Abstract
The aim of this study was to apply the combined thermomechanical–biological treatment for corn processing by-product (CPBP) valorization to added-value food and feed material. The mechanical–thermal pre-treatment was performed by applying the extrusion technique. Extruded CPBPs (14, 16, and 18% moisture) were further biodegraded with Lactiplantibacillus plantarum-LUHS122 (Lpl), Liquorilactobacillus uvarum-LUHS245 (Lu), Lacticaseibacillus casei-LUHS210 (Lc), and Lacticaseibacillus paracasei-LUHS244 (Lpa). Acidity parameters, microbial characteristics, sugars concentration, amino and fatty acids profile, biogenic amines (BA), and antibacterial and antifungal properties of CPBP were analyzed. Fermented CPBP had a reduced count of mould/yeast. A significantly lower (p ≤ 0.05) count of total enterobacteria was found in most of the extruded–fermented CPBP. Fermentation of extruded CPBP (moisture of 16 and 18%) increased valine and methionine content. Cadaverine and spermidine were not found after treatment of CPBP, and the lowest content of BA was found in the extruded–fermented (Lpa, moisture 18%) CPBP. Applied treatment had a significant effect on most of the fatty acids. CPBP fermented with Lpl, Lu, and Lpa displayed inhibition properties against 3 of the 10 tested pathogenic/opportunistic bacterial strains. Extruded–fermented (Lu, Lc, and Lpa moisture of 14 and 18%) CPBP showed antifungal activity against Rhizopus. Extruded–fermented (14% moisture, Lpl) CPBP inhibited Rhizopus and Aspergillus fumigatus. In conclusion, combined treatment can improve certain parameters and properties of CPBP in order to produce safer and more nutritious ingredients for food and feed industries.
1. Introduction
Cereal grains are staple crops and provide food and energy for the population year-round because they are easy to store, and maintain essential nutrients for humans and animals [1,2]. The most important part of the cereal is starchy endosperm; however, most of the functional compounds are generally located in the outer part of the grain. Despite that, the utilization of cereal grain outer part in the food industry is very low (on average, 7.5%) due to the negative effects on overall acceptability of the product [1]. In addition to wheat, rye, and rice, corn (Zea mays L.) is cultivated globally in many regions [3,4]. Corn grain outer layer matrix is very complex, and contains hemicellulose, cellulose, protein, starch, crude oil, and phenolic acids [5]. Various by-products are obtained after corn starch processing (e.g., corn bran, steep liquor, corn germ, gluten meal, etc.) and most of them are used as livestock or poultry feed or valorized into valuable products for food and medicine industry, such as edible oils, dietary fibre, sterol, ferulic acid, proteins, zeaxanthin, and active polysaccharides [6,7,8]. Chemical composition of the corn by-products is also suitable for biological treatment, because all the composites are good energy sources for microorganism biomass cultivation in the fermentation industry. Only 18% of corn production is used for human nutrition, while the rest is dedicated to animal feed [9]. However, some part of the non-starch by-products are still not fully utilized [10]. Moreover, the important point is the efficiency of the valorization of these by-products while maintaining the quality of the final product. Despite that some valorization technologies are developed and used for higher value product preparation and sustainable processing, which is based on economic efficiency and environmentally friendly production, is still challenging. Some methods for the preparation of high value-added products include extraction, and biorafination steps, which are not economically efficient, and could not be easily adapted at an industrial scale.
Extrusion is applied in the food industry due to low cost, high production rate, and energy efficiency [11]. Extrusion induces physicochemical changes in processed material, e.g., starch gelatinization, denaturation of proteins, amylose–lipid complex formation, inactivation of enzymes and microorganisms [12]. Studies on the extrusion of various corn products (flour, meal, grits, starch, and gluten meal) and their combination with other materials such as brewer’s spent grain, sugar beet pulp, apple pomace, sweet potato, and soybean flour, have been conducted [12]. Fermentation with lactic acid bacteria (LAB) is another promising technique, which leads to decreases in the level of fermentable carbohydrates, increases in total soluble solids, free amino acids, etc., functional compounds in fermented substrate, and can be used as a single treatment or in combination with other techniques [13]. Taking into consideration that microbial contamination of by-products could be a problem to ensure domination of the technological microorganisms in fermentable substrate, extrusion pre-treatment can be a valuable step to decontaminate them in order to ensure stability of the process. As well as extrusion, fermentation could improve the digestibility of protein and starch, and this would be beneficial for the production of low-cost, nutritionally enriched food and feed ingredients [1,14]. However, little data are available in the literature on the changes in such corn by-products as bran and germ after fermentation. To the best of our knowledge, there is no data about the combined technique of extrusion and fermentation for these by-products processing.
Therefore, our hypothesis is that the valorization of corn processing by-products could be designed in a more appropriate and sustainable manner by using whole by-product conversion, by combining extrusion and fermentation processes, as the latter are common and economically efficient processes in the food and feed industry. The appropriate selection of the technological microorganisms, which possess antimicrobial properties, for corn by-product fermentation could lead to the production of a functional material with additional desirable antibacterial and antifungal properties for the food and feed industry. In this study, combined thermomechanical–biological treatment for corn cereal grain processing by-product valorization to added-value food and feed material was tested. Extruded corn by-products with moisture content of 18, 16, and 14% were biodegraded with antimicrobial properties possessing Lactiplantibacillus plantarum-LUHS122, Liquorilactobacillus uvarum-LUHS245, Lacticaseibacillus casei-LUHS210, and Lacticaseibacillus paracasei-LUHS244. To select the most appropriate technique for corn by-product valorization, acidity parameters (pH, total titratable acidity, lactic acid concentration), microbial characteristics (LAB, mould/yeast, total bacteria count, total enterobacteria count), sugars concentration (fructose, glucose, sucrose, maltose), amino and fatty acids profile, biogenic amines concentration, and antibacterial and antifungal properties of the prepared samples were analyzed.
2. Materials and Methods
2.1. Corn By-Products and Technological Microorganisms Used in Experiments
The principal scheme for corn by-products valorization is given in Figure 1.
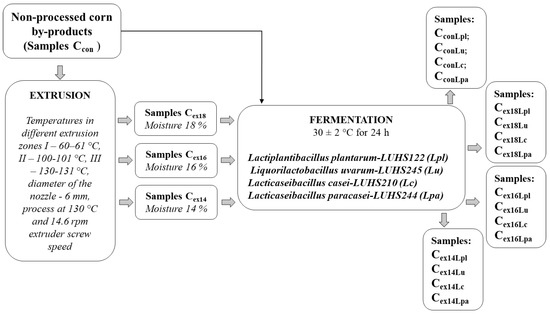
Figure 1.
