Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Conditions, and Treatments
2.2. Measurement of Plant Height, SPAD Value, and Biomass Accumulation
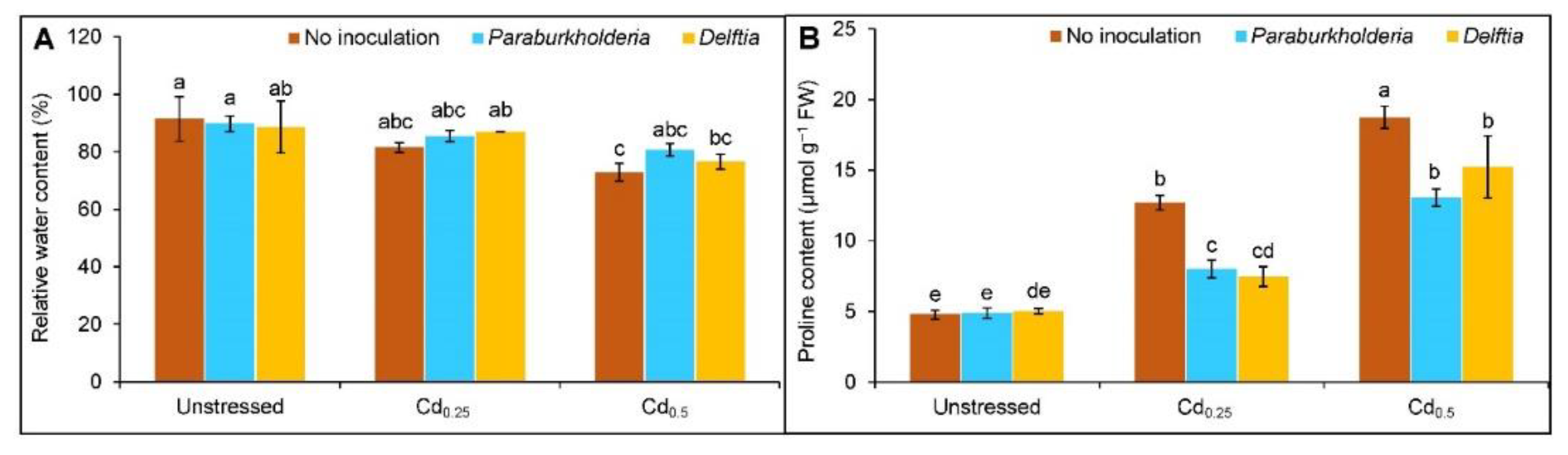
2.3. Estimation of Relative Water Content
2.4. Quantification of Proline Content
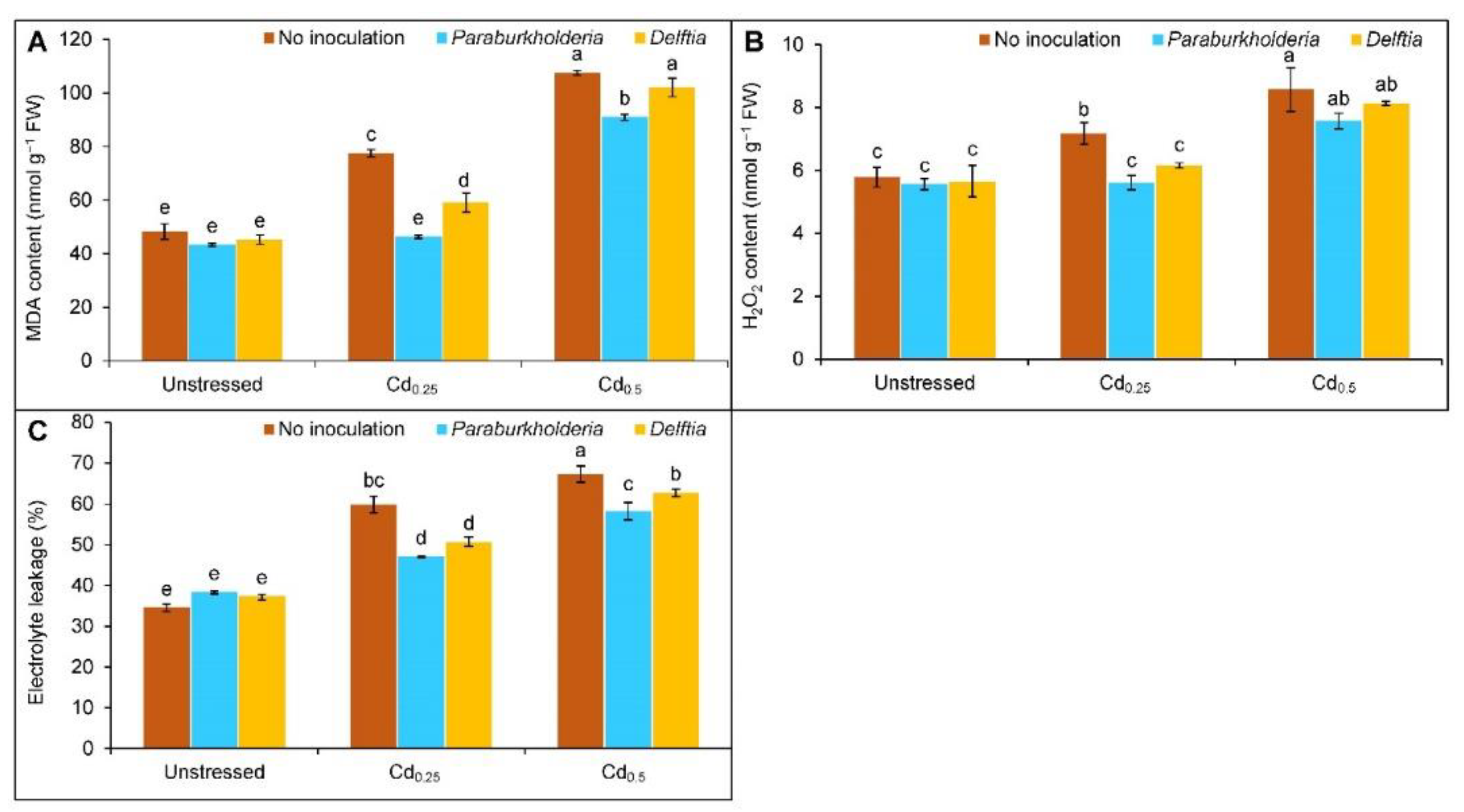
2.5. Estimation of Electrolyte Leakage
2.6. Determination of Stress Indicators
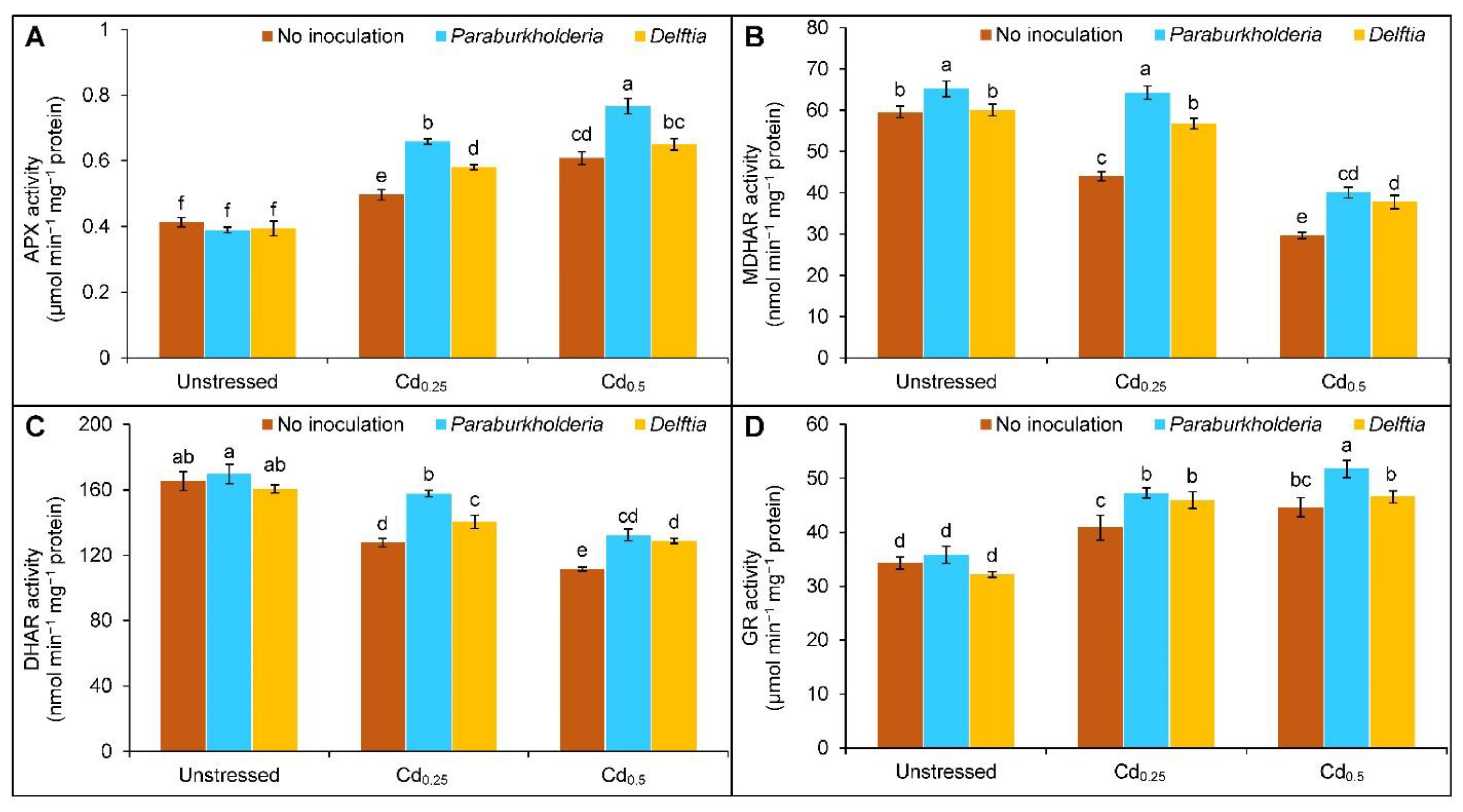
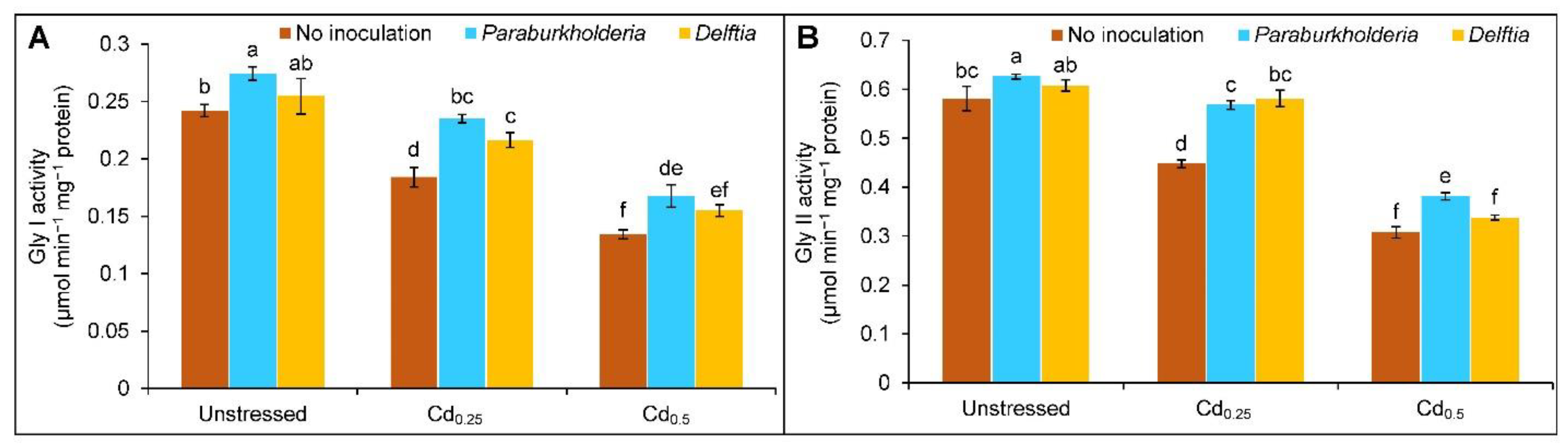
2.7. Extraction and Quantification of Protein Content and Enzymes Activity Assay
2.8. Statistical Analysis
3. Results
3.1. Growth Parameters and Photosynthetic Activity
3.2. Relative Water Content and Proline Accumulation
3.3. Oxidative Stress Indicators
3.4. Activities of Antioxidant Enzymes
3.5. Activities of Glyoxalase Enzymes
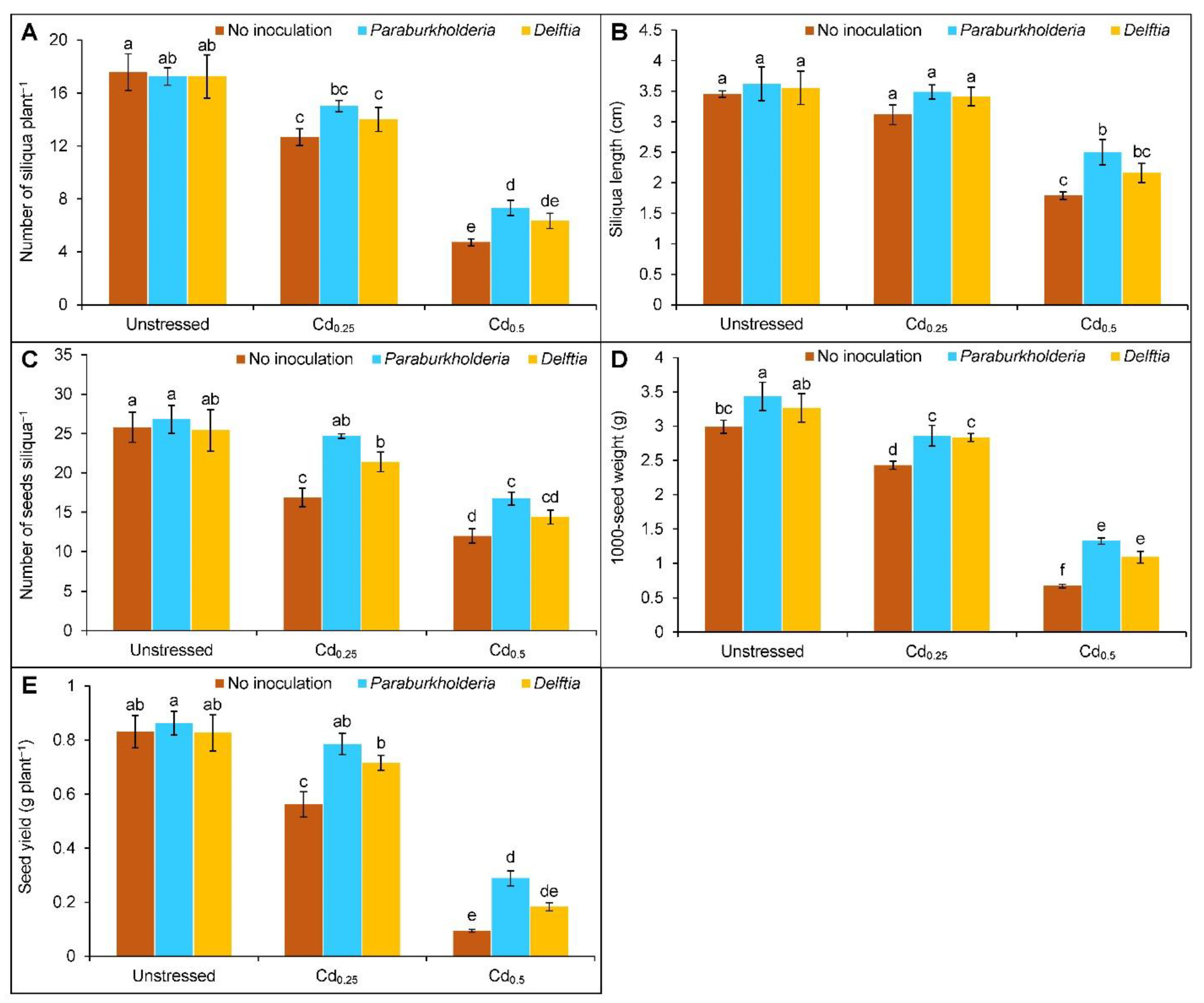
3.6. Yield Parameters
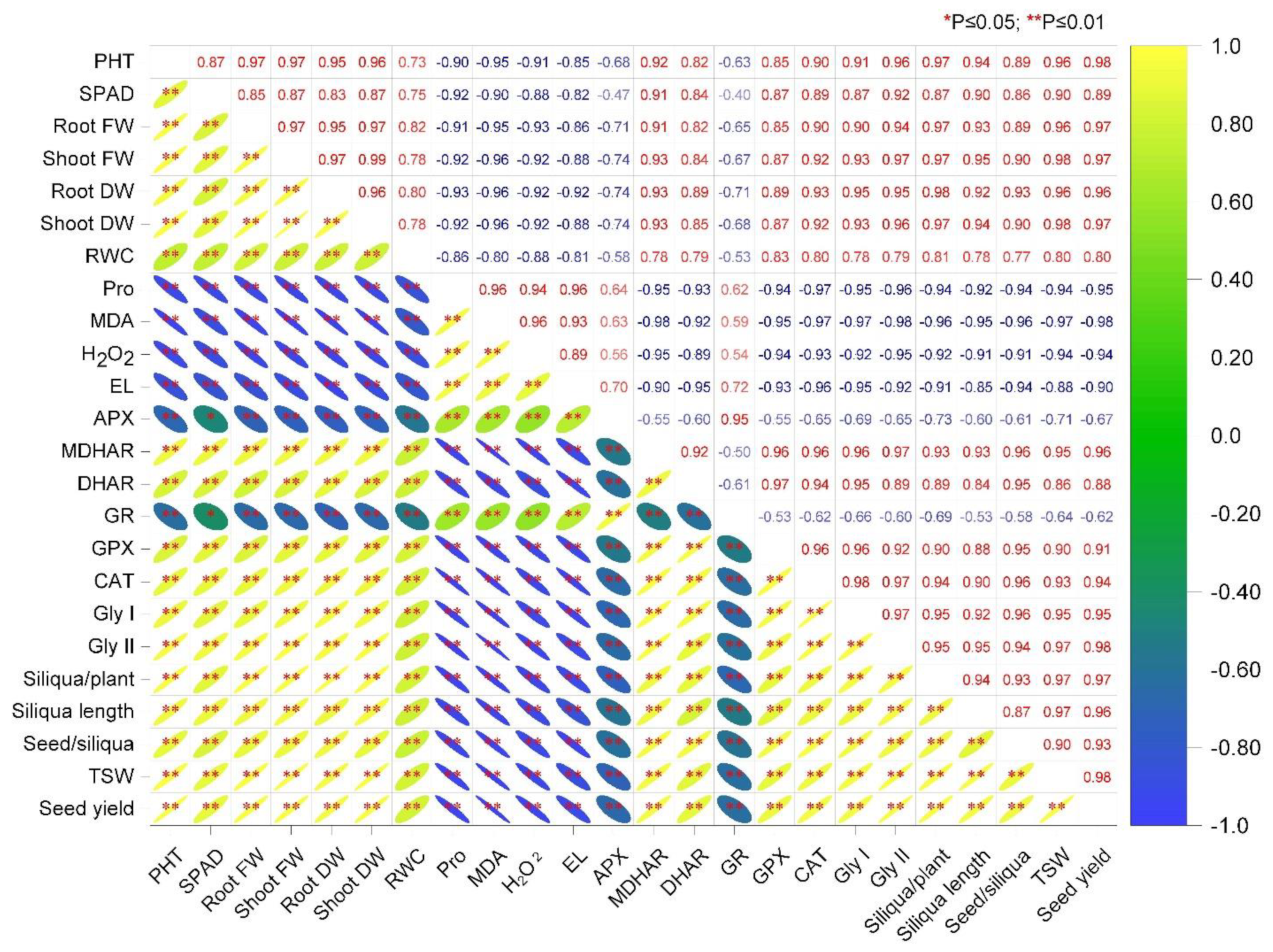
3.7. Correlation Analysis among the Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trived, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant growth-promoting actinobacterial inoculant assisted phytoremediation increases cadmium uptake in Sorghum bicolor under drought and heat stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.A.; Ahmed, H.M.I.; Misra, M.; Sharma, P.; Misra, A.N.; Hasanuzzaman, M. Nitric oxide alleviates cadmium-impeded growth by limiting ROS accumulation in pea seedlings. BIOCELL 2022, 46, 2583–2593. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Y.; Li, Y.; Lin, Q.; Bai, J.; Tang, L.; Wang, S.; Ying, R.; Qiu, R. Survival strategies of the plant-associated bacterium Enterobacter sp. strain EG16 under cadmium stress. Appl. Environ. Microbiol. 2016, 82, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of chromium-induced oxidative stress by combined treatment of selected plant-growth-promoting rhizobacteria and earthworms via modulating the expression of genes related to reactive oxygen species metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 802512. [Google Scholar] [CrossRef] [PubMed]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Mohapatra, P.K.D.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Chi, Y.; You, Y.; Wang, J.; Chen, X.; Chu, S.; Wang, R.; Zhang, X.; Yin, S.; Zhang, D.; Zhou, P. Two plant growth-promoting bacterial Bacillus strains possess different mechanisms in affecting cadmium uptake and detoxification of Solanum nigrum L. Chemosphere 2022, 305, 135488. [Google Scholar] [CrossRef]
- Saeed, Z.; Naveed, M.; Imran, M.; Bashir, M.A.; Sattar, A.; Mustafa, A.; Hussain, A.; Xu, M. Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 2019, 14, e0213016. [Google Scholar] [CrossRef]
- Tanwir, K.; Javed, M.T.; Abbas, S.; Shahid, M.; Akram, M.S.; Chaudhary, H.J.; Iqbal, M. Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111584. [Google Scholar] [CrossRef]
