Productive, Morpho-Physiological, and Postharvest Performance of Six Basil Types Grown in a Floating Raft System: A Comparative Study
Abstract
1. Introduction
2. Results
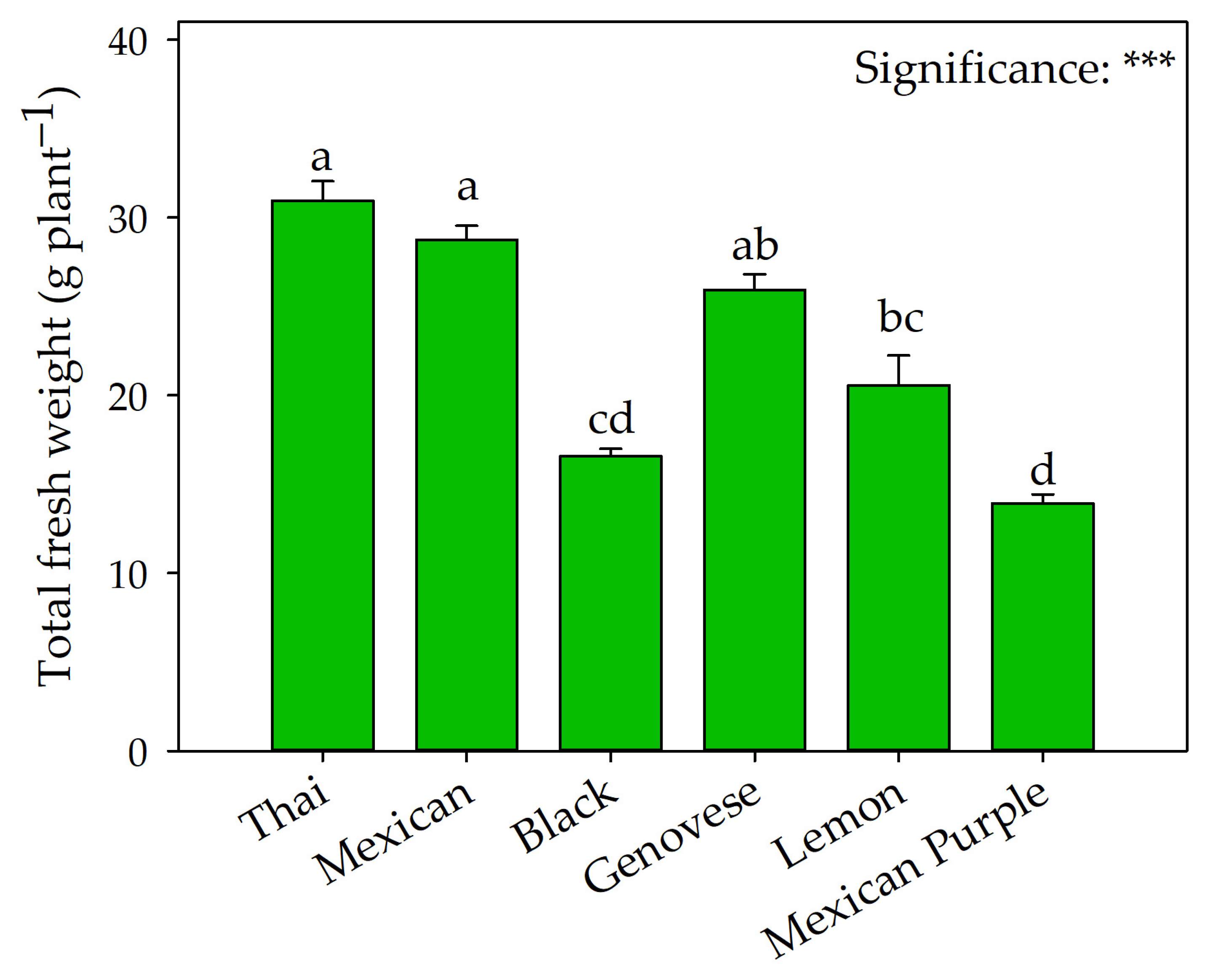
2.1. Yield and Biometric Indices
2.2. Morpho-Physiological Traits
2.3. Ascorbic Acid Concentration and Minerals
3. Discussion
3.1. Underexplored Basil Varieties Are Suitable for Floating Cultivation
3.2. The Morphophysiological Traits That Make the Difference
3.3. Mineral Profile, Pigments, and Secondary Metabolites Differ among Basil Varieties
4. Materials and Methods
4.1. Growth System, Plant Material, and Experimental Design
4.2. Collection, Processing, and Storage of Plant Samples
4.3. Shelf Life Evaluation
4.4. Determination of Leaf Gas Exchange and SPAD Index
4.5. Determination of Mineral Concentration
4.6. Determination of the Concentration of Pigments and Ascorbic Acid
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the drying kinetics and energy efficiency of purple basil (Ocimum basilicum L.) leaves using different drying methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.; Chaicumpa, W.; Michalak, I. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev. Int. 2021, 58, 1–29. [Google Scholar] [CrossRef]
- De Masi, L.; Siviero, P.; Esposito, C.; Castaldo, D.; Siano, F.; Laratta, B. Assessment of agronomic, chemical and genetic variability in common basil (Ocimum basilicum L.). Eur. Food Res. Technol. 2006, 223, 273–281. [Google Scholar] [CrossRef]
- Barátová, S.; Mezeyová, I.; Hegedúsová, A.; Andrejiová, A. Impact of biofortification, variety and cutting on chosen qualitative characteristic of basil (Ocimum basilicum L.). Acta Fytotech. Zootech. 2015, 18, 71–75. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M. Agro-morphological and phenotypic variability of sweet basil genotypes for breeding purposes. Crop Sci. 2021, 61, 621–642. [Google Scholar] [CrossRef]
- Bajomo, E.M.; Aing, M.S.; Ford, L.S.; Niemeyer, E.D. Chemotyping of commercially available basil (Ocimum basilicum L.) varieties: Cultivar and morphotype influence phenolic acid composition and antioxidant properties. NFS J. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Zeljković, S.Ć.; Komzáková, K.; Šišková, J.; Karalija, E.; Smékalová, K.; Tarkowski, P. Phytochemical variability of selected basil genotypes. Ind. Crops Prod. 2020, 157, 112910. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive harvests modulate the productive and physiological behavior of three genovese pesto basil cultivars. Agronomy 2021, 11, 560. [Google Scholar] [CrossRef]
- Srbinovska, A.; Gasparotto, L.; Conchione, C.; Ursol, L.M.; Lambertini, F.; Suman, M.; Moret, S. Mineral oil contamination in basil pesto from the Italian market: Ingredient contribution and market survey. J. Food Compos. Anal. 2023, 115, 104914. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Corrado, G.; Pannico, A.; De Pascale, S.; Rouphael, Y. Morpho-physiological responses and secondary metabolites modulation by preharvest factors of three hydroponically grown genovese basil cultivars. Front. Plant Sci. 2021, 12, 671026. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Vitaglione, P.; Chiaiese, P.; Rouphael, Y. Unraveling the Modulation of Controlled Salinity Stress on Morphometric Traits, Mineral Profile, and Bioactive Metabolome Equilibrium in Hydroponic Basil. Horticulturae 2021, 7, 273. [Google Scholar] [CrossRef]
