Abstract
The application of polyploidy in sustainable agriculture has already brought much appreciation among researchers. Polyploidy may occur naturally or can be induced in the laboratory using chemical or gaseous agents and results in complete chromosome nondisjunction. This comprehensive review described the potential of polyploidization on plants, especially its role in crop improvement for enhanced production and host-plant resistance development against pests and diseases. An in-depth investigation on techniques used in the induction of polyploidy, cytogenetic evaluation methods of different ploidy levels, application, and current research trends is also presented. Ongoing research has mainly aimed to bring the recurrence in polyploidy, which is usually detected by flow cytometry, chromosome counting, and cytogenetic techniques such as fluorescent in situ hybridization (FISH) and genomic in situ hybridization (GISH). Polyploidy can bring about positive consequences in the growth and yield attributes of crops, making them more tolerant to abiotic and biotic stresses. However, the unexpected change in chromosome set and lack of knowledge on the mechanism of stress alleviation is hindering the application of polyploidy on a large scale. Moreover, a lack of cost–benefit analysis and knowledge gaps on the socio-economic implication are predominant. Further research on polyploidy coupling with modern genomic technologies will help to bring real-world market prospects in the era of changing climate. This review on polyploidy provides a solid foundation to do next-generation research on crop improvement.
1. Introduction
The duplication of single or combined differentiated genomes is known as polyploidy. Autopolyploid arises from the doubling of structurally similar, homologous (AAAA) genomes within a single species, while allopolyploids arise through interspecific hybridization and subsequent doubling of nonhomologous (AABB) genomes [1,2,3]. Aneuploid genomes have incomplete chromosome sets, which occur naturally in plant populations and are induced by chemical and physical agents [4,5,6]. Differences in the number of chromosomes [7,8,9] and phenotypic differences [10,11,12] have been verified using molecular cytogenetic methods. These differences increase allergic diversity [13] and heterozygosity [14] by genome buffering, resulting in novel dose effects. For instance, wheat, canola, cotton, peanut, soybean, and tobacco [1,6,15,16] have been identified as domesticated crops.
Several chemical and gaseous agents are currently used to induce polyploidy. The most widely used agents are colchicine and oryzalin, though colchicine has been suggested to be avoided due to its carcinogenic properties [17]. The common consequences of induced polyploids are increased cell size as well as whole plant size, reduced fertility, and heterosis [18]. In modern breeding, cytoplasmic male sterility often occurs in F1 progenies, where N2O gas treatment (~6 bar, 48 h) plays a significant role in restoring fertility [19]. Similarly, pre- and post-fertilization barriers can be minimized through embryo rescue, particularly in flowering ornamentals, such as Lilium and Hibiscus [20,21,22,23]. Currently, polyploidization is vital in creating crop diversity and producing fruits, vegetables, and flowers most sought after by consumers.
Due to climate change and global warming, plants experience multiple abiotic stresses such as salinity [24,25,26,27], drought [28,29,30], temperature [31,32,33,34], and biotic stresses such as insect pests [35,36,37] and diseases. These stresses threaten crop production by disrupting plants’ physiological and biochemical processes. Polyploidy offers several advantages amid stress situations, which are described in previous studies. However, the underlying mechanism and holistic understanding are still missing.
Several studies assessed polyploidy, but cytogenetic evaluations of polyploids and their effects on crop physiology remain uncertain. Therefore, polyploidy’s roles in plant development, mechanisms and assessment processes, plant physiology changes, biotic-abiotic stresses, challenges, and prospects are outlined here. This study aims to help to recognize possible interactions between polyploids and to establish a consistent assessment.
2. Role of Polyploidy in Modern Plant Breeding
Plant breeders modify crop traits using multiple tools, including polyploidization, to satisfy market demand. This technique creates intense phenotypes and high vigor, making it one of the most potent crop enhancement methods [38]. In addition, some plants have a specific demand for their specific traits, such as seedless fruits in grapevine and banana, which can be achieved through polyploidization [39,40]. Polyploidy results in higher heterozygosity and genome redundancy that are considered advantageous for improving crop plants over conventional plant breeding tools [41,42].
