Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum
Abstract
1. Introduction
2. Results
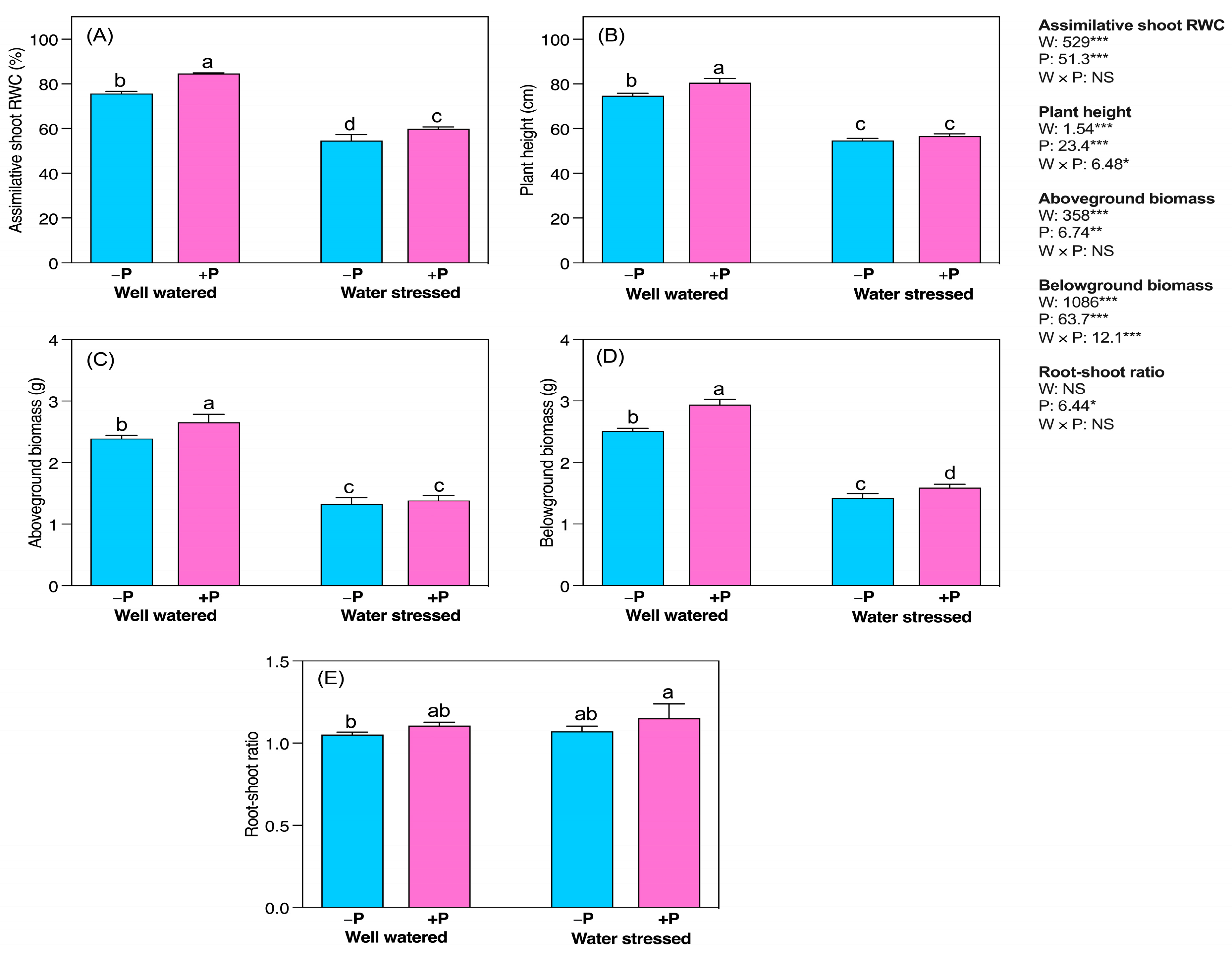
2.1. Changes in Growth Characteristics
2.2. Changes in Chlorophyll a and Chlorophyll b Pigments
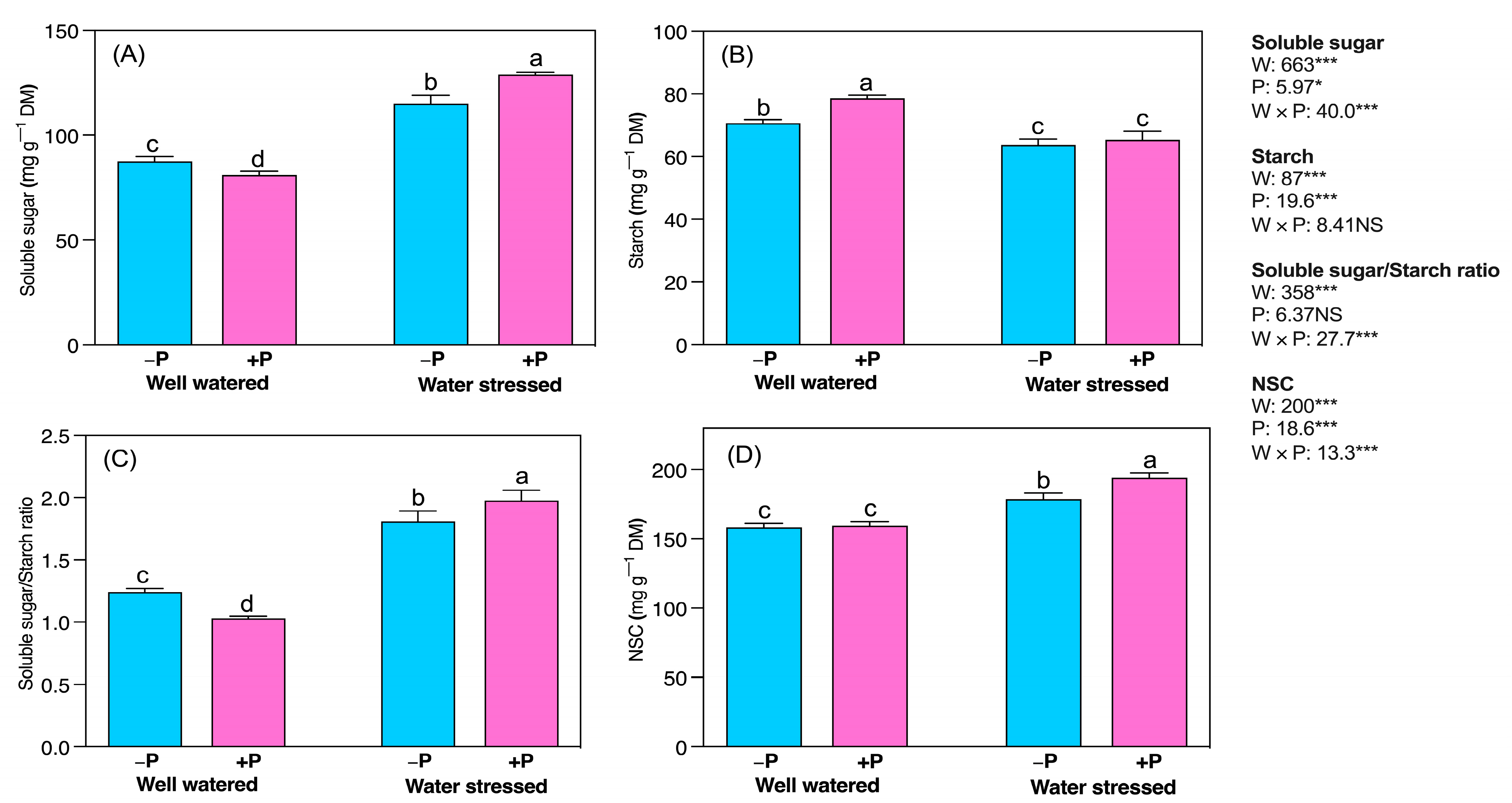
2.3. Biochemical Changes
2.4. Changes in ROS Accumulation and Lipid Peroxidation
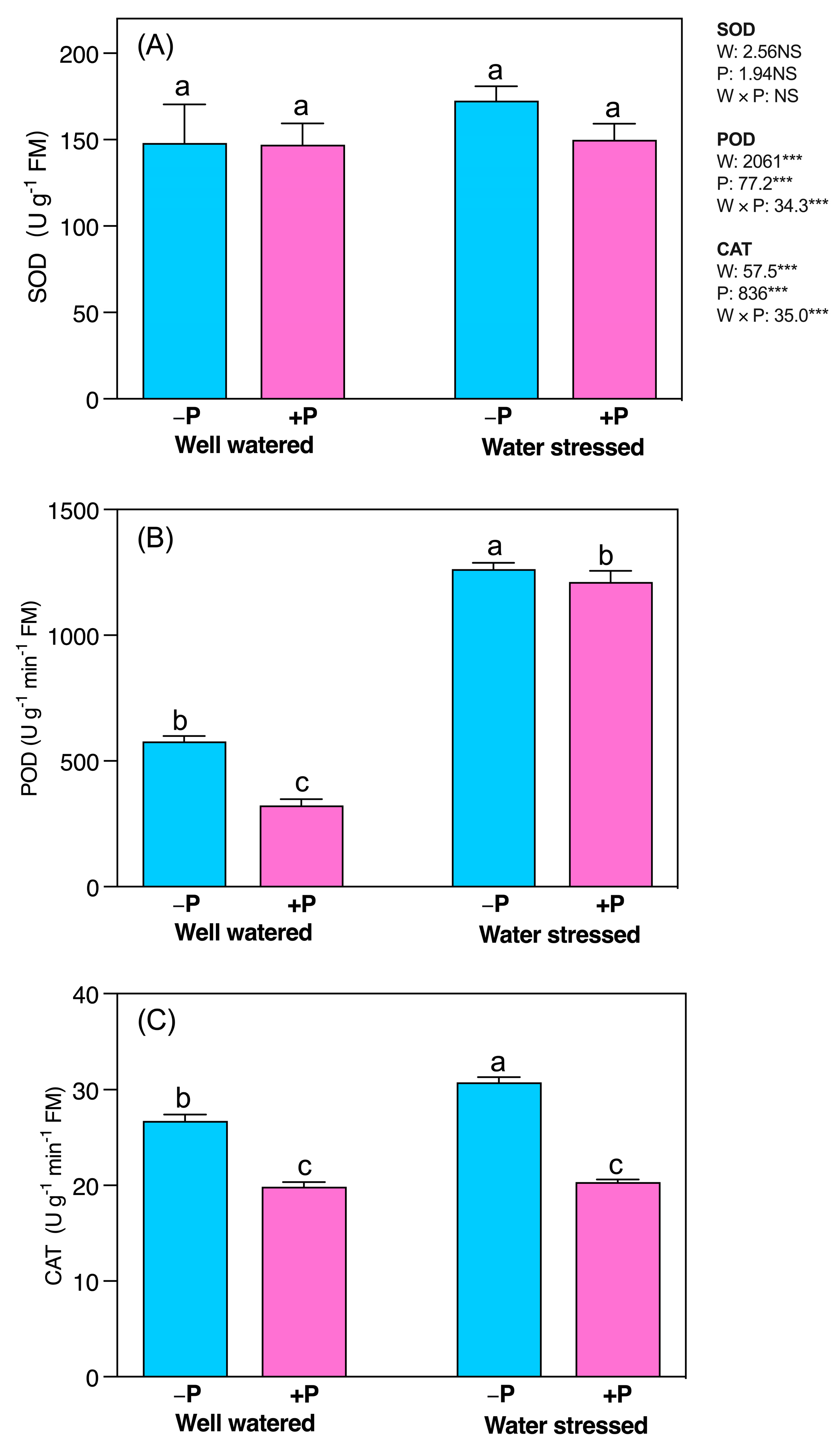
2.5. Changes in Activities of Antioxidant Enzymes
2.6. Changes in Enzymatic Activities of Nitrate Assimilation
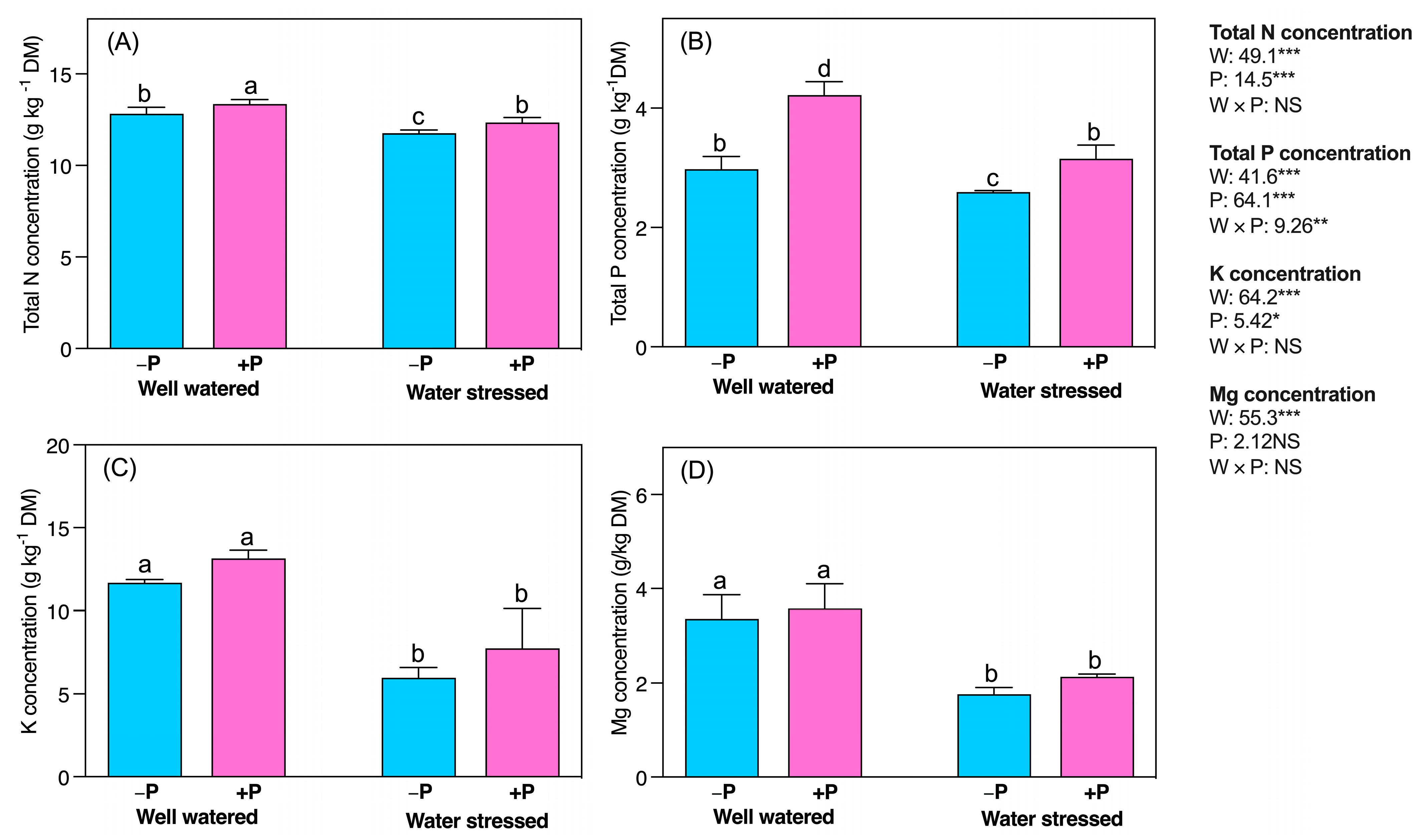
2.7. Changes in Mineral Nutrients
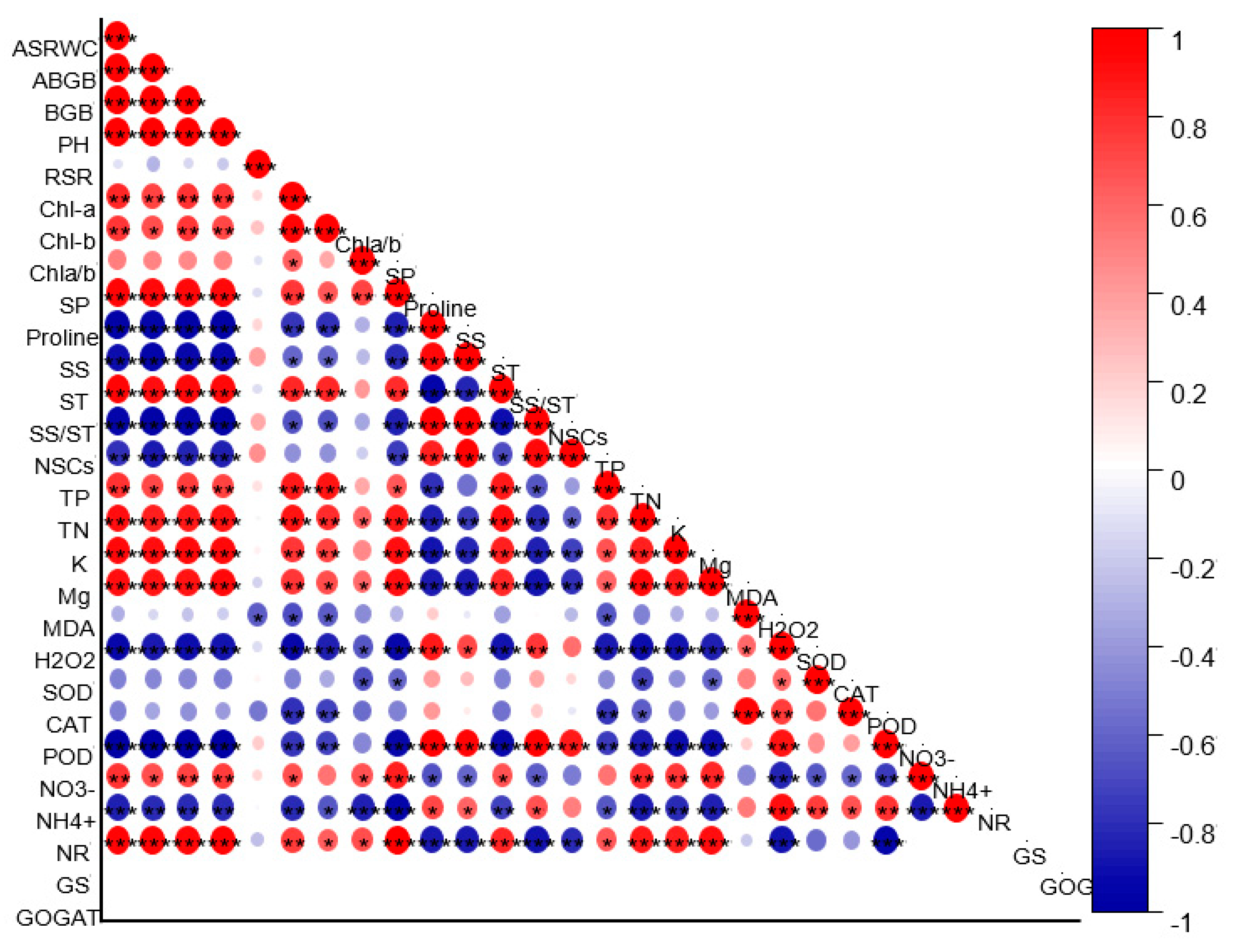
2.8. Relationship between the Studied Parameters
2.9. Results Summary
3. Discussion
3.1. P Fertilization Improvedthe Growth and Biomass of Young Seedlings
3.2. Improved Assimilative Shoots Relative Water Content (RWC)
3.3. P Fertilization Improved Photosynthetic Chlorophyll Pigments
3.4. P Fertilization Increased Soluble Protein, and Carbohydrate Concentrations
3.5. P Fertilization Reduced Lipid Peroxidation, ROS Production, and Antioxidant System
3.6. P Fertilization Increased N-Metabolism by Increasing Its Assimilative Enzymes
3.7. P Fertilization Improved Mineral Nutrients
4. Materials and Methods
4.1. Experiment Design
4.2. Treatments Application
4.3. Growth Parameters
4.3.1. Determination of Assimilative Shoot Relative Water Content
4.3.2. Measurement of Plant Growth and Biomass
4.4. Physio-Biochemical Analysis
4.4.1. Measurement of Photosynthetic Pigments
4.4.2. Determination of Nonstructural Carbohydrates
4.4.3. Determination of Soluble Protein and Proline
4.4.4. Oxidative Stress Indicators
4.4.5. Antioxidant Enzyme Activities
4.4.6. Determination of N-Metabolizing Enzymes
