The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency
Abstract
1. Introduction
2. Results and Discussion
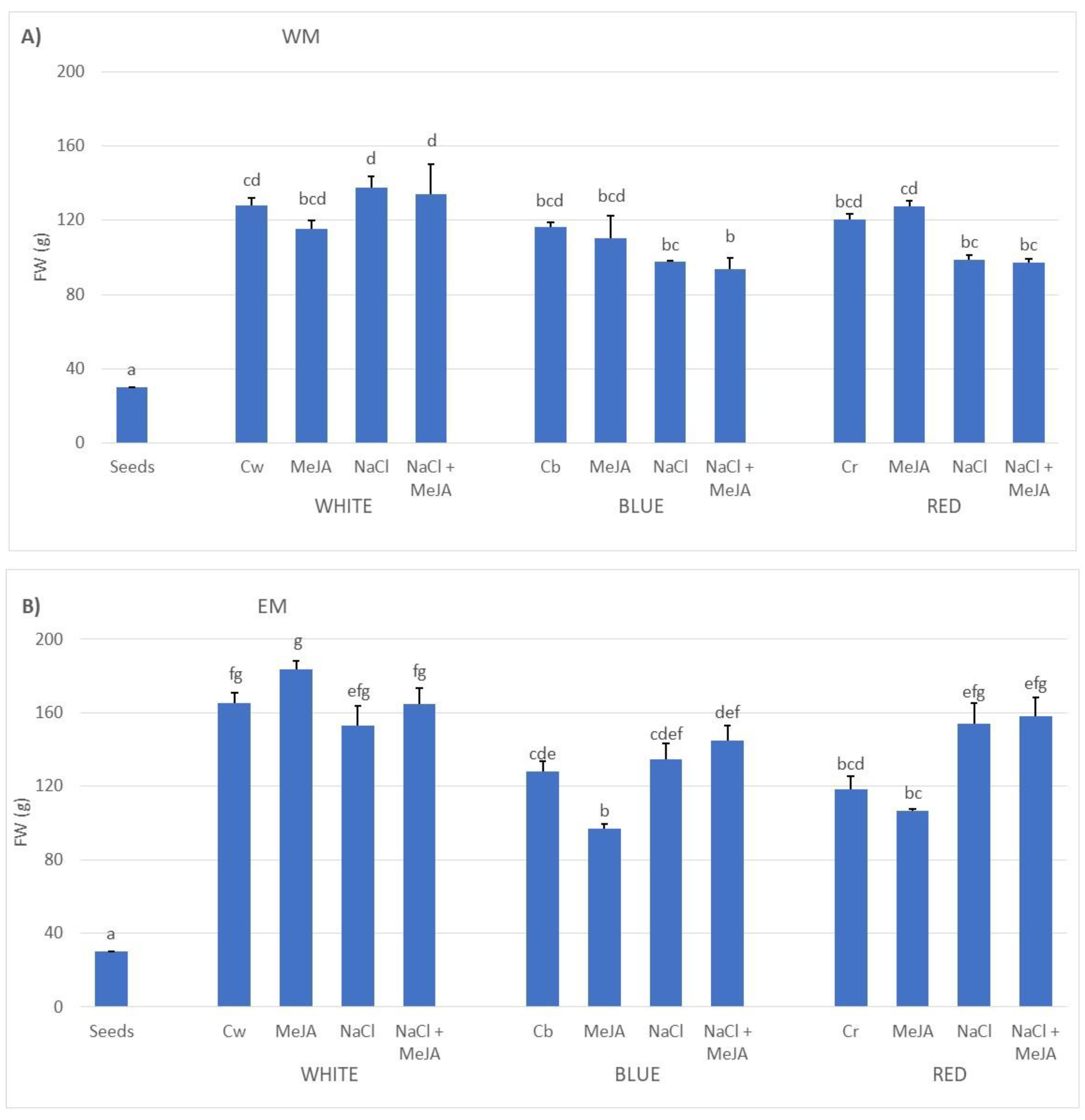
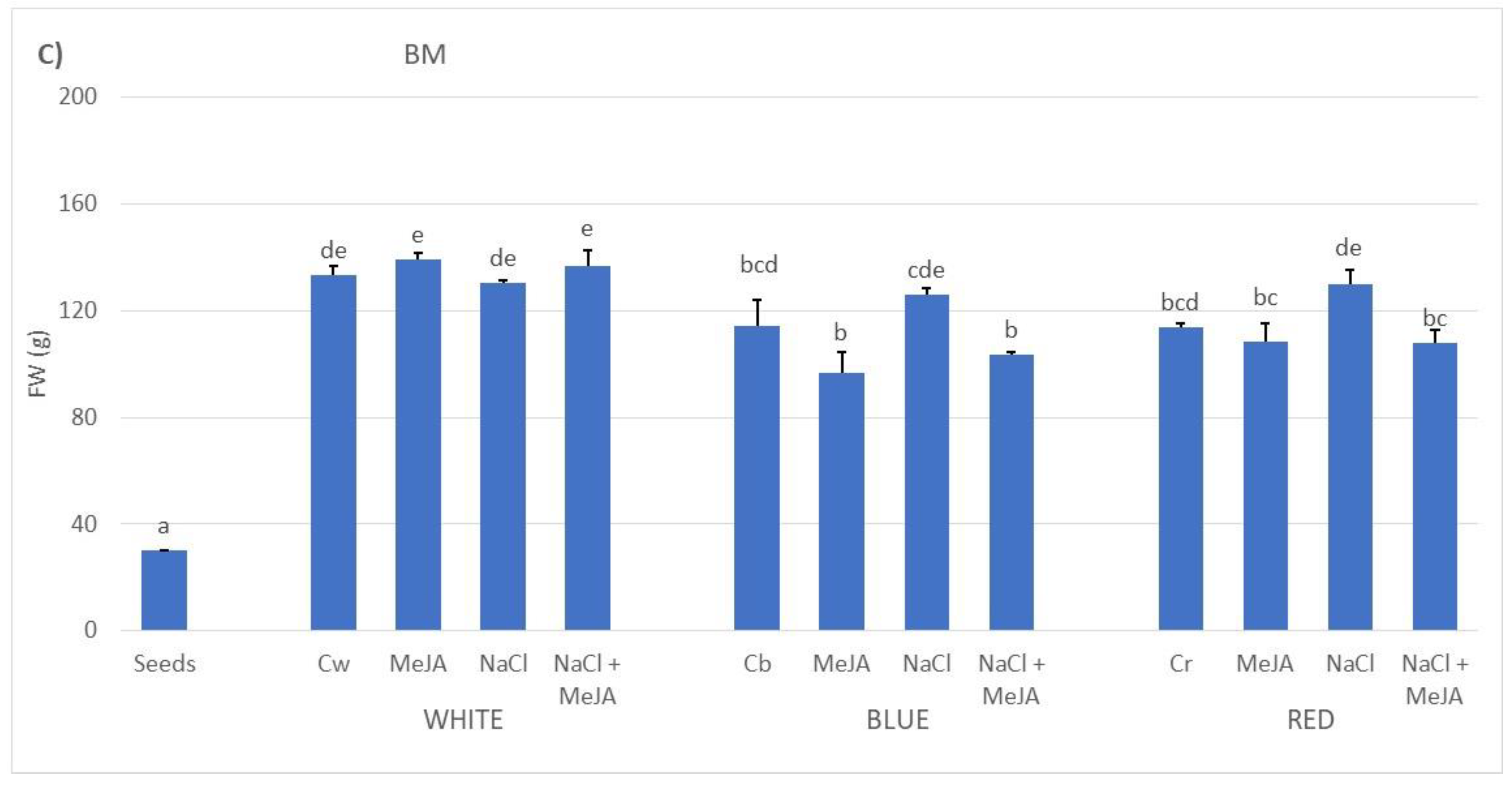
2.1. Effect of Treatments on the Biomass Production
2.2. Profile of GSLs in the Three Mustard Species
