Modulating Expression Levels of TCP Transcription Factors by Mentha x piperita Volatiles—An Allelopathic Tool to Influence Leaf Growth?
Abstract
1. Introduction
2. Results
2.1. Effects of Fumigations on the Arabidopsis Phenotype
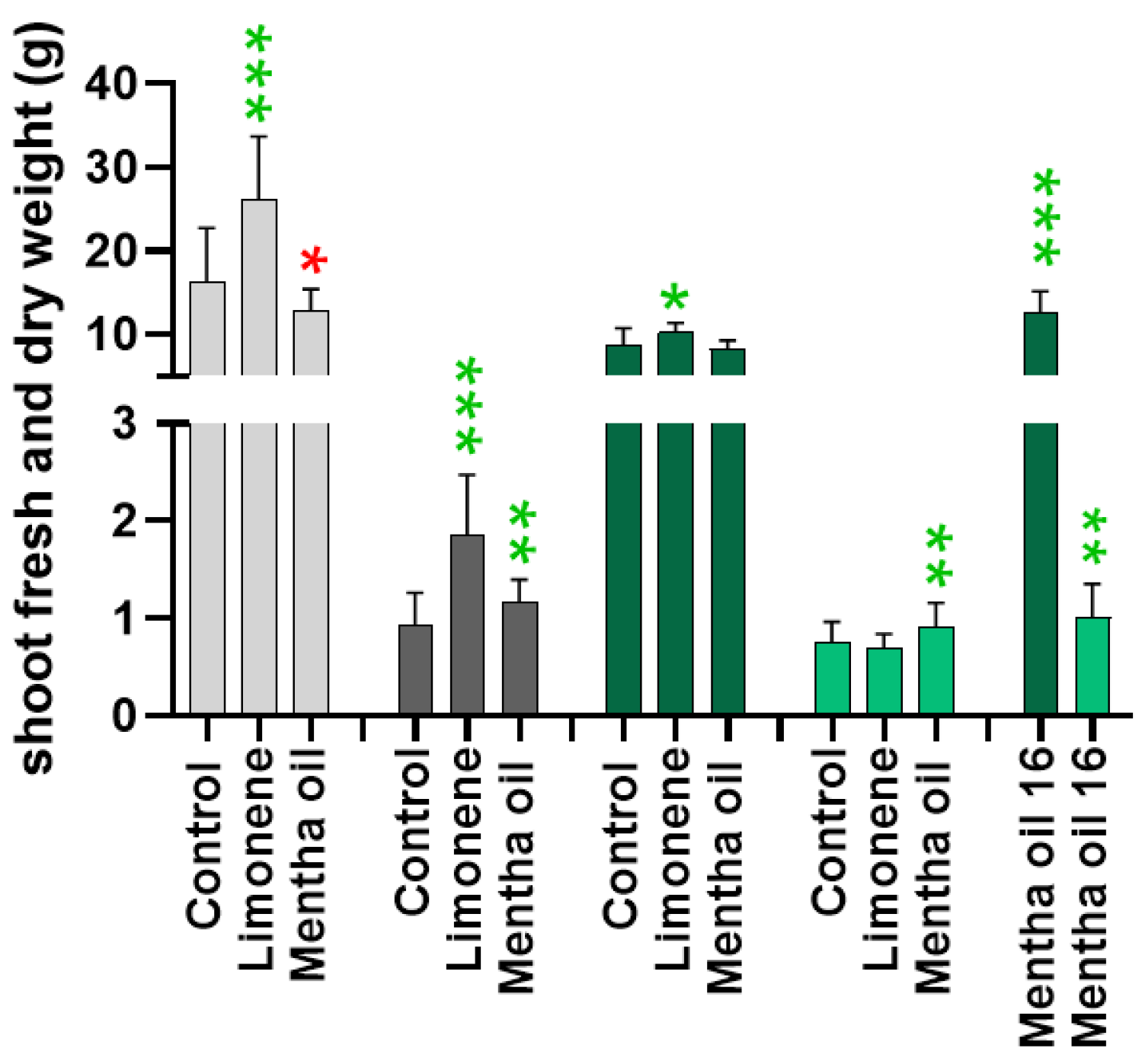
2.2. Determinations of White and Chinese Cabbage Fresh and Dry Weights after Fumigation with Limonene and Mentha Essential Oil
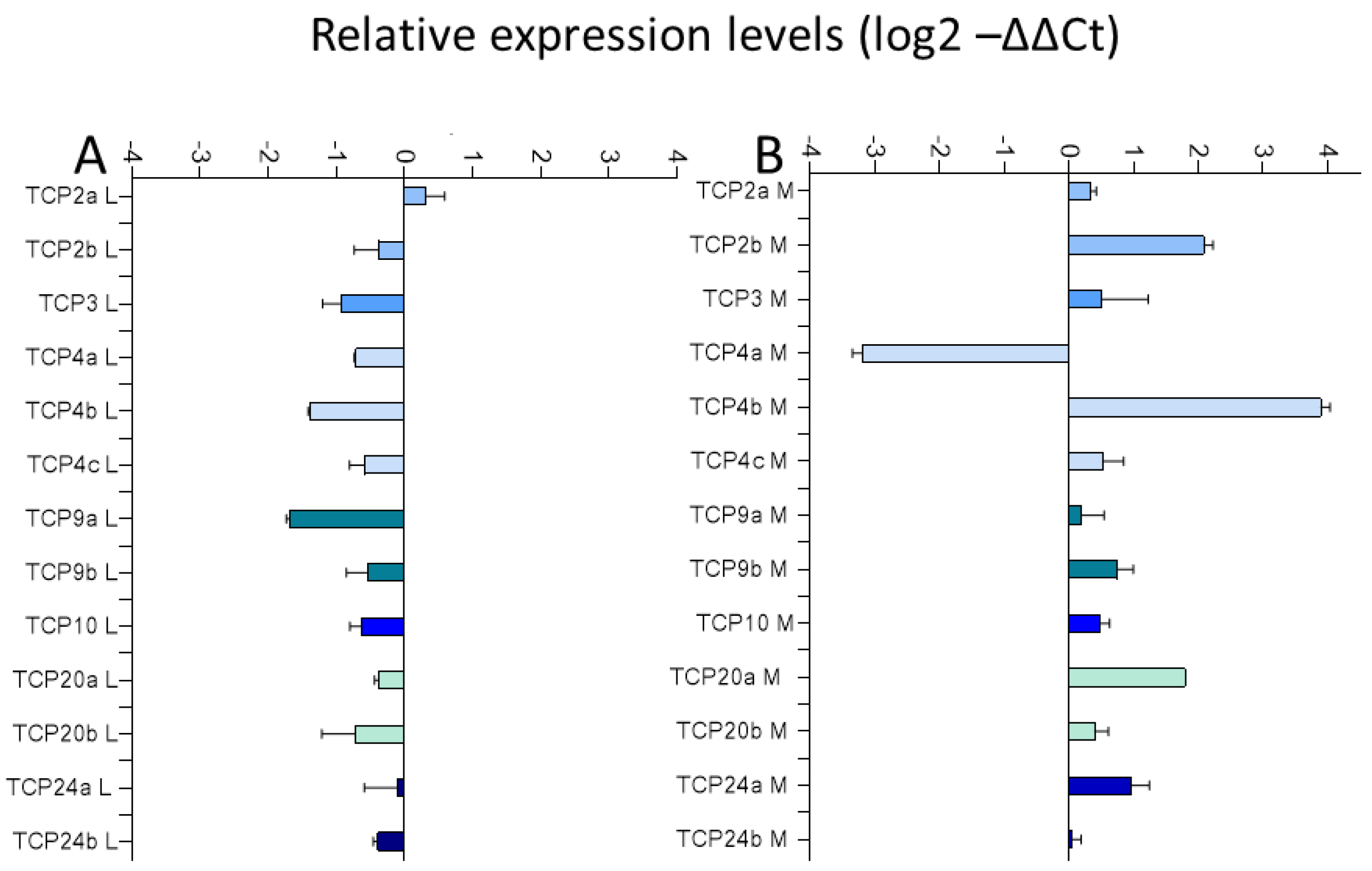
2.3. TCP Transcription Factors—Targets of Mentha x piperita Emitted Volatiles, Essential Oil Fraction, and Limonene
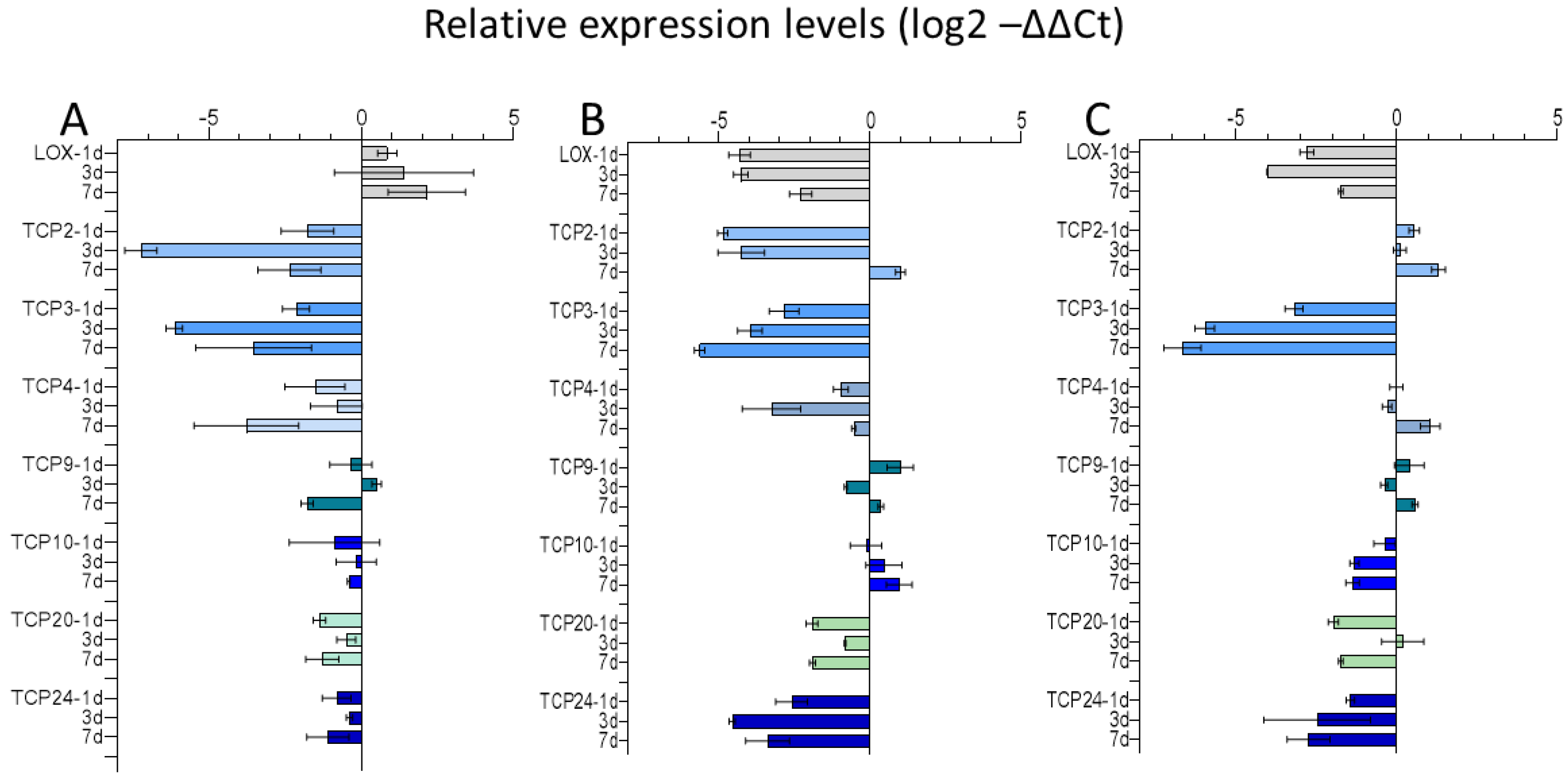
2.3.1. Expression of TCP Transcription Factors in Chinese Cabbage after Exposure to Mentha Essential Oil Fraction and Limonene
2.3.2. Choice of White Cabbage for Greenhouse and Polytunnel Trials
2.4. Greenhouse and Polytunnel Trials with Brassica oleracea convar. capitata var. alba and Mentha plants
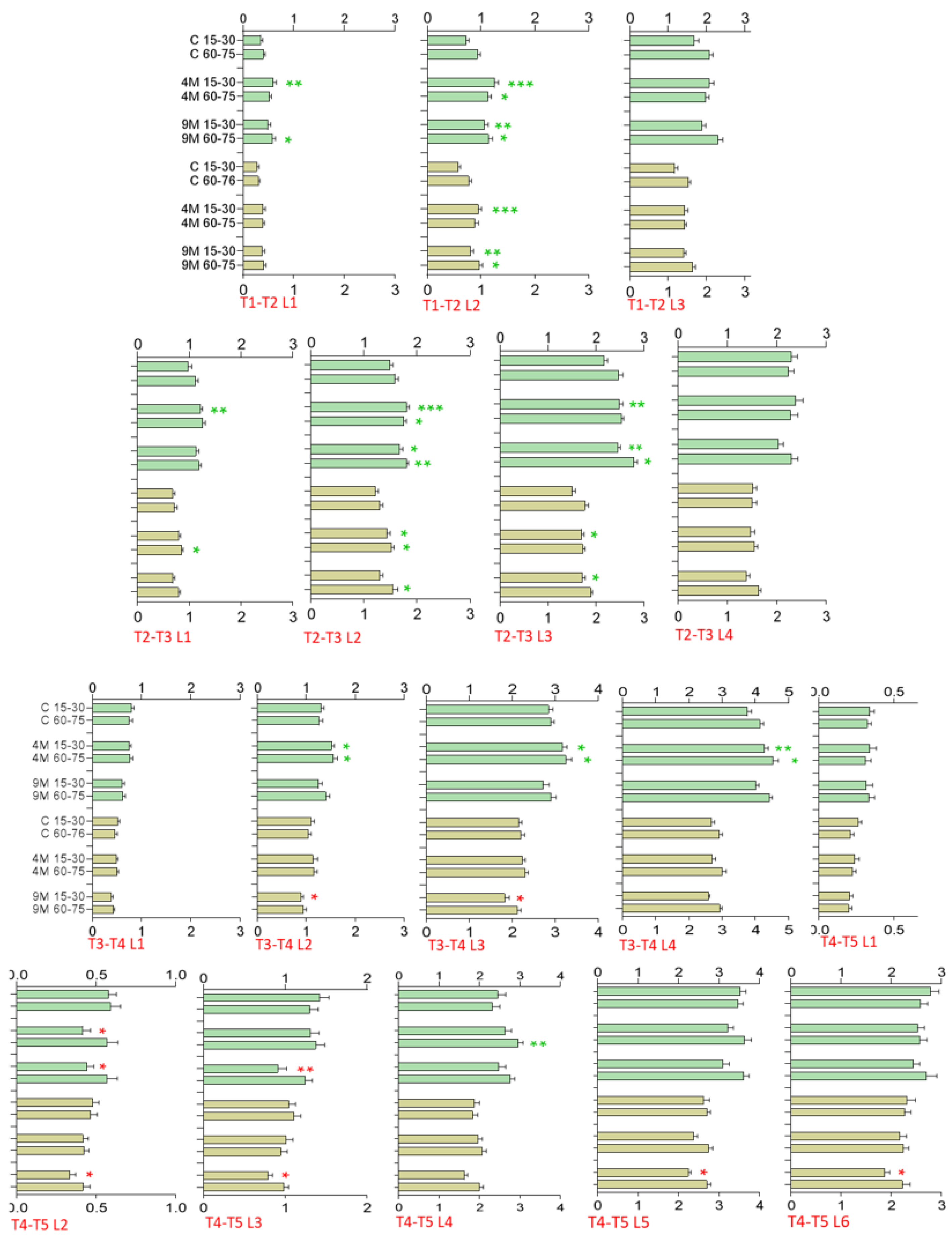
Greenhouse Trial—Leaf Length and Width, Growth Increments
2.5. Polytunnel Trial—Leaf Length and Width, Growth Increments
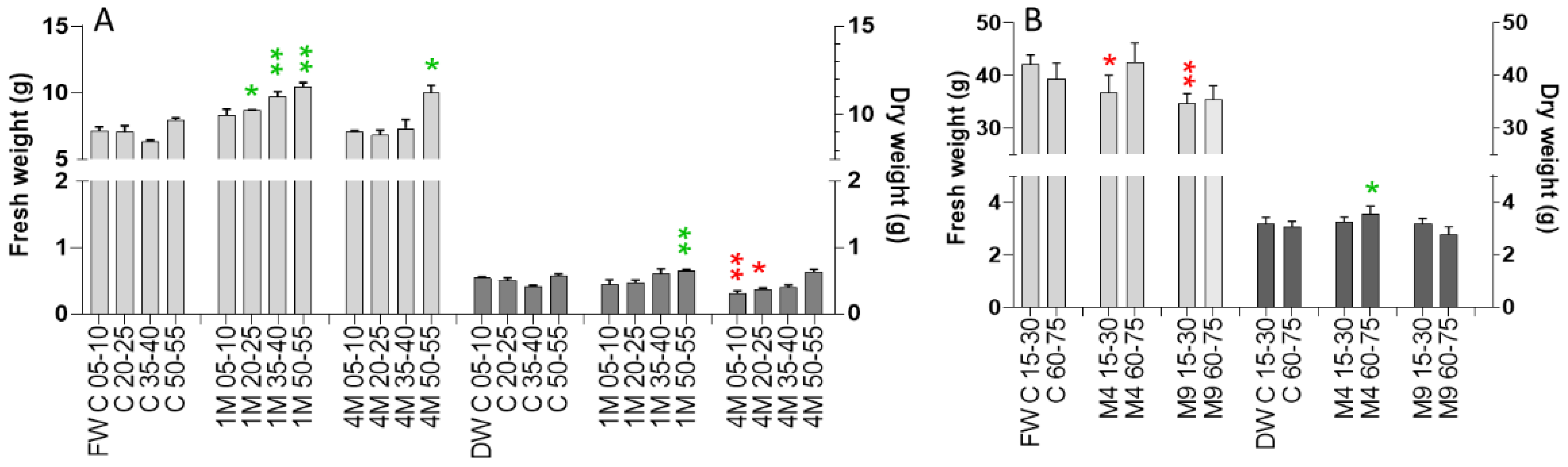
2.6. Greenhous and Polytunnel Trials—Fresh and Dry Weight
3. Discussion
4. Material and methods
4.1. Plant Material
4.1.1. Mentha x piperita Variety English Mint cv. “Mitcham”
4.1.2. Arabidopsis thaliana Col-0
4.1.3. Brassica oleracea cv. capitata var. alba convar. LENNOX F1 (white cabbage) and Brassica rapa (Chinese cabbage)
4.2. Fumigation Experiments
4.2.1. Fumigation of Arabidopsis thaliana with Mentha x piperita Mitcham