4.4.7. Determination of Mineral Elements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tariq, A.; Ullah, A.; Sardans, J.; Zeng, F.; Graciano, C.; Li, X.; Wang, W.; Ahmed, Z.; Ali, S.; Zhang, Z. Alhagi sparsifolia: An ideal phreatophyte for combating desertification and land degradation. Sci. Total Environ. 2022, 844, 157–228. [Google Scholar] [CrossRef] [PubMed]
- Spinoni, J.; Barbosa, P.; Cherlet, M.; Forzieri, G.; McCormick, N.; Naumann, G.; Vogt, J.V.; Dosio, A. How will the progressive global increase of arid areas affect population and land-use in the 21st century? Glob. Planet Change 2021, 205, 103597. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Huang, J.; Gao, Z.; Wang, G.; Li, D.; Yu, H.; Chen, X. Accelerated dryland expansion regulates future variability in dryland gross primary production. Nat. Commun. 2020, 11, 1665. [Google Scholar] [CrossRef]
- Sala, O.E.; StuartChapin, F., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Lian, X.; Piao, S.; Chen, A.; Huntingford, C.; Fu, B.; Li, L.Z.; Huang, J.; Sheffield, J.; Berg, A.M.; Keenan, T.F.; et al. Multifaceted characteristics of dryland aridity changes in a warming world. Multifaceted characteristics of dryland aridity changes in a warming world. Nat. Rev. Earth Environ. 2021, 2, 232–250. [Google Scholar] [CrossRef]
- Ibáñez, J.; Martínez, J.; Schnabel, S. Desertification due to overgrazing in a dynamic commercial livestock–grass–soil system. Ecol. Modell. 2007, 205, 277–288. [Google Scholar] [CrossRef]
- Arndt, S.K.; Arampatsis, C.; Foetzki, A.; Li, X.; Zeng, F.; Zhang, X. Contrasting patterns of leaf solute accumulation and salt adaptation in four phreatophytic desert plants in a hyperarid desert with saline groundwater. J. Arid Environ. 2004, 59, 259–270. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Li, N.; Song, D.; Sun, F.; Wu, X.; Dakhil, M.A. Impact of phosphorus application on drought resistant responses of Eucalyptus grandis seedlings. Physiol. Plant. 2019, 166, 894–908. [Google Scholar] [CrossRef]
