Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Tissue Culture
2.3. Experiment 1: Tissue Culture with AM1 and AM2
2.4. Experiment 2: Soil Culture with AM1 and AM2
2.5. Experiment 3: Soil Culture with AM3
2.6. Analysis of Heavy Metals
2.7. Data Analysis
3. Results
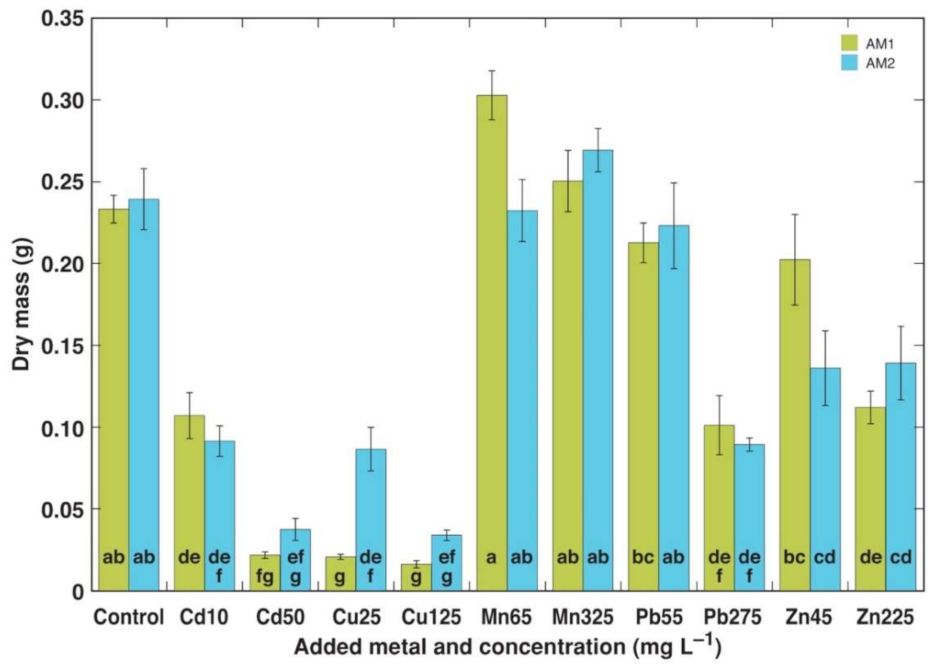
3.1. Experiment 1
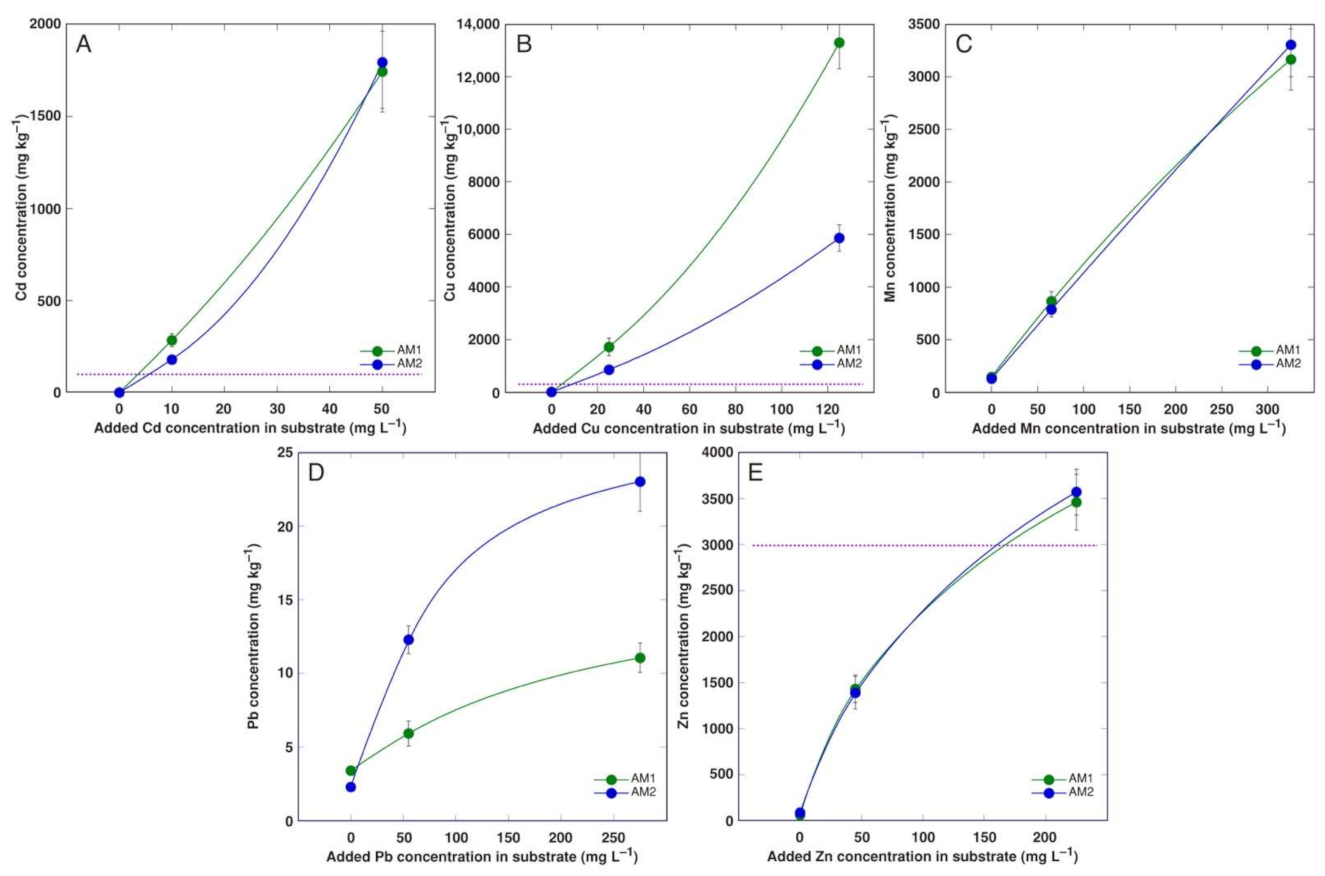
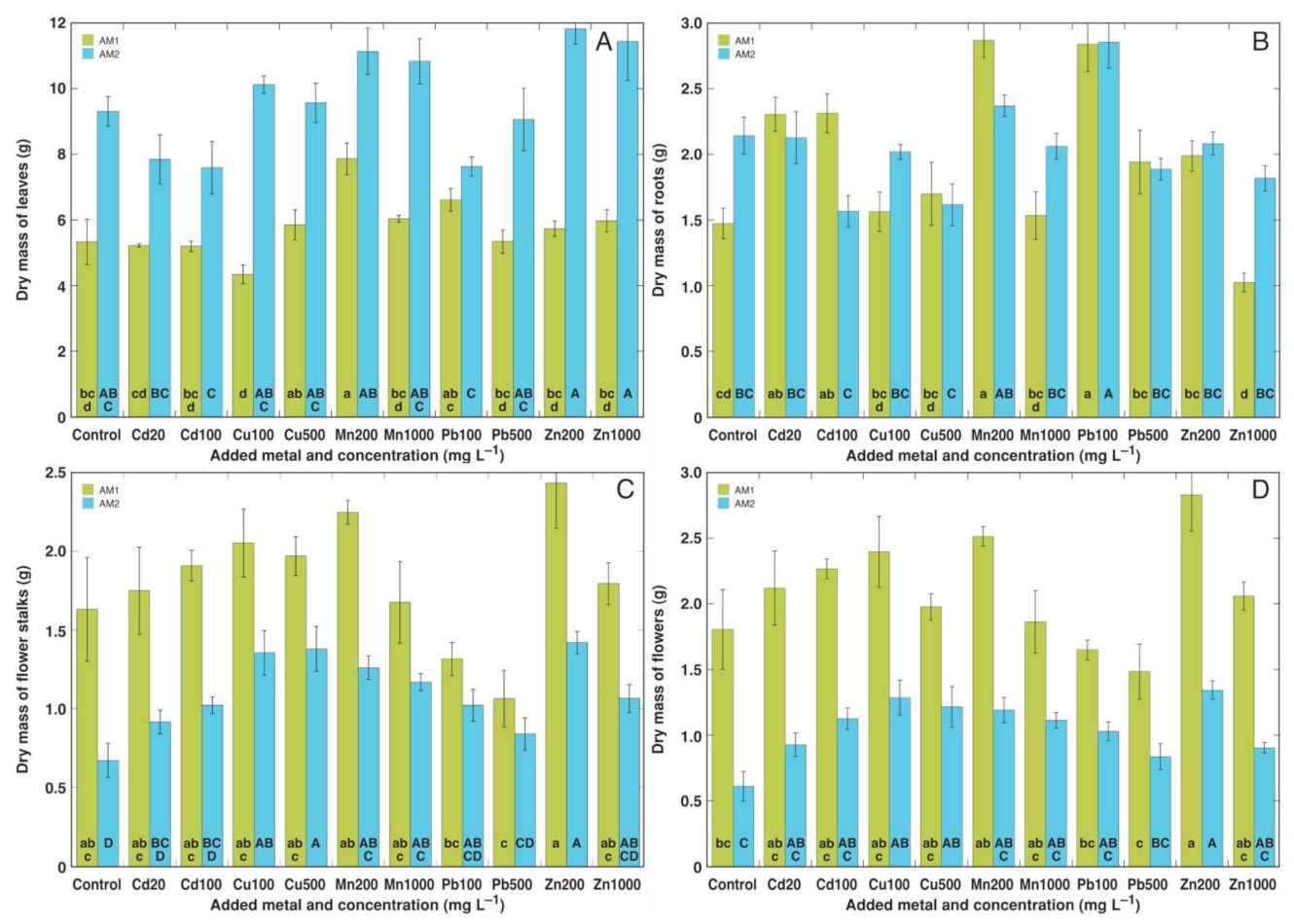
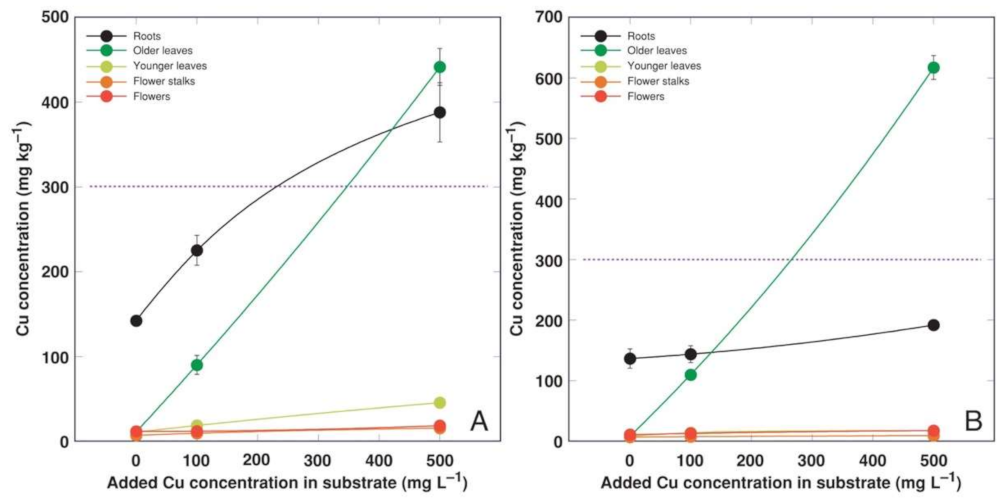
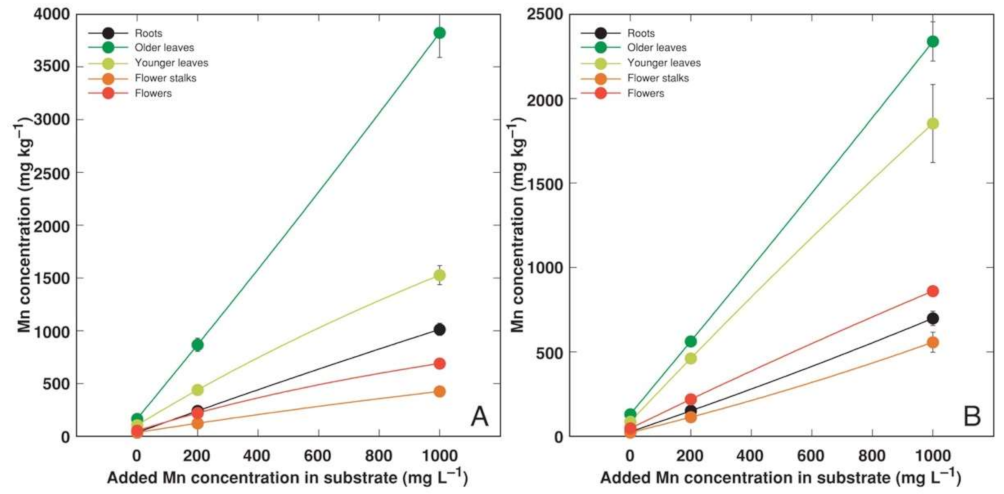
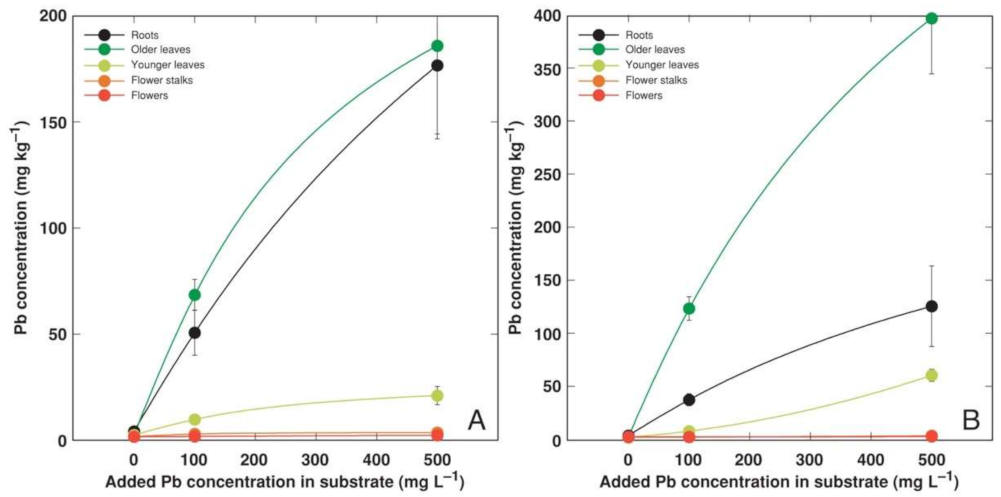
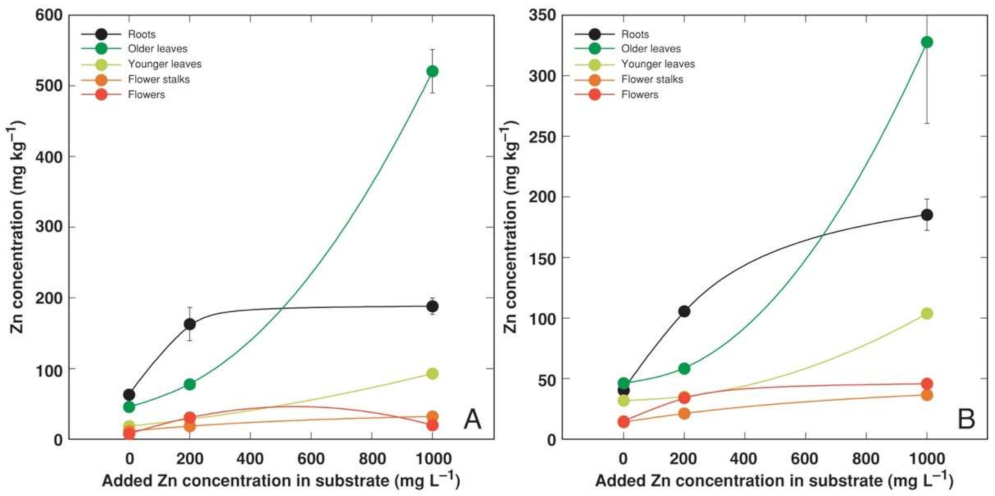
3.2. Experiment 2
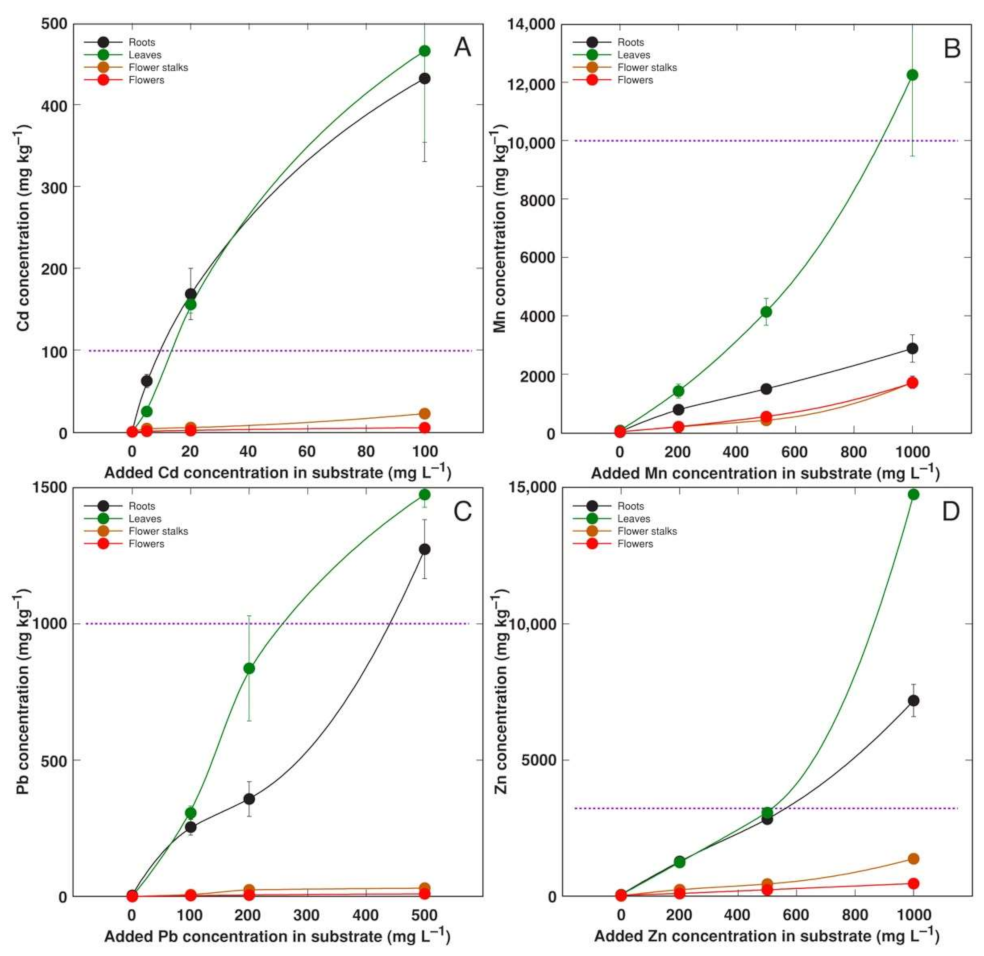
3.3. Experiment 3
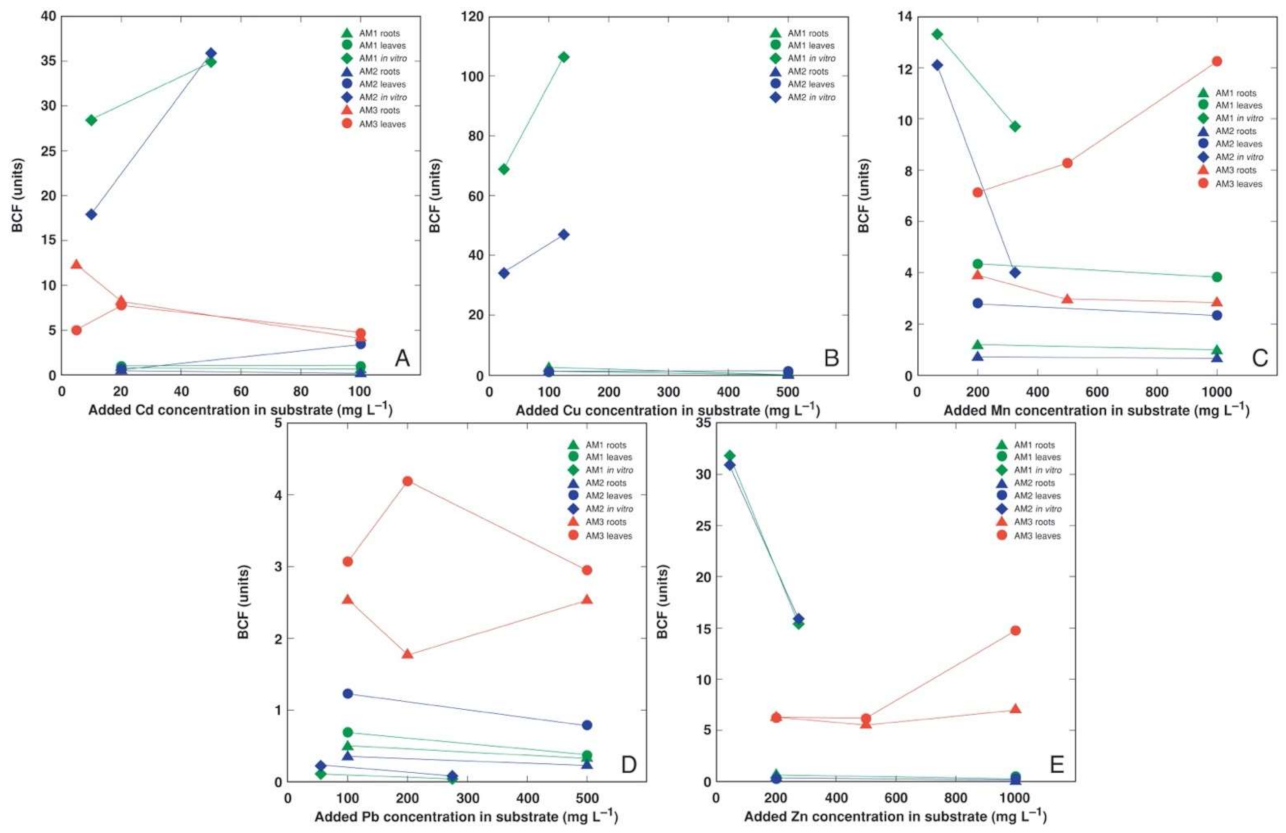
3.4. Comparison of Bioconcentration Efficiency
4. Discussion
4.1. Metal Tolerance of Soil-Grown Plants
4.2. Metal Accumulation Potential of Different Accessions
4.3. Comparison of In Vitro and In Planta Experimental Systems
4.4. Perspectives of High Metal Tolerance and Accumulation Potential in A. maritima
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goolsby, E.W.; Mason, C.M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of heavy metals: And indispensable contrivance in green remediation technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Baker, A.J.M.; Jaffre, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z. Metal accumulation and tolerance in wetland plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Ievinsh, G.; Andersone-Ozola, U.; Landorfa-Svalbe, Z.; Karlsons, A.; Osvalde, A. Wild plants from coastal habitats as a potential resource for soil remediation. In Soil Health. Soil Biology 59; Giri, B., Varma, A., Eds.; Springer Nature: Berlin, Germany, 2020; pp. 121–144. [Google Scholar]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Folsom, T.C.; Lee, T.; Park, F.; Price, C.; Roeder, D.; Schmitz, J.; Stockwell, C. Heavy metal tolerance in Typha latifolia without the evolution of tolerant races. Ecology 1974, 55, 1163–1165. [Google Scholar] [CrossRef]
- Lefèbvre, C. Population variation and taxonomy in Armeria maritima with special reference to heavy-metal-tolerant populations. New Phytol. 1974, 73, 209–219. [Google Scholar] [CrossRef]
- Baumbach, H.; Hellwig, F.H. Genetic differentiation of metallicolous and non-metallicolous Armeria maritima (Mill.) Willd. taxa (Plumbaginaceae) in Central Europe. Plant Syst. Evol. 2007, 269, 245–258. [Google Scholar] [CrossRef]
- Lefebvre, C.; Kakes, P. Note on some populations of Armeria maritima (Mill.) Willd. from the south and north of the Netherlands. Acta Bot. Neerl. 1978, 27, 17–26. [Google Scholar] [CrossRef]