Interspecific hybridization helps to increase the diversity of crops and helps them adapt to new environments [43]. For example, Allopolyploid Triticale is a manufactured crop developed by crossing hexaploid bread wheat and rye to achieve specific goals (e.g., high yield, grain quality, less disease, and stress tolerance) [44,45]. In addition, bridge hybridization is done to transfer genes from one ploidy stage to another if a direct crossover is not feasible. Creation of diversity is one of the most important tasks to develop a crop variety where polyploidy has the efficacy to enhance crop diversity [46,47].
Polyploidy is common in newly domesticated crops [48]. In many cultivated crops, polyploidy has been observed in the speciation process and is now commonly used to create new species selected for features [3]. Unreduced gametes result in plant polyploidy and are used in crop breeding. Polyploidy increases the chromosome number, which helps plants tolerate the mutation by allelic modifications [49,50]. Chromosome deletion-related polyploidy breeding and substitution can produce targeted traits. We have seen many examples of successful cultivation influenced by polyploidy breeding. For example, seedless triploid watermelons, tetraploid red clovers, ryegrass, rye, and many ornamental plants have been developed or improved using polyploid breeding [17,51].
In summary, the main benefits of polyploidy are related to improving the use of heterozygosity. It buffers the effect of gene redundancy in mutations and, in some cases, facilitates reproduction by self-fertilization or asexual means [52]. It has a significant influence on farmers and food security issues.
3. Induction of Polyploids
The recurrence and frequency of polyploidization in plant species make polyploidization an influential research area [53] in which a major step is to select traits in plants [54]. The occurrence of polyploidy in plants was discovered about a century ago. Because of the widespread occurrence of polyploids in wild and cultivated plants, it is important for plant breeders and evolutionary biologists. In the past, antimitotic reagents-induced polyploids have not directly contributed to crop improvement. On the other hand, sexual polyploids (unreduced 2n gametes) are more relevant for crop improvement in many cases. Two pathways cause polyploids: mitotic polyploidization and meiotic polyploidization [55].
Mitotic polyploidization depends on doubling somatic tissue where homoeologous chromosome recombination occurs [56]. The first mitotic polyploidization was introduced in 1930 [57]. This activation polyploidization was tested on plants in vitro [55]. Colchicine, oryzalin, trifluralin, amiprophos-methyl, N2O gas treatment, and caffeine have recently been used as antimitotic reagents [17]. Colchicine is an alkaloid from wild meadow saffron and was the most used as an antimitotic reagent. Oryzalin is a potent herbicide from the Dow AgroScience, USA toluidine chemical band [17,58]. Wetting roots or auxiliary buds or shoots with a colchicine solution of a specific concentration and duration resulted in the successful development of polyploids in many crop species [57,59], as shown in Figure 1. Previous studies successfully applied in vitro chromosomes doubling of colchicine and oryzalin for starch, fodder beet, ryegrass, oriental melon, watermelon, and red clover [17,60].
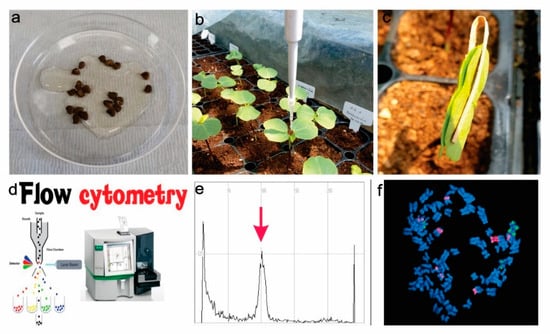
Figure 1.
Mechanism of in vivo polyploidization; (a). Seeds soaking (6–24) hours with colchicine (0.01–0.2)%, (b). Colchicine treatment (10–20 µL) 10 days in the young leaves, (c). Leaves binding with clips for maximum chemical attachment, (d). Flow cytometry analysis for ploidy level assessment, (e). Ploidy level assessment by a histogram, (f). Hibiscus ploidy assessment using chromosome number; and 5S rDNA (green) and 18 rDNA (red) signals.