- Hessini, K.; Martínez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.-B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Li, M.; Noor, J.; Tariq, A.; Liu, Y.; Shi, L. Effects of salinity on photosynthetic traits, ion homeostasis and nitrogen metabolism in wild and cultivated soybean. PeerJ 2019, 7, e8191. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, S.; Germon, F.; Aveline, A.; Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The influence of water stress on biomass and N accumulation, N partitioning between above and below ground parts and on N rhizodeposition during reproductive growth of pea (Pisum sativum L.). Soil Biol. Chem. 2009, 41, 380–387. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Above-and below-ground interactions of grass and pasture legume species when grown together under drought and low phosphorus availability. Plant Soil 2011, 348, 281–297. [Google Scholar] [CrossRef]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptusglobulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Kuhns, L.; Lynch, J.P.; Brown, K.M. Buffered phosphorus fertilizer improves growth and drought tolerance of woody landscape plants. J. Environ. Hortic. 2002, 20, 214–219. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Jin, Y.; Li, W.; Zhang, L. Effects of phosphorus application on photosynthetic carbon and nitrogen metabolism, water use efficiency and growth of dwarf bamboo (Fargesiarufa) subjected to water deficit. Plant Physiol. Biochem. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A. Phosphorous application improves drought tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D. Phosphorous fertilization alleviates drought effects on Alnuscremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Faustino, L.I.; Bulfe, N.M.L.; Pinazo, M.A.; Monteoliva, S.E.; Graciano, C. Dry weight partitioning and hydraulic traits in young Pinus taeda trees fertilized with nitrogen and phosphorus in a subtropical area. Tree Physiol. 