The principal scheme for corn by-products valorization.
Corn by-products, non-processed, and extruded in a Twin Screw extruder (Jinan Shengrun Machinery Co., Ltd., Jinan, China), were obtained from SME “Ustukiu malunas” (Pasvalys, Lithuania). The temperatures in the different extrusion zones were I—60–61 °C, II—100–101 °C, and III—130–131 °C. Different moisture contents of the corn by-products substrate during the extrusion were tested (18, 16, and 14%). Extruder feed rate (F) was 8.2 ± 0.3 kg/h, and the nozzle diameter was 6 mm. The moisture content of the final corn by-product samples (after extrusion) was 11%. The samples were extruded at 130 °C and 14.6 rpm extruder screw speed. Three extruded corn by-product sample groups were prepared (Cex18, Cex16, Cex14) and non-extruded corn by-product samples were used as a control (CCon).
The LAB strains Lactiplantibacillus plantarum-LUHS122, Liquorilactobacillus uvarum-LUHS245, Lacticaseibacillus casei-LUHS210, and Lacticaseibacillus paracasei-LUHS244 were used for the fermentation of CCon, Cex18, Cex16, Cex14. Characteristics, including carbohydrates metabolism, survival at low pH, gas production capacities, and antimicrobial and antifungal properties, of the LAB strains, used for corn by-product fermentation, are reported by Bartkiene et al. [15]. Prior to the experiments, LAB strains were multiplied in MRS broth (de Man–Rogosa–Sharpe, CM 0359, Oxoid Ltd., Hampshire, UK) at 30 ± 2 °C for 48 h. The corn by-products, water, and a suspension of LAB strain (3% of dry matter relative to the corn by-product mass) containing 8.9 log10 CFU/mL were incubated at 30 ± 2 °C for 24 h. For 100 g of corn by-product, 60 mL of water was used. Three parallel replicates of the fermentation were performed, and three parallel samples were analyzed.
2.2. Analysis of the Acidity Parameters and Microbiological Characteristics
The pH was measured using a pH electrode (PP-15; Sartorius, Goettingen, Germany). The total titratable acidity (TTA) was evaluated for a 10 g portion of sample mixed with 90 mL of water; the results were expressed as mL of 0.1 mol/L NaOH solution required to achieve a pH value of 8.2. The concentration of L-(+) and D-(−)-lactic acid isomers was evaluated using a specific Megazyme assay Kit (Megazyme, Bray, Ireland). LAB, total bacteria (TBC), enterobacteria (TEC), and mould/yeast (M/Y) counts in the samples were determined according to Bartkiene [16].
2.3. Analysis of the Amino Acids Profile and Biogenic Amines Concentration
For amino acid analysis, analytes were extracted from homogenized sample with aqueous 0.1 M HCl solution and dansylation were performed according to the method of Hua-Lin Cai et al. [17], with some modifications. The concentrations of analytes were determined using The Varian ProStar HPLC system (Varian Corp., Palo Alto, CA, USA) and Thermo Scientific LCQ Fleet Ion trap mass detector. The detailed description of the method is given in Supplementary Data S1.
Biogenic amines (BA) were analyzed according to the method of Ben-Gigirey et al. [18] with some modifications by Bartkiene et al. [19]. Following BAs were analyzed: tryptamine, phenylethylamine, cadaverine, putrescine, histamine, tyramine, spermine (SPER), and spermidine. The extraction of BA was performed by using 0.4 M perchloric acid. The derivatization was carried out with a dansyl chloride solution in acetonitrile (10 mg/mL). The content of each BA was analyzed with the Varian ProStar HPLC system (Varian Corp., Palo Alto, CA, USA). The detailed description of the method is given in Supplementary Data S1.
2.4. Determination of Sugars Concentration in Corn By-Product Samples
Sugars concentration analysis of the non-treated and treated corn by-products was carried out with an Ultra Performance Liquid Chromatography system (Shimadzu Corp., Kyoto, Japan). A 2 mg/mL standard solution of a sugar mixture (fructose, glucose, sucrose, and maltose) was used for sugar detection. The detailed description of the method is given in Supplementary Data S1.
2.5. Evaluation of Fatty Acids Profile
The fatty acid (FA) composition of the corn by-product samples was determined using GCMS-QP2010 (Shimadzu, Japan) gas chromatograph with a mass spectrometer. The FA methyl esters (FAME) concentration was determined using 3-point calibration curve method and results were expressed as the percentage of total FAME concentration in the sample. The detailed sample preparation and chromatographic conditions is given in Supplementary Data S1 and Supplementary Data S3.
2.6. Evaluation of Antimicrobial Properties
The antibacterial activity of the non-treated and treated corn by-products against a variety of pathogenic and opportunistic bacterial strains was assessed by measuring the diameter of inhibition zones (DIZ, mm) in agar well diffusion assays. The list of pathogenic and opportunistic bacterial strains and detailed description of the method is given in Supplementary Data S1.
The antifungal activities of the non-treated and treated corn by-products against 10 different mould species were determined by the agar well diffusion assay [20]. The list of mould species and detailed description of the method is given in Supplementary Data S1.
2.7. Statistical Analysis
The physico-chemical data were expressed as the mean values (n = 3) of each sample ± standard error (SE), and the microbiological data were expressed as the mean values (n = 5) of each sample ± standard error (SE). The effects of the different treatments were analyzed by multivariate analysis of variance (ANOVA) and Tukey’s honestly significant difference test (HSD) procedure, as post-hoc tests. A linear Pearson’s correlation was used to quantify the strength of relationships between variables. The correlation coefficients were calculated using the statistical package SPSS for Windows (v15.0, SPSS, Chicago, IL, USA). Correlation strength interpretation was performed in accordance with Evans et al. [21]. The results were recognized as statistically significant at p ≤ 0.05.
3. Results and Discussion
3.1. Acidity Parameters and Microbiological Characteristics of the Corn By-Products
The changes in acidity parameters of corn by-product after fermentation are shown in Table 1. After 24 h of fermentation, the significant reduction in pH values and increase in TTA values of all samples were observed, compared to non-fermented samples. The lowest pH after 24 h of fermentation was found in the CconLpl, CconLu, and CconLc samples (3.34, 3.35, and 3.30, respectively) and the highest TTA was found for Cex18Lpl (5.5° N).