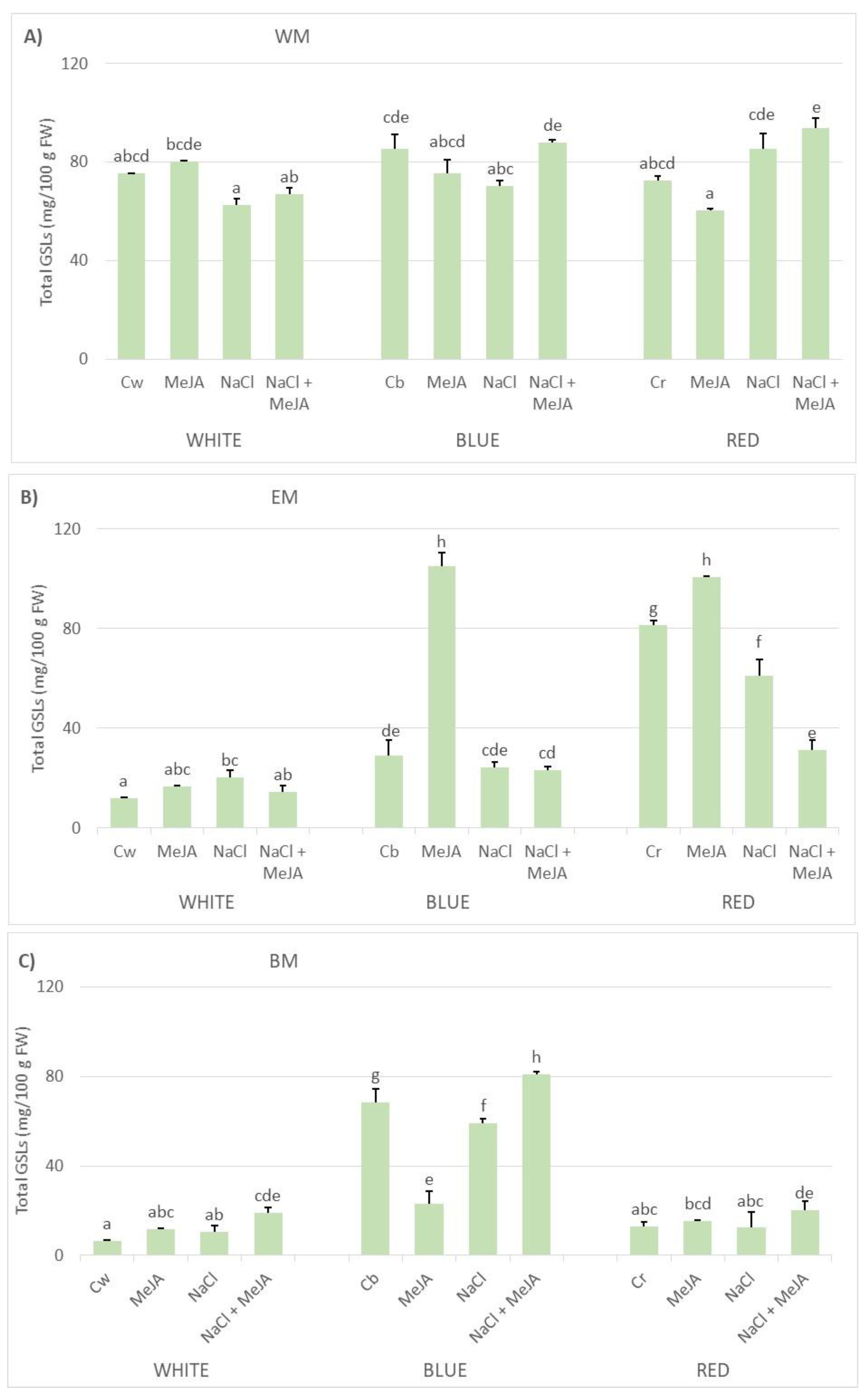
2.3. Effect of LED Lighting, Saline Stressor, and MeJA Elicitor on the Accumulation of GSLs
3. Materials and Methods
3.1. Plant Material and Experimental Conditions
- -
- Light factor. Three conditions for the development of sprouts were compared (Table 7): broad-spectrum LEDs, blue LEDs, and red LEDs. Broad-spectrum lighting (BS) was provided with the model Protect BioLED 100 W (SysLed Spain, S.L.) covering the spectrum 400–700 nm. The system was compared to the selective monochromatic blue (430–520 nm) or red (580–660 nm) experimental LED light lamps provided by Inbautek S.L. (Murcia, Spain).