2013, 33, 241–251. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ye, H.; Zhang, D.; Gu, J.D.; Deng, O. The dynamics of phosphorus fractions and the factors driving phosphorus cycle in Zoige Plateau peatland soil. Chemosphere 2021, 278, 130501. [Google Scholar] [CrossRef]
- Xu, D.; Wang, W.; Gao, T.; Fang, X.; Gao, X.; Li, J.; Bu, H.; Mu, J. Calcium alleviates decreases in photosynthesis under salt stress by enhancing antioxidant metabolism and adjusting solute accumulation in Calligonum mongolicum. Conserv. Physiol. 2017, 5, cox060. [Google Scholar] [CrossRef]
- Xu, S.; Ji, X.; Jin, B.; Zhang, J. Root distribution of three dominant desert shrubs and their water uptake dynamics. Plant Ecol. 2017, 10, 780–790. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Chai, X.; Graciano, C. Involvement of soluble proteins in growth and metabolic adjustments of drought-stressed Calligonum mongolicum seedlings under nitrogen addition. Plant Biol. 2021, 23, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Bhattachan, A.; D’Odorico, P.; Dintwe, K.; Okin, G.S.; Collins, S.L. Resilience and recovery potential of duneland vegetation in the southern Kalahari. Ecosphere 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Jiang, M.; Caldararu, S.; Zaehle, S.; Ellsworth, D.S.; Medlyn, B.E. Towards a more physiological representation of vegetation phosphorus processes in land surface models. New Phytol. 2019, 222, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Chtouki, M.; Laaziz, F.; Naciri, R.; Garré, S.; Nguyen, F.; Oukarroum, A. Interactive effect of soil moisture content and phosphorus fertilizer form on chickpea growth, photosynthesis, and nutrient uptake. Sci. Rep. 2022, 12, 6671. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, X.; Li, L.; Lu, Y.; Shareef, M.; Huang, C.; Liu, G.; Gui, D.; Zeng, F. Groundwater depth affects phosphorus but not carbon and nitrogen concentrations of a desert phreatophyte in Northwest China. Front. Plant Sci. 2018, 9, 338. [Google Scholar] [CrossRef]
