Abstract
Pumpkin seed flour is a promising raw material for use in the technology of various bakery products. It has a high biological value and valuable amino acid profile. During the technological process of making bread, there are conformational changes in the protein structure. The purpose of the study was to determine the effect of pumpkin seed flour on conformational changes in the structure of protein substances of dough and bread from wheat flour by near-infrared reflection spectroscopy. The protein profile changed to complete when replacing 10% or more of wheat flour because the score for all amino acids was higher than 100%. The utilitarian coefficient indicates the same balance of amino acids in proteins of all samples. As the percentage of substitution increases, the number of amino acids used for anabolic purposes decreases, and these are more fully utilized by the body.
1. Introduction
During recent years, the increasing interest of consumers in fitness regimes and a healthy body appearance contributed to a rise in the demand for healthy products. Furthermore, after the spread of COVID-19, consumers were forced to change their attitude toward food; by staying at home, a lot of people enhanced awareness about food waste and a healthy lifestyle, adopting a positive adaptive behavior related to healthy diets and food systems [1]. According to Brigagão et al. [2], modern consumers have changed their food preferences regarding bioactive nutrient compounds of fruits and vegetables and their inedible parts.
Thus, among other recent trends, consumer attention to the nutraceutical aspect of food products is becoming mainstream, providing both challenges and opportunities to farmers and other actors involved in the agrifood supply chain: innovative “product-rich-in” and “free-from” became very profitable segments, offering, respectively, fortified/functional characteristics (for example, antioxidant properties, high content of proteins, prebiotics, probiotics, etc.), and products with a low or no content of particular attributes such as fat, sugar, lactose, gluten, etc.
Health problems can influence these food choices; however, an increasing number of people consume these products because of their linkage with a healthier lifestyle.
Several studies stress the effect of cognitive factors, socio-economic status, food-related values, and traits that influence the interest of consumers in functional food [3,4,5].
In this context, the trend of using non-traditional raw materials to increase the nutritional value of products and provide them with healthy properties is rising in the bakery industry [6]. Different types of flour (rice, soy, buckwheat, and others) are used to replace part of the wheat flour in the technology of wheat bread. Pumpkin seed flour has a valuable chemical composition of up to 45% protein and 15% fiber content [7]. It is also a source of other macro- and micronutrients, such as carbohydrates, vitamins, and minerals [8]. Pumpkin seed flour also has a high antioxidant capacity thanks to the high content of bioactive compounds such as tocopherols, carotenoids, provitamins, pigments, triterpenoids, phenolic compounds, and other derivatives [9,10].
In addition to the nutritional components, in the case of pumpkin seeds, another aspect to consider is the reduction of agroindustry waste provided by reusing seeds flour in preparing bread, cake, and other foodstuffs. An important challenge of the food industry is using alternative plant parts, such as peels and seeds, with a significant nutritional value in the formulation of food products, enriching their functional value and reducing costs in waste treatment [11]. Indeed, pumpkin seed flour is used in the technology of various bakery products. Given the increasing popularity of ready-to-eat bakery products such as pan and pita, the enrichment of these products with vegetables high in protein, fiber, and bioactive ingredients is relevant [12]. The composite flours (wheat flour and pumpkin seed flour) showed better water holding capacity and softer gels than mono-flour products [12]. Consistency and viscoelasticity were lower compared to samples of bread from only wheat flour. Dough strength and stability were reduced, lowering bread volume, but the nutritional quality improved, enhancing antioxidant activity [12].
The addition of pumpkin seed flour in the amount of 5–15% to whole wheat flour in the technology of bread increased crude protein, ash, and crude fat content, but the addition of higher levels of pumpkin seed flour might cause rancidity [13].
The size of the particles of pumpkin seed flour is larger than wheat wholemeal flour, and the water absorption capacity exceeds such an indicator for wheat flour by 1.5 times [14]. The gas-forming capacity of the dough with this additive decreased, as well as the amount of formed sugars, but, on the contrary, the amount of fermented sugars increased [14].
The increased amount of added partially defatted pumpkin seed flour (5–15%) decreased the water absorption capacity of the dough, and the dough stability increased from 9.43 to 10.23 min [15].
A key role in forming the structure of the dough belongs to the gliadin and glutenin fractions of protein, which, in the hydrated state, form gluten, a continuous viscoelastic network. In the technological process of making bread, there are molecular conformations of gluten proteins altering the viscoelastic properties of dough systems. They can be evaluated by numerous techniques, e.g., infrared spectroscopy [16].
Glutenins are multi-chained proteins, polymerized by disulfide bonds and not cross-linked. They provide gluten with elastic properties. Gliadin proteins contain intra-molecular disulfide bonds [17]. The breaking of them causes the unfolding of the protein molecules. They provide gluten with cohesive properties [18]. Proteins of pumpkin seed flour differ from proteins of wheat flour, so there are differences in dough behavior during fermentation.
The amino acid profile and protein digestibility in vitro provide the quality of proteins. The in vitro protein digestibility of pumpkin seed kernels is 90%, and that of defatted pumpkin seed flour is 77.91% [19].
In dough, there are microstructural and molecular changes in gluten proteins. The amorphous matrix of gluten at the microstructural level experienced a different degree of depolymerization into laminar and fibrillar structures. The development of these elements was the result of conformational changes in the secondary structure of gluten proteins [20]. Because of the absence of gluten in pumpkin seeds, the structure of proteins in flour with the addition of pumpkin seed flour changes during the breadmaking process, and differs from changes in wheat flour bread.
The aim of the study was to determine the effect of pumpkin seed flour on conformational changes in the structure of protein substances of dough and bread from wheat flour.
2. Results and Discussion
2.1. Protein and Amino Acid Composition and Functional Properties of Wheat and Pumpkin Seed Flours
Flour plays a decisive role in forming the properties of dough for bakery products, especially for its protein content and composition [21]. The total content of proteins found in pumpkin seed flour was 40% lower than that of wheat flour (Figure 1).
