR 700; Report EUR 19774 EN; EU publications: Luxembourg, 2001. [Google Scholar]
- Leitão, N.; Dangeville, P.; Carter, R.; Charpentier, M. Nuclear calcium signatures are associated with root development. Nat. Commun. 2019, 10, 4865. [Google Scholar] [CrossRef]
- Qin, L.; Guo, S.; Ai, W.; Tang, Y.; Cheng, Q.; Chen, G. Effect of salt stress on growth and physiology in amaranth and lettuce: Implications for bioregenerative life support system. Adv. Space Res. 2013, 51, 476–482. [Google Scholar] [CrossRef]
- Jones, S.B.; Or, D. Microgravity effects on water flow and distribution in unsaturated porous media: Analyses of flight experiments. Water Resour. Res. 1999, 35, 929–942. [Google Scholar] [CrossRef]
- Heinse, R.; Jones, S.B.; Or, D.; Podolskiy, I.; Topham, T.S.; Poritz, D.; Bingham, G.E. Microgravity Oxygen Diffusion and Water Retention Measurements in Unsaturated Porous Media aboard the International Space Station. Vadose Zone J. 2015, 14, 135. [Google Scholar] [CrossRef]
- Kitaya, Y. Plant Factory and Space Development, “Space Farm”. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; pp. 363–379. [Google Scholar]
- Schlegel, A.J. Effect of Composted Manure on Soil Chemical Properties and Nitrogen Use by Grain Sorghum. J. Prod. Agric. 1992, 5, 153–157. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl. Soil Ecol. 2014, 78, 28–36. [Google Scholar] [CrossRef]
- Bie, Z.; Ito, T.; Shinohara, Y. Effects of sodium sulfate and sodium bicarbonate on the growth, gas exchange and mineral composition of lettuce. Sci. Hortic. 2004, 99, 215–224. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Cramer, G.R. Uptake and role of ions in salt tolerance. In Strategies for Improving Salt Tolerance in Higher Plants; Jaiwal, P.K., Singh, R.P., Gulati, A., Eds.; Oxford & IBH Publishing Co. Pvt. Ltd: New Delhi, India, 1997; pp. 55–86. [Google Scholar]
- Lazof, D.B.; Bernstein, N. The NaCl Induced Inhibition of Shoot Growth: The Case for Disturbed Nutrition with Special Consideration of Calcium. Adv. Bot. Res. 1998, 29, 113–189. [Google Scholar]
- Duri, L.G.; Pannico, A.; Petropoulos, S.A.; Caporale, A.G.; Adamo, P.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Bioactive Compounds and Antioxidant Activity of Lettuce Grown in Different Mixtures of Monogastric-Based Manure With Lunar and Martian Soils. Front. Nutr. 2022, 9, 890786. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Beare, M.H. Alkaline Persulfate Oxidation for Determining Total Nitrogen in Microbial Biomass Extracts. Soil Sci. Soc. Am. J. 1993, 57, 1007–1012. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 9780125138406. [Google Scholar]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef]