- Tesi, R. Orticoltura Mediterranea Sostenibile; Pàtron Editore: Bologna, Italy, 2010; pp. 1–503. [Google Scholar]
- Ciriello, M.; Pannico, A.; El-Nakhel, C.; Formisano, L.; Cristofano, F.; Duri, L.G.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Colla, G. Sweet basil functional quality as shaped by genotype and macronutrient concentration reciprocal action. Plants 2020, 9, 1786. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Couto, H.G.S.; Blank, A.F.; e Silva, A.M.D.O.; de Lima Nogueira, P.C.; de Fátima Arrigoni-Blank, M.; de Castro Nizio, D.A.; de Oliveira Pinto, J.A. Essential oils of basil chemotypes: Major compounds, binary mixtures, and antioxidant activity. Food Chem. 2019, 293, 446–454. [Google Scholar] [CrossRef]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C. Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum cv.‘Cinnamon’) and lemon basil (Ocimum× citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into composition of bioactive phenolic compounds in leaves and flowers of green and purple basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef]
- Costa, L.; Montano, Y.M.; Carrión, C.; Rolny, N.; Guiamet, J.J. Application of low intensity light pulses to delay postharvest senescence of Ocimum basilicum leaves. Postharvest Biol. Technol. 2013, 86, 181–191. [Google Scholar] [CrossRef]
- Larsen, D.H.; Li, H.; van de Peppel, A.C.; Nicole, C.C.; Marcelis, L.F.; Woltering, E.J. High light intensity at End-Of-Production improves the nutritional value of basil but does not affect postharvest chilling tolerance. Food Chem. 2022, 369, 130913. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Influence of packaging and storage conditions on biochemical quality and enzymatic activity in relation to shelf life enhancement of fresh basil leaf. J. Food Sci. Technol. 2018, 55, 3199–3211. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: A review. Plant Cell Tissue Organ Cult. 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Ciriello, M.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. An Appraisal of Critical Factors Configuring the Composition of Basil in Minerals, Bioactive Secondary Metabolites, Micronutrients and Volatile Aromatic Compounds. J. Food Compos. Anal. 2022, 111, 104582. [Google Scholar] [CrossRef]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Shah, Q.M.A.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L., var. longifolia) and comparison with protected soil-based cultivation. Agri. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef]