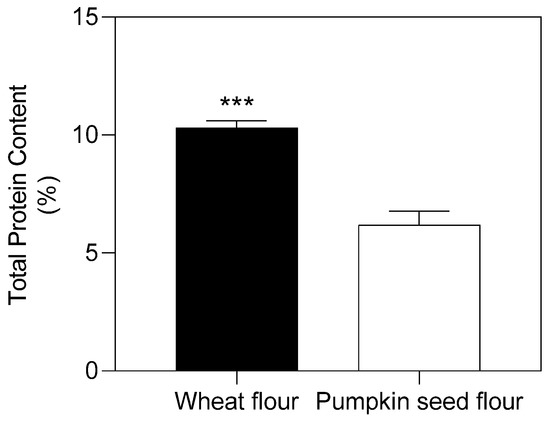
Figure 1.
Total protein content (% dry mass) of wheat and pumpkin seed flour. Data were compared with Student’s t-test (p ≤ 0.05). Significance ***: p ≤ 0.001.
Indeed, through the analysis of the fractional composition of proteins, higher albumin, glutenin, prolamin, and insoluble protein content in wheat flour was observed when compared with the pumpkin seed flour (Table 1). A higher presence of glutenin (gluten protein) in wheat flour than in pumpkin seed flour could represent a source of heterogeneity in the dough mixture, since more glutenins link together and form a heterogeneous mixture of polymers through disulfide bonded linkages of polypeptides [22]. For this reason, the presence of wheat flour in an enriched bread (with pumpkin seed, but also soya bean flour or others; [23,24]) is fundamental for the rheological characteristics of dough and, subsequently, of bread.

Table 1.
Fractional composition of proteins of wheat flour and pumpkin seed flour. Data were compared with Student’s t-test (p ≤ 0.05). Significance ***: p ≤ 0.001.
The highest water-soluble and salt-soluble protein fractions found in the pumpkin seed flour can affect chemical processes in the dough (proteolysis of proteins, changes in the redox potential in the dough, changes in the tertiary and quaternary structure of proteins), and its structural and mechanical characteristics [24].
The high biological value of pumpkin seed flour is due to a higher presence of EAA than that found in the wheat flour (Table 2).

Table 2.
The content of essential amino acids (EAA) in wheat flour and pumpkin seed flour. Data were compared with Student’s t-test (p ≤ 0.05). Significance ***: p ≤ 0.001.
The analysis of EAA showed a significantly higher EAA concentration in pumpkin seed flour when compared with the wheat flour. The predominant essential amino acid of pumpkin seed flour was leucine, whereas tryptophan was the lowest. This composition was confirmed by other authors [25], even though they found a higher concentration of all EAA when compared with the results of the present work.
The functional and technological properties of flours determine their behavior during processing, and characterize the ability to bind and retain moisture [26]. Indeed, these indicators influence the ability to form a viscoelastic dough, which is important in obtaining high quality bread, and provides its necessary structure and technological properties. To develop the required characteristics of the final product, the functional and technological properties of pumpkin seed flour were analyzed (Table 3).

Table 3.
Functional and technological properties of wheat flour and pumpkin seed flour. Data were compared with Student’s t-test (p ≤ 0.05). Significance ***: p ≤ 0.001.
Both moisture binding capacity and moisture retaining capacity were significantly higher in pumpkin seed flour than in wheat flour due to the higher content of fiber generally present in pumpkin seed [7]. This aspect results as positive in the dough and in the bread, since fiber and high moisture can positively influence the softness of grain-based products.
2.2. Near-Infrared Reflection Spectra of Flours and Doughs
Flour is a material which consists of a set of particles. Their size exceeds the wavelength of light. Therefore, there is a propagation of optical radiation in all directions (Tyndall effect), which forms a white reflection. To identify and analyze the components of such materials, it is advisable to use the reflection spectrum in the near infrared area [27]. The reflection spectra of wheat flour and pumpkin seed flour showed little differences (Figure 2). In the first part of spectra, the minimum reflection for the wheat flour sample was observed at a wavelength of 1460 nm, and for pumpkin seed flour, it was shifted to 1500 nm. In the wavelength range 1440–1480 nm, the spectra of both flours coincided. This aspect indicates approximately the same water content in their chemical composition [28]. At the wavelength of 2100 nm, which characterizes the content and behavior of starch [29], there was an interesting feature: both spectra had extremes, but for pumpkin seed flour, this was the maximum extreme, and, for wheat flour, this was the minimum extreme of the intensity of the reflection. Indicators at this wavelength showed the presence of starch [29]. This difference in extremes indicates that wheat flour has higher starch content than pumpkin seed flour. Near this region, in the spectrum of pumpkin seed flour, two other additional minima of intensity were observed at 2260 and 2180 nm. The intensity of reflection at 2180 nm indicates the presence of total proteins [30]. Their content in pumpkin seed flour was higher than in wheat flour, correlating with values of the total protein content in the investigated types of flour (Figure 1). The different intensity of reflection at this wavelength can be explained by the difference in the fractional composition of proteins of the studied types of flour, and by the different content between soluble and insoluble proteins in the flours under investigation (Table 1). The last extreme in the spectrum of pumpkin seed flour was observed at the wavelength of 2345 nm, which indicates a high content of dietary fiber in it [31].
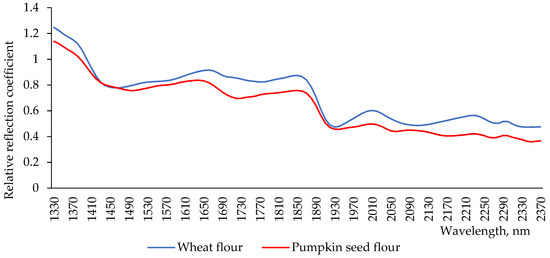
Figure 2.
