Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses
Abstract
1. Introduction
2. Results
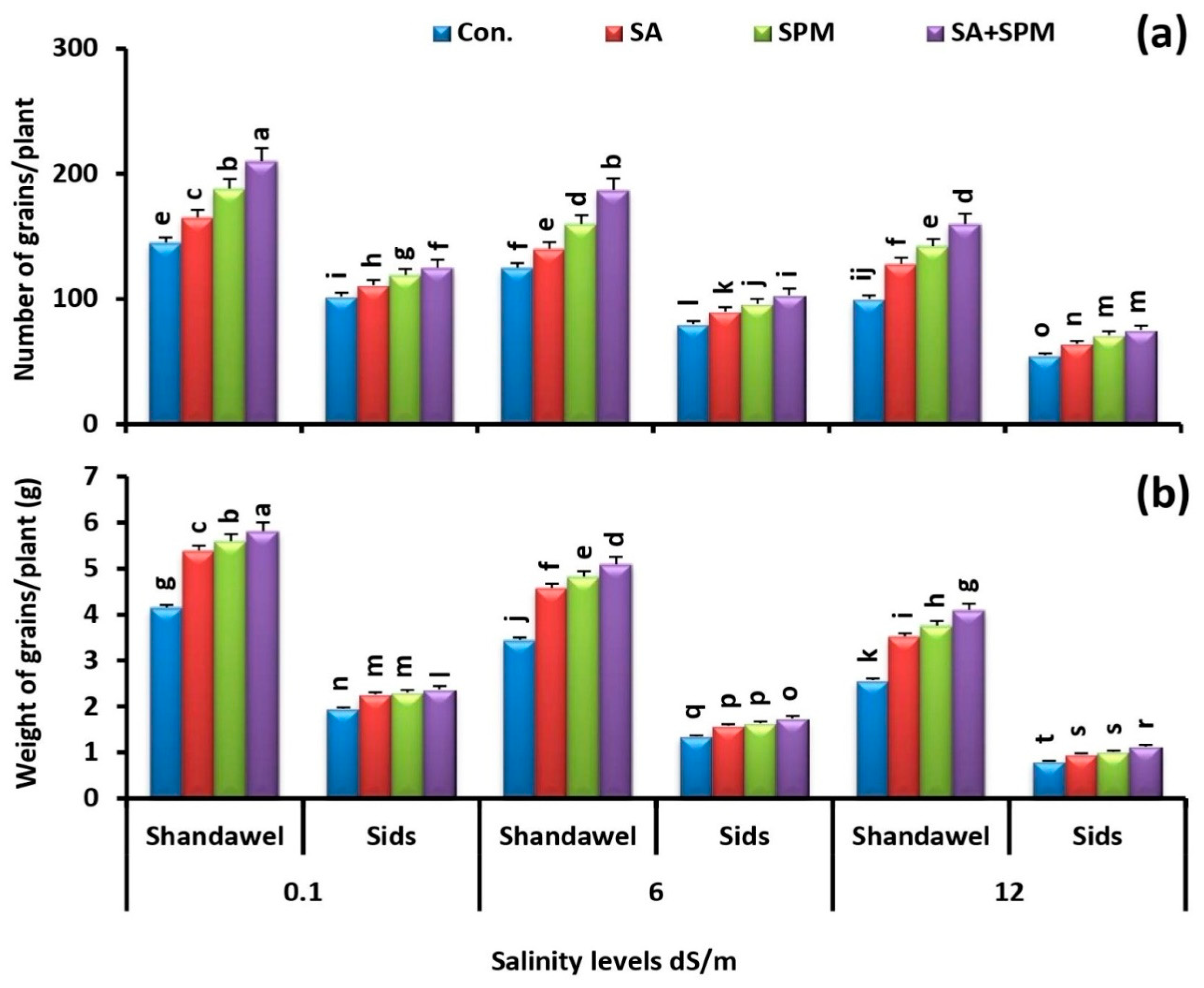
2.1. Foliar Applications of SA and/or SPM Ameliorate the Growth and Yield Reduction Caused by Salinity
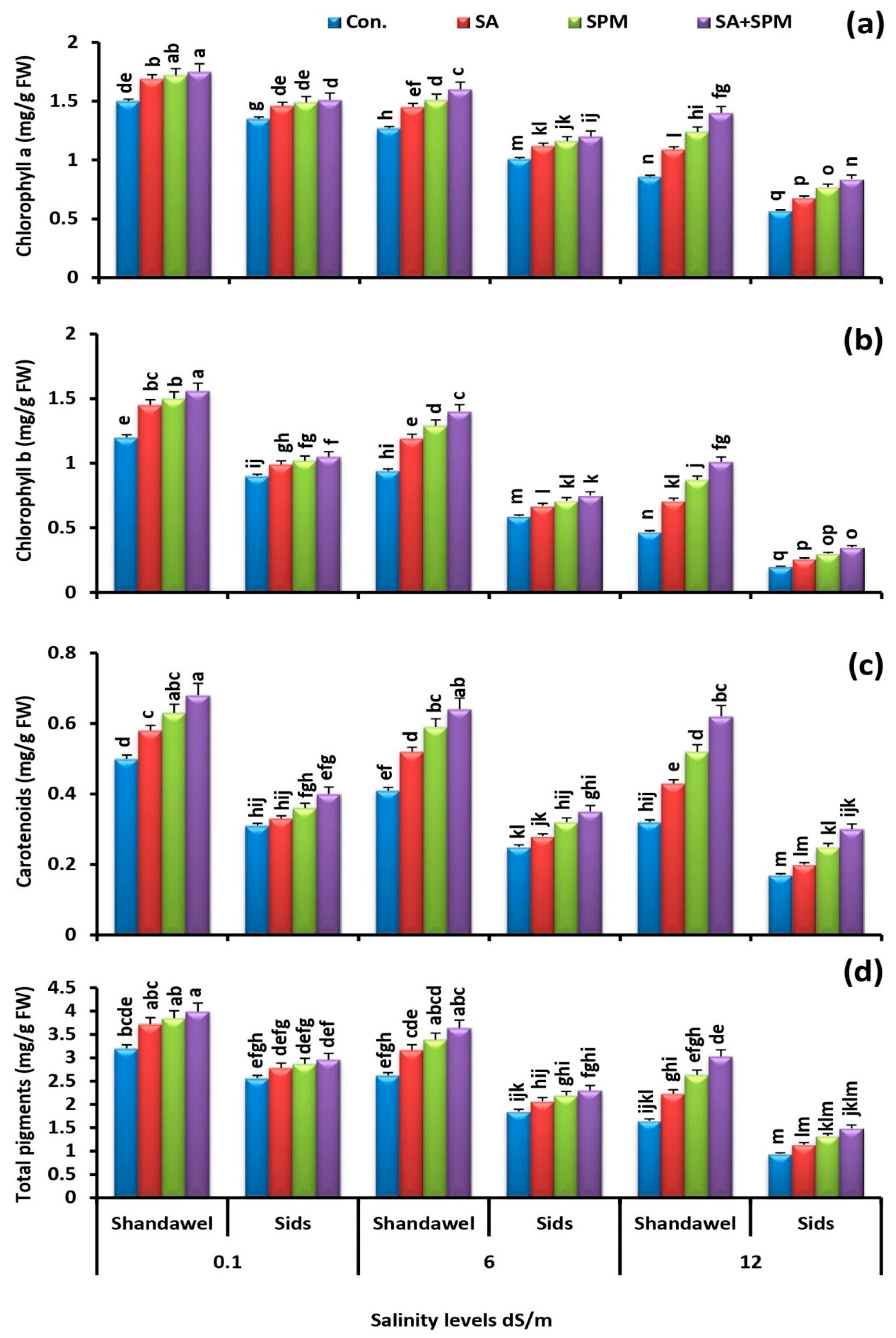
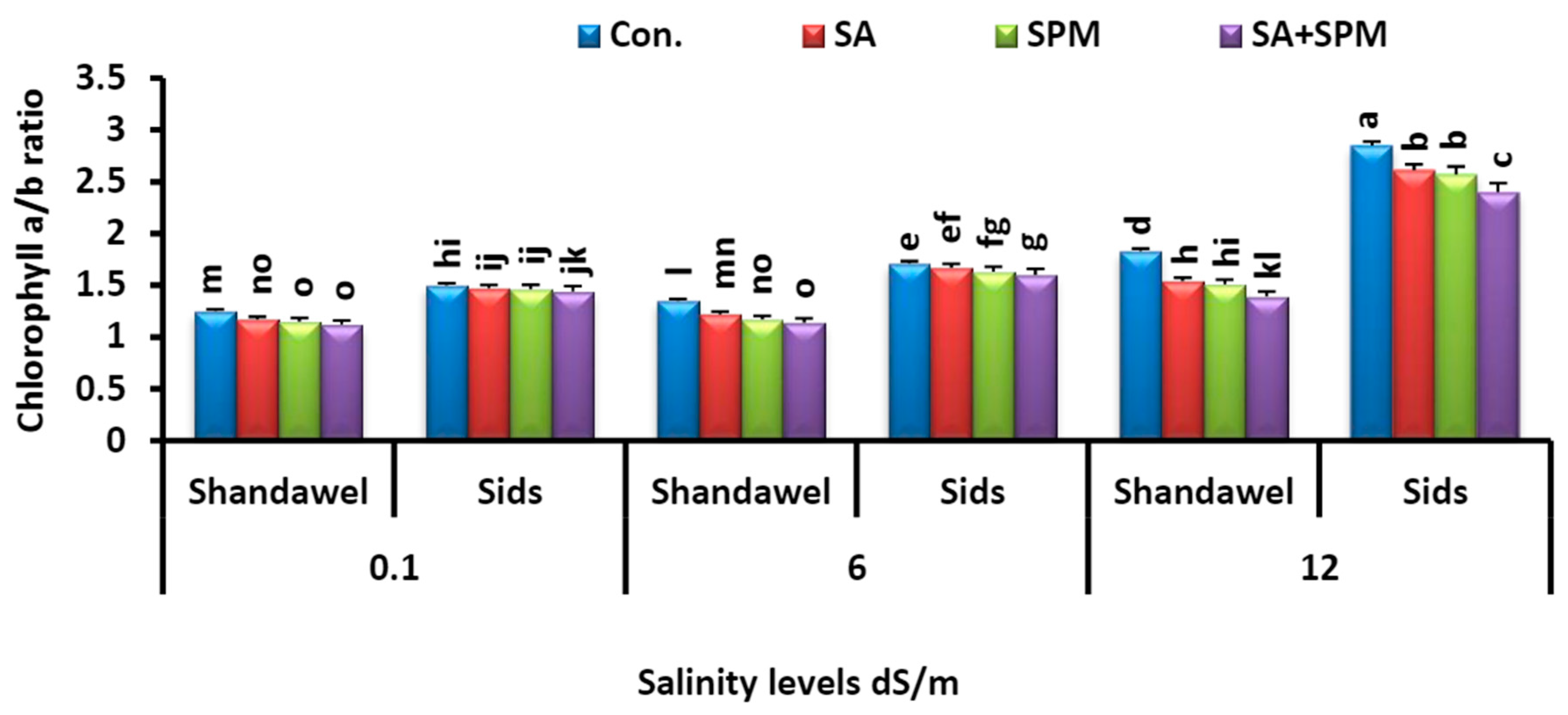
2.2. SA and/or SPM Treatments Enhance Photosynthetic Pigment Concentration in Salt-Stressed Wheat Plants
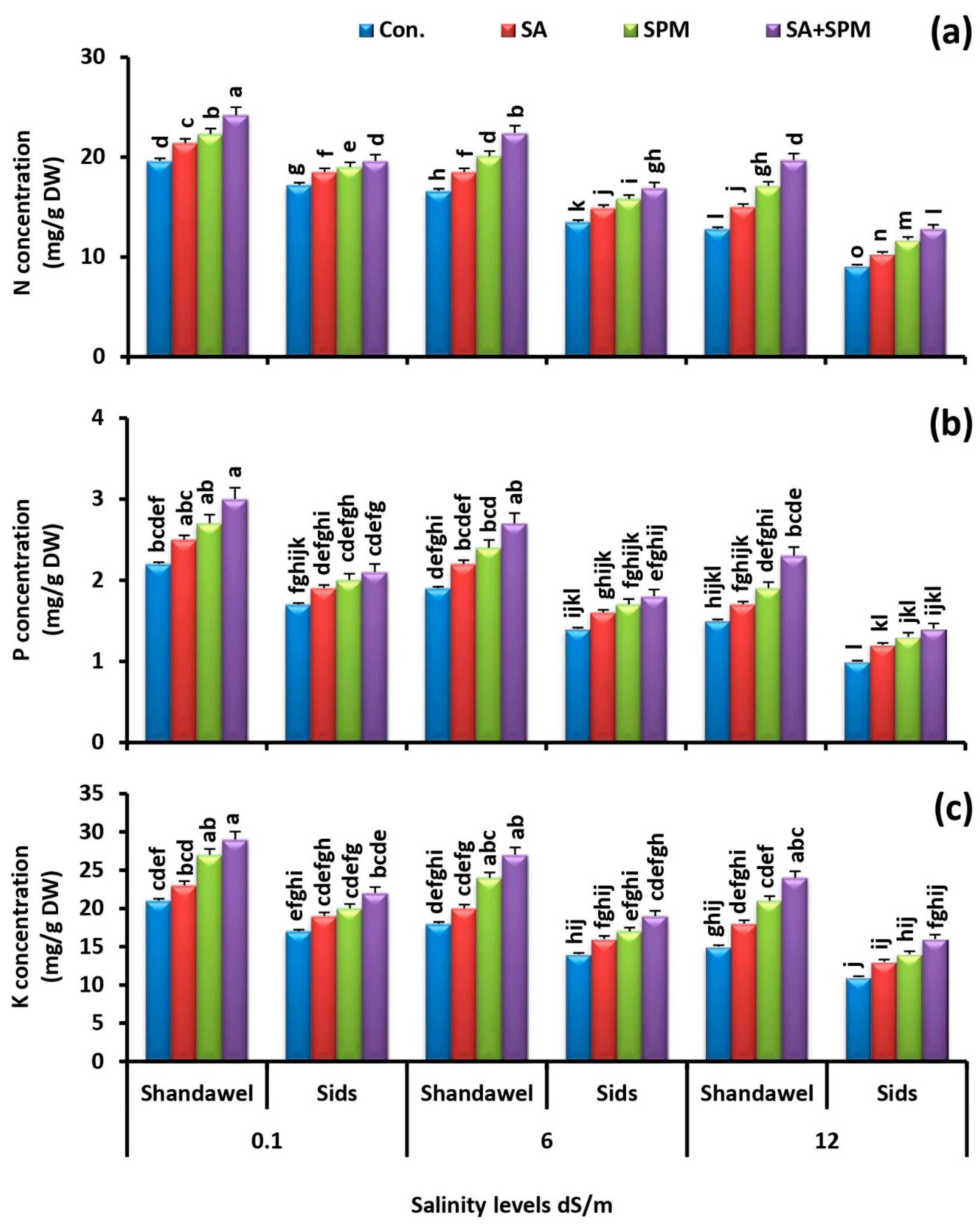
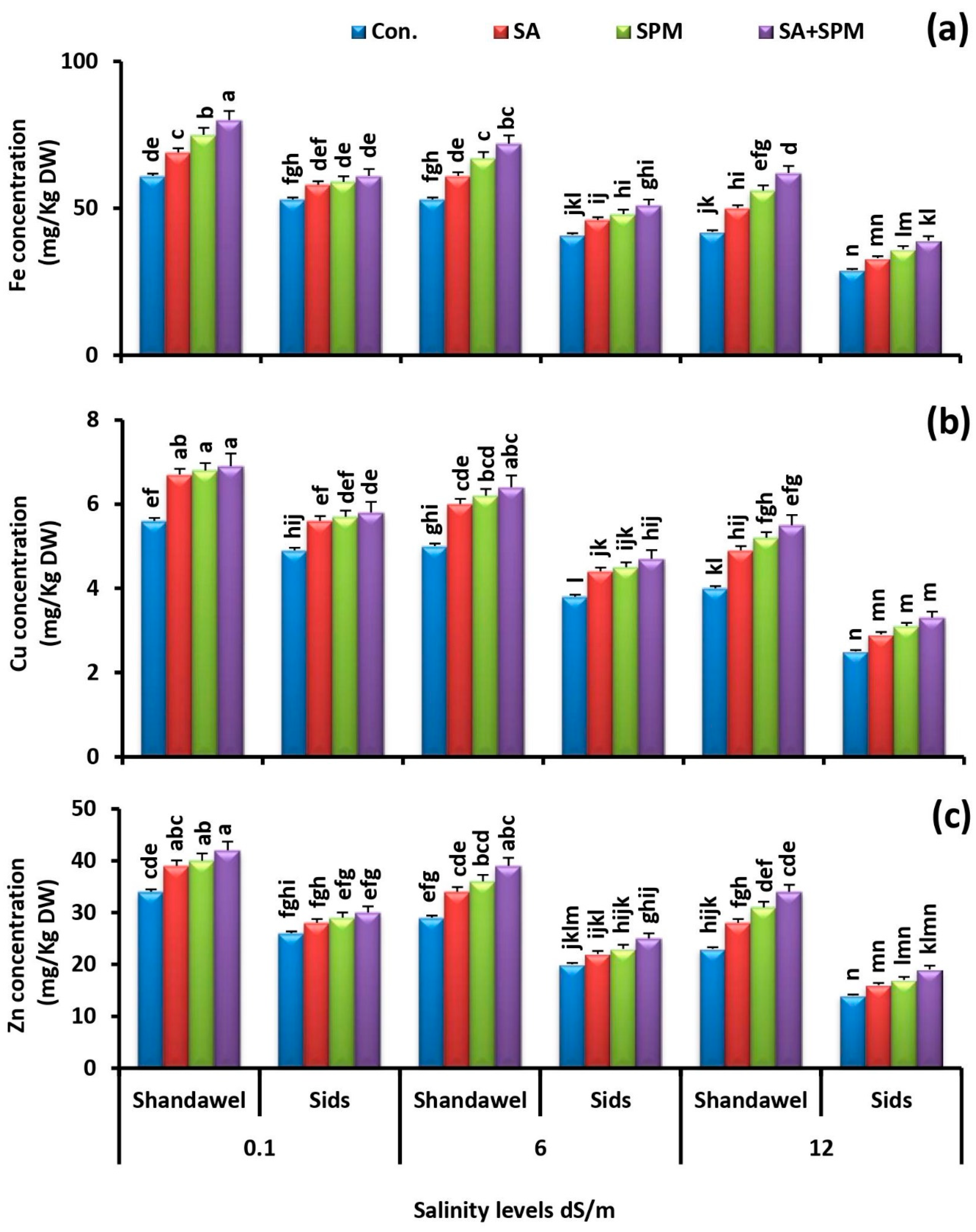
2.3. Spraying of SA and/or SPM Maintain Ionic Balance in Salt-Stressed Wheat Plants
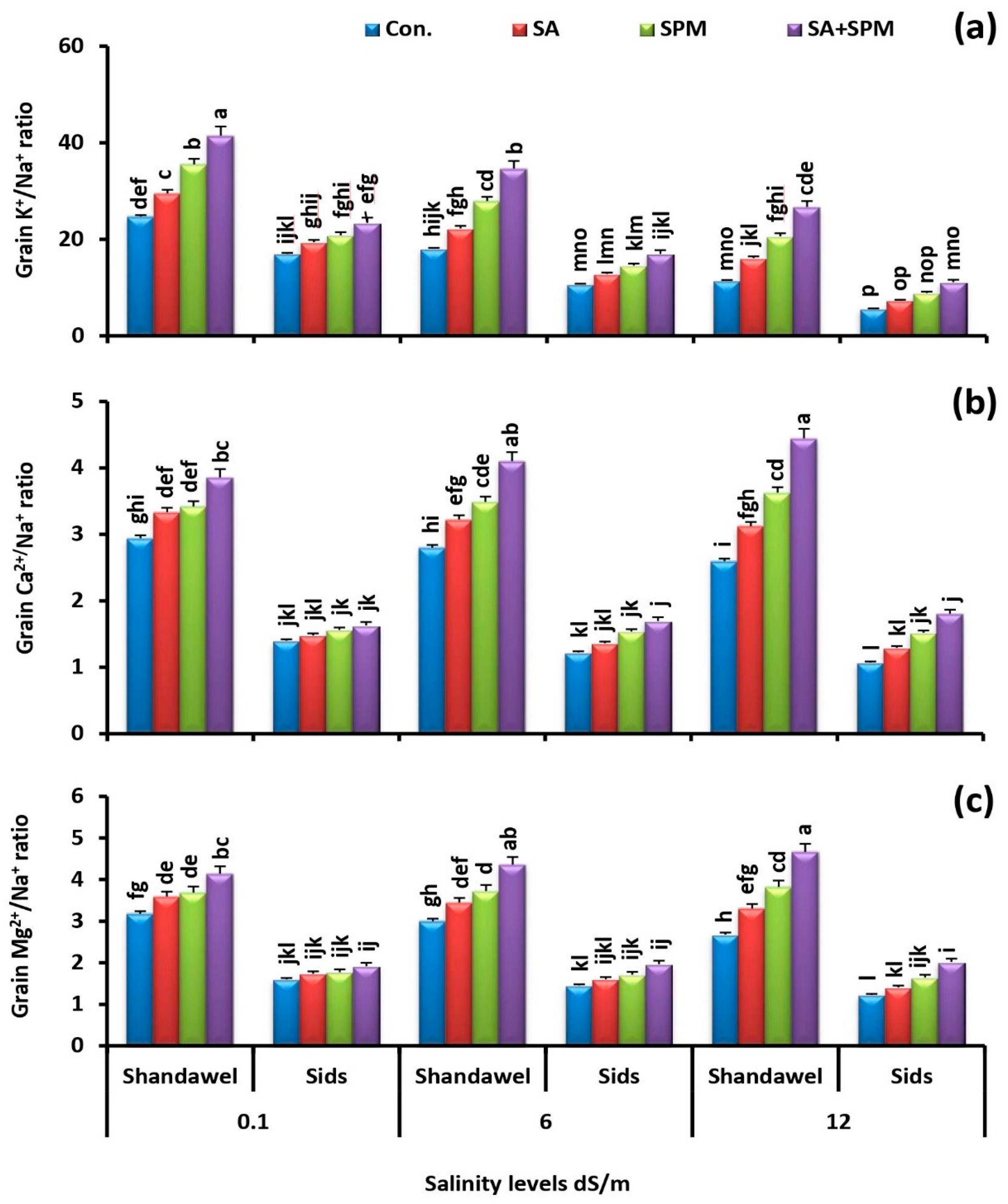
2.4. Exogenously Applied SA and/or SPM Preserve K+/Na+, Ca2+/Na+, and Mg2+/Na+ Ratios under Saline Conditions
2.5. SA and/or SPM Treatments Improve Organic Solutes Accumulation under Salt Stress
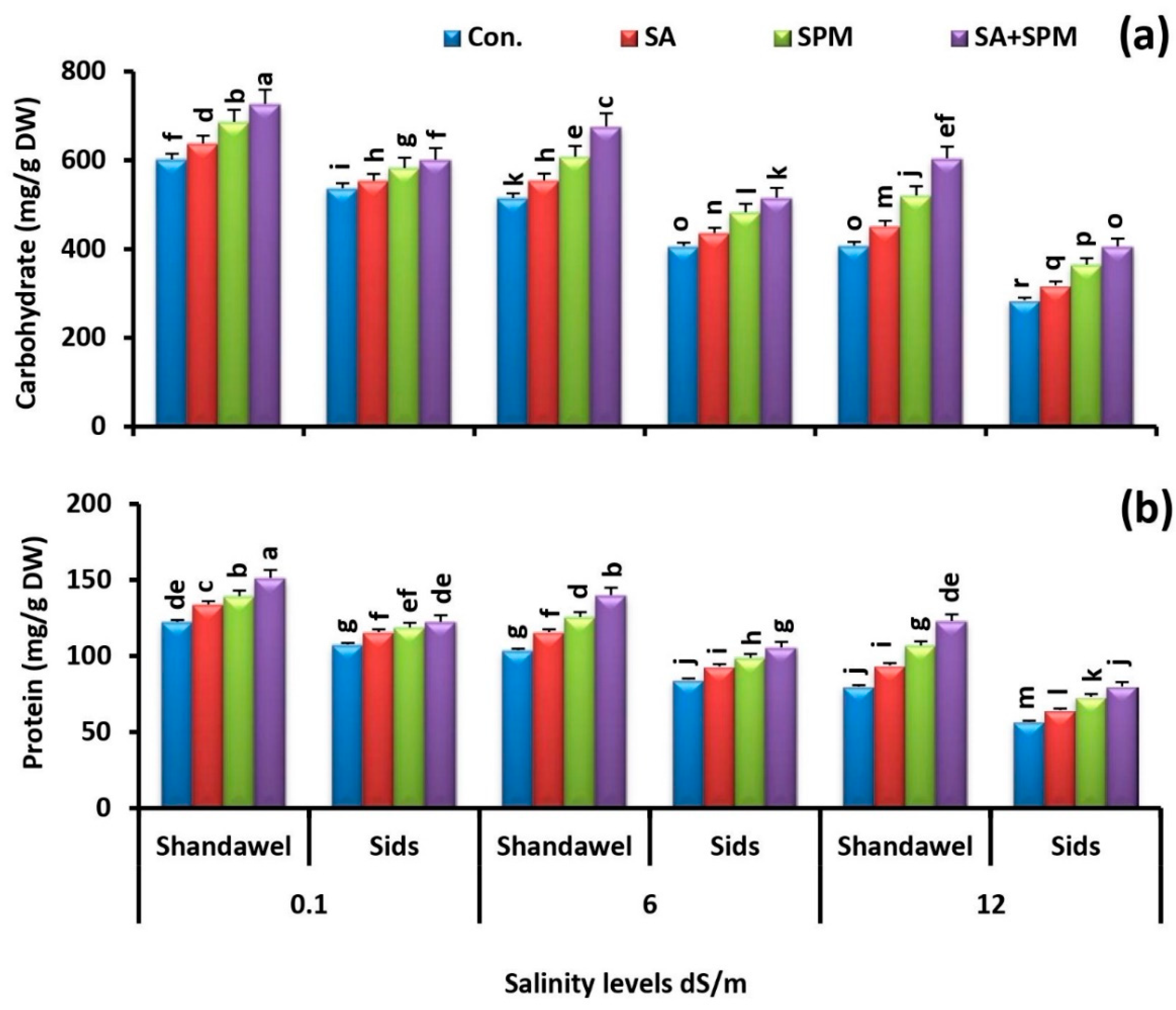
2.6. Spraying of SA and/or SPM Enhance Grain Carbohydrate and Protein Content under Saline Conditions
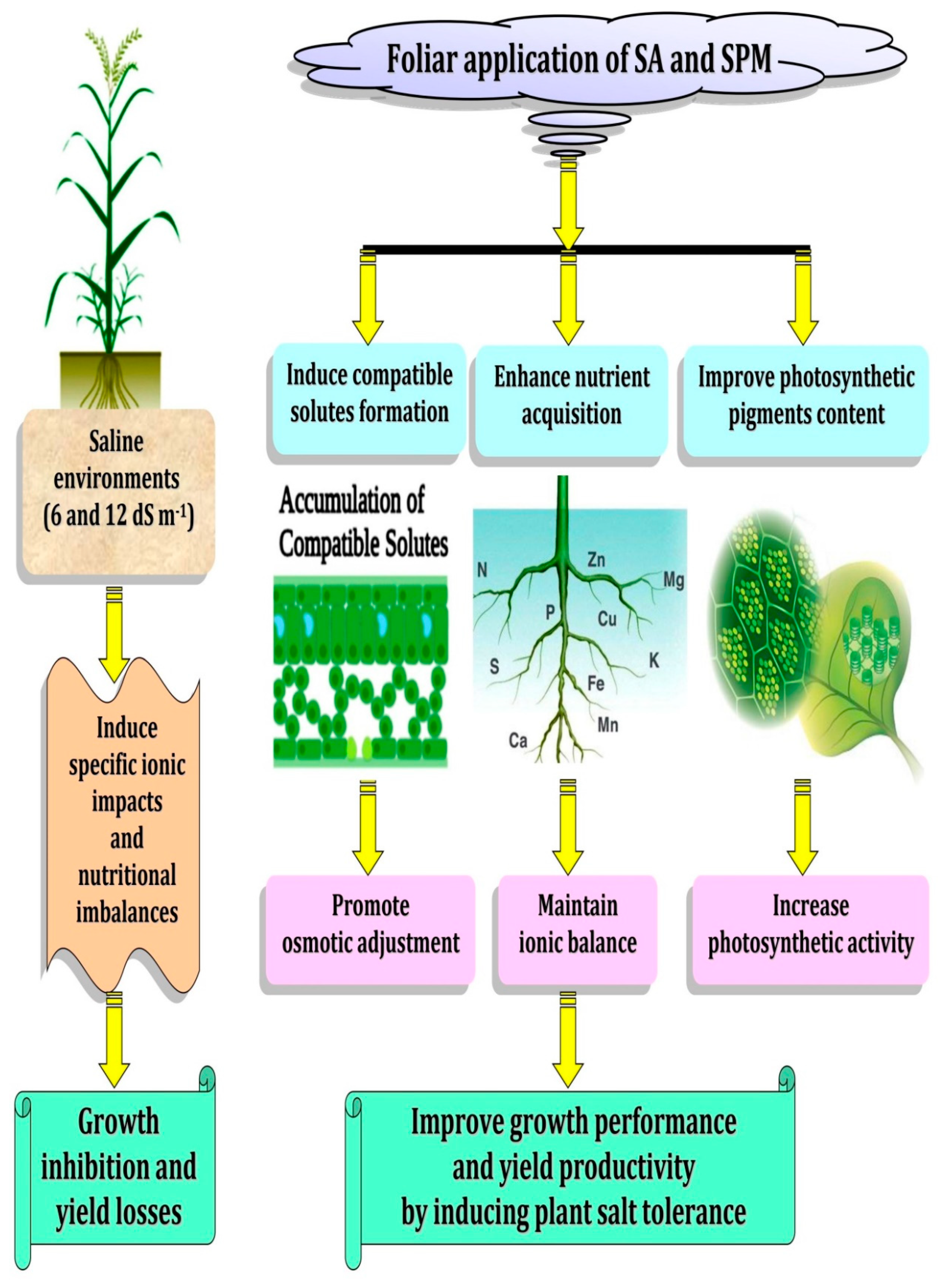
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Plant Growth and Productivity Analysis
4.3. Determination of Photosynthetic Pigments Concentration
4.4. Determination of N, P, K, Na, Ca, Mg, Fe, Zn, and Cu Concentrations
4.5. Determination of Compatible Solutes Accumulation
4.6. Estimation of Grain Carbohydrate and Protein Content
4.7. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zörb, C.; Geilffus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit. Rev. Plant Sci. 2016, 35, 146–189. [Google Scholar] [CrossRef]