| Source of Variance | Growth Index | Leaf Number | Leaf Area | Dry Biomass | Dry Matter |
|---|---|---|---|---|---|
| No. plant−1 | cm2 | g plant−1 | % | ||
| Simulants (S) | |||||
| MMS1 | 1458 ± 266 | 13.75 ± 1.14 | 450 ± 79.1 | 4.33 ± 0.75 | 16.1 ± 1.77 |
| LHS1 | 512 ± 109 | 8.91 ± 0.62 | 127 ± 28.1 | 1.38 ± 0.32 | 18.2 ± 2.19 |
| *** | *** | *** | *** | *** | |
| Amendment (%) (M) | |||||
| 0 | 31.8 ± 6.09 c | 6.69 ± 0.44 d | 16.8 ± 4.50 d | 0.43 ± 0.07 c | 27.7 ± 1.13 a |
| 10 | 1153 ± 208 b | 13.29 ± 1.25 b | 355 ± 74.2 b | 4.34 ± 0.81 a | 16.9 ± 0.63 b |
| 30 | 1645 ± 311 a | 14.08 ± 1.47 a | 490 ± 113 a | 4.56 ± 1.05 a | 13.3 ± 0.54 c |
| 50 | 1109 ± 352 b | 11.25 ± 1.28 c | 292 ± 99.0 c | 2.09 ± 0.76 b | 10.7 ± 0.48 d |
| *** | *** | *** | *** | *** | |
| S × M | |||||
| MMS1 × 0 | 45.0 ± 2.78 de | 7.56 ± 0.29 e | 26.5 ± 2.7 f | 0.58 ± 0.07 e | 25.3 ± 0.17 b |
| MMS1 × 10 | 1588 ± 153 b | 16.00 ± 0.67 b | 521 ± 14.2 b | 6.10 ± 0.30 b | 16.5 ± 0.32 c |
| MMS1 × 30 | 2332 ± 105 a | 17.33 ± 0.19 a | 740 ± 23.7 a | 6.88 ± 0.32 a | 12.8 ± 0.76 de |
| MMS1 × 50 | 1866 ± 218 b | 14.11 ± 0.11 c | 512 ± 24.1 b | 3.77 ± 0.25 c | 9.77 ± 0.29 f |
| LHS1 × 0 | 18.6 ± 1.80 e | 5.83 ± 0.36 f | 7.2 ± 0.2 f | 0.29 ± 0.02 e | 30.1 ± 0.80 a |
| LHS1 × 10 | 717 ± 56.2 c | 10.58 ± 0.22 d | 190 ± 7.2 d | 2.58 ± 0.29 d | 17.2 ± 1.33 c |
| LHS1 × 30 | 958 ± 44.3 c | 10.83 ± 0.44 d | 240 ± 17.6 c | 2.23 ± 0.20 d | 13.9 ± 0.76 d |
| LHS1 × 50 | 353 ± 39.0 d | 8.39 ± 0.20 e | 71.8 ± 4.7 e | 0.40 ± 0.02 e | 11.6 ± 0.48 ef |
| *** | *** | *** | *** | * |
| Source of Variance | Root Dry Mass | Root Length | Root Surface Area | Root Volume | Specific Root Surface |
|---|---|---|---|---|---|
| g plant−1 | m plant−1 | cm2 10−2 plant−1 | cm3 plant−1 | m2 g−1 | |
| Simulants (S) | |||||
| MMS1 | 0.84 ± 0.18 | 158.52± 29.92 | 17.28 ± 3.11 | 15.2 ± 2.53 | 0.23 ± 0.02 |
| LHS1 | 0.30 ± 0.08 | 57.21 ± 13.70 | 8.93 ± 2.23 | 11.3 ± 2.80 | 0.30 ± 0.05 |
| * | ** | * | n.s. | n.s. | |
| Amendment (%) (M) | |||||
| 0 | 0.07 ± 0.01 d | 8.74 ± 1.47 c | 1.39 ± 0.24 c | 1.85 ± 0.34 d | 0.18 ± 0.02 b |
| 10 | 0.40 ± 0.06 c | 116.26 ± 15.45 b | 15.11 ± 1.49 b | 16.28 ± 1.51 b | 0.43 ± 0.04 a |