Spectra of reflection of wheat flour and pumpkin seed flour.
After kneading the dough, the spectra of the obtained samples with different concentrations of pumpkin seed flour to replace part of the wheat flour differed slightly, even though all the extremes were identical (Figure 3). However, the spectrum of the control sample had a lower intensity of reflection than pumpkin seed flour-addicted samples, and an evident pumpkin seed flour-dependent intensity of reflection was observed in the other samples. At 1930 nm, the wavelength responsible for the moisture content in the product, the reflection intensity of all samples coincided. This could be due to the fact that the samples were dried. The similar nature of the obtained spectra indicated that the determining factor influencing the formation of the properties of the dough was wheat flour, since it was the mass fraction which remains the largest in the dough. However, during the formation of the dough, there were changes in the structural elements (in particular, the absorption of water by fiber contained in pumpkin seed flour) in dough with pumpkin seed flour when compared with control sample. For this reason, the intensity of the reflection of pumpkin seed flour, independently of the pumpkin seed flour percentage, tended to the spectrum of control dough, acquiring springy-elastic and viscous-plastic properties that are conferred in the control sample by the content of glutenins and gliadins of wheat flour.
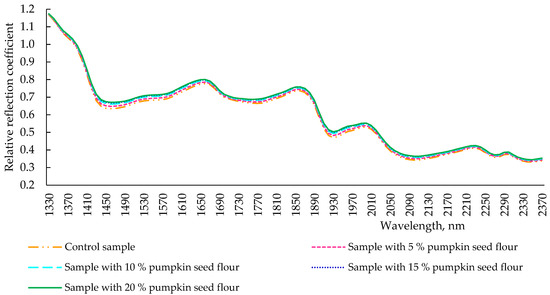
Figure 3.
Spectra of reflection of dough samples (after kneading) with pumpkin seed flour at different concentrations (0, 5, 10, 15, and 20 %).
The total time of fermentation and keeping of the dough is 3.5 h. The conformational changes in the structural substances of the dough were monitored after the completion of the fermentation (Figure 4). After kneading (3.5 h), all spectra of dough samples with different dosages of pumpkin seed flour changed their reflection intensity, but all extremes remained at the wavelength values observed in Figure 3. There was a clear pattern: the intensity of the reflection spectrum increased in a pumpkin seed flour-dependent way, and the control sample had the lowest location of its spectrum. The nature of the spectra of the dough of all samples after fermentation repeated the spectrum of wheat flour (Figure 2), but the intensity of the reflection of the samples tended to pumpkin seed flour. This was noticeable, especially at the maximum dosage of pumpkin seed flour (20%). This was due to the fact that the additive pumpkin seed flour contained much more dietary fiber in its composition, as reported for pumpkin seed flour-fortified muffins in the experiment conducted by Białek et al. [9], which observed an increasing fiber content dependent on the pumpkin seed flour concentration. Moreover, these changes in the reflection intensity can also be due to the redistribution of water in pumpkin seed flour dough samples, which led to partial dehydration of the gluten network, already reduced by the lower glutenin content in pumpkin seed flour when compared with wheat flour, and, thus, to the modifications of protein secondary structures [32]. Indeed, a loss of elastic α-helix structures was carried out, reducing the viscosity and the elasticity of the dough samples [32].
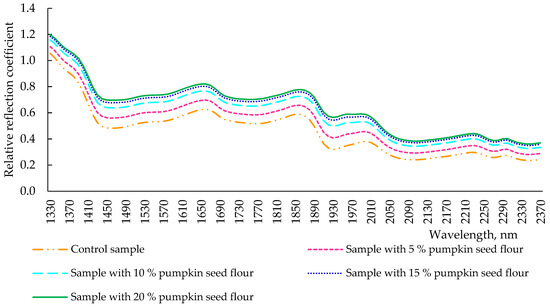
Figure 4.
Spectra of reflection of dough samples (after 3.5 h of fermentation) with pumpkin seed flour at different concentrations (0, 5, 10, 15, and 20%).
Comparing the control dough sample analyzed immediately after kneading (Figure 3) and after 3.5 h of fermentation (Figure 4), these samples had a similar nature, but a different intensity of reflection. Indeed, after the fermentation process, the reflection intensity of the dough decreased (Figure 3 and Figure 4). This was because, during fermentation, microbiological and biochemical processes occurred in the dough, and the hydration of gluten took place [33]. The wheat flour dough generally reveals a protein secondary structure characterized by α-helix conformations, which, during the fermentation process, helps to capture carbon dioxide bubbles, intensifying the gas formation and gas retention [14,34]. As already specified, adding pumpkin seed flour led to a gluten reduction in the dough and, thus, to lower hydration of the gluten network when compared with the control dough and, consequently, to a loss of the α-helices in the protein structure, reducing its viscosity and elasticity.
During the process of baking, under the influence of high temperatures (220 °C), changes in protein and other substances of the product occurred, uniforming the reflection intensity of the final products (Figure 5). Indeed, throughout the analyzed wavelength range (1330–2370 nm), the reflection spectra of the control sample of the bread and samples with different dosages of pumpkin seed flour resulted very similarly. However, the control sample had a slightly lower reflection intensity throughout the spectrum when compared with the additive bread samples. This might be due to the lower presence of α-helical structures, which are one of the structural elements responsible for the viscosity of the dough, in the control sample and in the samples with pumpkin seed flour addition.
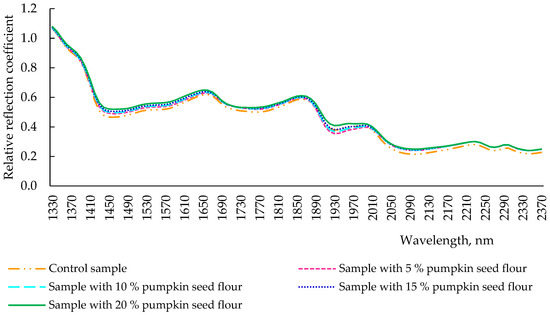
Figure 5.