- Arzani, A. Improving salinity tolerance in crop plants: A biotechnological view. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 373–383. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Influence of arbuscular mycorrhizae on root colonization, growth and productivity of two wheat cultivars under salt stress. Arch. Agron. Soil. Sci. 2012, 58, 85–100. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L.) plants by enhancing root H+-pump activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Hinai, M.S.A.; Ullah, A.; Al-Rajhi, R.S.; Farooq, M. Proline accumulation, ion homeostasis and antioxidant defence system alleviate salt stress and protect carbon assimilation in bread wheat genotypes of omani origin. Environ. Exp. Bot. 2022, 193, 104687. [Google Scholar] [CrossRef]
- Talaat, N.B.; Todorova, D. Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540. [Google Scholar] [CrossRef]
- Talaat, N.B.; Mostafa, A.A.; El-Rahman, S.N.A. A novel plant growth-promoting agent mitigates salt toxicity in barley (Hordeum vulgare L.) by activating photosynthetic, antioxidant defense, and methylglyoxal detoxification machineries. J. Soil Sci. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, M.K.; Swain, P.; Sarkar, R.K. Tissue tolerance coupled with ionic discrimination can potentially minimize the energy cost of salinity tolerance in rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Co-Application of melatonin and salicylic acid counteracts salt stress-induced damage in wheat (Triticum aestivum L.) photosynthetic machinery. J. Soil Sci. Plant Nutr. 2021, 21, 2893–2906. [Google Scholar] [CrossRef]
- Talaat, N.B. Polyamine and nitrogen metabolism regulation by melatonin and salicylic acid combined treatment as a repressor for salt toxicity in wheat (Triticum aestivum L.) plants. Plant Growth Regul. 2021, 95, 315–329. [Google Scholar] [CrossRef]
- Bukhat, S.; Manzoor, H.; Athar, H.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Hoang, H.L.; de Guzman, C.C.; Cadiz, N.M.; Hoang, T.T.H.; Tran, D.H.; Rehman, H. Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). J. Soil Sci. Plant Nutr. 2020, 20, 1759–1769. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Xianguo, C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar] [CrossRef]
- Islam, A.T.M.T.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Effect of salicylic acid seed priming on morpho-physiological responses and yield of baby corn under salt stress. Sci. Hortic. 2022, 304, 111304. [Google Scholar] [CrossRef]
- Todorova, D.; Talaat, N.B.; Katerova, Z.; Alexieva, V.; Shawky, B.T. Polyamines and brassinosteroids in drought stress responses and tolerance in plants. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; Volume 2, pp. 608–627. [Google Scholar]