Table 1.
Acidity parameters of non-treated and treated corn by-products.
The content of L(+)- and D (−)-lactic acid isomers after 24 h of fermentation varied from 0.274 to 0.360 and 0.321 to 0.6 g/100 g, respectively. The highest content of L (+) isomer was produced in CconLpa and Cex14Lpa (0.360 and 0.362 g/100 g, respectively). The lowest content of D (−) isomer was found in Cex18Lpa (0.056 g/100 g).
The results also showed that increased moisture content of extruded corn by-products increased the pH and decreased TTA of samples, as well as the concentration of lactic acid isomers. At 16 and 18% corn by-products moisture, the pH of the fermented samples was the highest, and the TTA was the lowest compared to corn extrudates with lower moisture content (Table 1). The concentration of lactic acid isomers was lower at higher moisture (18%) content of corn extrudates and varied from 0.095 to 0.221 g/100 g and 0.056 to 0.251 g/100 g, L (+) and D (−) content, respectively, compared to corn extrudates with moisture of 14% (Table 1).
Grain seeds fermentation is related with increased nutritional value, a high number of viable LAB, reduced pH, and a high concentration of organic acids [22,23,24]. In a previous study, the increase in TTA values with increasing fermentation time supported the decrease in pH performance, which was one of the most important changes during LAB fermentation [25,26]. Fast acidification by starter cultures, resulting in pH reduction, is considered critical from a food safety standpoint and plays a vital role in eliminating food pathogens and extending product quality [27,28].
Microbiological parameters of non-treated and treated corn by-products are shown in Table 2. Lactic acid bacteria (LAB) counts were significantly higher in the fermented (non-extruded) and extruded–fermented corn by-products, compared to the control samples. The highest LAB count was found in CconLc (9.39 log10 CFU/g). LAB count in the extruded (non-fermented) samples was lower or similar to that of the control samples. In corn by-products fermented with Lpl, Lu, and Lc, mould/yeast (M/Y) counts (3.72–3.80 log10 CFU/g) were significantly lower compared to the control. M/Y was significantly higher in most of the other extruded and extruded–fermented samples, compared to control. Significantly lower total bacteria count (TBC) was found in most of the fermented (CconLpl, CconLu, and CconLpa) and extruded–fermented (Cex14Lpl, Cex14Lu, Cex16Lpa, Cex18Lu, Cex18Lc, and Cex18Lpa) samples, compared to the control group, with the lowest (7.94–8.12 log10 CFU/g) being in CconLu, Cex18Lu, and Cex18Lpa. Total enterobacteria count (TEC) was significantly lower in all the fermented (non-extruded) samples and most of the extruded–fermented samples (Cex14Lc, Cex14Lu, Cex16Lpl, Cex16Lu, Cex16Lc, Cex18Lpl, Cex18Lu, Cex18Lc, and Cex18Lpa), with the lowest being in Cex16Lc and Cex18Lpl (5.45 and 5.32 log10 CFU/g). The extruded (non-fermented) samples had significantly higher TEC than control.

Table 2.
Microbiological parameters of the corn by-products.
The Lactobacillus group produces many antimicrobial compounds, including lactic and acetic acids, that reduce environmental pH and are antagonistic to a wide range of pathogenic and opportunistic microorganisms [29]. Organic acids, produced by LAB, lower environment pH, and limit the growth of bacterial pathogens [30]. Our results are similar with Ayyash et al. [31], who found that compared with day 0, all Lactobacillus spp. populations increased (p < 0.05) in all grain ferments. In general, Lactobacillus spp. increased by ~1.5 log (~7.0 logs to 9.0 logs) during 48 days of storage. The similar results were observed by Ferrero et al. [32], who reported that the yeast count decreased with fermentation and was below the detection limit at 250 days, while the mould count was under the detection limit after 30 days of fermentation, regardless of the treatment.
3.2. Amino Acids Profile and Biogenic Amines Formation in Corn By-Products
Essential amino acids (EAA) mass concentrations in corn by-products are presented in Table 3. The predominant EAA in control group were isoleucine (Ile), valine (Val), tryptophan (Trp), and threonine (Thr) and their content ranged from 0.15 to 0.36 g/100 g. Lysine was not found in all samples, while the presence of leucine (<0.02 g/100 g) was observed in all fermented (not extruded) samples, as well as extruded–fermented Cex14Lpl and Cex14Lu samples. Significant changes (p ≤ 0.05) were found in the contents of all EAA between the tested samples. Extrusion increased the content of Phe and Val in Cex14 and Cex18, respectively, as well as Met in Cex16 and Cex18, compared to the control samples. Fermentation of the control samples increased the contents of Phe and histamine (His). Fermentation of the extruded samples increased the contents of Phe, His, and Thr in Cex14Lpl; Val in Cex14Lpl and all extruded samples with moisture content of 16 and 18%; methionine in all extruded samples with moisture content of 16 and 18%, compared to control. The contents of Trp and Ile were reduced or similar in fermented, extruded, or extruded–fermented samples, compared to control.

Table 3.
Essential amino acids mass concentration (g/100 g) in corn by-products.
Non-essential amino acid (NEAA) concentrations in corn by-products are given in Table 4. The presence of arginine (<0.08 g/100 g) was observed in all fermented (not extruded) samples, as well as extruded–fermented Cex14Lpl, Cex14Lu, and Cex16Lc samples. The contents of alanine and proline were reduced in the fermented, extruded, or extruded–fermented samples, compared to the control. Tyrosine (Tyr) and glutamine (Glu) were reduced in all the extruded and extruded–fermented samples, compared to the control. However, fermented (non-extruded) corn by-products shared similar contents of Tyr and Glu with the control group. After treatment of corn by-products, arginine appeared in all fermented (non-extruded) samples, as well as in some extruded–fermented samples (Cex14Lpl, Cex14Lu, and Cex16Lc). Aspartic acid and glycine were significantly higher in fermented (non-extruded) samples and Cex14Lpl, compared to the control group. Serine was significantly higher in CconLu and Cex14Lpl, compared to the control group. Cysteine (Cys) was significantly higher in all extruded and extruded–fermented samples with moisture content of 16 and 18%, compared to the control group. The highest content (1.40–1.69 g/100 g) of Cys was found in the extruded–fermented corn by-products with a moisture content of 18%.

Table 4.
Non-essential amino acids mass concentration (g/100g) in corn by-products.