| 30 | 1.08 ± 0.13 a | 180.67 ± 22.67 a | 21.81 ± 1.71 a | 21.99 ± 1.47 a | 0.23 ± 0.02 b |
| 50 | 0.71 ± 0.2 b | 125.78 ±33.17 b | 14.11 ± 3.32 b | 12.87 ± 2.57 c | 0.22 ± 0.01 b |
| ** | ** | ** | ** | ** | |
| S × M | |||||
| MMS1 × 0 | 0.09 ± 0.01 e | 13.15 ± 0.70 f | 2.01 ± 0.22 d | 2.55 ± 0.40 de | 0.21 ± 0.02 c |
| MMS1 × 10 | 0.60 ± 0.01 c | 163.24 ± 2.23 c | 18.88 ± 0.51 b | 17.62 ± 0.98 bc | 0.32 ± 0.01. b |
| MMS1 × 30 | 1.46 ± 0.01 a | 243.83 ± 12.90 a | 25.44 ± 1.02 a | 21.28 ± 0.64 a | 0.17 ± 0.03 d |
| MMS1 × 50 | 1.18 ± 0.21 b | 213.8 ±28.38 b | 22.79 ± 2.96 a | 19.3 ± 2.45 b | 0.21 ±0.01 c |
| LHS1 × 0 | 0.05 ± 0.00 e | 4.33 ± 0.30 f | 0.77 ± 0.04 e | 1.17 ± 0.09 e | 0.16 ± 0.01 d |
| LHS1 × 10 | 0.21 ± 0.21 d | 69.29 ± 6.24 e | 11.35 ± 1.32 c | 14.94 ± 2.09 c | 0.54 ± 0.01 a |
| LHS1 × 30 | 0.69 ± 0.06 c | 117.51 ± 10.94 d | 18.18± 1.73 b | 22.70 ± 2.20 a | 0.28 ± 0.03 bc |
| LHS1 × 50 | 0.24 ± 0.02 d | 37.71 ± 1.34. f | 5.44 ± 0.09 d | 6.39 ± 0.04 d | 0.24 ± 0.01 c |
| ** | ** | ** | ** | ** |
| Source of Variance | SPAD Index | Fluorescence |
|---|---|---|
| Fv/Fm Ratio | ||
| Simulants (S) | ||
| MMS1 | 12.40 ± 0.53 | 0.738 ± 0.02 |
| LHS1 | 10.51 ± 0.57 | 0.721 ± 0.03 |
| *** | ns | |
| Amendment (%) (M) | ||
| 0 | 9.27 ± 0.57 c | 0.579 ± 0.04 b |
| 10 | 11.63 ± 0.35 b | 0.756 ± 0.01 a |
| 30 | 13.48 ± 0.53 a | 0.803 ± 0.00 a |
| 50 | 11.42 ± 0.93 b | 0.781 ± 0.01 a |
| *** | *** | |
| S × M | ||
| MMS1 × 0 | 10.12 ± 0.59 | 0.612 ± 0.03 |
| MMS1 × 10 | 11.79 ± 0.71 | 0.744 ± 0.00 |
| MMS1 × 30 | 14.31 ± 0.48 | 0.800 ± 0.01 |
| MMS1 × 50 | 13.36 ± 0.10 | 0.798 ± 0.01 |
| LHS1 × 0 | 8.41 ± 0.75 | 0.547 ± 0.08 |
| LHS1 × 10 | 11.46 ± 0.30 | 0.767 ± 0.00 |
| LHS1 × 30 | 12.66 ± 0.70 | 0.806 ± 0.00 |
| LHS1 × 50 | 9.49 ± 0.75 | 0.765 ± 0.01 |
| ns | ns |
| Source of Variance | Extr. N | Extr. C | MBN | MBC |
|---|---|---|---|---|
| mg kg−1 DW | ||||
| Simulants (S) | ||||
| MMS1 | 78.0 ± 56.4 | 152.6 ± 101.0 | 77.8 ± 50.3 | 397.4 ± 350.2 |
| LHS1 | 75.0 ± 52.7 | 185.2 ± 120.2 | 92.0 ± 67.6 | 523.2 ± 548.4 |
| *** | *** | *** | *** | |
| Amendment % (M) | ||||
| 0 | 11.6 ± 2.9 d | 32.5 ± 10.6 d | 11.4 ± 3.5 d | 46.2 ± 17.2 d |
| 10 | 39.9 ± 4.3 c | 111.3 ± 15.0 c | 57.5 ± 9.9 c | 152.6 ± 42.7 c |
| 30 | 112.6 ± 11.8 b | 218.6 ± 48.9 b | 110.4 ± 34 b | 500.6 ± 152.3 b |
| 50 | 141.7 ± 20.9 a | 313.1 ± 40.0 a | 160.2 ± 20.9 a | 1141.8 ± 304.1 a |
| *** | *** | *** | *** | |
| Rhizo vs. bulk soil (RB) | ||||
| RH | 81.7 ± 60.9 | 166.4 ± 110.5 | 94.9 ± 66.4 | 450.6 ± 427.6 |