In vitro polyploidization showed better performance than the success rate of in vivo polyploidization in sugar and fodder beet, ryegrass, and red clover [55,59]. Table 1 provides a list of crops, vegetables, and ornamental and medicinal plants treated with chromosome antimitotic agents for chromosome duplication using different methods and protocols.

Table 1.
Commonly used methods for polyploidization in vitro.
Meiotic polyploidization produces 2n gametes due to the incomplete division of chromosomes [86]. Polyploids that originate through the functioning of 2n gametes are called sexual polyploids, and their usefulness for crop improvement has been demonstrated in potato, alfalfa, and red clover. Introgression can be accomplished by recombination due to genetic crossing-over between alien chromosomes as well as the addition of alien chromosomes in the case of sexual polyploidization in allopolyploids, which is exceedingly difficult or unlikely in the case of colchicine or oryzalin induced allopolyploids. This deviation can occur in plants with normal chromosome pairing as well as in those with disturbed chromosome pairing such as homoeologous recombination of meiotic replication that was seen in Alstroemeria [87], Lilium [88] and Gasteria lutzii × Aloe aristate [89]. The process leading to the formation of 2n gamete is called meiotic nuclear restitution during micro- or megasporogenesis. Depending on the meiotic stage at which nuclear restitution occurs, different restitution mechanisms have been recognized, such as first division restitution (FDR), second division restitution (SDR) [90], and novel intermediate meiosis restitution [88]. In FDR, the non-sister chromatids are heterozygous from the centromere to the first convergence point, while preserving heterozygosity in both parents [91]. In SDR, the two sister chromatids are homozygous between the centromere and the first crossover point, and the resulting gametes have lowered heterozygosity levels compared to the parents [92]. In some cases, 2n gametes restitution cannot be classified as FDR or SDR; the word “indeterminate meiotic restitution” (IMR) has been coined to describe it [88]. Furthermore, IMR might be a widespread occurrence in allotriploids, where both bivalents and univalents are most produced.
4. Cytogenetic Evaluation of Induced Polyploids
Traditionally, polyploids have been assessed by morphological examination. Advanced cytogenetic methods such as flow cytometry, genomic in situ hybridization (GISH), and fluorescence in situ hybridization (FISH) are currently used for polyploid evaluation [11,20,22,93].
4.1. Flow Cytometry
Counting chromosomes in an individual cell is the most efficient and accurate way to confirm ploidy. However, the basic number of chromosomes must be identified before counting. Furthermore, chromosomes are confusing regarding mixoploidy because of the smaller size with a higher number of chromosomes, e.g., taxa Hibiscus [94] and taxa Chrysanthemum.
Flow cytometry may also be the unique method incorporating strong analytical utility to calculate the cell nucleus’s physical size and genome [95,96]. Flow cytometry is a comparatively simple and easy method of calculating a polyploid’s nuclear DNA material. By measuring the relative DNA content using flow cytometry [97,98], the ploidy level of mediated polyploids can be easily verified (Figure 2). However, flow cytometry has some limitations as it is not precise enough to estimate the exact chromosomes number and is unable to differentiate the variation in chromosomes number compared to the ploidy level.
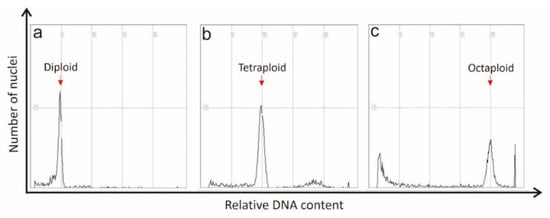
Figure 2.
Histograms show the flow cytometry analysis of comparative changes in ploidy levels in watermelon. (a) Diploid (2n = 2x = 24), (b) tetraploid (2n = 4x = 48), and (c) octaploid (2n = 8x = 96). Red arrows indicate ploidy levels of diploid, tetraploid and octaploid.