- Nicoletto, C.; Santagata, S.; Bona, S.; Sambo, P. Influence of cut number on qualitative traits in different cultivars of sweet basil. Ind. Crops Prod. 2013, 44, 465–472. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Opačić, N.; Žutić, I.; Voća, S.; Poštek, M.; Radman, S.; Benko, B.; Uher, S.F. Valorization of nutritional potential and specialized metabolites of basil cultivars depending on cultivation method. Agronomy 2021, 11, 1048. [Google Scholar] [CrossRef]
- Lange, D.D.; Cameron, A.C. Postharvest shelf life of sweet basil (Ocimum basilicum). HortScience 1994, 29, 102–103. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in basil (Ocimum basilicum L.). J. Agri. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Ptushenko, V.V.; Ptushenko, E.A.; Samoilova, O.P.; Tikhonov, A.N. Chlorophyll fluorescence in the leaves of Tradescantia species of different ecological groups: Induction events at different intensities of actinic light. Biosystems 2013, 114, 85–97. [Google Scholar] [CrossRef]
- Dumbrava, D.; Moldovan, C.; Raba, D.-N.; Popa, M.-V. Vitamin C, chlorophylls, carotenoids and xanthophylls content in some basil (Ocimum basilicum L.) and rosemary (Rosmarinus officinalis L.) leaves extracts. J. Agroaliment. Process. Technol. 2012, 18, 253–258. [Google Scholar]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. HortScience 2005, 40, 1230–1233. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Comp. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Muráriková, A.; Neugebauerová, J. Seasonal variation of ascorbic acid and nitrate levels in selected basil (Ocimum basilicum L.) varieties. Horti. Sci. 2018, 45, 47–52. [Google Scholar] [CrossRef]
- Saadatian, M.; Peyvast, G.; Olfati, J.; Ramezani-Kharaz, P. Different species of basil need different ammonium to nitrate ratio in hydroponics’ system. Acta Agric. Slov. 2015, 103, 223–232. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Calcium and bone. Clinic. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, S. Sources and deficiency diseases of mineral nutrients in human health and nutrition: A review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Soteriou, G.A.; Kyratzis, A.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Differential Response to NaCl Osmotic Stress in Sequentially Harvested Hydroponic Red and Green Basil and the Role of Calcium. Front. Plant Sci. 2022, 13, 799213. [Google Scholar] [CrossRef]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Xu, G.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in chloride nutrition of plants. Adv. Agron. 1999, 68, 97–150. [Google Scholar]
- Yao, Y.-F.; Wang, C.-S.; Qiao, J.; Zhao, G.-R. Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway. Metab. Eng. 2013, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; De Pascale, S.; Rouphael, Y. Dataset on the effects of anti-insect nets of different porosity on mineral and organic acids profile of Cucurbita pepo L. fruits and leaves. Data 2021, 6, 50. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]




| Variables | Thai | Mexican | Black | Genovese | Lemon | Mexican Purple | Significance |
|---|---|---|---|---|---|---|---|
| Mean ± Standard Error | |||||||
| Leaf fw (g pt−1) | 14.36 ± 0.31 b | 14.02 ± 0.26 b | 10.42 ± 0.37 c | 16.52 ± 0.53 a | 11.08 ± 0.70 c | 9.60 ± 0.36 c | *** |