Spectra of reflection of bread with pumpkin seed flour at different concentrations (0, 5, 10, 15, and 20%).
A variety of functional groups (carbonyl, hydroxyl, carboxyl, amide, amino, and cyano) can be quickly and reliably identified using infrared spectroscopy. In particular, most food products contain OH, CH, and NH functional groups, which are determined by the formed spectra (by analyzing the frequency and amplitude). Spectra arising from the oscillations of various groups in the near-infrared region have characteristic bands and characteristic frequencies [35]. In the near-infrared region, overtones of normal oscillations are used to analyze food products and technological mixtures [35]. The spectral index lg (1/R) was used to assess food quality parameters.
Analyzing the reflection spectra of the dough after kneading, after 3.5 h of fermentation, and of bread (both control sample and sample with 20% pumpkin seed flour), the highest reflection was noted at wavelengths of 1460 nm, 1760 nm, 1930 nm, 2100 nm, 2270 nm, and 2350 nm (Figure 6), with the extremum for all analyzed samples at 1460 nm, corresponding to the first overtone of valence vibrations of the OH group [36]. The functional groups of the first overtones, C-H and S-H, appear at 1760 nm [36]. Water is characterized by reflection at 1930 nm (OH deformation oscillations, the second overtone) [36]. To determine the content of gluten, the wavelength of 2100 nm was used, which corresponds to the second overtone of N-H deformation vibrations [36]. The wavelength of 2270 nm (O-H valence vibrations/C-O valence combination) is characteristic of lignin [36]. The maximum reflection at 2350 nm (the second overtone of C-H deformation vibrations) is responsible for the presence of lipids, which give products an oily taste [36].
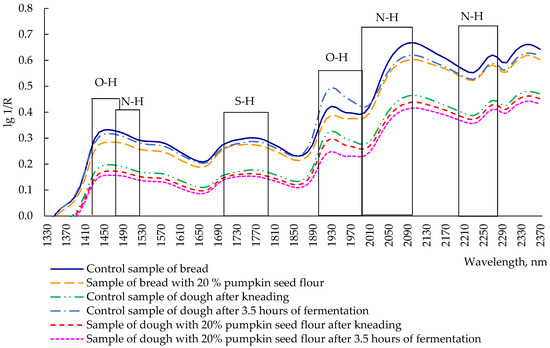
Figure 6.
Changes and redistribution of structural groups in bread and dough after kneading and after 3.5 h of fermentation.
The evaluation of the protein content in food products is usually carried out at 1510 nm (N-H valence vibrations, first overtone), 2060 nm (N-H deformation vibrations, second overtone or N-H deformation/N-H valence combination), and 2180 nm (N-H deformation oscillations, second overtone); however, these spectra did not show any extreme reflection.
The spectrum of the control sample of bread was the highest among all samples, except at 1930 nm, where the control sample of dough after 3.5 h of fermentation reported a reflection intensity of 0.5 lg 1/R. Bread with 20% pumpkin flour had a slightly lower intensity than the control bread, but, in some areas, both the spectra were overlapping, in particular at 1730, 2050, and 2250 nm. The other three spectra (the control sample of the dough after kneading, and the dough with 20% pumpkin seed flour after kneading and after 3.5 h of fermentation) were located in a separate group with lower reflection intensity when compared with bread samples and control dough after fermentation. The spectra of the dough with 20% pumpkin seed flour had the lowest reflection intensity among all samples, but the sample after kneading was located above the fermented sample. Regardless of the type of used flour, the samples of bread had a higher intensity of reflection than dough. This can be explained by the effect of high temperature in the baking process, which causes significant changes in protein and starch, fat, and other substances [37]. Indeed, the baking process can lead to damaged starch, through the formation of swollen granules or amylase leaching, affecting the rheological properties of dough and, consequently, the interaction with other chemical compounds [38]. Regarding the protein content, high baking temperatures and the low moisture percentage can lead to the aggregation of gluten polymers (in the case of wheat bread), or to an influence of sugars of the little amount of gluten present in pumpkin seed bread [39].
2.3. EAA Composition and Biological Value of Bread
The EAA profile of bread with different percentages of pumpkin seed flour is reported in Table 4. The results showed an increase of EAA dependent to the increase of pumpkin seed flour added to the wheat flour in the bread preparation. These results agree with the findings of other authors that tried the enrichment with pumpkin seed flour and other vegetable flours [40,41]. For example, Carlson et al. [40] reported that supplementation of the wheat flour with 10–20% tomato seed flour increased lysine content by 40–69% in breads, and El-Souccary [41] observed an increase of sulfur amino acid in enriched bread with pumpkin seed flour, especially after the roasting of bread.

Table 4.
Essential amino acid profile (EAA) of bread with the replacement of part of wheat flour with pumpkin seed flour at different percentages (0, 5, 10, 15, and 20%).
Finally, the utilitarian coefficient of the EAA composition, characterizing the balance of EAA in relation to the physiologically necessary norm, was calculated (Table 5). To characterize the total mass of EAA for anabolic needs in human beings, the indicator of comparative excess of EAA (redundancy coefficient) was calculated, and the average value of excess EAA composition (an indicator of the coefficient of difference of amino acid score—DCAS indicator) and biological value were also determined (Table 5).

Table 5.
Utilitarian coefficient, redundancy coefficient, coefficient of difference of amino acid score (DCAS), and biological value of bread with the replacement of part of wheat flour with pumpkin seed flour at different percentages (0, 5, 10, 15, and 20%).