The increased content of some amino acid in fermented corn by-products could be explained by the proteolytic activity of LAB and endogenous proteases which are activated under the acidic conditions [33]. Moreover, certain amino acids and peptides are also used by LAB for their metabolism. Our results partly agree with Onyango et al. [34], that aspartic acid, glycine, cystine, and methionine increased after fermentation, while contents of all other amino acids showed no significant changes. Another study showed that fermentation of corn milling by-products improved the content of free amino acids and polypeptides [35]. The increased content of free amino acids after wheat bran fermentation with LAB was also observed [36]. Protein structure could be destroyed due to the conditions of the extrusion process as high temperature and pressure [37]. Thermal degradation of lysine, valine, leucine, threonine, and isoleucine was reported [14]. It was found that the extrusion of wheat bran led to a quantitative decrease in amino acids and the protein and moisture content of raw material, while the barrel temperature had no significant influence on cysteine and methionine content in rice-based snacks [38,39]. Moisture levels also influence lysine retention, but conflicting results have been observed [40]. However, the increase in the concentration of amino acids (for arginine, histidine, proline, and alanine by 80, 3, 13, and 11%, respectively) in corn after extrusion, relative to the native form, was observed by Kholodilina et al. [38]. It was also reported that extrusion increased all amino acids, except Lys and Pro in corn [41].
The concentrations of BAs in corn by-products that were extruded and fermented by LAB, are presented in Figure 2. Histamine, tryptamine, putrescine, tyramine, and spermine were not found in all the tested samples. Phenylethylamine (PHE) was found in all corn by-products, while cadaverine and spermidine were found only in non-treated samples. Results showed that treatment had a significant effect (p ≤ 0.05) on the concentration of PHE in corn by-products. Fermentation with Lpl and Lc strains, as well as extrusion (moisture content of 14 and 16%) of control samples significantly increased PHE content by on average 18.4%. However, after extrusion, PHE was significantly lower in the Cex18 sample, compared to the control group. In most of cases, fermentation of the extruded samples led to the increased content of PHE, compared to only extruded corn by-products or control group. Fermentation with L. paracasei-LUHS244 significantly reduced the content of PHE in CconLpa and Cex18Lpa samples, compared to non-treated corn by-products and that was the lowest concentration of BA found between all samples.
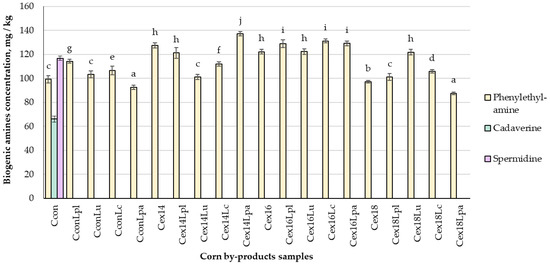
Figure 2.
Biogenic amines mass concentration in corn by-products C—corn by-product samples; con—control samples (non-extruded non-fermented); Lpl, Lu, Lc, Lpa—fermented with L. plantarum-LUHS122, L. uvarum-LUHS245, L. casei-LUHS210, and L. paracasei-LUHS244 strains, respectively; ex—extruded samples; 14, 16, 18—moisture content of the corn by-product samples. Data are represented as means (n = 3) ± SE. a–j—mean values denoted with different letters are significantly different between samples (p ≤ 0.05).
Biogenic amines (BA) are low-molecular-weight nitrogenous organic bases, which can accumulate in high concentration in food due to microbial activity and cause toxic effects in consumers. Some food microorganisms are able to degrade BA once they have been synthesized in the food matrix [42,43]. Corn can also be a source of biogenic amines. Some biogenic amines can be naturally present in corn whereas others can be introduced during production, processing, and storage. They can be formed by thermal or microbial decarboxylation of amino acids and may be used as an index of quality or hygienic conditions of products [44]. Cadaverine and spermidine can be naturally found in raw plant foods, while PHE can be produced by microorganisms from phenylalanine [45,46].
The reports on the effect of extrusion processing or LAB fermentation on biogenic amine changes in corn products are scarce. Previous research reported that no significant changes in the formation of amines occurred during brewing fermentation, except for tryptamine and tyramine [47]. In the another study, the content of PHE in hemp (Cannabis sativa L.) seed paste fermented with L. uvarum-LUHS245 and L. casei-LUHS210 increased by 5% and decreased by 52%, respectively, compared with untreated samples [48].
3.3. Sugars Concentration in Corn By-Product Samples
The contents of fructose, glucose, sucrose, and maltose in non-treated and treated corn by-products were analyzed and the results are given in Figure 3. There was a significant effect (p ≤ 0.05) of treatment on the fructose, glucose, and sucrose concentration in corn by-product samples. Significantly lower (p ≤ 0.05) contents of fructose and glucose (on average by 42.2 and 47%, respectively) were found in all fermented (non-extruded) corn by-products, compared to non-treated samples. The same tendency was also observed with fructose content in Cex14, Cex14Lpl, and Cex16Lpl samples (lower, on average, by 53.1%), while fructose was not determined in the rest of samples. Glucose was not found in all extruded and extruded–fermented samples. The presence of sucrose was observed after extrusion in Cex14, Cex16, and Cex18. After fermentation of the extruded samples, the content of this sugar was found to be significantly lower (p ≤ 0.05) in Cex14Lu and Cex16Lpl by 66.1 and 48.6%, respectively, compared to only extruded samples. Maltose was not found in any of the tested samples.
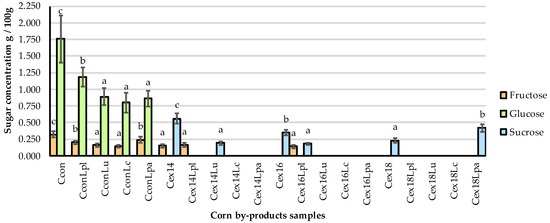
Figure 3.
The sugars mass concentration in corn by-products. C—corn by-product samples; con—control samples (non-extruded, non-fermented); Lpl, Lu, Lc, Lpa—fermented with L. plantarum-LUHS122, L. uvarum-LUHS245, L. casei-LUHS210, and L. paracasei-LUHS244 strains, respectively; ex—extruded samples; 14, 16, 18—moisture content of the corn by-product samples. Data are represented as means (n = 3) ± SE. a–c—mean values denoted with different letters are significantly different between samples (p ≤ 0.05).