- Sepehri, M.; Khatabi, B. Combination of siderophore-producing bacteria and Piriformospora indica provides an efficient approach to improve cadmium tolerance in alfalfa. Microbial. Ecol. 2021, 81, 717–730. [Google Scholar] [CrossRef]
- Mourato, M.P.; Moreira, I.N.; Leitão, I.; Pinto, F.R.; Sales, J.R.; Martins, L.L. Effect of heavy metals in plants of the genus Brassica. Int. J. Mol. Sci. 2015, 16, 17975–17998. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.P. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar] [CrossRef]
- Saghafi, D.; Ghorbanpour, M.; Ajiloo, H.S.; Lajayer, B.A. Enhancement of growth and salt tolerance in Brassica napus L. seedlings by halotolerant Rhizobium strains containing ACC-deaminase activity. Ind. J. Plant Physiol. 2019, 24, 225–235. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Amelioration of salt stress tolerance in rapeseed (Brassica napus) cultivars by seed inoculation with Arthrobacter globiformis. Plant Biosyst. 2022, 156, 370–383. [Google Scholar] [CrossRef]
- Braña, V.; Cagide, C.; Morel, M.A. The sustainable use of Delftia in agriculture, bioremediation, and bioproducts synthesis. In Microbial Models: From Environmental to Industrial Sustainability; Castro-Sowinski, S., Ed.; Springer: Singapore, 2016; pp. 227–250. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Haque, E.; Paul, N.C.; Khaleque, M.A.; Al-Garni, S.M.; Rahman, M.; Islam, M.T. Enhancement of growth and grain yield of rice in nutrient deficient soils by rice probiotic bacteria. Rice Sci. 2017, 24, 264–273. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.; Alam, M.; Mohi-Ud-Din, M.; Miah, M.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Hu, X.; Cao, Y.; Pang, W.J.; Huang, J.Y.; Guo, P.; Huang, L. Biodegradation of phenanthrene and heavy metal removal by acid-tolerant Burkholderia fungorum FM-2. Front. Microbiol. 2019, 10, 408. [Google Scholar] [CrossRef]
- Morel, M.A.; Ubalde, M.C.; Braña, V.; Castro-Sowinski, S. Delftia sp. JD2: A potential Cr(VI)-reducing agent with plant growth-promoting activity. Arch. Microbiol. 2011, 193, 63–68. [Google Scholar] [CrossRef] [PubMed]
- BARI (Bangladesh Agricultural Research Institute). Krishi Projukti Hatboi; Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2021. (In Bengali) [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dorr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef]
- Principato, G.B.; Rosi, G.; Talesa, V.; Govannini, E.; Uolila, L. Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochim. Biophys. Acta 1987, 911, 349–355. [Google Scholar] [CrossRef]
- CoStat—Statistics Software; Version 6.400; CoHort Software: Monterey, CA, USA, 2008.
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-growth-promoting rhizobacteria emerging as an effective bioinoculant to improve the growth, production, and stress tolerance of vegetable crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef]