- Talaat, N.B. 24-Epibrassinolide and spermine combined treatment sustains maize (Zea mays L.) drought tolerance by improving photosynthetic efficiency and altering phytohormones Profile. J. Soil Sci. Plant Nutr. 2020, 20, 516–529. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Xu, Z.; Zhao, D.; Hu, X. Exogenous spermine-induced expression of SlSPMS gene improves salinity–alkalinity stress tolerance by regulating the antioxidant enzyme system and ion homeostasis in tomato. Plant Physiol. Biochem. 2020, 157, 79–92. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Ahangera, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Islam, M.A.; Jin-huan, P.; Fan-wei, M.; Ya-wen, L.; Ning, X.; Chao, Y.; Jun, L. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J. Integr. Agric. 2020, 19, 643–655. [Google Scholar] [CrossRef]
- Geng, W.; Qiu, Y.; Peng, Y.; Zhang, Y.; Li, Z. Water and oxidative homeostasis, Na+/K+ transport, and stress-defensive proteins associated with spermine-induced salt tolerance in creeping bentgrass. Environ. Exp. Bot. 2021, 192, 104659. [Google Scholar] [CrossRef]
- Talaat, N.B.; Ibrahim, A.S.; Shawky, B.T. Enhancement of the expression of ZmBZR1 and ZmBES1 regulatory genes and antioxidant defense genes triggers water stress mitigation in maize (Zea mays L.) plants treated with 24-epibrassinolide in combination with spermine. Agronomy 2022, 12, 2517. [Google Scholar] [CrossRef]