| BK | 71.3 ± 47.0 | 171.4 ± 113.9 | 74.8 ± 50.9 | 470.0 ± 498.4 |
| *** | ns | *** | ns | |
| S × M × RB | ||||
| MMS1 × 0 × RH | 10.3 ± 1.0 f | 32.1 ± 7.0 | 12.5 ± 2.2 k | 51.0 ± 15.9 |
| MMS1 × 0 × BK | 10.4 ± 1.0 f | 21.5 ± 8.0 | 12.6 ± 2.8 k | 37.0 ± 12.6 |
| MMS1 × 10 × RH | 35.0 ± 2.2 e | 96.4 ± 10.2 | 69.1 ± 4.0 h | 146.4 ± 26.4 |
| MMS1 × 10 × BK | 43.1 ± 2.6 d | 112.0 ± 15.3 | 59.6 ± 2.8 i | 183.8 ± 43.9 |
| MMS1 × 30 × RH | 118.7 ± 5.1 b | 181.2 ± 25.5 | 92.6 ± 3.4 f | 483.6 ± 137.9 |
| MMS1 × 30 × BK | 122.7 ± 5.0 b | 201.9 ± 35.5 | 78.0 ± 6.5 g | 470.0 ± 138.8 |
| MMS1 × 50 × RH | 162.7 ± 5.0 a | 287.0 ± 29.7 | 163.3 ± 8.6 b | 843.0 ± 158.2 |
| MMS1 × 50 × BK | 120.2 ± 6.5 b | 288.7 ± 32.8 | 134.0 ± 5.9 d | 964.5 ± 128.3 |
| LHS1 × 0 × RH | 14.1 ± 4.2 g | 44.0 ± 7.1 | 10.0 ± 4.7 k | 63.4 ± 11.6 |
| LHS1 × 0 × BK | 11.5 ± 2.9 g | 32.4 ± 7.1 | 10.4 ± 3.5 k | 33.6 ± 11.7 |
| LHS1 × 10 × RH | 37.7 ± 2.0 e | 119.7 ± 12.9 | 58.2 ± 1.5 i | 140.2 ± 42.6 |
| LHS1 × 10 × BK | 43.5 ± 2.6 d | 117.4 ± 11.7 | 42.9 ± 1.2 j | 140.2 ± 48.5 |
| LHS1 × 30 × RH | 114.3 ± 5.2 b | 216.0 ± 30.3 | 164.7 ± 5.6 b | 618.8 ± 171.2 |
| LHS1 × 30 × BK | 94.7 ± 2.6 c | 275.3 ± 48.0 | 106.1 ± 4.4 e | 429.8 ± 121.9 |
| LHS1 × 50 × RH | 160.0 ± 4.9 a | 355.0 ± 21.9 | 188.6 ± 5.8 a | 1258.7 ± 202.1 |
| LHS1 × 50 × BK | 123.7 ± 5.8 b | 321.8 ± 34.7 | 154.7 ± 4.4 c | 1500.8 ± 165.0 |
| *** | ns | *** | ns | |
| S × M | *** | ** | *** | *** |
| Source of Variance | Ca | K | Mg | P | Fe | Na | Mn | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 DW | |||||||||
| Simulants (S) | |||||||||
| MMS1 | 2215 | 768 | 416 | 11.8 | 0.69 | 45.2 | 1.57 | 0.15 | 0.10 |
| LHS1 | 1102 | 490 | 246 | 8.46 | 1.45 | 69.2 | 0.67 | 0.17 | 0.13 |
| *** | *** | *** | *** | *** | * | *** | * | *** | |
| Amendment % (M) | |||||||||
| 0 | 1201 d | 90.3 d | 156 d | 0.10 d | 0.04 c | 31.3 b | 0.24 b | 0.04 d | 0.03 d |
| 10 | 1551 c | 338 c | 227 c | 6.08 c | 0.84 b | 27.5 b | 1.48 a | 0.14 c | 0.10 c |
| 30 | 1869 b | 707 b | 388 b | 14.9 b | 1.52 a | 39.4 b | 1.39 a | 0.19 b | 0.14 b |
| 50 | 2012 a | 1382 a | 553 a | 19.5 a | 1.87 a | 130 a | 1.37 a | 0.27 a | 0.19 a |
| *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Rhizo vs. bulk soil (RB) | |||||||||
| RH | 1634 | 527 | 303 | 9.82 | 1.12 | 51 | 1.13 | 0.16 | 0.11 |
| BK | 1683 | 731 | 359 | 10.5 | 1.01 | 63 | 1.11 | 0.16 | 0.12 |
| ns | ** | *** | ns | ns | ns | ns | ns | ns | |
| S × M × RB | |||||||||