- Köhl, K.I. The effect of NaCl on growth, dry matter allocation and ion uptake in salt marsh and inland populations of Armeria maritima. New Phytol. 1997, 135, 213–225. [Google Scholar] [CrossRef]
- Brown, G. The effects of lead and zinc on the distribution of plant species at former mining areas of Western Europe. Flora 1995, 190, 243–249. [Google Scholar] [CrossRef]
- Abratowska, A.; Wasowicz, P.; Bednarek, P.T.; Telka, J.; Wierzbicka, M. Morphological and genetic distinctiveness of metallicolous and non-metallicolous populations of Armeria maritima s.l. (Plumbaginaceae) in Poland. Plant Biol. 2012, 14, 586–595. [Google Scholar] [CrossRef]
- Buckland, S.M.; Price, A.H.; Hendry, G.A.F. The role of ascorbate in drought-treated Cochlearia atlantica Pobed. and Armeria maritima (Mill.) Willd. New Phytol. 1991, 119, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Köhl, K.I. Do Armeria maritima (Mill.) Willd. ecotypes from metalliferous soils and non-metalliferous soils differ in growth response under Zn stress? A comparison by a new artificial soil method. J. Exp. Bot. 1997, 48, 1959–1967. [Google Scholar] [CrossRef]
- Lange, K.; Viklander, M.; Blecken, G.-T. Effects of plant species and traits on metal treatment and phytoextraction in stormwater bioretention. J. Environ. Manag. 2020, 276, 111282. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Gérard, E.; Perronnet, K.; Morel, J.L. Measurement of in situ phytoextraction of zinc by spontaneous metallophytes growing on a former smelter site. Sci. Total Environ. 2001, 279, 215–221. [Google Scholar] [CrossRef]
- Becker, T.; Brändel, M. Vegetation-environment relationships in a heavy metal-dry grassland complex. Folia Geobot. 2007, 42, 11–28. [Google Scholar] [CrossRef]
- Olko, A.; Abratowska, A.; Żyłkowska, J.; Wierzbicka, M.; Tukiendorf, A. Armeria maritima from a calamine heap—Initial studies on physiologic–metabolic adaptations to metal-enriched soil. Ecotox. Environ. Saf. 2008, 69, 209–218. [Google Scholar] [CrossRef]
- Gourguillon, L.; Rustenholz, C.; Lobstein, A.; Gondet, L. Callus induction and establishment of cell suspension cultures of the halophyte Armeria maritima (Mill.) Willd. Sci. Hortic. 2018, 233, 407–411. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E. Remediation of soil-free grounds contaminated by zinc, lead and cadmium with use of metallophytes. Polish J. Environ. Stud. 2008, 17, 707–712. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro selection and biochemical characterisation of zinc and manganese adapted callus lines in Brassica spp. Plant Sci. 1999, 137, 89–100. [Google Scholar] [CrossRef]