4.2.2. Fumigation of A. thaliana and Brassica plants with Mentha essential oil and limonene for Gene Expression Experiments
4.3. Real Time PCR
4.4. Determination of Cabbage Dry and Fresh Weights after Fumigation with High Mentha Essential Oil and Limonene Concentrations
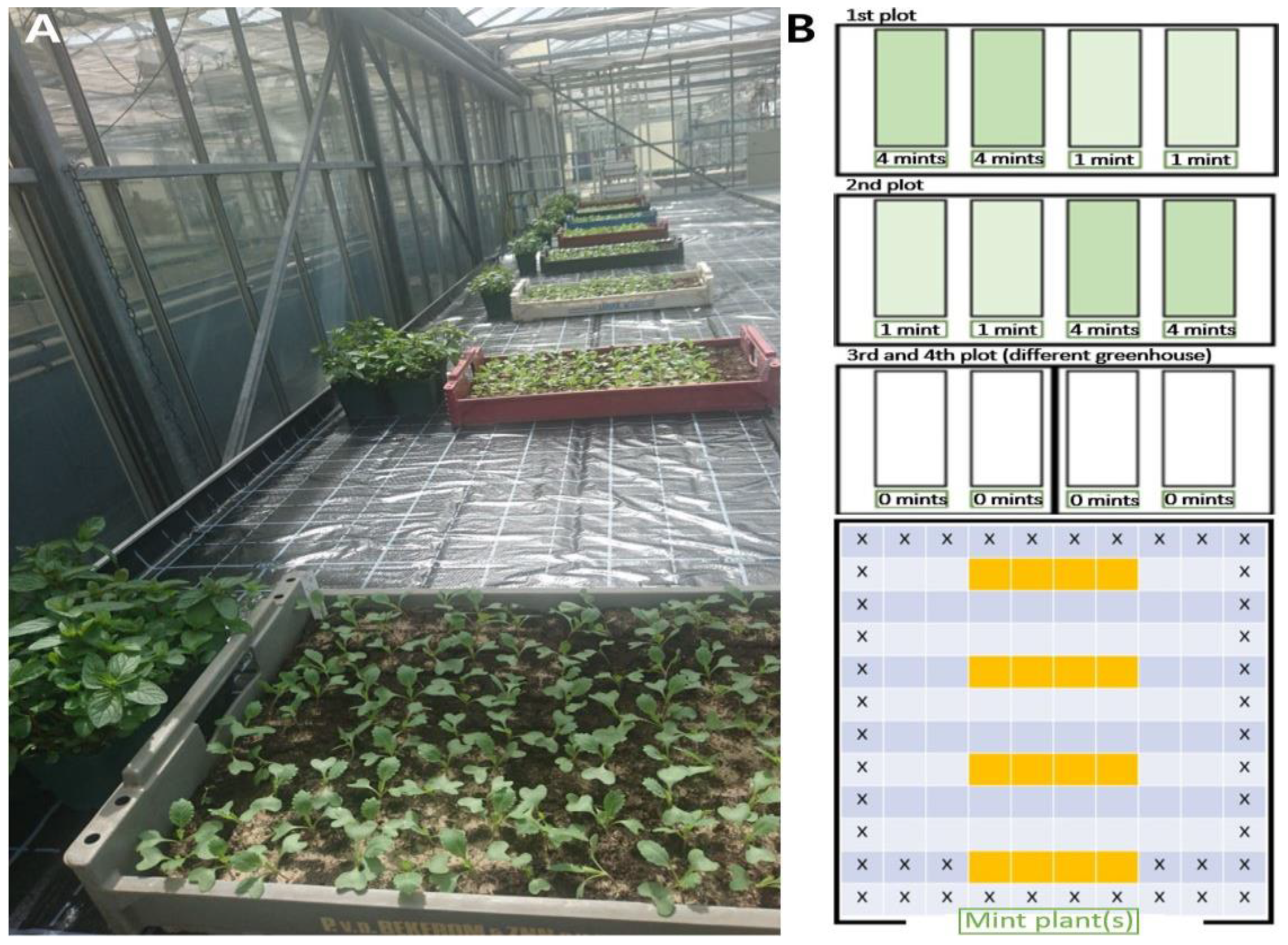
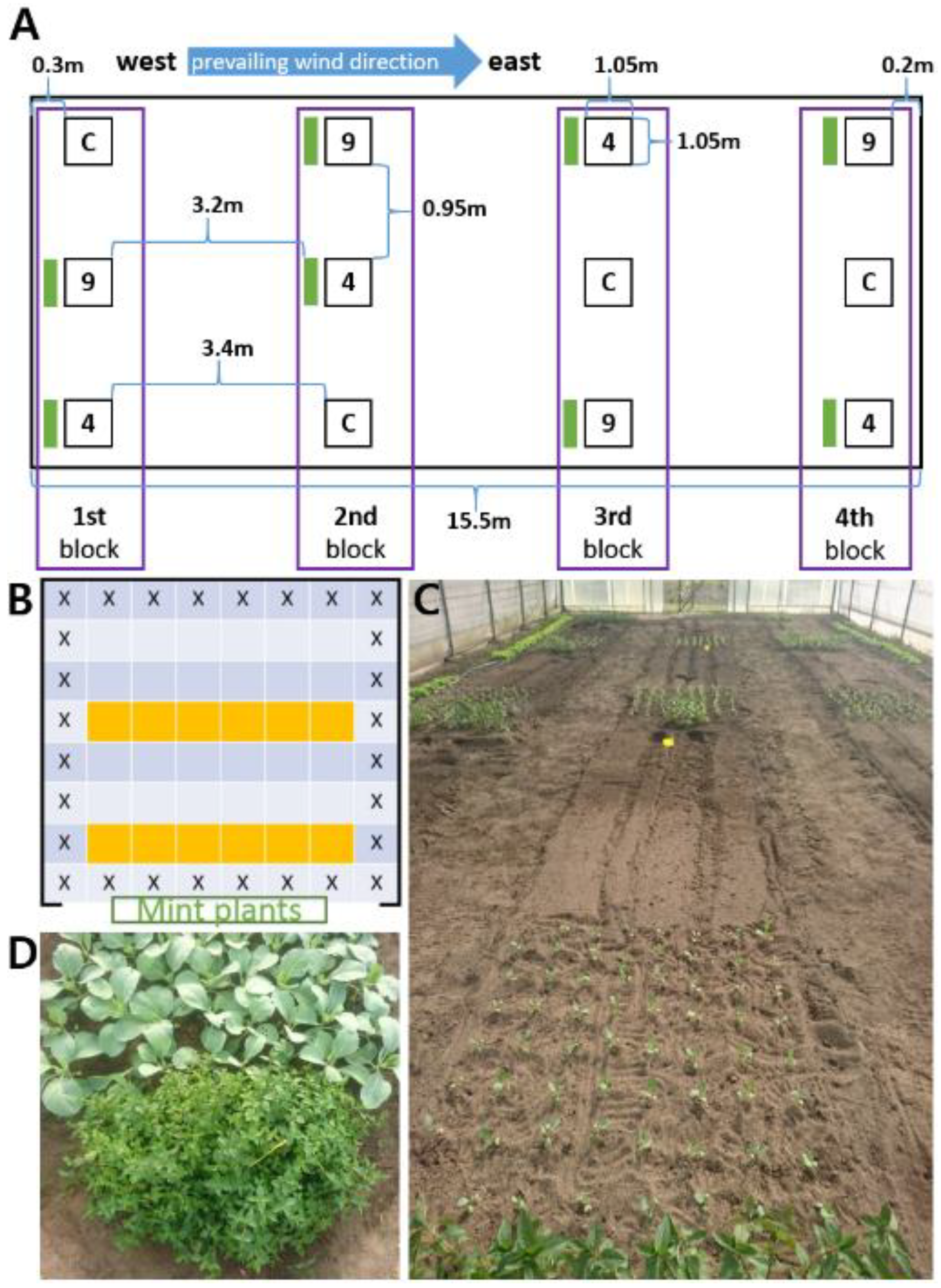
4.5. Greenhouse Trial with Mentha piperita Plants
4.6. Polytunnel Trial
4.7. Measurements of Leaf Parameters
4.8. Fresh and Dry Weight
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Bruno, L.; Sunseri, F.; Pacenza, M.; Forgione, I.; Bitonti, M.B.; Abenavoli, M.R. The allelochemical farnesene affects Arabidopsis thaliana root meristem altering auxin distribution. Plant Physiol. Biochem. 2017, 121, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Graña, E.; Díaz-Tielas, C.; Sánchez-Moreiras, A.M.; Reigosa, M.J.; Celeiro, M.; Abagyan, R.; Teijeira, M.; Duke, M.V.; Clerk, T.; Pan, Z.; et al. Transcriptome and binding data indicate that citral inhibits single strand DNA-binding proteins. Physiol. Plant. 2020, 169, 99–109. [Google Scholar] [CrossRef]
- Sarheed, M.M.; Rajabi, F.; Kunert, M.; Boland, W.; Wetters, S.; Miadowitz, K.; Kazmierczak, A.; Sahi, V.P.; Nick, P. Cellular Base of Mint Allelopathy: Menthone Affects Plant Microtubules. Front. Plant Sci. 2020, 11, 546345. [Google Scholar] [CrossRef] [PubMed]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, F.; Dudai, N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017, 65, 19–30. [Google Scholar] [CrossRef]