- Amirbakhtiar, N.; Ismaili, A.; Ghaffari, M.-R.; Mirdar Mansuri, R.; Sanjari, S.; Shobbar, Z.-S. Transcriptome analysis of bread wheat leaves in response to salt stress. PLoS ONE 2021, 16, e0254189. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M.; Rahimmalek, M.; Razavi, K. Morpho-physiological and gene expression responses of wheat by Aegilops cylindrica amphidiploids to salt stress. Plant Cell Tissue Organ Cult. 2021, 144, 619–639. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuno, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef]
- Saha, P.; Sade, N.; Arzani, A.; Wilhelmi, M.R.; Coe, K.M.; Li, B.; Blumwald, E. Effects of abiotic stress on physiological plasticity and water use of Setaria viridis (L.). Plant Sci. 2016, 251, 128–138. [Google Scholar] [CrossRef]
- Talaat, N.B. RNAi based simultaneous silencing of all forms of light-dependent NADPH:protochlorophyllide oxidoreductase genes result in the accumulation of protochlorophyllide in tobacco (Nicotiana tabacum). Plant Physiol. Biochem. 2013, 71, 31–36. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol Plant. 2018, 40, 91. [Google Scholar] [CrossRef]
- Collins, A.R. Carotenoids and genomic stability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 475, 21–28. [Google Scholar] [CrossRef]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing. J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Aasfar, A.; Joutei, K.A. Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem. Biol. Technol. Agric. 2021, 8, 25. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A.; Pottosin, I.I. Polyamines prevent NaCl induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007, 581, 1993–1999. [Google Scholar] [CrossRef]