- -
- Saline stressor. Trays were irrigated daily from Day 3 with the non-saline solution (5 g L−1 sodium hypochlorite) (0 mM NaCl) or with 100 mM NaCl in 5 g L−1 sodium hypochlorite (100 mM NaCl) to maintain the humidity of the inert media.
- -
- Foliar elicitation. From Day 3, sprouts were sprayed daily with 0 or 250 µM methyl jasmonate (MeJA, Sigma-Aldrich, Análisis Vínicos SL, Tomelloso, Spain) in 0.1% ethanol (96% v/v). As a control, non-elicited trays were sprayed daily with 0.1% ethanol. A combination of elicitation and saline stress treatment was also done. As a result, 12 treatments were applied:
- -
- Control (non-saline irrigation and 0 µM MeJA);
- -
- Elicitation with 250 µM MeJA;
- -
- Saline irrigation with 100 mM NaCl;
- -
- Combined elicitation + saline irrigation (250 µM MeJA + 100 mM NaCl), for each light system.
3.2. Extraction and Determination of Glucosinolates (GSLs)
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Kestwal, R.M.; Lin, J.C.; Bagal-Kestwal, D.; Chiang, B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Solovyeva, A.E.; Shelenga, T.V.; Konarev, A.V.; Kurina, A.B.; Kornyukhin, D.L.; Fateev, D.A.; Artemyeva, A.M. Nutritional and biologically active compounds in Russian (VIR) Brassicaceae vegetable crops. Turk. J. Agric. For. 2021, 45, 541–556. [Google Scholar] [CrossRef]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of Long-Term Consumption of Broccoli Sprouts on Inflammatory Markers in Overweight Subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive Activity of Sulforaphane. Drug Des. Devel. Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, 1800079. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; Mesa-Plata, O.; Cartea-González, M.E.; de Haro-Bailón, A. Quantification and in Vitro Bioaccessibility of Glucosinolates and Trace Elements in Brassicaceae Leafy Vegetables. Food Chem. 2021, 339, 127860. [Google Scholar] [CrossRef]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic Variation of Glucosinolates and Their Breakdown Products in Leaves of Brassica Rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of Cabbage (Brassica Oleracea) Accession and Growing Conditions on Myrosinase Activity, Glucosinolates and Their Hydrolysis Products. Foods 2021, 10, 2903. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli Sprouts: An Exceptionally Rich Source of Inducers of Enzymes That Protect against Chemical Carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Domagała, D.; Kusznierewicz, B. Young Shoots of Red Cabbage Are a Better Source of Selected Nutrients and Glucosinolates in Comparison to the Vegetable at Full Maturity. Eur. Food Res. Technol. 2020, 246, 2505–2515. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica Oleracea Varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Sávio, A.L.V.; da Silva, G.N.; Salvadori, D.M.F. Inhibition of Bladder Cancer Cell Proliferation by Allyl Isothiocyanate (Mustard Essential Oil). Mutat. Res. 2015, 771, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Holley, R.A. Control of Salmonella Enterica and Listeria Monocytogenes in Hummus Using Allyl Isothiocyanate. Int. J. Food Microbiol. 2018, 278, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing Elicitation and Seed Priming to Enrich Broccoli and Radish Sprouts in Glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of Seed Priming, Salinity and Methyl Jasmonate Treatment on Bioactive Composition of Brassica Oleracea Var. Capitata (White and Red Varieties) Sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Kozłowska, H. Effect of Light Conditions on the Contents of Glucosinolates in Germinating Seeds of White Mustard, Red Radish, White Radish, and Rapeseed. J. Agric. Food Chem. 2008, 56, 9087–9093. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of Light on Health-Promoting Phytochemicals of Broccoli Sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Yepes, L.; Moreno, D.A.; Carvajal, M. Evaluation of Elicitation Effect in Understudied Cruciferous Vegetables. J. Agron. Technol. Eng. Manag. 2018, 1, 78–83. [Google Scholar]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of Broccoli Sprouts (Brassica Oleracea L. Var. Italica) Growth and Quality by KCl Seed Priming and Methyl Jasmonate under Salinity Stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Hernández-Cánovas, L.; Abellán-Victorio, Á.; Moreno, D.A. The Quality and Glucosinolate Composition of Cruciferous Sprouts under Elicitor Treatments Using MeJA and LED Lights. Proceedings 2021, 70, 67. [Google Scholar] [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to Red, Blue Monochromatic Light Improves the Bioactive Compound Content in Broccoli Sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Vaštakaite-Kairiene, V.; Kelly, N.; Runkle, E.S. Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage. Plants 2021, 10, 549. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The Effects of Red, Blue, and White Light-Emitting Diodes on the Growth, Development, and Edible Quality of Hydroponically Grown Lettuce (Lactuca Sativa L. Var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal Red:Blue Ratio in Led Lighting for Nutraceutical Indoor Horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light Added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Cho, J.Y.; Jung, D.S.; Park, J.E.; Nho, C.W.; Bakari, G.; Misinzo, G.; Jung, J.H.; Yang, S.H.; et al. Exposure to Salinity and Light Spectra Regulates Glucosinolates, Phenolics, and Antioxidant Capacity of Brassica Carinata L. Microgreens. Antioxidants 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting Sprouts of Brassicaceae for Optimum Phytochemical Composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef] [PubMed]
- Artés–Hernández, F.; Miranda-Molina, F.D.; Klug, T.V.; Martínez–Hernández, G.B. Enrichment of Glucosinolate and Carotenoid Contents of Mustard Sprouts by Using Green Elicitors during Germination. J. Food Compos. Anal. 2022, 110, 104546. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W.J. Developmental Changes of Sinigrin and Glucoraphanin in Three Brassica Species (Brassica Nigra, Brassica Juncea and Brassica Oleracea Var. Italica). Sci. Hortic. 2002, 96, 11–26. [Google Scholar] [CrossRef]
- Schreiner, M.; Beyene, B.; Krumbein, A.; Stützel, H. Ontogenetic Changes of 2-Propenyl and 3-Indolylmethyl Glucosinolates in Brassica Carinata Leaves as Affected by Water Supply. J. Agric. Food Chem. 2009, 57, 7259–7263. [Google Scholar] [CrossRef]
- Bellostas, N.; Sørensen, J.C.; Sørensen, H. Profiling Glucosinolates in Vegetative and Reproductive Tissues of Four Brassica Species of the U-Triangle for Their Biofumigation Potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated in Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