- Gupta, A.; Rai, S.; Bano, A.; Khanam, A.; Sharma, S.; Pathak, N. Comparative evaluation of different salt-tolerant plant growth promoting bacterial isolates in mitigating the induced adverse effect of salinity in Pisum sativum. Biointerface Res. Appl. Chem. 2021, 11, 13141–13154. [Google Scholar]
- Xu, Q.; Pan, W.; Zhang, R.; Lu, Q.; Xue, W.; Wu, C.; Song, B.; Du, S. Inoculation with Bacillus subtilis and Azospirillum brasilense produces abscisic acid that reduces Irt1-mediated cadmium uptake of roots. J. Agric. Food Chem. 2018, 66, 5229–5236. [Google Scholar] [CrossRef]
- Li, Y.; Mo, L.; Zhou, X.; Yao, Y.; Ma, J.; Liu, K.; Yu, F. Characterization of plant growth-promoting traits of Enterobacter sp. and its ability to promote cadmium/lead accumulation in Centella asiatica L. Environ. Sci. Pollut. Res. 2021, 29, 4101–4115. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Ramzan, N.; Mustafa, A.; Samad, A.; Niamat, B.; Yaseen, M.; Ahmad, Z.; Hasanuzzaman, M.; Sun, N.; Shi, W.; et al. Alleviation of salinity induced oxidative stress in Chenopodium quinoa by Fe biofortification and biochar—Endophyte interaction. Agronomy 2020, 10, 168. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wang, B. Adaptation mechanism of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.H.M.B.; Parvin, K.; Mohsin, S.M.; Mahmud, J.A.; Hasanuzzaman, M.; Fujita, M. Modulation of cadmium tolerance in rice: Insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 2020, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar]