- Hamamoto, S.; Horie, T.; Hauser, F.; Deinlein, U.; Schroeder, J.I.; Uozumi, N. HKT transporters mediate salt stress resistance in plants:from structure and function to the field. Curr. Opin. Biotechnol. 2015, 32, 113–120. [Google Scholar] [CrossRef]
- Suma, A.; Granata, D.; Thomson, A.S.; Carnevale, V.; Rothberg, B.S. Polyamine blockade and binding energetics in the MthK potassium channel. J. Gen. Physiol. 2020, 152, 1085–1098. [Google Scholar] [CrossRef]
- Inada, M.; Ueda, A.; Shi, W.; Takabe, T. A Stress-inducible plasma membrane protein 3 (AcPMP3) in a monocotyledonous halophyte, Aneurolepidium chinense, regulates cellular Na+ and K+ accumulation under salt stress. Planta 2005, 220, 395–402. [Google Scholar] [CrossRef]
- Gharbi, E.; Lutts, S.; Dailly, H.; Quinet, M. Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanumlycopersicum) responses to salinity. Plant Signal Behav. 2018, 13, e1469361. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef]
- Zhao, F.; Song, C.; He, J.; Zhu, H. Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 2007, 145, 1061–1072. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize effects resistance mechanisms and management: A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; Gonzlez, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mung bean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462–494. [Google Scholar] [CrossRef]