- Arroyo, F.T.; Arcos, R.R.; Araujo, A.J.; Bejarano, R.G.; Basallote, M.J.; Barrau, C. Inhibitory Effect of the Glucosinolate–Myrosinase System on Phytophthora Cinnamomi and Pythium Spiculum. Plant Prot. Sci. 2019, 55, 93–101. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.; Zhang, F.; Wang, Q. Effects of Plant Hormones on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale. Food Res. Int. 2012, 48, 359–366. [Google Scholar] [CrossRef]
- López-Berenguer, C.; Martínez-Ballesta, M.D.C.; Moreno, D.A.; Carvajal, M.; García-Viguera, C. Growing Hardier Crops for Better Health: Salinity Tolerance and the Nutritional Value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef]
- Bennett, R.N.; Kiddle, G.; Wallsgrove, R.M. Lnvolvement of Cytochrome P450 in Glucosinolate Biosynthesis in White Mustard. Plant Physiol. 1997, 114, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica Juncea Sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of Different LED Lights on Aliphatic Glucosinolates Metabolism and Biochemical Characteristics in Broccoli Sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
| Species | Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| L | S | E | L × S | L × E | S × E | L × S × E | R | |
| White mustard | 34.0 *** | 7.9 * | 1.0 ns | 24.1 *** | 1.7 ns | 0.0 ns | 1.1 ns | 30.2 |
| Ethiopian mustard | 41.8 *** | 11.5 *** | 0.0 ns | 21.0 *** | 4.1 ns | 2.4 ns | 3.3 ns | 15.8 |
| Black mustard | 48.1 *** | 2.3 ns | 8.6 ** | 2.9 ns | 12.8 ** | 1.3 ns | 1.3 ns | 22.7 |
| RT (min.) | Compound | Parental Ion (ESI-) M- | GLS Semi-Systematic Name | Class | S. alba White Mustard | B. nigra Black Mustard | B. carinata Ethiopian Mustard |
|---|---|---|---|---|---|---|---|
| 6.3 | Sinigrin (SIN) | 358 | 2-propenyl-GSL | Aliphatic | √ | √ | |
| 11.4 | Glucosinalbin (GSB) | 424 | 4-hydroxybenzyl-GSL | Aromatic | √ | ||
| 16.3 | 4-Hydroxiglucobrassicin (4-HGB) | 463 | 4-hydroxy-3-indolylmethyl-GSL | Indolic | √ | √ | |
| 20.6 | Glucobrassicin (GB) | 447 | 3-indolylmethyl-GSL | Indolic | √ | √ | |
| 25.5 | 4-Methoxyglucobrassicin (4-MGB) | 477 | 4-methoxy-3-indolylmethyl-GSL | Indolic | √ | √ | √ |
| 27.3 | Neoglucobrassicin (NGB) | 477 | N-methoxy-3-indolylmethyl-GSL | Indolic | √ | √ |
| Species | Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| L | S | E | L × S | L × E | S × E | L × S × E | R | |
| White mustard | 10.7 ** | 1.6 ns | 1.0 ns | 45.3 *** | 1.6 ns | 12.5 *** | 7.1 * | 20.1 |
| Ethiopian mustard | 42.5 *** | 18.4 *** | 2.5 *** | 11.4 *** | 8.4 *** | 11.9 *** | 4.2 *** | 0.7 |
| Black mustard | 72.0 *** | 4.7 *** | 0.0 ns | 3.8 *** | 2.9 *** | 6.5 *** | 9.1 *** | 1.0 |
| Treatment | Glucosinolates (mg 100 g−1 FW) | ||||
|---|---|---|---|---|---|
| GSB | GB | 4-MGB | Aromatic | Indolic | |
| White | |||||
| Cw | 58.87 abc | 6.38 abc | 10.06 ab | 58.87 abc | 16.44 abc |
| 250 µM MeJA | 64.73 bcd | 7.58 c | 8.04 a | 64.73 bcd | 15.62 abc |
| 100 mM NaCl | 50.29 a | 5.57 abc | 6.61 a | 50.29 a | 12.18 a |
| NaCl + MeJA | 52.59 ab | 6.95 bc | 7.39 a | 52.59 ab | 14.35 abc |
| Blue LEDs | |||||
| Cb | 64.64 bcd | 7.17 bc | 13.59 bc | 64.64 bcd | 20.77 c |
| 250 µM MeJA | 59.40 abc | 5.34 abc | 10.63 ab | 59.40 abc | 15.96 abc |
| 100 mM NaCl | 52.36 ab | 3.80 ab | 14.24 bc | 52.36 ab | 18.05 abc |
| NaCl + MeJA | 67.51 cd | 6.19 abc | 14.11 bc | 67.51 cd | 20.30 c |
| Red LEDs | |||||
| Cr | 58.93 abc | 3.17 a | 10.39 ab | 58.93 abc | 13.56 ab |
| 250 µM MeJA | 47.01 a | 5.52 abc | 7.96 a | 47.01 a | 13.48 ab |
| 100 mM NaCl | 68.51 cd | 3.83 ab | 12.86 bc | 68.51 cd | 16.69 abc |
| NaCl + MeJA | 74.17 d | 4.27 abc | 15.23 c | 74.17 d | 19.50 bc |