The variations of fructose, glucose, and sucrose concentrations in fermented corn by-products are related with the LAB activity. During fermentation, the conversion of available carbohydrates by LAB, which could also possess polysaccharide-degrading activity, yields such compounds as lactic, and acetic acid [49,50]. As carbohydrates undergo a series of changes under the extrusion conditions, the lower concentrations of fructose, the absence of glucose and a higher content of sucrose in extruded corn by-products could be explained by the influence of extrusion processes. Due to the impact of shear forces, as well as feed moisture content and temperature, the insoluble fibres in corn by-products could be broken down into soluble compounds with reduced molecular weight [51]. Lower moisture content in feed causes a more intensive decomposition of insoluble dietary fibres [11]. Moreover, the formation of Maillard browning products during extrusion lowers the content of reducing sugars in corn by-products.
3.4. Corn By-Products Fatty Acids Profile
The fatty acids (FA) profile of non-treated and treated corn by-products is given in Table 5 and Supplementary Data S2. The FA profile of samples revealed that linoleic acid (C18:2 n6, 41–53%) was the highest followed by oleic (C18:1 n9, 32–33%), palmitic (C16:0, 9–13%), stearic (C18:0, 2–4%), and α-linolenic (C18:3 n3, 1–3%) acids (Supplementary Data S2). Similar results have been reported for predominant FA by other authors [52,53]. The rest of FA were observed at low levels (less than 1%). It was found that treatment had a significant effect (p ≤ 0.05) on most of the FA (C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C15:0, C15:1, C16:0, C16:1, C17:0, C18:0, C18:3, C20:1, C21:0, C20:2, C22:0, C20:3, C24:0, and C22:6) in corn by-products. Palmitic and stearic acids were significantly higher in Cex18Lpl and Cex18Lc, compared to the rest of the samples. Compared to non-treated corn-by products, α-linolenic was significantly higher in extruded and extruded–fermented samples, with Cex18Lu, Cex18Lc, and Cex18Lpa being the highest. In the case of other FA, clearer tendencies cannot be drawn. However, significant differences in the content of oleic and linoleic acid between samples were not found.

Table 5.
The fatty acids percentage profile of the non-treated and treated corn by-products.
The FA profile of all corn by-products was dominated by polyunsaturated FA (PFA, 45–54%), followed by monounsaturated FA (MFA, 33–35%), and then saturated FA (SFA, 13–21%) (Table 5). Significantly higher content of SFA was found in several extruded–fermented Cex14Lc, Cex18Lpl Cex18Lc, compared to the rest of the samples (p ≤ 0.05), while MFA content was similar in all tested corn by-products. PFA content was significantly lower (p ≤ 0.05) only in Cex16Lu, compared to other samples. The higher content of PFA is favourable in food because they improve blood sugar level, possess blood cholesterol and pressure lowering abilities, and fight against inflammatory reactions and various cancers [54]. The PFA/SFA ratio ranged from 2.2 to 4.2 with the highest being for CconLpl, CconLpa, and Cex14Lpl and the lowest being for Cex18Lpl and Cex18Lc. Ratios of all tested samples were higher than 0.4 as recommended by the World Health Organization [55]. The group of omega 6 FA was the highest (41–53%) followed by omega 9 (32–34%), and omega 3 (1–4%) in all tested samples (Table 5). Significant changes in the content of omega 6 and omega 9 were not observed in all tested samples. However, compared to non-treated samples, the content of omega 3 was significantly higher in all extruded and extruded–fermented corn by-products, except Cex14Lu. The omega 6/omega 3 FA ratio ranged from 11.4 to 48.2 with the highest being for CconLpa, and the lowest being for Cex18Lc. The decrease in omega 6/omega 3 ratio is desirable for the prevention of cardiovascular diseases, diabetes, obesity, and cancer but there are no recommended specific values [56]. Some fermented samples (CconLpl, CconLu, CconLc, CconLpa, and Cex18Lpl) contained a very small amount (lower than 0.08%) of trans FA.
Availability of data is limited to being on the effect of fermentation and extrusion on FA profile of corn by-products. Wani et al. [57] reported that FA composition, including PFA and MFA content, of corn-based snacks, was not significantly affected by extrusion. Contrarily, Ramos Diaz et al. [58] found that after extrusion, the content of palmitic, linoleic, oleic, and linolenic acid was reduced in corn-based extrudates, compared to those non-extruded. The slight loss of some lipids during extrusion occurs due to the high temperature, which could also cause the essential reduction in PFA stability, and the formation of amylose–lipid complexes [37,58]. Lower conditions of temperature and moisture in the extrusion process increase the stability of PFA in extruded products during storage [14]. Changes in the FA profile after fermentation could be attributed to the lipolytic activity of LAB [59]. In addition, LAB abilities to generate FA and modify their saturation and desaturation are reported [60].
3.5. Antimicrobial Characteristics of Corn By-Products
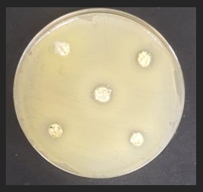
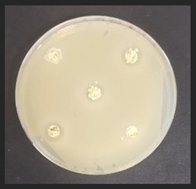
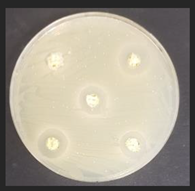
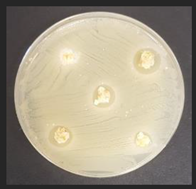
Diameter inhibition zones (DIZ) of the non-treated and treated corn by-products against pathogenic and opportunistic microorganisms are given in Table 6. Fermented corn by-products CconLpl, CconLu, and CconLpa displayed inhibition properties against 3 of the 10 tested pathogenic/opportunistic bacterial strains: Acinetobacter johnsonii, Staphylococcus aureus, Aeromonas veronii. Sample CconLc showed inhibition properties only against Acinetobacter johnsonii and Staphylococcus aureus. Most of samples, except Ccon, Cex14, Cex16, Cex18, Cex18Lpl, Cex18Lpa, showed inhibition properties against Acinetobacter johnsonii (DIZ of 12.5 mm on average). No efficiency in inhibiting Salmonella enterica Infantis, E. coli, Bacillus pseudomycoides, Cronobacter sakazakii, Hafnia alvei, Enterococcus durans, Kluyvera cryocrescens was observed by any of the samples.

Table 6.
Antibacterial properties of non-treated and treated corn by-products.