- Graciano, C.; Guiamét, J.J.; Goya, J.F. Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptusgrandis seedlings. For. Ecol. Manag. 2005, 212, 40–49. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G. Influence of soil water regime on nutrient mobility and uptake by Dalbergia sissoo seedlings. Trop. Ecol. 2004, 45, 337–340. [Google Scholar]
- Yin, C.; Pang, X.; Chen, K.; Gong, R.; Xu, G.; Wang, X. The water adaptability of Jatropha curcas is modulated by soil nitrogen availability. Biomass Bioenergy. 2012, 47, 71–81. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef]
- Meng, L.S.; Yao, S.Q. Transcription co-activator Arabidopsis ANGUSTIFOLIA 3 (AN 3) regulates water-use efficiency and drought tolerance by modulating stomatal density and improving root architecture by the transrepression of YODA (YDA). Plant Biotechnol. J. 2015, 13, 893–902. [Google Scholar] [CrossRef]
- Canham, C.A.; Froend, R.H.; Stock, W.D. Rapid root elongation by phreatophyte seedlings does not imply tolerance of water table decline. Trees 2015, 29, 815–824. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. Sci. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Dayal, J.; Goswami, C.L.; Munjal, R. Influence of phosphorus application on water relations, biochemical parameters and gum content in cluster bean under water deficit. Biol. Plant 2004, 48, 445–448. [Google Scholar]
- Singh, V.; Pallaghy, C.K.; Singh, D. Phosphorus nutrition and tolerance of cotton to water stress: II. Water relations, free and bound water and leaf expansion rate. Field Crop. Res. 2006, 96, 199–206. [Google Scholar] [CrossRef]