Analysis of the utilitarian coefficient, redundancy coefficient, and biological value of bread enriched with different percentages of pumpkin seed flour indicates a similar balance of EAA in proteic structures in all samples. However, as the percentage of pumpkin seed flour enrichment increased, the DCAS decreased, subsequently increasing the use of EAA by the human body, which was dependent on the pumpkin seed flour percentage.
3. Materials and Methods
3.1. Preparation of Flour, Dough, and Bread Samples
Protein composition and functional and technological properties (total protein content, protein fractional composition, essential amino acid composition, moisture binding capacity, and moisture retaining capacity) of premium wheat flour and pumpkin seed flour were analyzed following the methods described in paragraphs 2.2–2.5 and compared. During the flour preparation, 500 g seeds of wheat and of pumpkin bought in a local market were milled in a heavy grinder, sifted through a mesh sieve (200 μm aperture size) to obtain a fine powder, and kept at 4 °C until further use. Then, dough samples were prepared, each with 200 g premium wheat flour, 5 g salt, 7 g pressed baker’s yeast, 300 g water, and pumpkin seed flour in the amount of 5%, 10%, 15%, and 20% to replace wheat flour, and the prepared dough samples were fermented for 3.5 h at room temperature (25 °C). A sample without pumpkin seed flour was used as a control sample. After the investigation of semi-finished products, bread from doughs prepared with different concentrations of pumpkin seed flour and wheat flour for the control sample was prepared, cooking at 220 °C for 25 min in a ventilated oven (Sveba-Dahlen AB, Fristad, Sweden). Three breads for each treatment (each from 500 g of dough) were obtained and stored at room temperature (25 °C) for one week, until the analysis. Flours were analyzed for total protein content, mass functional composition of protein, essential amino acid profile, and their biological and technological value. The reflection spectroscopic profile of doughs was analyzed immediately after kneading and after 3.5 h of fermentation. Finally, the bread was analyzed spectroscopically for its EAA composition and biological value.
3.2. Total Protein Content
Flour mineralization with a strong acid releasing nitrogen can determine N content by the titration technique, following the Kjeldahl method. An amount of 1 g of raw material was hydrolyzed with 15 mL concentrated sulfuric acid containing two copper catalyst tablets for 2 h in a heat block at 420 °C. After cooling, distilled H2O was added to the hydrolysates before neutralization and titration [42]. The amount of present protein was calculated from the nitrogen concentration in the product. Total N was expressed as g proteins per 100 g of flour.
3.3. Protein Fractional Composition
The seed flour was defatted with pentane. The flour/solvent slurry was stirred at a 1:10 w/v ratio for 24 h, and the solvent was then removed by centrifugation. The mixture was dried at room temperature and stored in airtight sample bottles at 4 °C until subsequent use. Then, pumpkin proteins were fractionated from pentane defatted meal according to the Osborne differential extraction procedure described by Horax et al. [43]. The meal/water suspensions (20 g of meal into 100 mL of deionized water) were stirred for 2 h at room temperature and centrifuged for 30 min at 20,000× g to separate the supernatant from the pellet, thus obtaining the deionized water extract (DWE). The same extraction/separation conditions were kept for the next successive protein extraction steps. The water extract pellet was resuspended into 100 mL of 1 M NaCl solution and stirred as mentioned above. The supernatant obtained after centrifugation, designated as salt extract (SE), was then extracted in 100 mL of deionized water adjusted at pH 11 with 0.5 M NaOH solution, leading to the alkaline extract (AE). Each extraction was repeated twice. After each extraction, the pellets were washed twice using 20 mL of solvent to collect the residual protein entrapped in the insoluble residues. The DWE, SE, and AE were precipitated for isolation by adjusting the pH of the obtained supernatant to the pH corresponding to the minimum of solubility (pHms) determined from the turbidity experiment [44]. The pH was adjusted by 1 M HCl or 1 M NaOH solutions in acidic or alkaline pH. After centrifugation at 15,000× g for 15 min, the isolated protein precipitates were washed twice using deionized water at their respective pHms and recentrifuged. Finally, the resulting protein fractions were resolubilized by adjusting the pH to 7.0, freeze-dried, and stored at 4 °C until further analysis [45].
3.4. Essential Amino Acid (EAA) Composition
The determination of amino acid composition was conducted in accordance with the method of ion exchange chromatography [22]. The quality and quantitative determination of amino acids consisted of the hydrolysis of proteins and the determination of their quantitative estimation with the help of an automatic analyzer of amino acids, T-339 (Mikrotechna Praha a.s., Praha, Czech Republic), using polystyrene sulfonate ion exchange resins of “Ostion LJ ANB” in Li-citrate buffer one-column mode. The elution of amino acids from the column was conducted, in turn, by Li-citrate buffers from pH 2.75 ± 0.01; pH 2.95 ± 0.01; pH 3.2 ± 0.02; pH 3.8 ± 0.02; pH 5.0 ± 0.2. Amino acids were detected at a wavelength of 560 nm by rectification with a ninhydrin solution on a photometer (Unicam SP 800, Unicam Instruments, Cambridge, Britain). The results of detection were registered by a variplotter in the form of the peaks of absorption of light of ninhydrin-positive substances in an eluent that number the direct ratio concentrations of this substance in solution. The correlation of the solution of ninhydrin reagent and eluents was 1 to 2; the temperature of thermostatic T1 = 38.5 °C; T2 = 65 °C. The prototype was diluted in Li-citrate buffer by pH 2.2 ± 0.02 and inflicted on an ion exchange column. The quantitative estimation of chromatograms of the pre-production model settled in relation to the Bio-Rad standard mixture of amino acids. The mass of every amino acid, expressed as g per 100 g protein (Ai), in the investigated solution was calculated by the following formula:
where Mi is molecular mass of each amino acid; Si is an area of peak of each amino acid on an aminogram from the investigated solution; Se is an area of peak of each amino acid on an aminogram from the solution of the standard mixture of amino acids which accords to one micromole.