The natural bio preservatives in foods and feed or their ingredients not only improve the microbiological safety of these products but are a sustainable approach to protect human or animal health [61]. The antimicrobial effects of fermented corn by-products are highly related to the presence of LAB and their active metabolites [62]. In the fermentable substrate, LAB can synthesize several or more antimicrobial compounds, including bacteriocins, bacteriocin like substances (BLIS), organic acids (lactic, acetic, and propionic acids), acetoin, hydrogen peroxide, acetaldehyde, carbon dioxide, and diacetyl [63]. In order to suspend the growth of pathogenic bacteria, sufficient concentrations of antimicrobial metabolites should be released by LAB and that happens when particular levels of LAB are reached in fermented substrate [64]. However, the composition of substrates (carbohydrates, amino acids, vitamins, fatty acids, and minerals) and fermentation conditions (pH, temperature, aeration, and agitation) could strongly affect the growth of LAB, as well as the accumulation and profile of antimicrobial compounds excreted by LAB [61,62]. Our previous study showed that in this study used L. plantarum LUHS122, L. uvarum LUHS245, L. casei LUHS210, and L. paracasei LUHS244 displayed good inhibition properties against pathogenic and opportunistic bacterial strains (Klebsiella pneumoniae, Salmonella enterica 24 SPn06, Pseudomonas aeruginosa 17-331, Acinetobacter baumanni 17-380, Proteus mirabilis, MRSA M87fox-MRSA–Methicillin-resistant, Enterococcus faecalis 86, Enterococcus faecium 103, Bacillus cereus 18 01, 10–Streptococcus mutans, Enterobackter cloacae, 12–Citrobacter freundii, Staphylococcus epidermidis, Staphylococcus haemolyticus, Pastaurella multocida) [15].
The antifungal activities of the non-treated and treated corn by-products against the species of Aspergillus niger, Memnoniella echinate, Chrysosporium merdarium, Aspergillus fumigatus, Trichoderma viride, Rhizopus, Fusarium nivale, Penicillium viridicatum, Aspergillus versatile, and Aspergillus ferenczii were tested and the results are given in Table 7. Delay of Rhizopus spore formation was obtained with extruded and fermented samples of Cex14Lu, Cex14Lc, Cex14Lpa, Cex14Lpl, Cex18Lu, Cex18Lc, and Cex18Lpa. A delay of Aspergillus fumigatus spore formation was found with the Cex14Lpl sample. However, the lack of inhibitory ability was detected for the rest of the samples against all tested moulds.

Table 7.
Antifungal properties of non-treated and treated corn by-products.
The antifungal activity of extruded and fermented corn by-products could result from the synergistic activities of several antifungal metabolites of LAB, for which profile and concentrations depend on strain, species, as well as on LAB growth conditions (availability of nutrients, temperature, pH, atmosphere, and viscosity) [63,65]. Antifungal metabolites include organic acids (lactic acid, acetic acid, 3-phenyllactic acid, etc.), fatty acids (3-hydroxydecanoic acid, ricinoleic acid, decanoic acid, etc.), cyclic dipeptides, reuterin, hydrogen peroxide, and diacetyl [66]. In this study used LAB strains already showed antifungal properties against Aspergillus fischeri, Aspergillus nidulans, Penicillium oxalicum, Penicillium funiculosum, Fusarium poae, Alternaria alternate, and Fusarium graminearum [15].
4. Conclusions
The economic efficiency and environmentally friendly production are important aspects in the sustainable valorization of cereals processing by-products, and this is still challenging. The valorization of corn processing by-products could be designed in a more appropriate and sustainable manner by using whole by-product conversion, and by combining extrusion and fermentation processes, as the latter are common and economically efficient processes in the food and feed industry. This study indicated that fermentation with antimicrobial properties possessing LAB strains or the combined technique of extrusion and fermentation improved the microbiological safety of corn by-products. The latter technique increased the content of certain amino acids (e.g., valine, methionine) in most of the samples. Such biogenic amines as cadaverine and spermidine were not found after treatments of corn by-products, while the lowest content of biogenic amines was found in extruded–fermented (with L. paracasei-LUHS244, moisture 18%) samples. Applied treatments affected the content of most fatty acids. The level of omega 3 was significantly higher in extruded and extruded–fermented corn by-products. However, the contents of saturated, monounsaturated, and polyunsaturated fatty acids were similar between most of the samples. Corn by-products fermented with L. plantarum-LUHS122, L. uvarum-LUHS245, and L. paracasei-LUHS244 showed antibacterial activity against Acinetobacter johnsonii, Staphylococcus aureus, Aeromonas veronii. Extruded (14% moisture) and fermented with L. plantarum-LUHS122 corn by-products inhibited Rhizopus and Aspergillus fumigatus. In sum, combining extrusion and fermentation processes for corn by-product valorization can improve certain parameters and properties of these products, and they can be recommended as safer and more nutritious ingredients for food and feed production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11223080/s1. Supplementary Data S1: Description of Methods; Supplementary Data S2. Table S1: The fatty acids profile of the corn by-products; Supplementary Data S3. Table S2: Limit of detection values (LOD) for analyzed fatty acids.