- Malá, J.; Máchová, P.; Cvrčková, H.; Vaněk, T. Heavy metals uptake by the hybrid aspen and rowan-tree clones. J. Forest Sci. 2007, 63, 491–497. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Capuana, M.; Arneroli, M.; Gabbrielli, R.; Gonelli, C. Exploring the metal phytoremediation potential of three Populus alba L. clones using an in vitro screening. Environ. Sci. Pollut. Res. 2011, 18, 82–90. [Google Scholar] [CrossRef]
- Lombardi, L.; Sebastiani, L. Copper toxicity in Prunus cerasifera: Growth and antioxidant enzyme responses of in vitro grown plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kļaviņa, D.; Gailīte, A.; Ievinsh, G. Initial responses of explants from rare and endangered coastal plant species during initiation of tissue culture. Acta Univ. Latv. 2006, 710, 81–91. [Google Scholar]
- Pan, J.; Cai, R.; Liu, Y.; He, H.; Wu, A. Propagation in vitro and karyotype analysis of Armeria maritima. Guangxi Zhiwu 2004, 24, 33–36. [Google Scholar]
- Szarek-Łukaszewska, G.; Słysz, A.; Wierzbicka, M. Response of Armeria maritima (Mill.) Willd. to Cd, Zn and Pb. Acta Biol. Cracov. Ser. Bot. 2004, 46, 19–24. [Google Scholar]
- Dahmani-Muller, H.; van Ort, F.; Gélie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Envrion. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Brewin, L.E.; Mehra, A.; Lynch, P.T.; Farago, M.E. Mechanisms of copper tolerance by Armeria martitima in Dolfrwynog Bog, North Wales—Initial studies. Environ. Geochem. Health 2003, 25, 147–156. [Google Scholar] [CrossRef]
- Visioli, G.; Marmiroli, N. The proteomics of heavy metal hyperaccumulation in plants. J. Proteom. 2013, 79, 133–145. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metaloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- dos Reis, R.A.; Hendrix, S.; Mourato, M.P.; Martins, L.L.; Vangronsveld, J.; Cuypers, A. Efficient regulation of copper homeostasis underlies accession-specific sensitivities to excess copper and cadmium in roots of Arabidopisis thaliana J. Plant Physiol. 2021, 261, 153434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Yang, X.E.; He, Z.L. Growth response and phytoextraction of copper at different soil levels by Elsholtzia splendens. Chemosphere 2004, 55, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Reichman, S. The Responses of Plant to Metal Toxicity: A Review Focusing on Copper, Manganese and Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002; p. 54. [Google Scholar]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef]