4.2. In Situ Hybridization
Molecular cytogenetic research approaches such as FISH and GISH are commonly used and well-respected tools to investigate plant genetics. FISH and GISH have often been used to identify information surrounding chromosomal mutations, structure, and genomic evolution [99,100]. Oligos specific to a repetitive sequence or a particular genomic region can be visualized using fluorescence in situ hybridization [8,101,102,103]. For example, different ribosomal DNA (rDNA) signals are doubled in a tetraploid compared to those in a diploid. A study of 5S rDNA in cotton plants revealed that most diploids have two 5S rDNA signals and all allotetraploid species have four 5S rDNA signals [104]. The same result was found in a woody species of the genus Rubus [105]. The 45S, 18S, 25S, and 5S rDNA are commonly used as FISH markers for cytogenetic study. This method has been used intensely in gene duplication methods and amplification in intraspecific and interspecific polyploids. A brief working directory of FISH and GISH is shown in Figure 3.
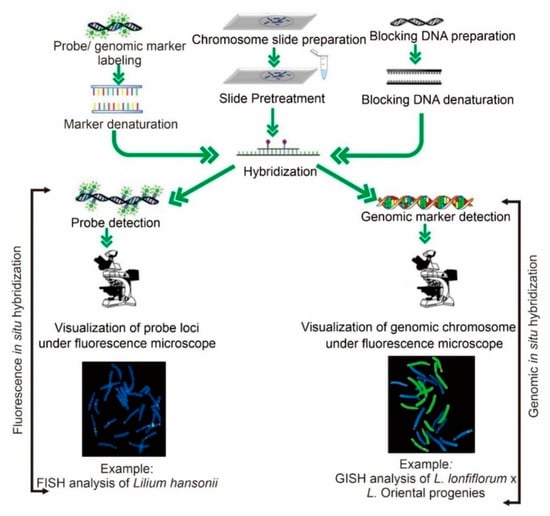
Figure 3.
Working steps for fluorescent and genomic in situ hybridization were used for the cytogenetic study of horticultural modified crops. Different methods, such as nick translation, random primed labeling, and PCR, are used to label the probe during marker labeling. Various methods, such as autoclaving, shearing the DNA with a tiny needle in a syringe, or sonicating, are used to prepare to block DNA. Chromosome slide preparation is the selection of well-spread chromosomes prepared from a young root tip using an enzyme mixture at 37 °C. Slide pretreatment is the enzymatic digestion of the chromosomes in order to unmask the DNA prior to hybridization. Hybridization involves the attachment of blocking and probe/genomic markers with chromosomes to identify the specific loci/origin of the genome of the respective chromosome. During detection, attachment of the designed antibody against the target marker along with blocking buffer to detect the specific fluorochrome.
GISH is also useful for studying cytogenetics and determining hybridity status, particularly in the case of interspecific plant hybridization [22,106,107,108,109]. Within an interspecific polyploid, GISH distinguishes the genomic structure, chromosomal constituents, crossing over, aneuploidy, and alien genes introgression. For example, in diploid interspecific Lilium (L. longiflorum × Asiatic lily; 2n = 2x = 24), 12 L. longiflorum and 12 Asiatic chromosomes can be identified using GISH (Figure 4). The number of L. longiflorum and Asiatic chromosomes are doubled in the induced tetraploids and can be visualized through GISH. Therefore, GISH is an advanced multicolor detection technique that plays a gratuitous role in the chromosomal and genomic investigation of induced polyploids.
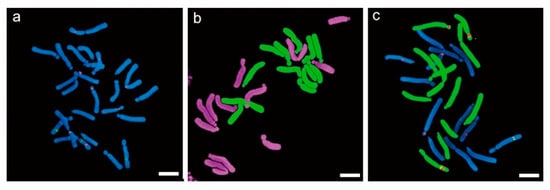
Figure 4.