Author Contributions
Conceptualization, E.B.; methodology, E.B., V.B., M.R., R.R. and A.V.; formal analysis and data curation, G.Z., E.Z. and V.S.; validation, V.S. and E.Z.; investigation, V.S., E.Z., D.K. and D.C.; resources, E.B.; writing—original draft preparation E.B., D.K., D.C., V.S. and E.Z.; writing—review and editing, E.B., V.B., M.R., R.R. and A.V.; visualization, V.S., E.Z. and D.K.; supervision, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the EUREKA Network Project E!13309 “SUSFEETECH” (Nr. 01.2.2-MITA-K-702-05-0001), COST Action CA18101 ‘SOURDOugh biotechnology network towards novel, healthier and sustainable food and bIoproCesseS’ and LSMU Science Foundation, support No. 0101010202/010302.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luithui, Y.; Baghya Nisha, R.; Meera, M.S. Cereal By-Products as an Important Functional Ingredient: Effect of Processing. J. Food Sci. Technol. 2019, 56, 1–11. [Google Scholar] [CrossRef]
- Tsadik, Y.Y.G.; Emire, S.A. Development of Value Added Products from Byproducts of Ethiopian Wheat Milling Industries. J. Food Process. Technol. 2015, 6, 1. [Google Scholar]
- Staller, J. Maize Cobs and Cultures: History of Zea mays L.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 3-642-04506-5. [Google Scholar]
- Scott, M.P.; Emery, M. Maize: Overview. Encyclopedia of Food Grains, 2nd ed.; Faubion, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780123944375. [Google Scholar]
- Wang, L.; Liu, H.-M.; Xie, A.-J.; Zhu, C.-Y.; Qin, G.-Y. Dietary Fiber Extraction from Defatted Corn Hull by Hot-Compressed Water. Pol. J. Food Nutr. Sci. 2018, 68, 133–140. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Jane, J. Macronutrients in Corn and Human Nutrition. Compr. Rev. Food Sci. Food Saf. 2016, 15, 581–598. [Google Scholar] [CrossRef]
- Ostrander, B.M. Maize Starch for Industrial Applications. In Industrial Crops; Springer: Berlin/Heidelberg, Germany, 2015; pp. 171–189. [Google Scholar]
- Decimo, M.; Quattrini, M.; Ricci, G.; Fortina, M.G.; Brasca, M.; Silvetti, T.; Manini, F.; Erba, D.; Criscuoli, F.; Casiraghi, M.C. Evaluation of Microbial Consortia and Chemical Changes in Spontaneous Maize Bran Fermentation. AMB Express 2017, 7, 205. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Li, L.; Zhang, M.; Tian, S.; Wang, D.; Liu, K.; Liu, H.; Zhu, W.; Wang, X. Comprehensive Utilization of Corn Starch Processing By-Products: A Review. Grain Oil Sci. Technol. 2021, 4, 89–107. [Google Scholar] [CrossRef]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of Food Processing By-Products in Extrusion Processing: A Review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Xu, M.; Zhang, X.; Cao, S.; Hu, Y.; Luan, G. Physicochemical Properties and Protein Structure of Extruded Corn Gluten Meal: Implication of Temperature. Food Chem. 2022, 399, 133985. [Google Scholar] [CrossRef]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Gulati, P.; Brahma, S.; Rose, D. Impacts of Extrusion Processing on Nutritional Components in Cereals and Legumes: Carbohydrates, Proteins, Lipids, Vitamins, and Minerals. In Extrusion Cooking; Woodhead Publishing: Amsterdam, The Netherlands, 2020; pp. 415–436. ISBN 978-0-12-815360-4. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic Acid Bacteria Combinations for Wheat Sourdough Preparation and Their Influence on Wheat Bread Quality and Acrylamide Formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-L.; Zhu, R.-H.; Li, H.-D. Determination of Dansylated Monoamine and Amino Acid Neurotransmitters and Their Metabolites in Human Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry. Anal. Biochem. 2010, 396, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Gruzauskas, R.; Ruzauskas, M.; Zokaityte, E.; Starkute, V.; Klupsaite, D.; Vadopalas, L.; Badaras, S.; Özogul, F. Changes in the Microbial Community and Biogenic Amine Content in Rapeseed Meal during Fermentation with an Antimicrobial Combination of Lactic Acid Bacteria Strains. Fermentation 2022, 8, 136. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Belmont, CA, USA, 1996; ISBN 0-534-23100-4. [Google Scholar]
- Nnam, N.M.; Obiakor, P.N. Effect of Fermentation on the Nutrient and Antinutrient Composition of Baobab (Adansonia Digitata) Seeds and Rice (Oryza Sativa) Grains. Ecol. Food Nutr. 2003, 42, 265–277. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The Potential of Fermentation on Nutritional and Technological Improvement of Cereal and Legume Flours: A Review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Ma, S.; Tian, X.; Sun, B.; Huang, J.; Li, L.; Wang, X.; Bao, Q. Effect of Wheat Bran Dietary Fiber on Structural Properties of Wheat Starch after Synergistic Fermentation of Lactobacillus Plantarum and Saccharomyces Cerevisiae. Int. J. Biol. Macromol. 2021, 190, 86–92. [Google Scholar] [CrossRef]
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Alzbergaite, G.; Zadeike, D.; Bartkiene, E.; Ozogul, F.; Rueller, L.; Robert, J.; Rocha, J.M.F. Bio-Refinery of Plant Drinks Press Cake Permeate Using Ultrafiltration and Lactobacillus Fermentation into Antimicrobials and Its Effect on the Growth of Wheatgrass in Vivo. Food Biosci. 2022, 46, 101427. [Google Scholar] [CrossRef]
- Adebo, O.; Njobeh, P.; Adeboye, A.; Adebo, J.; Sobowale, S.; Ogundele, O.M.; Kayitesi, E. Advances in Fermentation Technology for Novel Food Products. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018; pp. 71–87. ISBN 978-3-319-74819-1. [Google Scholar]
- Adebo, O.A.; Njobeh, P.B.; Kayitesi, E. Fermentation by Lactobacillus Fermentum Strains (Singly and in Combination) Enhances the Properties of Ting from Two Whole Grain Sorghum Types. J. Cereal Sci. 2018, 82, 49–56. [Google Scholar] [CrossRef]
- Gunasekaran, Y.K.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Zokaityte, E.; Klupsaite, D.; Bartkevics, V.; Guiné, R.P.; Bartkiene, E. Plant-based Proteinaceous Snacks: Effect of Fermentation and Ultrasonication on End-product Characteristics. Food Sci. Nutr. 2020, 8, 4746–4756. [Google Scholar] [CrossRef]