- Kriegs, B.; Jansen, M.; Hahn, K.; Peisker, H.; Samajova, O.; Beck, M.; Braun, M.; Ulbrich, A.; Baluska, F.; Schulz, M. Cyclic monoterpene mediated modulations of Arabidopsis thaliana phenotype—Effects on the cytoskeleton and on the expression of selected genes. Plant Signal. Behav. 2010, 5, 832–838. [Google Scholar] [CrossRef]
- Schulz, M.; Kussmann, P.; Knop, M.; Kriegs, B.; Gresens, F.; Eichert, T.; Ulbrich, A.; Marx, F.; Fabricius, H.; Goldbach, H.; et al. Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant Signal. Behav. 2007, 2, 231–239. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. Int. J. Mol. Sci. 2020, 21, 50. [Google Scholar] [CrossRef]
- Sukegawa, S.; Shiojiri, K.; Higami, T.; Suzuki, S.; Arimura, G.-I. Pest management using mint volatiles to elicit resistance in soy: Mechanism and application potential. Plant J. 2018, 96, 910–920. [Google Scholar] [CrossRef]
- Grulova, D.; De Martino, L.; Mancini, E.; Salamon, I.; De Feo, V. Seasonal variability of the main components in essential oil of Mentha × piperita L. J. Sci. Food Agric. 2015, 95, 621–627. [Google Scholar] [CrossRef]
- Kjonaas, R.; Croteau, R. Demonstration that Limonene is the First Cyclic Intermediate in the Biosynthesis of Oxygenated p-Menthane Monoterpenes in Mentha piperita and Other Mentha Species. Arch. Biochem. Biophys. 1983, 220, 79–89. [Google Scholar] [CrossRef]
- Ulbrich, A.; Kahle, H.; Kramer, P.; Schulz, M. Mentha x piperita volatiles promote Brassica oleracea—A pilot study for sustainable vegetable production. Allelopath. J. 2018, 43, 93–104. [Google Scholar] [CrossRef]
- Lan, J.; Qin, G. The Regulation of CIN-like TCP Transcription Factors. Int. J. Mol. Sci. 2020, 21, 4498. [Google Scholar] [CrossRef]