In situ hybridization of diploid (2n = 2x = 24) Lilium. (a). FISH analysis of intraspecific F1 using 5S and 45S ribosomal DNA; (b). GISH analysis of interspecific (L. longiflorum × L. hansonii) F1 using genomic DNA; and (c). FISH and GISH combined analysis of interspecific (L. longiflorum × L. Oriental hybrid) F1 using 5S, 45S rDNA, and genomic DNA.
5. Effect of Polyploidization at the Morphological and Molecular Level
Polyploidization results in morphological changes in plants due to whole genome duplication, changes in chromosomal structure, nuclear enlargement along with gene dosage and epigenetic consequences, as well as an increased number of larger cells [18,110,111,112,113,114]. Further, due to the changes in different levels, several morphological traits such as plant height, root length and number, leaf number, area and size, pollen size and number, stomata number and size, and flowers and fruits number and size as summarized in Table 2.

Table 2.
Effect of polyploidization on plant morphology and yield attributes.
With the increase of ploidy level, the plant height, width and length of flower, flower size, and the number of internodes in dendrobium increased [17,126,127,128]. Polyploidization affects the floral traits such as flowering time, flower diameter, shape, size, and color, as well as different parts of flowers in kiwifruit and salvia [9,129,130,131,132,133,134,135,136,137]. These shreds of evidence suggest that polyploidy can be applied in plant breeding by targeting the flower size, shape, color, modifications in size, and the number of floral parts. Fruit size and fruits number, along with other fruit characteristics such as fruit weight, fruit peel, flesh weight and seed number are affected by the increase of ploidy number [130,138,139,140,141,142,143,144]. Due to variations in cell size and chromosome size (Figure 5), polyploidy changes the characters of the leaf [145,146,147,148,149,150,151,152,153]. Stomata number, density, size, and area are the important traits of leaves that are affected by the change of ploidy number [154,155], and this effect (Figure 5) has been observed in citrus [120].

Figure 5.
Leaf morphology and stomata size of watermelon induced by oryzalin. (a). diploid leaf, (b). tetraploid leaf, (c). stomata of diploid, and (d). stomata of a tetraploid leaf, respectively. Scale bar= 10 μm [17].
Changes in fruits, leaves, flowers, and color can be considered from the application point of view. Targeted traits can be achieved along with higher variation with the changes of ploidy level. A change in ploidy level also affects molecular and gene expression. Changing the ploidy level due to changes in nuclear DNA, chromosome number, and structure can manipulate genetic diversity, genome replication, gene expression, and heterosis [156]. Changes in ploidy level affect DNA content and the number of chromosomes [157,158,159]. During replication, polyploidization often induces epigenetic changes such as transposon simulation and chromatin modification, as well as the extension or loss of chromosomal fragments. The polyploidization effect at the plant morphology, physiology, and molecular levels needs extensive research to reveal the mechanisms that will help plant breeders for directed modification and crop improvement.
6. Effect of Polyploidization on Abiotic Stresses
6.1. Salinity Induced Stress Alleviation
H2O2 and malondialdehyde (MDA) concentrations increase in salinized tissues due to the generation of reactive oxygen species (ROS) [160,161]. Proline plays a pivotal role in alleviating salt-induced stress by maintaining cell turgor (i.e., as osmolyte) [162]. Polyploids reduce the H2O2 and MDA concentrations, increase proline concentration, and tolerate salinity stress. Higher proline concentration and lower H2O2 and MDA concentration (Table 3) in polyploid plants are reported in the studies [163,164]. Due to MDA’s lower concentration in tetraploids-maintained cell membrane integrity, and Na+ hardly reached the cells. Conversely, higher H+ transport through cells in tetraploid rice cultivars may be attributed to salt tolerance. Interestingly, Tu and coworkers [163] noticed that a defensive space between the pericycle and cortex contributes to more salt tolerance. Further, Jinag et al. [164] reported that the mortality rate of tetraploids in saline stress was 12.3–12.6% lower than that of diploid ones (Table 3). Meng et al. [165] reported that tetraploids show a stable K+/Na+ ratio (16:10 and 15:10, respectively, in roots and shoots), while K+ decreased in diploid turnips (46:100, and 48:100, respectively, in roots and shoots). Diploid turnips also experienced a significant reduction in chlorophyll content (40.3% versus 11.9% in tetraploids). Furthermore, seed germination, root, and shoot growth were enhanced in polyploid during salt-induced stress (Table 3) [163,165,166,167]. Although salinity has a more extreme effect on diploids than on their corresponding tetraploids, the underlying mechanism in tetraploid plants is unclear. Besides, no study was conducted on actual saline containing different salt solution mixtures in different concentrations. Thus, tetraploid behavior in natural conditions is difficult to predict. Table 3 shows the salinity-inducing methods, test crop, polyploidy adaptation, and effect on test crop.