- Sato, A.M.; Catuchi, T.A.; Ribeiro, R.V.; Souza, G.M. The use of network analysis to uncover homeostatic responses of a drought-tolerant sugarcane cultivar under severe water deficit and phosphorus supply. Acta Physiol. Plant 2010, 32, 1145–1151. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop. Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Campbell, C.D.; Sage, R.F. Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.). Plant Cell Environ. 2006, 29, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Badgujar, G.; Reddy, V.R.; Fleisher, D.H.; Bunce, J.A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. [Google Scholar] [CrossRef]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lu, Q.; Verma, D.P.S. Reciprocal regulation of Δ 1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol. Gen. Genet. 1996, 253, 334–341. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Klein, R.A.; Ratliff, K.A.; Vianello, M.; Adams, R.B., Jr.; Bahník, Š.; Bernstein, M.J.; Bocian, K.; Brandt, M.J.; Brooks, B.; Brumbaugh, C.C. Investigating variation in replicability: A “many labs” replication project. Soc. Psychol. 2014, 45, 142. [Google Scholar] [CrossRef]
- Rao, I.M.; Terry, N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet (IV. Changes with time following increased supply of phosphate to low-phosphate plants). Plant Physiol. 1995, 107, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Fletcher, J.M. Plant antioxidants: Colour me healthy. Biologist 2001, 48, 115–120. [Google Scholar] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef]
- Zhong, C.; Bai, Z.G.; Zhu, L.F.; Zhang, J.H.; Zhu, C.Q.; Huang, J.L.; Jin, Q.Y.; Cao, X.C. Nitrogen-mediated alleviation of photosynthetic inhibition under moderate water deficit stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2019, 157, 269–282. [Google Scholar] [CrossRef]