Amino acid SCORE is expected according to the certificate scale of THEO/WHO [46].
3.5. Moisture Binding and Retaining Capacity
Moisture binding capacity was determined by the centrifugation method [47], and was calculated as follows:
Moisture binding capacity (%) = (weight of precipitate/weight of original flour) × 100
The determination of the moisture retaining capacity was carried out by mixing the sample with water and determining the amount of separated liquid after a centrifugation [48], and was calculated as follows:
Moisture retaining capacity (%) = [(weight of tube with flour and water retained − weight of tube with flour)/(weight of flour)] × 100
3.6. Near-Infrared Reflection Spectroscopy
The reflection spectra from a smooth surface and shredded samples were researched by using an Infrapid spectrometer (Labor-Mim, Hungary) in the near-infrared range from 1330 to 2370 nm. The spectrometer initially recorded the reflectance spectrum from reference I0 (component part of the instrument); then, a reflection spectrum from the researched sample I was obtained. The spectra are represented as the reflectivity of R in relative units (the ratio of the intensities I/I0 = R), depending on the wavelength in nm [49,50,51]. The intensity of reflection was measured in wheat and pumpkin flours, in wheat dough immediately after kneading and after 3.5 h of fermentation, and in bread. The reflection intensity was expressed through the relative reflection coefficient.
3.7. Biological Value of Bread
The utilitarian coefficient (U) characterizes the balance of all EAA proteins with respect to the physiological norm. It is used to compare the protein composition of various food products based on their amino acid composition and the inadequacy of their use in the body. It was calculated by the formula:
where Cmin is Cmin—score of the first limited acid EAA units; Aj is the mass fraction of the j-th of EAA in the product, mg/g protein; Aej is the mass fraction of the j-th of EAA in the protein model, mg/g protein, according to FAO/WHO scale.
The redundancy coefficient (σred) shows the mass fraction of EAA in 100 g of the product, which is not fully used by the body. It was calculated by the formula:
DCAS is the average excess of EAA in comparison with the smallest amino acid score of the limiting amino acid. It was calculated by the formula:
where ΔDAS—the difference in amino acid score for each EAA compared to the AS of a limiting amino acid expressed as %; n—the number of amino acids.
The biological value of protein (BV), expressed as % [52], was calculated by the formula:
3.8. Statistical Analysis
The results of the analyses of different flours (from wheat and from pumpkin seed) were compared with a two-tailed Student’s t-test using a significance level of 0.05. The results of the analyses of different breads were subjected to a one-way analysis of variance (ANOVA), using the different concentrations of pumpkin seed flour (0, 5, 10, 15, 20 %) as the factor of variation. All the means were separated by an LSD (Least Significant Difference) post-hoc test (p < 0.05). The normality of data was tested using the Shapiro–Wilk test, whereas the homoscedasticity was tested using Bartlett’s test. Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad (GraphPad, La Jolla, CA, USA).
4. Conclusions
Conformational changes in the structure of protein substances of dough and bread from wheat flour occurred when adding pumpkin seed flour, due to their different protein compositions. The total content of proteins found in pumpkin seed flour was 40% lower than that of wheat flour, with a higher presence of EAA.
Near-infrared reflection spectroscopy confirmed the difference in the fractional composition of proteins of the studied flours, and the different content between soluble and insoluble proteins, as well as the presence of OH, CH, SH, and NH groups.
The protein profile was complete after the replacement of 10% or more of wheat flour with pumpkin seed flour; this could be because the score for all amino acids was higher than 100%. As the percentage of the substitution of wheat flour with pumpkin seed flour increases, the number of amino acids used for anabolic purposes decreases, and they are fully utilized by the human body. Further research must be conducted in order to determine the best content of pumpkin seed flour to introduce in wheat bread. Concerning further research to be carried out in light of the results of this research, taking into consideration the variations that different cultivars and phenotypes of pumpkin may have in terms of production and nutritional characteristics [53], from a food technology and marketing perspective, it would be interesting to investigate which specific types of pumpkins are more suitable for processing seeds into flour for baked products.
Furthermore, sensory acceptance analysis might explore the level of preference and the purchase intention of consumers in order to position pumpkin seed flour products in the right target market.