- Schommer, C.; Debernardi, J.M.; Bresso, E.G.; Rodriguez, R.E.; Palatnik, J.F. Repression of cell proliferation by miR319-regulated TCP4. Mol. Plant. 2014, 7, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Koyama, T.; Gonzalez, N.; Inzé, D.; Yamaguchi-Shinozaki, K.; Shinozaki, K. CIN-like TCP13 is essential for plant growth regulation under dehydration stress. Plant Mol. Biol. 2022, 108, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Lou, P.; Han, Y.; Chen, Z.; Chen, J.; Ni, J.; Yang, Y.; Jiang, Z.; Xu, M. GrTCP11, a Cotton TCP Transcription Factor, Inhibits Root Hair Elongation by Down-Regulating Jasmonic Acid Pathway in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 769675. [Google Scholar] [CrossRef]
- Baulies, J.L.; Bresso, E.G.; Goldy, C.; Palatnik, J.F.; Schommer, C. Potent inhibition of TCP transcription factors by miR319 ensures proper root growth in Arabidopsis. Plant Mol. Biol. 2022, 108, 93–103. [Google Scholar] [CrossRef]
- Danisman, S. TCP transcription factors at the interface between environmental challenges and the plant’s growth responses. Front. Plant Sci. 2016, 7, 1930. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Allen, E.; Wu, X.; Schommer, C.; Schwab, R.; Carrington, J.C.; Weigel, D. Control of leaf morphogenesis by microRNAs. Nature 2003, 425, 257–263. [Google Scholar] [CrossRef]