- Cao, X.; Zhong, C.; Zhu, C.; Zhu, L.; Zhang, J.; Wu, L.; Jin, Q. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant Physiol. Biochem. 2018, 132, 128–137. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pang, Y.; Zhao, Z. Drought, salinity, and low nitrogen differentially affect the growth and nitrogen metabolism of Sophora japonica (L.) in a semi-hydroponic phenotyping platform. Front. Plant Sci. 2021, 12, 715456. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Xu, T.; Zhang, J.; Shen, K.; Li, Z.; Liu, J. Drought-induced responses of nitrogen metabolism in Ipomoea batatas. Plants 2020, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, X.; Li, X.; Lin, L.; Zeng, F.; Gui, D.; Lu, Y. Nitrogen (N) and phosphorus (P) resorption of two dominant alpine perennial grass species in response to contrasting N and P availability. Environ. Exp. Bot. 2016, 127, 37–44. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar]
- Yang, H. Effects of nitrogen and phosphorus addition on leaf nutrient characteristics in a subtropical forest. Trees 2018, 32, 383–391. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Yue, Z.; Zeng, F.; Li, X.; Li, L. Effects of short-term nitrogen and phosphorus addition on leaf stoichiometry of a dominant alpine grass. PeerJ 2021, 9, e12611. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Plant carbon budgets. In Principles of Terrestrial Ecosystem Ecology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 157–181. [Google Scholar]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I.B. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Effect of light on lipid peroxidation in chloroplasts. Biochem. Biophys. Res. Commun. 1965, 19, 716–720. [Google Scholar] [CrossRef]
- Giannospolitis, C.N.; Ries, S.K. Superoxide dismutase. Plant Physiol. 1977, 59, 309–314. [Google Scholar]