- Loneragan, J.F. Distribution and movement of manganese in plants. In Manganese in Soils and Plants; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 33, pp. 113–124. [Google Scholar]
- Singer, C.E.; Havill, D.C. Manganese as an ecological factor in salt marshes. Vegetatio 1985, 62, 287–292. [Google Scholar] [CrossRef]
- Singer, C.E.; Havill, D.C. Resistance to divalent manganese of salt marsh plants. J. Ecol. 1993, 81, 797–806. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, response to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Zhao, E.J.; Jiang, R.F.; Dunham, S.J.; McGrath, S.P. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol. 2006, 172, 6467–6654. [Google Scholar] [CrossRef]
- Heumann, H.-G. Ultrastructural localization of zinc in zinc-tolerant Armeria maritima ssp. halleri by autometallography. J. Plant Physiol. 2002, 159, 191–203. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; van der Ent, A.; Baker, A.J.M. Global distribution and ecology of hyperaccumulating plants, Mineral Resource Reviews. In Agromining: Farming for Metals; Van der Ent, A., Baker, A.J.M., Echevarria, G., Simonnot, M.-O., Morel, J.L., Eds.; Springer: Cham, Switzerland, 2018; pp. 75–92. [Google Scholar]
- Jarvis, M.D.; Leung, D.W.M. Chelated lead transport in Pinus radiata: An ultrastructural study. Environ. Exp. Bot. 2002, 48, 21–32. [Google Scholar] [CrossRef]
- Pourutt, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: New York, NY, USA, 2011; Volume 213, pp. 113–136. [Google Scholar]
- Collin, S.; Baskar, A.; Geevarbhese, D.M.; Ali, M.N.V.S.; Senatov, F.; Koppala, S.; Swamiappan, S. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Kovačević, B.; Miladinović, D.; Orlović, S.; Katanić, M.; Kebert, M.; Kovinčić, J. Lead tolerance and accumulation in white poplar cultivated in vitro. South-East Eur. For. 2013, 4, 3–12. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef]
- Parys, E.; Wasilewska, W.; Siedlecka, M.; Zienkiewicz, M.; Drożak, A.; Romanowska, E. Metabolic responses to lead of metallicolous and nonmetallicolous populations of Armeria maritima. Arch. Environ. Contam. Toxicol. 2014, 67, 565–577. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010, 68, 1–13. [Google Scholar] [CrossRef]
- Neumann, D.; Nieden, U.Z.; Lichtenberger, O.; Leopold, I. How does Armeria maritima tolerate high heavy metal concentrations? J. Plant Physiol. 1995, 146, 704–717. [Google Scholar] [CrossRef]