- Ji, C.; Tian, H.; Wang, X.; Song, X.; Ju, R.; Li, H.; Gao, Q.; Li, C.; Zhang, P.; Li, J.; et al. Bacillus subtilis HG-15, a halotolerant rhizoplane bacterium, promotes growth and salinity tolerance in wheat (Triticum aestivum). BioMed Res. Int. 2020, 2022, 9506227. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Ullah, S.; Kolo, Z.; Egbichi, I.; Keyster, M.; Ludidi, N. Nitric oxide influences glycine betaine content and ascorbate peroxidase activity in maize. S. Afr. J. Bot. 2016, 105, 218–225. [Google Scholar] [CrossRef]
- Zeng, X.; Pang, L.; Chen, Y.; Kong, X.; Chen, J.; Tian, X. Bacteria Sphingobium yanoikuyae Sy310 enhances accumulation capacity and tolerance of cadmium in Salix matsudana Koidz roots. Environ. Sci. Pollut. Res. 2020, 27, 19764–19773. [Google Scholar] [CrossRef]
- Ullah, I.; Al-Johny, B.O.; Al-Ghamdi, K.M.; Al-Zahrani, H.A.; Anwar, Y.; Firoz, A.; Naser, A.K.; Almatry, M.A.A. Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicol. Environ. Saf. 2019, 174, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Puthiyottil, P.; Akkara, Y. Pre-treatment with Bacillus subtilis mitigates drought induced photo-oxidative damages in okra by modulating antioxidant system and photochemical activity. Physiol. Mol. Biol. Plants 2021, 27, 945–957. [Google Scholar] [CrossRef]