- Bresso, E.G.; Chorostecki, U.; Rodriguez, R.E.; Palatnik, J.F.; Schommer, C. Spatial Control of Gene Expression by miR319-Regulated TCP Transcription Factors in Leaf Development. Plant Physiol. 2018, 176, 1694–1708. [Google Scholar] [CrossRef]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, S.; Cheng, Q.; Zong, Y.; Chen, W.; Guo, W.; Zhang, L. Analysis of Evolution, Expression and Genetic Transformation of TCP Transcription Factors in Blueberry Reveal That VcTCP18 Negatively Regulates the Release of Flower Bud Dormancy. Front. Plant Sci. 2021, 12, 697609. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Mou, M.; Chen, Y.; Xiang, S.; Chen, L.; Yu, D. Arabidopsis Class II TCP Transcription Factors Integrate with the FT–FD Module to Control Flowering. Plant Physiol. 2019, 181, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wu, F.; Yu, X.; Bai, J.; Zhong, W.; He, Y. microRNA319a-Targeted Brassica rapa ssp. pekinensis TCP Genes Modulate Head Shape in Chinese Cabbage by Differential Cell Division Arrest in Leaf Regions. Plant Physiol. 2014, 164, 710–720. [Google Scholar] [CrossRef]
- Du, J.; Hu, S.; Yu, Q.; Wang, C.; Yang, Y.; Sun, H.; Yang, Y.; Sun, X. Genome-Wide Identification and Characterization of BrrTCP Transcription Factors in Brassica rapa ssp. rapa. Front. Plant Sci. 2017, 8, 1588. [Google Scholar] [CrossRef]
- Efroni, I.; Blum, E.; Goldshmidt, A.; Eshed, Y. A Protracted and Dynamic Maturation Schedule Underlies Arabidopsis Leaf Development. Plant Cell 2008, 20, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 2010, 22, 3574–3588. [Google Scholar] [CrossRef]
- Xiong, Y.; Jiao, Y. The Diverse Roles of Auxin in Regulating Leaf Development. Plants 2019, 23, 243. [Google Scholar] [CrossRef]