- Khataar, M.; Mohammadi, M.H.; Shabani, F. Soil salinity and matric potential interaction on water use, water use efficiency and yield response factor of bean and wheat. Sci. Rep. 2018, 8, 2679. [Google Scholar]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Fernandez-Figares, I.; Marinetto, J.; Royo, C.; Ramos, J.M.; Del Moral, L.G. Amino-acid composition and protein and carbohydrate accumulation in the grain of triticale grown under terminal water stress simulated by a senescing agent. J. Cereal Sci. 2000, 32, 249–258. [Google Scholar] [CrossRef]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velghe, G.; Camerlynck, R. Chemical analysis of plants and soils. In Laboratory of Analytical and Agrochemistry; State University: Ghent, Belgium, 1982; pp. 14–24. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Pregl, F. Quantitative Organic Micro Analysis, 4th ed.; A. Churchill Ltd.: London, UK, 1945. [Google Scholar]
- Kacar, B.; Inal, A. Plant analysis. Nobel publication No: 1241. Appl. Sci. 2008, 63, 879. [Google Scholar]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Dıaz, M. Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for The photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bates, L.S.; Aldren, R.P.; Teare, L.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yih, R.Y.; Clark, H.E. Carbohydrate and protein content of boron deficient tomato root tips in relation to anatomy and growth. Plant Physiol. 1965, 40, 312–315. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Aarr, A.L.; Randaal, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]



| Salinity Levels EC (dS m−1) | pH | HCO3− + CO32− (mg kg−1) | Cl− (mg kg−1) | SO42− (mg kg−1) | Ca2+ (mg kg−1) | Mg2+ (mg kg−1) | Na+ (mg kg−1) | K+ (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 7.2 | 213.5 | 324.0 | 430.7 | 92.2 | 41.4 | 3.7 | 31.4 |
| 6.0 | 7.5 | 263.6 | 1173.4 | 996.9 | 398.5 | 173.9 | 306.7 | 39.7 |
| 12.0 | 7.8 | 275.4 | 1987.8 | 1686.1 | 886.5 | 314.5 | 808.6 | 52.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talaat, N.B.; Hanafy, A.M.A. Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses. Plants 2022, 11, 3198. https://doi.org/10.3390/plants11233198
Talaat NB, Hanafy AMA. Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses. Plants. 2022; 11(23):3198. https://doi.org/10.3390/plants11233198
Chicago/Turabian StyleTalaat, Neveen B., and Alaa M. A. Hanafy. 2022. "Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses" Plants 11, no. 23: 3198. https://doi.org/10.3390/plants11233198
APA StyleTalaat, N. B., & Hanafy, A. M. A. (2022). Plant Growth Stimulators Improve Two Wheat Cultivars Salt-Tolerance: Insights into Their Physiological and Nutritional Responses. Plants, 11(23), 3198. https://doi.org/10.3390/plants11233198