| Treatment | Glucosinolates (mg 100 g−1 FW) | |||||
|---|---|---|---|---|---|---|
| SIN | 4-HGB | 4-MGB | NGB | Aliphatic | Indolic | |
| White | ||||||
| Cw | 5.05 a | 1.27 a | 5.70 a | - | 5.05 a | 6.98 a |
| 250 µM MeJA | 8.53 ab | 1.28 a | 4.97 a | 1.99 a | 8.53 ab | 8.24 a |
| 100 mM NaCl | 9.67 ab | 1.46 a | 6.08 a | 3.26 ab | 9.67 ab | 10.80 a |
| NaCl + MeJA | 6.12 a | 1.03 a | 5.25 a | 2.15 a | 6.12 a | 8.43 a |
| Blue LEDs | ||||||
| Cb | 13.18 bc | 1.77 a | 11.32 cd | 2.83 ab | 13.18 bc | 15.92 b |
| 250 µM MeJA | 58.02 f | 13.94 e | 14.19 e | 18.73 f | 58.02 f | 46.87 f |
| 100 mM NaCl | 8.94 ab | 2.10 a | 10.48 c | 2.72 ab | 8.94 ab | 15.30 b |
| NaCl + MeJA | 8.46 ab | 0.98 a | 10.02 c | 3.85 b | 8.46 ab | 14.85 b |
| Red LEDs | ||||||
| Cr | 48.20 e | 8.77 c | 10.28 c | 14.23 e | 48.20 e | 33.27 d |
| 250 µM MeJA | 56.95 f | 9.96 d | 12.58 d | 21.10 g | 56.95 f | 43.65 e |
| 100 mM NaCl | 34.52 d | 7.17 b | 8.16 b | 11.15 d | 34.52 d | 26.49 c |
| NaCl + MeJA | 15.11 c | 1.78 a | 7.67 b | 6.53 c | 15.11 c | 15.99 b |
| Treatment | Glucosinolates (mg 100 g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|
| SIN | 4-HGB | GB | 4-MGB | NGB | Aliphatic | Indolic | |
| White | |||||||
| Cw | 2.97 a | 1.40 a | - | 2.14 a | - | 2.97 a | 3.55 a |
| 250 µM MeJA | 5.77 ab | 2.00 ab | - | 3.86 b | - | 5.77 ab | 5.86 ab |
| 100 mM NaCl | 4.90 a | 1.14 a | - | 4.55 bc | - | 4.90 a | 5.69 ab |
| NaCl + MeJA | 12.14 b | 2.86 b | - | 3.92 b | - | 12.14 b | 6.79 ab |
| Blue LED | |||||||
| Cb | 38.96 c | 4.75 c | 3.65 b | 6.21 c | 14.97 c | 38.96 c | 29.59 e |
| 250 µM MeJA | 11.06 b | 2.36 ab | - | 7.69 d | 2.00 a | 11.06 b | 12.05 c |
| 100 mM NaCl | 34.96 c | 4.36 c | 2.38 a | 5.25 bc | 12.19 b | 34.96 c | 24.18 d |
| NaCl + MeJA | 48.67 d | 6.26 d | 3.79 b | 5.24 bc | 16.83 c | 48.67 d | 32.12 f |
| Red LED | |||||||
| Cr | 6.36 ab | 0.98 a | - | 4.79 bc | 0.74 a | 6.36 ab | 6.51 ab |
| 250 µM MeJA | 7.61 ab | 1.14 a | - | 5.12 bc | 1.54 a | 7.61 ab | 7.80 b |
| 100 mM NaCl | 7.17 ab | 1.51 a | - | 3.62 b | 0.40 a | 7.17 ab | 5.52 ab |
| NaCl + MeJA | 11.74 b | 3.08 b | - | 5.55 bc | - | 11.74 b | 8.63 b |
| LED Treatment | Wavelength Spectra (nm) | Electric Intensity (mA) | Luminous Flux per Unit Area (lx) | PPFD |
|---|---|---|---|---|
| White light (control) | 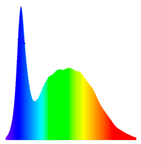 (380–780 nm) | 20 mA | 225 | 7 |
| Red light |  (580–660 nm) | 80 mA | 35 | 7 |
| Blue light |  (430–520 nm) | 60 mA | 210 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guijarro-Real, C.; Hernández-Cánovas, L.; Abellán-Victorio, Á.; Ben-Romdhane, O.; Moreno, D.A. The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency. Plants 2022, 11, 2961. https://doi.org/10.3390/plants11212961
Guijarro-Real C, Hernández-Cánovas L, Abellán-Victorio Á, Ben-Romdhane O, Moreno DA. The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency. Plants. 2022; 11(21):2961. https://doi.org/10.3390/plants11212961
Chicago/Turabian StyleGuijarro-Real, Carla, Lorena Hernández-Cánovas, Ángel Abellán-Victorio, Oumaima Ben-Romdhane, and Diego A. Moreno. 2022. "The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency" Plants 11, no. 21: 2961. https://doi.org/10.3390/plants11212961
APA StyleGuijarro-Real, C., Hernández-Cánovas, L., Abellán-Victorio, Á., Ben-Romdhane, O., & Moreno, D. A. (2022). The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency. Plants, 11(21), 2961. https://doi.org/10.3390/plants11212961