- Küpper, H.; Andersen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269. [Google Scholar] [CrossRef]
- Brej, T.; Fabiszewski, J. Plants accumulating heavy metals in the Sudety mts. Acta Soc. Bot. Polon. 2006, 75, 61–68. [Google Scholar] [CrossRef][Green Version]
- Bedell, J.-P.; Ferro, Y.; Bazin, C.; Perrodin, Y. Selection of a halophytic plant for assesing the phytotoxicity of dredged seaport sediment stored on land. Environ. Monit. Assess. 2014, 186, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Laffont-Schwob, I.; Rabier, J.; Masotti, V.; Foizer, H.; Tosini, L.; Vassalo, L.; Salducci, M.-D.; Prudent, P. Functional trait-based screening of Zn-Pb tolerant wild plant species at an abandoned mine site in Gard (France) for rehabilitation of Mediterranean metal-contaminated soils. Int. J. Environ. Res. Public Health 2020, 17, 5506. [Google Scholar] [CrossRef] [PubMed]
- Tomović, G.; Buzurović, U.; Ðurović, S.; Vicić, D.; Mihailović, N.; Jakovljević, K. Strategies of heavy metal uptake by three Armeria species growing on different geological substrates in Serbia. Environ. Sci. Pollut. Res. 2018, 25, 507–522. [Google Scholar] [CrossRef]
- Smykalova, I.; Vrbova, M.; Tejklova, E.; Vetrovcova, M.; Griga, M. Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Industr. Crops Prod. 2010, 32, 527–533. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Kafi, M.; Moghadam, M.R.F. Breeding by in vitro culture to improve tolerance and accumulation of lead in Cynodon dactylon L. J. Agric. Sci. Technol. 2015, 17, 1851–1860. [Google Scholar]
- Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E.; Smoleń, S. Comparative assessment of response to cadmium in heavy metal-tolerant shrubs cultured in vitro. Water Air Soil Pollut. 2017, 228, 304. [Google Scholar] [CrossRef]
- Salas-González, I.; Reyt, G.; Flis, P.; Custódio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef]
- Singha, S.; Townsend, E.C.; Oberly, H. Relationship between calcium and agar on vitrification and shoot-tip necrosis of quince (Cydonia oblonga Mill.) shoots in vitro. Plant Cell Tissue Organ Cult. 1990, 23, 135–142. [Google Scholar] [CrossRef]
- Gribble, K.; Conroy, J.P.; Holford, P.; Milham, P.J. In vitro uptake of minerals by Gypsophila paniculata and hybrid eucalypts, and relevance to media mineral formulation. Austr. J. Bot. 2002, 50, 713–723. [Google Scholar] [CrossRef]