Author Contributions
Conceptualization and methodology, A.S., O.G. and S.L.; investigation, A.S., O.G. and S.L.; writing—original draft preparation, A.S. and S.L.; writing—review and editing, A.S., O.G., C.C., A.C., L.G., C.M. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Meara, L.; Turner, C.; Coitinho, D.C.; Oenema, S. Consumer experiences of food environments during the COVID-19 pandemic: Global insights from a rapid online survey of individuals from 119 countries. Glob. Food Sec. 2022, 32, 100594. [Google Scholar] [CrossRef]
- Brigagão, T.C.S.; Fortes, R.R.; Lourenço, C.O.; Carvalho, E.E.N.; Cirillo, M.Â.; Nachtigall, A.M.; Vilas Boas, B.M. Optimization of gluten-free muffins made with pineapple peel, banana peel, and pumpkin seed flours. J. Food Process Preserv. 2021, 45, e16037. [Google Scholar] [CrossRef]
- Sääksjärvi, M.; Holmlund, M.; Tanskanen, N. Consumer knowledge of functional foods. Int. Rev. Retail Distrib. Consum. Res. 2009, 19, 135–156. [Google Scholar] [CrossRef]
- Annunziata, A.; Vecchio, R.; Kraus, A. Factors affecting parents’ choices of functional foods targeted for children. Int. J. Consum. Stud. 2016, 40, 527–535. [Google Scholar] [CrossRef]
- Nystrand, B.T.; Olsen, S.O. Relationships between functional food consumption and individual traits and values: A segmentation approach. J. Funct. Foods 2021, 86, 104736. [Google Scholar] [CrossRef]
- Ivanov, V.; Shevchenko, O.; Marynin, A.; Stabnikov, V.; Gubenia, O.; Stabnikova, O.; Shevchenko, A.; Gavva, O.; Saliuk, A. Trends and expected benefits of the breaking edge food technologies in 2021–2030. Ukr. Food J. 2021, 10, 7–36. [Google Scholar] [CrossRef]
- Jurgita, K.; Jariene, E.; Danilcenko, H.; Černiauskienė, J.; Wawrzyniak, A.; Hamulka, J.; Juknevičienė, E. Chemical composition of pumpkin (Cucurbita maxima D.) flesh flours used for food. J. Food Agric. Environ. 2014, 12, 61–64. [Google Scholar]
- Millar, K.; Barry-Ryan, C.; Burke, R.; McCarthy, S.; Gallagher, E. Dough properties and baking characteristics of white bread, as affected by addition of raw, germinated and toasted pea flour. Innov. Food Sci. Emerg. Technol. 2019, 56, 102189. [Google Scholar] [CrossRef]
- Bialek, M.; Rutkowska, J.; Adamska, A.; Bajdalow, E. Partial replacement of wheat flour with pumpkin seed flour in muffins offered to children. CyTA J. Food 2016, 14, 391–398. [Google Scholar] [CrossRef]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Batista, J.E.R.; Braga, L.P.; Oliveira, R.C.D.; Silva, E.P.; Damiani, C. Partial replacement of wheat flour by pumpkin seed flour in the production of cupcakes filled with carob. Food Sci. Technol. Res. 2018, 38, 250–254. [Google Scholar] [CrossRef]
- Aljahani, A.H. Wheat-yellow pumpkin composite flour: Physico-functional, rheological, antioxidant potential and quality properties of pan and flat bread. Saudi J. Biol. Sci. 2022, 29, 3432–3439. [Google Scholar] [CrossRef]
- Jeevitha, G.R.; Bhuvana, S. Study on the impact of pumpkin seed flour on whole wheat bread characteristics. Int. J. Chem. Stud. 2019, 7, 273–277. [Google Scholar]
- Shevchenko, A.; Drobot, V.; Galenko, O. Use of pumpkin seed flour in preparation of bakery products. Ukr. Food J. 2022, 11, 90–101. [Google Scholar] [CrossRef]
- Apostol, L.; Mosoiu, C.; Iorga, C.S.; Martinez, S.A. Effect of the addition of pumpkin powder on the physicochemical qualities and rheological properties of wheat flour. Rom. Biotechnol. Lett. 2020, 25, 1594–1600. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymanska-Chargot, M.; Mis, A.; Kowalski, R.; Gruszecki, W.I. Raman studies of gluten proteins aggregation induced by dietary fibers. Food Chem. 2016, 194, 86–94. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Dodig, D.; Ignjatović-Micić, D. Characterization of proteins from grain of different bread and durum wheat genotypes. Int. J. Mol. Sci. 2011, 12, 5878–5894. [Google Scholar] [CrossRef]
- Luchian, M.I.; Csatlos, C. Research on change in protein composition during dough processing. Bull. Transilv. Univ. Bras. II For. Wood Ind. Agric. Food Eng. 2011, 4, 109–114. [Google Scholar]
- Atuonwu, A.C.; Akobundu, E.N.T. Nutritional and sensory quality of cookies supplemented with defatted pumpkin (Cucubita pepo) seed flour. Pak. J. Nutr. 2010, 9, 672–677. [Google Scholar] [CrossRef]
- Nutter, J.; Saiza, A.; Iurlina, M. Microstructural and conformational changes of gluten proteins in wheat-rye sourdough. J. Cereal Sci. 2019, 87, 91–97. [Google Scholar] [CrossRef]
- Amjid, M.R.; Shehzad, A.; Hussain, S.; Shabbir, M.A.; Khan, M.R.; Shoaib, M. A comprehensive review on wheat flour dough rheology. Pak. J. Food Sci. 2013, 23, 105–123. [Google Scholar]
- Huang, S.C.; Tsai, Y.F.; Chen, C.M. Effects of wheat fiber, oat fiber on sensory and physico-chemical properties of Chinese-style sausages. Asian Aust. J. Anim. Sci. 2011, 24, 875–880. [Google Scholar] [CrossRef]
- Sanful, R.E.; Darko, S. Utilization of soybean flour in the production of bread. Pak. J. Nutr. 2010, 9, 815–818. [Google Scholar] [CrossRef]
- Costa, L.; Jardim, P.; Silva, F.; Castilho, V.; Damasceno, E.; Campagnol, P. Physicochemical and rheological characterization of pan bread made with pumpkin seed flour. Int. Food Res. J. 2018, 25, 1489–1496. [Google Scholar]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of watermelon, pumpkin, and paprika seed oils and flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef]
- Berton, B.; Scher, J.; Villiéras, F.; Hardy, J. Measurement of hydration capacity of wheat flour: Influence of composition and physical characteristics. Powder Technol. 2002, 128, 326–331. [Google Scholar] [CrossRef]