| Stem fw (g pt−1) | 16.59 ± 0.81 a | 14.74 ± 0.66 a | 6.14 ± 0.06 c | 9.40 ± 0.38 b | 9.48 ± 1.06 b | 4.21 ± 0.18 c | *** |
| Leaf-to-stem ratio fw | 0.87 ± 0.03 d | 0.96 ± 0.05 cd | 1.70 ± 0.05 b | 1.76 ± 0.03 b | 1.20 ± 0.10 c | 2.29 ± 0.06 a | *** |
| Leaf dw (g pt−1) | 1.61 ± 0.03 a | 1.52 ± 0.08 ab | 1.12 ± 0.04 cd | 1.70 ± 0.08 a | 1.32 ± 0.08 bc | 1.00 ± 0.04 d | *** |
| Stem dw (g pt−1) | 1.10 ± 0.03 a | 0.96 ± 0.07 ab | 0.39 ± 0.01 de | 0.57 ± 0.03 cd | 0.77 ± 0.07 bc | 0.24 ± 0.01 e | *** |
| Root dw (g pt−1) | 0.19 ± 0.01 ab | 0.23 ± 0.02 ab | 0.04 ± 0.01 c | 0.27 ± 0.04 a | 0.14 ± 0.03 b | 0.03 ± 0.00 c | *** |
| Total dw (g pt−1) | 2.71 ± 0.06 a | 2.48 ± 0.14 ab | 1.52 ± 0.05 c | 2.27 ± 0.11 ab | 2.09 ± 0.15 b | 1.24 ± 0.04 c | *** |
| Leaf dm (%) | 11.64 ± 0.27 ab | 11.11 ± 0.18 bcd | 11.37 ± 0.30 abc | 10.24 ± 0.22 d | 12.32 ± 0.30 a | 10.44 ± 0.11 cd | *** |
| Stem dm (%) | 6.67 ± 0.14 b | 6.51 ± 0.20 b | 6.61 ± 0.19 b | 6.04 ± 0.15 bc | 8.15 ± 0.13 a | 5.47 ± 0.13 c | *** |
| Total dm (%) | 8.77 ± 0.15 b | 8.62 ± 0.40 b | 9.16 ± 0.09 b | 8.73 ± 0.19 b | 10.18 ± 0.19 a | 8.97 ± 0.07 b | *** |
| LMA (g m−2) | 33.31 ± 0.95 a | 34.44 ± 1.72 a | 25.60 ± 0.56 b | 28.72 ± 0.98 b | 26.60 ± 0.81 b | 27.97 ± 0.68 b | *** |
| Leaf area (cm2) | 394.43 ± 10.01 ab | 444.46 ± 24.32 a | 402.42 ± 11.66 ab | 418.00 ± 23.23 a | 369.08 ± 41.25 ab | 299.61 ± 16.57 b | ** |
| Leaf number | 51.50 ± 0.63 a | 52.09 ± 1.48 a | 33.25 ± 1.06 c | 30.06 ± 0.76 c | 46.63 ± 1.26 b | 22.53 ± 0.91 d | *** |
| Height (cm) | 37.42 ± 0.68 a | 33.61 ± 1.14 b | 24.36 ± 0.33 d | 27.09 ± 0.98 cd | 28.25 ± 0.45 c | 19.22 ± 0.29 e | *** |
| Root length (cm) | 49.75 ± 5.70 | 44.63 ± 2.68 | 42.81 ± 9.12 | 50.69 ± 3.25 | 41.88 ± 2.36 | 37.46 ± 2.83 | n.s. |
| Treatment | E | gs | ACO2 | WUEi |
|---|---|---|---|---|
| mol H2O m−2 s−1 | mol H2O m−2 s−1 | μmol CO2 m−2 s−1 | μmol CO2 mol−1 H2O | |
| Thai | 5.48 ± 0.23 ab | 0.25 ± 0.01 | 22.71 ± 0.65 ab | 4.16 ± 0.19 a |
| Mexican | 5.70 ± 0.19 a | 0.26 ± 0.01 | 24.40 ± 0.54 a | 4.30 ± 0.23 a |
| Black | 5.12 ± 0.07 ab | 0.25 ± 0.01 | 10.83 ± 0.25 c | 2.12 ± 0.05 b |
| Genovese | 5.04 ± 0.08 b | 0.23 ± 0.01 | 20.52 ± 0.64 b | 4.08 ± 0.14 a |
| Lemon | 5.26 ± 0.12 ab | 0.25 ± 0.00 | 22.52 ± 0.54 ab | 4.29 ± 0.13 a |
| Mexican Purple | 5.42 ± 0.08 ab | 0.26 ± 0.01 | 6.62 ± 0.27 d | 1.22 ± 0.05 c |
| Significance | * | n.s. | *** | *** |
| Treatment | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids |
|---|---|---|---|---|
| mg g−1 fw | ||||
| Thai | 1.09 ± 0.03 ab | 0.62 ± 0.04 ab | 1.7 ± 0.07 ab | 0.34 ± 0.02 a |
| Mexican | 1.12 ± 0.03 ab | 0.68 ± 0.04 ab | 1.79 ± 0.07 ab | 0.32 ± 0.02 ab |
| Black | 1.02 ± 0.03 b | 0.61 ± 0.04 b | 1.62 ± 0.07 b | 0.34 ± 0.01 a |
| Genovese | 1.02 ± 0.00 b | 0.57 ± 0.01 b | 1.59 ± 0.01 b | 0.34 ± 0.00 a |
| Lemon | 1.18 ± 0.02 a | 0.80 ± 0.04 a | 1.97 ± 0.05 a | 0.27 ± 0.01 b |
| Mexican Purple | 1.09 ± 0.02 ab | 0.75 ± 0.07 ab | 1.83 ± 0.09 ab | 0.30 ± 0.03 ab |
| Significance | ** | ** | ** | * |
| Treatment | P | K | Ca | Mg | Na | Cl |