- Williams, R.R. Towards a model of mineral nutrition in vitro. In Transplant Production Systems; Kurata, K., Kozai, T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 213–229. [Google Scholar]
- Leifert, C.; Murphy, K.P.; Lumsden, P.J. Mineral and carbohydrate nutrition of plant cell and tissue cultures. Crit. Rev. Plant Sci. 1995, 14, 83–109. [Google Scholar] [CrossRef]
- Wilms, F.H.A.; Sassen, M.M.A. Origin and development of floral buds in tobacco explants. New Phytol. 1987, 105, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. The components of plant tissue culture media I: Macro- and micro-nutrients. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Berlin, Germany, 2008; pp. 65–113. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Berlin, Germany, 2008; pp. 115–173. [Google Scholar]
- Martins, J.P.R.; Conde, L.T.; Falqueto, A.R.; Gontijo, A.B.P.L. Selenium biofortified Aechmea blanchetiana (Bromeliaceae) can resist lead-induced toxicity during in vitro culture. Acta Physiol. Plant. 2021, 43, 149. [Google Scholar] [CrossRef]
- Reeves, R.D. Hyperaccumulation of trace elements by plants. In Phytoremediation of Metal-Contaminated Soils; Morel, J.-L., Echevarria, G., Goncharova, N., Eds.; Springer: Berlin, Germany, 2006; pp. 25–52. [Google Scholar]
- Nawaz, I.; Iqbal, M.; Bliek, M.; Schat, H. Salt and heavy metal tolerance and expression levels of candidate tolerance genes among four extremophile Cochlearia species with contrasting habitat preferences. Sci. Total Environ. 2017, 584–595, 731–741. [Google Scholar] [CrossRef]

| Code | Associated Water Reservoir | Habitat | Location | Coordinates | Type of Experiment | Heavy Metals Tested |
|---|---|---|---|---|---|---|
| AM1 | River Vecdaugava | Dry shore meadow | City of Riga, Ziemeļu District, Vecdaugava | 57°03′29″ N 24°05′47′′ E | Tissue culture, soil culture | Cd, Cu, Mn, Pb, Zn |
| AM2 | River Buļļupe | Salt-affected shore meadow | City of Riga, Kurzeme District, Island of Buļļu Sala, Vakarbuļļi | 56°59′54″ N 23°57′31″ E | Tissue culture, soil culture | Cd, Cu, Mn, Pb, Zn |
| AM3 | The Baltic Sea | Dry coastal meadow | Nybrostrand, Ystad Municipality, Skåne County, Sweden | 55°25′40″ N 13°57′27″ E | Soil culture | Cd, Mn, Pb, Zn |
| Heavy Metal | Salt | Added Metal Concentration (mg L−1) | ||
|---|---|---|---|---|
| Tissue Culture (Experiment 1) | Soil Culture (Experiment 2) | Soil Culture (Experiment 3) | ||
| Cd | CdCl2 2.5H2O | 10, 50 | 20, 100 | 5, 20, 100 |
| Cu | CuSO4 5H2O | 25, 125 | 100, 500 | – |
| Mn | MnSO4 H2O | 65, 325 | 200, 1000 | 200, 500, 1000 |