Table 3.
Effect of polyploidy on abiotic stress management.
6.2. Drought Stress Alleviation
Previous ploidy-level research has suggested that enhancing ploidy can successfully alleviate or help plants better adapt to drought stress, as depicted in Table 3. Due to osmotic stress effects, cell plants usually incur damage by producing MDA and other superoxide’s that cause cell membrane disintegration. Tetraploid plants showed lower MDA concentrations for drought tolerance than diploid plants [168,171,173]. Although diploids and tetraploids experience increased ROS production due to drought stress, ROS scavenging and ROS homeostasis increased in tetraploids [171,172]. Moreover, Yang et al. [131] found more superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) in tetraploid ones during drought stress. Similarly, 30–40% more phenolic content and higher antiradical activity are found in tetraploid cultivars than in diploid cultivars, indicating ROS homeostasis and better tetraploid stress adaptation [172].
To mitigate drought stress, plants undergo stomatal leaf closure to reduce transpiration. Enhanced ABA in plants, especially in leaves, reduces leaf turgor, resulting in decreased stomatal pore aperture, which reduces the incidence of leaf water loss. Eventually, plants can conserve water within themselves. Polyploid plants show more ABA synthesis than diploids when under drought. Detailed work has been carried out by Rao et al. [169], establishing that polyploid plants show more drought stress synthesis than diploids. In addition, some ABA expresser enzymes such as 9-cis-epoxycarotenoid dioxygenase 1 (NCED1), NCED2, and gene expression ABRE binding factor 5-like (ABF5-like) were observed; such phenomena in tetraploids are responsible for increasing ABA synthesis and signaling pathways for stress adaptation. On the other hand, aquaporin genes such as MdPIP1;1 and MdTIP1;1, which are responsible for cell-to-cell water transportation, are expressed less in polyploid plants ([168] Table 3). Overall, morphological growth and chlorophyll content in tetraploids were higher than in diploids during drought stress [168,169,170,171,172] (Table 3). Nevertheless, consideration should be given to the relative fitness of different ploidy levels at different drought levels. In addition, to evaluate the efficacy of polyploidy in drought tolerance, other environmental factors associated with drought stress are also important.