| MMS1 × 0 × RH | 1950 | 147 | 260 | 0.13 | 0.04 | 27.3 | 0.08 | 0.06 | 0.02 |
| MMS1 × 0 × BK | 2124 | 187 | 296 | 0.13 | 0.04 | 36.9 | 0.06 | 0.06 | 0.03 |
| MMS1 × 10 × RH | 2258 | 421 | 334 | 7.39 | 0.19 | 29.5 | 2.22 | 0.11 | 0.06 |
| MMS1 × 10 × BK | 2176 | 558 | 375 | 9.28 | 0.59 | 30.8 | 2.39 | 0.12 | 0.09 |
| MMS1 × 30 × RH | 2339 | 671 | 406 | 18.1 | 0.85 | 29.0 | 2.01 | 0.17 | 0.11 |
| MMS1 × 30 × BK | 2183 | 1106 | 484 | 18.8 | 0.99 | 38.2 | 1.94 | 0.17 | 0.12 |
| MMS1 × 50 × RH | 2358 | 1254 | 528 | 19.5 | 1.61 | 66.3 | 2.00 | 0.25 | 0.16 |
| MMS1 × 50 × BK | 2330 | 1801 | 644 | 21.4 | 1.18 | 103 | 1.87 | 0.23 | 0.17 |
| LHS1 × 0 × RH | 330 | 15.9 | 45.8 | 0.09 | 0.04 | 29.7 | 0.45 | 0.03 | 0.03 |
| LHS1 × 0 × BK | 398 | 12.0 | 24.4 | 0.05 | 0.03 | 31.4 | 0.39 | 0.03 | 0.03 |
| LHS1 × 10 × RH | 796 | 147 | 78.4 | 2.80 | 1.16 | 26.7 | 0.58 | 0.13 | 0.08 |
| LHS1 × 10 × BK | 976 | 225 | 122 | 4.87 | 1.42 | 23.1 | 0.72 | 0.19 | 0.16 |
| LHS1 × 30 × RH | 1377 | 472 | 289 | 12.5 | 2.59 | 36.8 | 0.85 | 0.23 | 0.18 |
| LHS1 × 30 × BK | 1576 | 579 | 372 | 10.4 | 1.65 | 53.8 | 0.77 | 0.18 | 0.15 |
| LHS1 × 50 × RH | 1663 | 1092 | 486 | 18.1 | 2.52 | 163 | 0.88 | 0.30 | 0.22 |
| LHS1 × 50 × BK | 1697 | 1379 | 553 | 18.9 | 2.17 | 189 | 0.71 | 0.28 | 0.20 |
| ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| S × M | *** | ns | *** | *** | * | ** | *** | ** | ns |
| Source of Variance | PO4 | K | Mg | Ca | SO4 | Na |
|---|---|---|---|---|---|---|
| g kg−1 DW | ||||||
| Simulants (S) | ||||||
| MMS1 | 4.10 ± 0.73 | 26.53 ± 2.97 | 6.32 ± 0.60 | 1.69 ± 0.14 | 0.72 ± 0.14 | 1.89 ± 0.20 |
| LHS1 | 3.54 ± 0.59 | 24.82 ± 1.79 | 5.69 ± 0.43 | 2.10 ± 0.11 | 0.66 ± 0.08 | 2.17 ± 0.37 |
| ns | ns | * | *** | ns | ns | |
| Amendment % (M) | ||||||
| 0 | 0.53 ± 0.02 c | 16.01 ± 0.33 c | 3.65 ± 0.15 c | 1.65 ± 0.11 bc | 0.21 ± 0.02 c | 1.41 ± 0.21 b |
| 10 | 3.89 ± 0.15 b | 24.50 ± 0.93 b | 5.78 ± 0.28 b | 1.55 ± 0.10 c | 0.67 ± 0.09 b | 1.48 ± 0.19 b |
| 30 | 4.67 ± 0.21 b | 25.94 ± 0.34 b | 7.04 ± 0.42 a | 1.90 ± 0.19 b | 0.72 ± 0.03 b | 1.83 ± 0.27 b |
| 50 | 6.19 ± 0.64 a | 36.25 ± 3.33 a | 7.56 ± 0.61 a | 2.49 ± 0.11 a | 1.14 ± 0.13 a | 3.41 ± 0.33 a |
| *** | *** | *** | *** | *** | *** | |
| S × M | ||||||
| MMS1 × 0 | 0.56 ± 0.02 | 15.93 ± 0.26 d | 3.49 ± 0.21 e | 1.44 ± 0.12 | 0.20 ± 0.02 d | 1.21 ± 0.29 d |
| MMS1 × 10 | 3.83 ± 0.29 | 23.07 ± 1.19 b | 6.29 ± 0.35 bcd | 1.35 ± 0.02 | 0.50 ± 0.11 c | 1.47 ± 0.24 cd |