| Pb | Pb(CH3COO)2 3H2O | 55, 275 | 100, 500 | 100, 200, 500 |
| Zn | ZnSO4 H2O | 45, 225 | 200, 1000 | 200, 500, 1000 |
| Heavy Metal | Concentration (mg L−1) | Water Content (g g−1 DM) | Multiplication Rate (Relative Units) | Necrotic Explants (%) | |||
|---|---|---|---|---|---|---|---|
| AM1 | AM2 | AM1 | AM2 | AM1 | AM2 | ||
| Control | 0 | 8.0 ± 0.3 abcd | 8.0 ± 0.3 abcd | 2.88 ± 0.08 ab | 2.76 ± 0.08 abc | 0 | 0 |
| Cd | 10 | 4.5 ± 0.6 h | 5.9 ± 0.3 efgh | 0.92 ± 0.20 e | 0.52 ± 0.14 ef | 0 | 0 |
| 50 | 5.6 ± 0.1 fgh | 6.3 ± 0.4 cdefgh | 0 f | 0.08 ± 0.05 f | 96 | 96 | |
| Cu | 25 | 5.0 ± 0.2 gh | 5.8 ± 0.3 efgh | 0.40 ± 0.06 ef | 1.60 ± 0.30 de | 100 | 36 |
| 125 | 5.2 ± 0.4 gh | 5.2 ± 0.2 fgh | 0 f | 0 f | 100 | 92 | |
| Mn | 65 | 8.7 ± 0.4 ab | 8.2 ± 0.2 abc | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 0 | 0 |
| 325 | 9.5 ± 0.5 a | 9.4 ± 0.4 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 0 | 0 | |
| Pb | 55 | 7.7 ± 0.2 abcde | 7.1 ± 0.4 bcdef | 2.84 ± 0.10 ab | 2.92 ± 0.05 a | 0 | 0 |
| 275 | 6.2 ± 0.3 defgh | 6.2 ± 0.2 defgh | 2.16 ± 0.20 bcd | 2.04 ± 0.23 cd | 0 | 0 | |
| Zn | 45 | 8.5 ± 0.7 ab | 7.6 ± 0.6 abcde | 2.68 ± 0.17 abc | 2.56 ± 0.29 abc | 0 | 0 |
| 225 | 6.1 ± 0.3 defgh | 6.4 ± 0.2 cdefg | 1.72 ± 0.05 d | 2.56 ± 0.20 abc | 8 | 0 | |
| Heavy Metal | Concentration (mg L−1) | Total Dry Mass (g) | Inflorescences (n) | Total Length of Flower Stalks (cm) | Water Content in Older Leaves (g g−1 DM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| AM1 | AM2 | AM1 | AM2 | AM1 | AM2 | AM1 | AM2 | ||
| Control | 0 | 10.3 ± 1.3 b | 12.7 ± 0.3 bcd | 13.5 ± 1.8 bc | 7.7 ± 0.8 a | 3275 ± 551 abc | 1670 ± 232 c | 4.4 ± 0.1 ab | 4.5 ± 0.1 abc |
| Cd | 20 | 11.4 ± 0.4 b | 11.8 ± 1.0 cd | 16.7 ± 1.9 abc | 10.7 ± 1.0 a | 4302 ± 966 ab | 2383 ± 190 abc | 4.2 ± 0.1 ab | 4.5 ± 0.1 abc |
| 100 | 10.6 ± 0.2 ab | 11.3 ± 1.0 d | 14.7 ± 0.8 abc | 10.8 ± 0.8 a | 3504 ± 178 abc | 2640 ± 146 abc | 4.6 ± 0.3 a | 4.1 ± 0.2 c | |
| Cu | 100 | 10.4 ± 0.7 b | 14.8 ± 0.1 abcd | 17.0 ± 1.5 abc | 12.5 ± 1.1 a | 3720 ± 274 abc | 3325 ± 382 ab | 4.3 ± 0.2 ab | 4.8 ± 0.2 abc |
| 500 | 10.5 ± 0.5 ab | 13.8 ± 1.0 abcd | 14.0 ± 1.1 abc | 11.8 ± 2.0 a | 3604 ± 188 abc | 3096 ± 410 ab | 3.1 ± 0.4 c | 4.7 ± 0.1 abc | |
| Mn | 200 | 15.5 ± 0.5 a | 16.0 ± 0.6 ab | 20.0 ± 1.0 a | 11.7 ± 1.3 a | 4646 ± 202 a | 3031 ± 203 ab | 4.4 ± 0.1 ab | 5.1 ± 0.3 ab |
| 1000 | 11.1 ± 0.7 b | 15.2 ± 0.8 abc | 15.0 ± 1.1 abc | 10.3 ± 0.9 a | 3195 ± 445 abc | 2697 ± 91 abc | 3.9 ± 0.2 abc | 5.2 ± 0.1 a | |