6.3. Temperature Stress Alleviation
Extreme temperature escape by polyploidy has certain trade-offs. Chen et al. [177] experimented on the effects of heat stress on diploid and tetraploid Asparagus officinalis by putting both cultivars under extended stress. They placed both cultivars at 45 °C for 96 h and observed better adaptation of tetraploid plants than diploids (Table 3). The tetraploid cultivar also had higher photosynthetic pigments and lower stomatal densities than the diploid. Zhang et al. [53] worked on diploid and tetraploid Dioscorea zingiberensis and found similar results from tetraploids under temperature stress. Usually, in temperature stress, due to ROS production, plants experience decreases in the level of ascorbate (AsA) and glutathione (GSH). Polyploid Dioscorea zingiberensis showed a gradual instead of a drastic reduction in diploids in these antioxidant compounds [53]. This result is strong evidence of tetraploid heat-stress alleviation. However, Godfree et al. [174] proposed that polyploidy is not solely responsible for stress adaptation. They suggested that both polyploidy and reproductive homeostasis contribute to heat stress alleviation. They found distinct morphological differences, consistently heavier seeds, and decreased seed sizes in tetraploids than diploid, which they considered stressful reproductive production homeostatic maintenance. To understand how polyploidy affects transcriptomic responses to temperature stress, Yin et al. [178] experimented with a diploid and a tetraploid Dioscorea zingiberensis. They found “Activation Transcriptomic Reaction” in tetraploids, in which 19 bands were silenced and 47 bands were activated in diploids, 32 bands were silenced, and 28 bands were activated under temperature stress. They reported that enhanced transcriptomic responses to activation could confer tolerance in tetraploids during heat stress. Alternatively, Liu et al. [175] observed varied heat and cold stress responses from tetraploids. They calculated the LT50 (lethal time to 50% plant mortality under stress) and found that tetraploid LT50 was 2.40 times lower than that of diploids in cold conditions. Although in heat stress, diploid LT50 was 1.20 times higher than tetraploid, which suggests lower tetraploid heat stress tolerance. Such varied results and findings from various research indicate the need for further research on temperature stress. Information regarding polyploidy-induced abiotic stress alleviation is presented in Table 3.
7. Effect of Polyploidization on Plant Biotic Stresses
7.1. Polyploid-Insect Interaction
While plant polyploidy on insect abundance and dispersal is uncertain, two major events are observed in insect physiology, such as i. polyploidy caused novel insect defense (herbivores), and ii. co-opt counter-insect defense and extended host selection [178,179,180]. One example of a polyploidy-induced defense mechanism can be seen in Brassicales, in which genome duplication contributes to the development of glucosinolate compounds to establish protection against butterflies [180]. However, Edger et al. [180] also stated that, in some cases, herbivores are drawn to polyploids through coevolutionary mechanisms. Polyploidy shows different effects on herbivory, often divided into attraction and escape. Concerning attraction, Arvanitis et al.’s [178] findings provide adequate evidence. In a common garden where the corresponding tetraploid and octoploid Cardamine pratensis were grown, bud gall midge Dasineura cardamine preferred octoploid over tetraploid where tetraploid cardamines rarely struck. Does higher polyploidy attract insects? Herbivores are typically fond of polyploids. Their argument is backed by Thompson et al. [181], who recorded higher infestations of prodoxy moth Greya politella in Heuchera grossulariifolia tetraploids compared to its diploid ones. Similarly, in tetraploid Arnica cordifolia [182], tephritid fly Campiglossa footeorum displayed higher attack rates than triploid cultivars.
On the other hand, Nuismer and Thompson [183] recorded frequent attacks by a stem borer moth Greya piperella in Heuchera grossulariifolia diploids rather than in tetraploids. Likewise, diploid Gymnadenia conopsea orchids [184] were more frequently attacked by aphids than their corresponding tetraploids. These contrasting findings indicate that polyploidy does not inhibit the behavior of herbivores. However, it has been suggested that insect herbivory is not a cytotype-dependent habitat selection but plays a key role in host-seeking. In open fields and natural ecosystems, insects typically prefer the most common host in that habitat. Alternatively, in a typical garden (Figure 6) where all cytotypes are grown, insects forage the hosts equally [185]. Although the results show that polyploidy has various implications concerning insect attacks, it can provide some trade-offs. Generally, polyploids produce higher growth and reproductive ability. Therefore, polyploids can help avoid economic injury and an herbivory-induced economic threshold, which can be difficult in diploids.
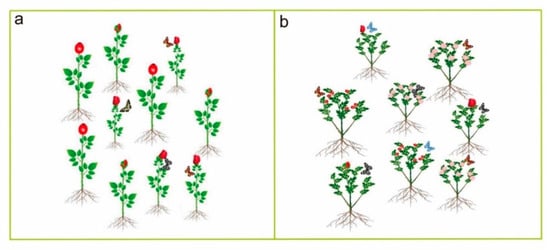
Figure 6.