- Das, B.K.; Kumar, A.; Maindola, P.; Mahanty, S.; Jain, S.K.; Reddy, M.K.; Arockiasamy, A. Non-native ligands define the active site of Pennisetum glaucum (L.) R. Br dehydroascorbate reductase. Biochem. Biophys. Res. Commun. 2016, 473, 1152–1157. [Google Scholar]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Khan, A.L.; Bilal, S.; Halo, B.A.; Al-Harrasi, A.; Khan, A.R.; Waqas, M.; Al-Thani, G.S.; Al-Amri, I.; Al-Rawahi, A.; Lee, I.-J. Bacillus amyloliquefaciens BSL16 improves phytoremediation potential of Solanum lycopersicum during copper stress. J. Plant Interact. 2017, 12, 550–559. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental selenium and boron mitigate salt-induced oxidative damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J. Plant Growth Regul. 2020, 39, 1488–1502. [Google Scholar] [CrossRef]
- Hernández, J.A.; Barba-Espín, G.; Diaz-Vivancos, P. Glutathione-mediated biotic stress tolerance in plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M., Mostofa, M., Diaz-Vivancos, P., Burritt, D., Fujita, M., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 309–319. [Google Scholar]
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, Q.; Khan, A.; Nawaz, A.; Baig, K.S.; Chen, J.T. Enhancing cadmium tolerance and pea plant health through Enterobacter sp. MN17 inoculation together with biochar and gravel sand. Plants 2020, 9, 530. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Jia, X.; Yang, F.; Wang, Z. Assimilation and translocation of dry matter and phosphorus in rice genotypes affected by salt-alkaline stress. Sustainability 2016, 8, 568. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Zhao, J.; Guan, F.; Yao, D.; Wu, N.; Tian, J. The endophytic bacterium Bacillus koreensis 181–22 promotes rice growth and alleviates cadmium stress under cadmium exposure. Appl. Microbiol. Biotechnol. 2021, 105, 8517–8529. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raihan, M.R.H.; Rahman, M.; Mahmud, N.U.; Adak, M.K.; Islam, T.; Fujita, M.; Hasanuzzaman, M. Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants 2022, 11, 2738. https://doi.org/10.3390/plants11202738
Raihan MRH, Rahman M, Mahmud NU, Adak MK, Islam T, Fujita M, Hasanuzzaman M. Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants. 2022; 11(20):2738. https://doi.org/10.3390/plants11202738
Chicago/Turabian StyleRaihan, Md. Rakib Hossain, Mira Rahman, Nur Uddin Mahmud, Malay Kumar Adak, Tofazzal Islam, Masayuki Fujita, and Mirza Hasanuzzaman. 2022. "Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems" Plants 11, no. 20: 2738. https://doi.org/10.3390/plants11202738
APA StyleRaihan, M. R. H., Rahman, M., Mahmud, N. U., Adak, M. K., Islam, T., Fujita, M., & Hasanuzzaman, M. (2022). Application of Rhizobacteria, Paraburkholderia fungorum and Delftia sp. Confer Cadmium Tolerance in Rapeseed (Brassica campestris) through Modulating Antioxidant Defense and Glyoxalase Systems. Plants, 11(20), 2738. https://doi.org/10.3390/plants11202738