| Pb | 100 | 12.4 ± 0.4 ab | 12.5 ± 0.2 bcd | 12.7 ± 1.0 c | 8.3 ± 0.7 a | 2549 ± 210 bc | 2218 ± 128 bc | 3.9 ± 0.0 abc | 4.7 ± 0.1 abc |
| 500 | 9.8 ± 0.7 b | 12.7 ± 0.9 bcd | 11.5 ± 0.7 c | 10.0 ± 1.6 a | 2011 ± 268 c | 2191 ± 287 bc | 4.0 ± 0.1 abc | 4.5 ± 0.1 bc | |
| Zn | 200 | 13.0 ± 0.9 ab | 16.7 ± 0.5 a | 19.0 ± 1.8 ab | 12.7 ± 0.8 a | 4516 ± 487 ab | 3382 ± 166 a | 4.4 ± 0.1 ab | 5.0 ± 0.1 abc |
| 1000 | 10.9 ± 0.5 b | 15.2 ± 1.4 abc | 17.8 ± 1.3 abc | 9.7 ± 1.0 a | 3722 ± 236 abc | 2368 ± 218 abc | 3.7 ± 0.1 bc | 4.5 ± 0.2 bc | |
| Heavy Metal | Concentration (mg L−1) | Inflorescences (n) | Flower Stalk Length (mm) | Leaf Dry Mass (g) | Flower Stalk Dry Mass (g) | Root Dry Mass (g) | Leaf Water Content (g g−1 DM) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 4.8 ± 1.0 | 303 ± 17 | 1.69 ± 0.20 | 1.16 ± 0.14 | 0.87 ± 0.15 | 2.54 ± 0.07 |
| Cd | 20 | 5.2 ± 1.2 | 313 ± 11 | 1.53 ± 0.24 | 1.13 ± 0.16 | 0.80 ± 0.09 | 2.40 ± 0.20 |
| 100 | 4.8 ± 0.3 | 262 ± 34 | 2.06 ± 0.28 | 1.00 ± 0.10 | 0.65 ± 0.29 | 2.32 ± 0.19 | |
| Cu | 100 | 6.0 ± 1.7 | 277 ± 7 | 2.50 ± 0.73 | 1.24 ± 0.14 | 0.81 ± 0.19 | 2.38 ± 0.11 |
| 500 | 6.0 ± 0.7 | 279 ± 14 | 1.90 ± 0.23 | 1.14 ± 0.18 | 0.67 ± 0.13 | 2.45 ± 0.11 | |
| Mn | 200 | 5.0 ± 0.4 | 282 ± 29 | 1.78 ± 0.28 | 0.90 ± 0.13 | 0.58 ± 0.19 | 2.71 ± 0.14 |
| 1000 | 6.0 ± 1.1 | 263 ± 13 | 1.55 ± 0.28 | 0.82 ± 0.15 | 0.77 ± 0.07 | 2.42 ± 0.26 | |
| Pb | 100 | 4.3 ± 0.5 | 287 ± 12 | 1.80 ± 0.29 | 0.97 ± 0.12 | 0.83 ± 0.14 | 2.27 ± 0.26 |
| 500 | 4.3 ± 0.6 | 321 ± 18 | 1.62 ± 0.12 | 1.03 ± 0.14 | 1.04 ± 0.50 | 2.71 ± 0.14 | |
| Zn | 200 | 5.6 ± 0.8 | 286 ± 6 | 1.64 ± 0.13 | 1.17 ± 0.09 | 0.79 ± 0.11 | 2.59 ± 0.06 |
| 1000 | 6.3 ± 1.4 | 286 ± 8 | 1.43 ± 0.13 | 1.05 ± 0.15 | 0.47 ± 0.12 | 2.46 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants 2022, 11, 2104. https://doi.org/10.3390/plants11162104
Purmale L, Jēkabsone A, Andersone-Ozola U, Karlsons A, Osvalde A, Ievinsh G. Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants. 2022; 11(16):2104. https://doi.org/10.3390/plants11162104
Chicago/Turabian StylePurmale, Līva, Astra Jēkabsone, Una Andersone-Ozola, Andis Karlsons, Anita Osvalde, and Gederts Ievinsh. 2022. "Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow" Plants 11, no. 16: 2104. https://doi.org/10.3390/plants11162104
APA StylePurmale, L., Jēkabsone, A., Andersone-Ozola, U., Karlsons, A., Osvalde, A., & Ievinsh, G. (2022). Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants, 11(16), 2104. https://doi.org/10.3390/plants11162104