- Wang, J.; Zhong, X.M.; Lv, X.L.; Shi, Z.S.; Li, F.H. Photosynthesis and physiology responses of paired near-isogenic lines in waxy maize (Zea mays L.) to nicosulfuron. Photosynthetica 2018, 56, 1059–1068. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Christov, K.N.; Popova, L.P. Antioxidative enzymes in barley plants subjected to soil flooding. Environ. Exp. Bot. 2004, 51, 93–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, X.; Tariq, A.; Zeng, F.; Graciano, C.; Li, X.; Gao, Y.; Ullah, A. Coordinated Patterns in the Allocation, Composition, and Variability of Multiple Elements among Organs of Two Desert Shrubs under Nitrogen Addition and Drought. J. Soil Sci. Plant Nutr. 2021, 22, 47–58. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Tariq, A.; Zeng, F.; Sardans, J.; Graciano, C.; Ullah, S.; Chai, X.; Zhang, Z.; Keyimu, M.; Asghar, M.A.; et al. Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum. Plants 2022, 11, 3054. https://doi.org/10.3390/plants11223054
Ullah A, Tariq A, Zeng F, Sardans J, Graciano C, Ullah S, Chai X, Zhang Z, Keyimu M, Asghar MA, et al. Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum. Plants. 2022; 11(22):3054. https://doi.org/10.3390/plants11223054
Chicago/Turabian StyleUllah, Abd, Akash Tariq, Fanjiang Zeng, Jordi Sardans, Corina Graciano, Sami Ullah, Xutian Chai, Zhihao Zhang, Maierdang Keyimu, Muhammad Ahsan Asghar, and et al. 2022. "Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum" Plants 11, no. 22: 3054. https://doi.org/10.3390/plants11223054
APA StyleUllah, A., Tariq, A., Zeng, F., Sardans, J., Graciano, C., Ullah, S., Chai, X., Zhang, Z., Keyimu, M., Asghar, M. A., Javed, H. H., & Peñuelas, J. (2022). Phosphorous Supplementation Alleviates Drought-Induced Physio-Biochemical Damages in Calligonum mongolicum. Plants, 11(22), 3054. https://doi.org/10.3390/plants11223054