- Baslar, M.; Ertugay, M.F. Determination of protein and gluten quality-related parameters of wheat flour using near-infrared reflectance spectroscopy (NIRS). Turk. J. Agric. For. 2011, 35, 139–144. [Google Scholar] [CrossRef]
- Munyendo, L.; Njoroge, D.; Hitzmann, B. The Potential of Spectroscopic Techniques in Coffee Analysis—A Review. Processes 2022, 10, 71. [Google Scholar] [CrossRef]
- Buresta, N.F.; Sayyah, A.H.A.; Taghinezhad, E. Prediction of some quality properties of rice and its flour by near-infrared spectroscopy (NIRS) analysis. Food Sci. Nutr. 2020, 9, 1099–1105. [Google Scholar] [CrossRef]
- Bruun, S.W.; Søndergaard, I.; Jacobsen, S. Analysis of protein structures and interactions in complex food by near-infrared spectroscopy. 1. Gluten powder. J. Agric. Food Chem. 2007, 55, 7234–7243. [Google Scholar] [CrossRef]
- Nyam, K.L.; Lau, M.; Tan, C.P. Fibre from pumpkin (Cucurbita pepo L.) seeds and rinds: Physico-chemical properties, antioxidant capacity and application as bakery product ingredients. Malays. J. Nutr. 2013, 19, 99–109. [Google Scholar] [PubMed]
- Wellner, N.; Mills, E.N.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J.; Halford, N.G.; Shewry, P.R.; Belton, P.S. Changes in protein secondary structure during gluten deformation studied by dynamic Fourier transform infrared spectroscopy. Biomacromolecules 2005, 6, 255–261. [Google Scholar] [CrossRef]
- Thiele, C.; Grassl, S.; Gänzle, M. Gluten hydrolysis and depolymerization during sourdough fermentation. J. Agric. Food Chem. 2004, 52, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.E.; Damodaran, S. Bran-induced changes in water structure and gluten conformation in model gluten dough studied by Fourier transform infrared spectroscopy. Food Hydrocoll. 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Eldin, A.I.B. Near Infra Red Spectroscopy. Wide Spectra Qual. Control 2011, 237–248. [Google Scholar] [CrossRef]
- Beć, K.B.; Karczmit, D.; Kwaśniewicz, M.; Ozaki, Y.; Czarnecki, M.A. Overtones of νC≡N Vibration as a Probe of Structure of Liquid CH3CN, CD3CN, and CCl3CN: Combined Infrared, Near-Infrared, and Raman Spectroscopic Studies with Anharmonic Density Functional Theory Calculations. J. Phys. Chem. 2019, 123, 4431–4442. [Google Scholar] [CrossRef]
- Chevallier, S.; Della Valle, G.; Colonna, P.; Broyart, B.; Trystram, G. Structural and chemical modifications of short dough during baking. J. Cereal Sci. 2002, 35, 1–10. [Google Scholar] [CrossRef]
- Arya, S.; Sadawarte, P.; Ashish, W. Importance of damaged starch in bakery products-a review. Starch 2015, 1, 2019. [Google Scholar]
- Storck, C.R.; da Rosa Zavareze, E.; Gularte, M.A.; Elias, M.C.; Rosell, C.M.; Dias, A.R.G. Protein enrichment and its effects on gluten-free bread characteristics. LWT-Food Sci. Technol. 2013, 53, 346–354. [Google Scholar] [CrossRef]
- Carlson, B.L.; Knorr, D.; Watkins, T.R. Influence of tomato seed addition on the quality of wheat flour breads. J. Food Sci. 1981, 46, 1029–1031. [Google Scholar] [CrossRef]
- El-Soukkary, F.A.H. Evaluation of pumpkin seed products for bread fortification. Plant Foods Hum. Nutr. 2001, 56, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Horax, H.; Hettiarachchy, N.; Over, K.; Chen, P.; Gbur, E. Extraction, fractionation and characterization of bitter melon seed proteins. J. Agric. Food Chem. 2010, 58, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita maxima) seed proteins: Sequential Extraction Processing and Fraction Characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Riaublanc, A.; Chouaibi, M.; Guéguen, J.; Hamdi, S. Functional properties of protein fractions obtained from pumpkin (Cucurbita maxima) seed. Int. J. Food Prop. 2015, 19, 172–186. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Park, J.H.; Lee, S.Y.; Choi, M.S.; Choi, J.H.; Kim, C.J. Effects of pumpkin (Cucurbita maxima Duch.) fiber on physicochemical properties and sensory characteristics of chicken frankfurters. Food Sci. Anim. Resour. 2012, 32, 174–183. [Google Scholar] [CrossRef]
- Unbehend, I.; Unbehend, G.; Slrelec, I. Water Absorption Determination of Wheat Flours applying Centrifuge and its Relationship to Farinograph Water Absorption Determination Deutsche Lebensmittel-Rundschau. Jahrgang 2006, 1, 11–16. [Google Scholar]
- Raikos, V.; Neacsu, M.; Russell, W.; Duthie, G. Comparative study of the functional properties of lupin, green pea, fava bean, hemp, and buckwheat flours as affected by pH. Food Sci. Nutr. 2014, 2, 802–810. [Google Scholar] [CrossRef]
- Niewitetzki, O.; Tillmann, P.; Becker, H.C.; Mollers, C. A new near-infrared reflectance spectroscopy method for high-throughput analysis of oleic acid and linolenic acid content of single seeds in oilseed rape (Brassica napus L.). J. Agric. Food Chem. 2010, 58, 94–100. [Google Scholar] [CrossRef]
- Yip, W.L.; Gausemel, I.; Sande, S.A.; Dyrstad, K. Strategies for multivariate modeling of moisture content in freeze-dried mannitol-containing products by near-infrared spectroscopy. J. Pharm. Biomed. Anal. 2012, 70, 202–211. [Google Scholar] [CrossRef]
- Vyshniak, V.; Dimitriev, O.; Litvynchuk, S.; Dombrovskiy, V. Identification of beeswax and its falsification by the method of infrared spectroscopy. Ukr. Food J. 2018, 7, 421–433. [Google Scholar] [CrossRef]
- Grek, O.; Onopriichuk, O.; Tymchuk, A. Biological value in milk-protein concentrates with malt ingredients. Ukr. Food J. 2019, 8, 571–583. [Google Scholar] [CrossRef]
- Meru, G.; Fu, Y.; Leyva, D.; Sarnoski, P.; Yagiz, Y. Phenotypic relationships among oil, protein, fatty acid composition and seed size traits in Cucurbita pepo. Sci. Hortic. 2018, 233, 47–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).