- Kim, C.; Ndegwa, E. Influence of PH and Temperature on Growth Characteristics of Leading Foodborne Pathogens in a Laboratory Medium and Select Food Beverages. Austin Food Sci. 2018, 1, 1031. [Google Scholar]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In Vitro Investigation of Bioactivities of Solid-State Fermented Lupin, Quinoa and Wheat Using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, F.; Piano, S.; Tabacco, E.; Borreani, G. Effects of Conservation Period and Lactobacillus Hilgardii Inoculum on the Fermentation Profile and Aerobic Stability of Whole Corn and Sorghum Silages. J. Sci. Food Agric. 2019, 99, 2530–2540. [Google Scholar] [CrossRef]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize Milling By-Products: From Food Wastes to Functional Ingredients Through Lactic Acid Bacteria Fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Onyango, C.; Noetzold, H.; Bley, T.; Henle, T. Proximate Composition and Digestibility of Fermented and Extruded Uji from Maize–Finger Millet Blend. LWT-Food Sci. Technol. 2004, 37, 827–832. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zhao, Y.; Zhu, Y.; Bai, J.; Fan, S.; Zhu, L.; Song, C.; Xiao, X. Recent Developments in Fermented Cereals on Nutritional Constituents and Potential Health Benefits. Foods 2022, 11, 2243. [Google Scholar] [CrossRef]
- Zhao, H.-M.; Guo, X.-N.; Zhu, K.-X. Impact of Solid State Fermentation on Nutritional, Physical and Flavor Properties of Wheat Bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, K.; Li, F.; Cao, H.; Duan, Q.; Zhu, M.; Wang, P.; Gao, Y.; Duan, Y. Effect of Extrusion on Physical and Chemical Properties and Storage Stability of Corn Germ. Cereal Chem. 2021, 98, 1135–1145. [Google Scholar] [CrossRef]
- Kholodilina, T.N.; Atlanderova, K.N.; Kurilkina, M.Y. Effect of the Extrusion Process on the Amino Acid Components Profile of the Broilers Diet. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012056. [Google Scholar] [CrossRef]
- Chaiyakul, S.; Jangchud, K.; Jangchud, A.; Wuttijumnong, P.; Winger, R. Effect of Extrusion Conditions on Physical and Chemical Properties of High Protein Glutinous Rice-Based Snack. LWT Food Sci. Technol. 2009, 42, 781–787. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional Aspects of Food Extrusion: A Review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Lee, S.A.; Jones, C.K.; Htoo, J.K.; Stein, H.H. Digestibility of Amino Acids, Fiber, and Energy by Growing Pigs, and Concentrations of Digestible and Metabolizable Energy in Yellow Dent Corn, Hard Red Winter Wheat, and Sorghum May Be Influenced by Extrusion. Anim. Feed Sci. Technol. 2020, 268, 114602. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The Problem of Biogenic Amines in Fermented Foods and the Use of Potential Biogenic Amine-Degrading Microorganisms as a Solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Kongkiattikajorn, J. Potential of Starter Culture to Reduce Biogenic Amines Accumulation in Som-Fug, a Thai Traditional Fermented Fish Sausage. J. Ethn. Foods 2015, 2, 186–194. [Google Scholar] [CrossRef]
- Bandeira, C.M.; Evangelista, W.P.; Gloria, M.B.A. Bioactive Amines in Fresh, Canned and Dried Sweet Corn, Embryo and Endosperm and Germinated Corn. Food Chem. 2012, 131, 1355–1359. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C. Fermented Foods, Part I: Biochemistry and Biotechnology; Taylor & Francis Group: Abingdon, UK, 2020; ISBN 978-0-367-73745-0. [Google Scholar]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Izquierdo-Pulido, M.; Mariné-Font, A.; Vidal-Carou, M.C. Biogenic Amines Formation during Malting and Brewing. J. Food Sci. 1994, 59, 1104–1107. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Bendoraitiene, J.; Navikaite-Snipaitiene, V.; Ruzauskas, M. Technology and Characterisation of Whole Hemp Seed Beverages Prepared from Ultrasonicated and Fermented Whole Seed Paste. Int. J. Food Sci. Technol. 2020, 55, 406–419. [Google Scholar] [CrossRef]
- Mao, M.; Wang, P.; Shi, K.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lv, F. Effect of Solid State Fermentation by Enterococcus Faecalis M2 on Antioxidant and Nutritional Properties of Wheat Bran. J. Cereal Sci. 2020, 94, 102997. [Google Scholar] [CrossRef]
- Matthews, A.; Grbin, P.R.; Jiranek, V. A Survey of Lactic Acid Bacteria for Enzymes of Interest to Oenology. Aust. J. Grape Wine Res. 2006, 12, 235–244. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Masatcioglu, M.T.; Köksel, H. Effect of Extrusion Treatment on Enzymatic Hydrolysis of Wheat Bran. J. Cereal Sci. 2020, 93, 102941. [Google Scholar] [CrossRef]
- Sanjeev, P.; Chaudhary, D.P.; Sreevastava, P.; Saha, S.; Rajenderan, A.; Sekhar, J.C.; Chikkappa, G.K. Comparison of Fatty Acid Profile of Specialty Maize to Normal Maize. J. Am. Oil Chem. Soc. 2014, 91, 1001–1005. [Google Scholar] [CrossRef]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of Sourdough Fermented with Corn Oil and Lactic Acid Bacteria on Bread Flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916, ISBN 92-4-120916-X. [Google Scholar]
- Kouřimská, L.; Sabolová, M.; Horčička, P.; Rys, S.; Božik, M. Lipid Content, Fatty Acid Profile, and Nutritional Value of New Oat Cultivars. J. Cereal Sci. 2018, 84, 44–48. [Google Scholar] [CrossRef]
- Wani, S.A.; Ganie, N.A.; Kumar, P. Quality Characteristics, Fatty Acid Profile and Glycemic Index of Extrusion Processed Snacks Enriched with the Multicomponent Mixture of Cereals and Legumes. Legume Sci. 2021, 3, e76. [Google Scholar] [CrossRef]
- Ramos Diaz, J.M.; Sundarrajan, L.; Kariluoto, S.; Lampi, A.-M.; Tenitz, S.; Jouppila, K. Effect of Extrusion Cooking on Physical Properties and Chemical Composition of Corn-based Snacks Containing Amaranth and Quinoa: Application of Partial Least Squares Regression. J. Food Process Eng. 2017, 40, e12320. [Google Scholar] [CrossRef]
- SILVA, E.O.O.; Nespolo, C.R.; Sehn, C.P.; Pinheiro, F.C.; Stefani, L.M. Lactic Acid Bacteria with Antimicrobial, Proteolytic and Lipolytic Activities Isolated from Ovine Dairy Products. Food Sci. Technol. 2019, 40, 293–299. [Google Scholar] [CrossRef]
- König, H.; Fröhlich, J. Lactic Acid Bacteria. Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Bashokouh, F.; Ramakrishnan, N.R.; Mustafa, S.; Ariff, A.B. Fermentation Factors Influencing the Production of Bacteriocins by Lactic Acid Bacteria: A Review. Rsc Adv. 2017, 7, 29395–29420. [Google Scholar] [CrossRef]
- Szutowska, J. Functional Properties of Lactic Acid Bacteria in Fermented Fruit and Vegetable Juices: A Systematic Literature Review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and Probiosis: Antimicrobial Activity of Lactic Acid Bacteria with a Focus on Their Antiviral and Antifungal Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Daliri, E.B.-M.; Wang, J.U.N.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Inhibitory Effect of Lactic Acid Bacteria on Foodborne Pathogens: A Review. J. Food Prot. 2019, 82, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, É.; Daga, M.; Arora, G.; Fliss, I. Antifungal Activity of Lactic and Propionic Acid Bacteria and Their Potential as Protective Culture in Cottage Cheese. Food Control 2017, 78, 350–356. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-Activity-Producing Lactic Acid Bacteria as Biocontrol Agents in Plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).