- Saini, K.; Markakis, M.N.; Zdanio, M.; Balcerowicz, D.M.; Beeckman, T.; De Veylder, L.; Prinsen, E.; Beemster, G.T.S.; Vissenberg, K. Alteration in Auxin Homeostasis and Signaling by Overexpression of PINOID Kinase Causes Leaf Growth Defects in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1009. [Google Scholar] [CrossRef]
- Li, S.; Zachgo, S. TCP3 interacts with R2R3-MYB proteins, promotes flavonoid biosynthesis and negatively regulates the auxin response in Arabidopsis thaliana. Plant J. 2013, 76, 901–913. [Google Scholar] [CrossRef]
- Kong, Q.; Low, P.M.; Lim, A.R.Q.; Yang, Y.; Yuan, L.; Ma, W. Functional Antagonism of WRI1 and TCP20 Modulates GH3.3 Expression to Maintain Auxin Homeostasis in Roots. Plants 2022, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, Y.; Yang, J.; He, Y. TCP24 modulates secondary cell wall thickening and anther endothecium development. Front. Plant Sci. 2015, 6, 436. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef]
- Godard, K.-A.; White, R.; Bohlmann, J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 2008, 69, 1838–1849. [Google Scholar] [CrossRef]
- Onosato, H.; Fujimoto, G.; Higami, T.; Sakamoto, T.; Yamada, A.; Suzuki, T.; Ozawa, R.; Matsunaga, S.; Seki, M.; Ueda, M.; et al. Sustained defense response via volatile signaling and its epigenetic transcriptional regulation. Plant Physiol. 2022, 189, 922–933. [Google Scholar] [CrossRef]