| MMS1 × 30 | 5.07 ± 0.12 | 25.32 ± 0.43 bc | 6.64 ± 0.67 bc | 1.54 ± 0.13 | 0.78 ± 0.03 b | 2.18 ± 0.00 bc |
| MMS1 × 50 | 6.95 ± 0.82 | 41.80 ± 3.59 a | 8.87 ± 0.18 a | 2.45 ± 0.13 | 1.40 ± 0.11 a | 2.70 ± 0.16 b |
| LHS1 × 0 | 0.51 ± 0.04 | 16.10 ± 0.68 d | 3.80 ± 0.21 e | 1.85 ± 0.07 | 0.23 ± 0.03 d | 1.61 ± 0.30 cd |
| LHS1 × 10 | 3.95 ± 0.15 | 25.93 ± 0.93 bc | 5.27 ± 0.12 d | 1.76 ± 0.07 | 0.85 ± 0.04 b | 1.50 ± 0.35 cd |
| LHS1 × 30 | 4.26 ± 0.18 | 26.56 ± 0.06 bc | 7.44 ± 0.53 b | 2.26 ± 0.18 | 0.67 ± 0.04 bc | 1.47 ± 0.48 cd |
| LHS1 × 50 | 5.43 ± 0.90 | 30.69 ± 3.39 b | 6.25 ± 0.29 cd | 2.53 ± 0.21 | 0.88 ± 0.10 b | 4.11 ± 0.18 a |
| ns | ** | *** | ns | *** | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporale, A.G.; Amato, M.; Duri, L.G.; Bochicchio, R.; De Pascale, S.; Simeone, G.D.R.; Palladino, M.; Pannico, A.; Rao, M.A.; Rouphael, Y.; et al. Can Lunar and Martian Soils Support Food Plant Production? Effects of Horse/Swine Monogastric Manure Fertilisation on Regolith Simulants Enzymatic Activity, Nutrient Bioavailability, and Lettuce Growth. Plants 2022, 11, 3345. https://doi.org/10.3390/plants11233345
Caporale AG, Amato M, Duri LG, Bochicchio R, De Pascale S, Simeone GDR, Palladino M, Pannico A, Rao MA, Rouphael Y, et al. Can Lunar and Martian Soils Support Food Plant Production? Effects of Horse/Swine Monogastric Manure Fertilisation on Regolith Simulants Enzymatic Activity, Nutrient Bioavailability, and Lettuce Growth. Plants. 2022; 11(23):3345. https://doi.org/10.3390/plants11233345
Chicago/Turabian StyleCaporale, Antonio G., Mariana Amato, Luigi G. Duri, Rocco Bochicchio, Stefania De Pascale, Giuseppe Di Rauso Simeone, Mario Palladino, Antonio Pannico, Maria A. Rao, Youssef Rouphael, and et al. 2022. "Can Lunar and Martian Soils Support Food Plant Production? Effects of Horse/Swine Monogastric Manure Fertilisation on Regolith Simulants Enzymatic Activity, Nutrient Bioavailability, and Lettuce Growth" Plants 11, no. 23: 3345. https://doi.org/10.3390/plants11233345
APA StyleCaporale, A. G., Amato, M., Duri, L. G., Bochicchio, R., De Pascale, S., Simeone, G. D. R., Palladino, M., Pannico, A., Rao, M. A., Rouphael, Y., & Adamo, P. (2022). Can Lunar and Martian Soils Support Food Plant Production? Effects of Horse/Swine Monogastric Manure Fertilisation on Regolith Simulants Enzymatic Activity, Nutrient Bioavailability, and Lettuce Growth. Plants, 11(23), 3345. https://doi.org/10.3390/plants11233345