|---|---|---|---|---|---|---|
| g kg−1 dw | ||||||
| Thai | 6.66 ± 0.29 ab | 42.25 ± 1.46 c | 8.58 ± 0.50 a | 2.46 ± 0.13 ab | 0.20 ± 0.03 d | 2.86 ± 0.28 bc |
| Mexican | 7.17 ± 0.22 ab | 51.53 ± 0.72 a | 8.23 ± 0.36 ab | 2.82 ± 0.13 a | 0.24 ± 0.05 cd | 4.37 ± 0.46 b |
| Black | 6.96 ± 0.09 ab | 50.10 ± 0.23 ab | 6.69 ± 0.27 c | 2.26 ± 0.03 bc | 0.31 ± 0.01 bcd | 7.07 ± 0.32 a |
| Genovese | 7.40 ± 0.21 a | 52.17 ± 0.45 a | 7.09 ± 0.16 bc | 1.90 ± 0.08 cd | 0.53 ± 0.01 a | 2.49 ± 0.29 c |
| Lemon | 6.18 ± 0.40 b | 35.50 ± 2.66 d | 5.70 ± 0.20 cd | 1.67 ± 0.20 d | 0.42 ± 0.04 ab | 3.24 ± 0.28 bc |
| Mexican Purple | 4.92 ± 0.23 c | 43.45 ± 1.79 bc | 4.71 ± 0.29 d | 1.64 ± 0.08 d | 0.36 ± 0.03 bc | 7.21 ± 0.49 a |
| Significance | *** | *** | *** | *** | *** | *** |
| Common Name | Scientific Name | Family | Leaf Characteristic | Plant Habitus |
|---|---|---|---|---|
| Thai basil | Ocimum basilicum L. var thyrsiflora | Lamiaceae | Small, pointed leaves with a very strong and intense aroma, slightly spicy between mint, clove, anise, and licorice. | Erect |
| Mexican basil | Ocimum basilicum L. cv Cinnamon | Lamiaceae | Oval, slightly serrated, and pointed leaves with a cinnamon aroma | Erect |
| Black basil | Ocimum basilicum L. cv Dark Opal | Lamiaceae | Oval, slightly serrated, and pointed leaves with a | Erect |
| Genovese basil | Ocimum basilicum L. cv Italiano Classico | Lamiaceae | Light green, slightly blistered, intensely fragrant leaves with no mint smell. | Erect |
| Lemon basil | Ocimum × citriodorum | Lamiaceae | Purple-colored, slightly boiling, intensely fragrant leaves without mint smell. | Erect |
| Mexican purple basil | Ocimum basilicum L. cv Purple Ruffle | Lamiaceae | Dark purple leaves curved along the midrib, serrated margin | Erect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciriello, M.; Cirillo, V.; Formisano, L.; El-Nakhel, C.; Pannico, A.; De Pascale, S.; Rouphael, Y. Productive, Morpho-Physiological, and Postharvest Performance of Six Basil Types Grown in a Floating Raft System: A Comparative Study. Plants 2023, 12, 486. https://doi.org/10.3390/plants12030486
Ciriello M, Cirillo V, Formisano L, El-Nakhel C, Pannico A, De Pascale S, Rouphael Y. Productive, Morpho-Physiological, and Postharvest Performance of Six Basil Types Grown in a Floating Raft System: A Comparative Study. Plants. 2023; 12(3):486. https://doi.org/10.3390/plants12030486
Chicago/Turabian StyleCiriello, Michele, Valerio Cirillo, Luigi Formisano, Christophe El-Nakhel, Antonio Pannico, Stefania De Pascale, and Youssef Rouphael. 2023. "Productive, Morpho-Physiological, and Postharvest Performance of Six Basil Types Grown in a Floating Raft System: A Comparative Study" Plants 12, no. 3: 486. https://doi.org/10.3390/plants12030486
APA StyleCiriello, M., Cirillo, V., Formisano, L., El-Nakhel, C., Pannico, A., De Pascale, S., & Rouphael, Y. (2023). Productive, Morpho-Physiological, and Postharvest Performance of Six Basil Types Grown in a Floating Raft System: A Comparative Study. Plants, 12(3), 486. https://doi.org/10.3390/plants12030486