- Nie, Y.; Han, F.; Ma, J.; Chen, X.; Song, Y.-T.; Niu, S.-H.; Wu, H.X. Genome-wide TCP transcription factors analysis provides insight into their new functions in seasonal and diurnal growth rhythm in Pinus tabuliformis. BMC Plant Biol. 2022, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Laila, R.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Suh, M.C.; Kim, J.; Nou, I.-S. Developmental and Genotypic Variation in Leaf Wax Content and Composition, and in Expression of Wax Biosynthetic Genes in Brassica oleracea var. capitata. Front. Plant Sci. 2017, 7, 1972. [Google Scholar] [CrossRef]
- Martelanc, M.; Vovk, I.; Simonovska, B. Determination of three major triterpenoids in epicuticular wax of cabbage (Brassica oleracea L.) by high-performance liquid chromatography with UV and mass spectrometric detection. J. Chromatogr. A 2007, 1164, 145–152. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition and Physiological Function of the Wax Layers Coating Arabidopsis Leaves: β-Amyrin Negatively Affects the Intracuticular Water Barrier. Plant Physiol. 2012, 160, 1120–1129. [Google Scholar] [CrossRef]
- Hadacek, F. Cuticular Wax Terpenoids in Plants: Functional Diversity of. In Encyclopedia of Lipidomics; Wenk, M., Ed.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Shahnejat Bushehri, A.A.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Kato-Noguchi, H. Mentha sylvestris: A Potential Allelopathic Medicinal Plant. Int. J. Agric. Biol. 2013, 15, 1313–1318. [Google Scholar]
- Maes, C.; Meersmans, J.; Lins, L.; Bouquillon, S.; Fauconnier, M.-L. Essential Oil-Based Bioherbicides: Human Health Risks Analysis. Int. J. Mol. Sci. 2021, 22, 9396. [Google Scholar] [CrossRef]
- Girel, S.; Schütz, V.; Bigler, L.; Dörmann, P.; Schulz, M. Bioactive Nitrosylated and Nitrated N-(2-hydroxyphenyl)acetamides and Derived Oligomers: An Alternative Pathway to 2-Amidophenol-Derived Phytotoxic Metabolites. Molecules 2022, 27, 4786. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S.; van Dijk, A.D.J.; Bimbo, A.; van der Wal, F.; Hennig, L.; de Folter, S.; Angenent, G.C.; Immink, R.G.H. Analysis of functional redundancies within the Arabidopsis TCP transcription factor family. J. Exp. Bot. 2013, 64, 5673–5685. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preusche, M.; Vahl, M.; Riediger, J.; Ulbrich, A.; Schulz, M. Modulating Expression Levels of TCP Transcription Factors by Mentha x piperita Volatiles—An Allelopathic Tool to Influence Leaf Growth? Plants 2022, 11, 3078. https://doi.org/10.3390/plants11223078
Preusche M, Vahl M, Riediger J, Ulbrich A, Schulz M. Modulating Expression Levels of TCP Transcription Factors by Mentha x piperita Volatiles—An Allelopathic Tool to Influence Leaf Growth? Plants. 2022; 11(22):3078. https://doi.org/10.3390/plants11223078
Chicago/Turabian StylePreusche, Matthias, Marvin Vahl, Johanna Riediger, Andreas Ulbrich, and Margot Schulz. 2022. "Modulating Expression Levels of TCP Transcription Factors by Mentha x piperita Volatiles—An Allelopathic Tool to Influence Leaf Growth?" Plants 11, no. 22: 3078. https://doi.org/10.3390/plants11223078
APA StylePreusche, M., Vahl, M., Riediger, J., Ulbrich, A., & Schulz, M. (2022). Modulating Expression Levels of TCP Transcription Factors by Mentha x piperita Volatiles—An Allelopathic Tool to Influence Leaf Growth? Plants, 11(22), 3078. https://doi.org/10.3390/plants11223078