Can insects identify different ploidy level plants? According to Segraves and Anneberg [185], insects forage more on predominant cytotypes in a natural habitat where different ploidy plants coexist ((a); where we imagine that small plants are predominant here, insect will forage more on small plants rather than flowers of bigger plants, i.e., irrespective of ploidy level). Contrarily, insects forage equally in a common garden where mixed cytotypes are grown ((b); imagine there are different ploidy levels flower in the common garden. Insects generally fail to detect different ploidy levels; thus, they forage equally in a common garden).
7.2. Polyploidy and Pathogen Resistance
Pathogens, including fungi, bacteria, and viruses, are the most daunting factor for crop cultivation worldwide. The grower must account for high economic losses incurred by yield loss and the application of pesticides to combat diseases [186]. Developing pathogen-resistant crop varieties through selective breeding is crucial to address this problem. The induction of polyploidy may be a promising solution [149]. Naturally occurring varieties of different crop species, including banana, strawberry, and watermelon, have been reported to resist a broad range of pathogens [186,187]. Allotriploid cultivars of banana (AAB) and polyploid watermelon germplasm are fusarium-resistant [187]. Many cultivars of octoploid Fragaria × ananassa Duchesne are resistant to anthracnose, fusarium wilt, crown rot, red core, verticillium wilt, and angular leaf spot [186]. Polyploid strawberries (US4808 and US4809) reported resistance to four Xanthomonas fragariae groups [186].
Several studies have reported that pathogen resistance in the polyploid genotype is higher than its diploid generation [187,188]). Autotetraploid and autotriploid watermelon demonstrated higher fusarium resistance than diploid watermelon [187]. Diploid apple cultivars are more susceptible to Alternaria alternata, and Colletotrichum gloeosporioides than autotetraploid apple cultivars Hanfu and Gala [188]. Allopolyploid tobacco prevents plant viruses better than diploid tobacco [189]. Tetraploid wheat avoids more powdery mildew and leaf rust than diploid wheat [190,191]. Allelic diversity, gene expression (over), and physiological state are the key factors determining a host plant’s ability to withstand various pathogens.
Polyploidy may influence plant species’ allelic diversity, gene expression (over), and physiological condition (Figure 7). The extra alleles at a given locus in polyploids increase allelic diversity due to the high probability of heterozygosity and enhance resistance. Multiple polyploid chromosome sets increase gene expression [192,193]. In contrast, it has been proposed that gene expression is downregulated (in certain loci or in the whole genome and sometimes even silenced) with increased ploidy level [10]. Polyploid plants can adapt to a wide range of environmental conditions by developing stress tolerance [194]. Invasive plant species are more tolerant of diseases than individuals suffering from environmental stressors [195]. Therefore, the polyploid with higher allelic diversity at resistance genes, higher expression levels of immune genes should select for cultivar development. The positive relationship between the disease resistance of the plant and environmental stressors also needs to be considered.
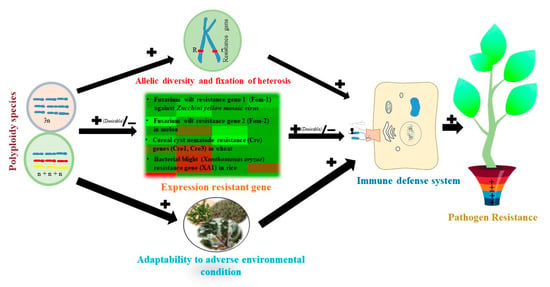
Figure 7.
Effect of polyploidy on pathogen resistance. The sign next to the arrow gives the direction of the effect. The sign “+’’ means the higher ploidy level increases the probability of effects, while “−” means the higher ploidy level decreases the probability of effects. Pot represents the combined effect of the polyploidy plant. The gene-for-gene model is the general mechanism of pathogen resistance. In the polyploidy host, high allelic diversity with dominant allele, fixed heterosis, and high expression (desirable) of resistance gene directly influence the pathogen resistance. The validated disease resistance genes and their target pathogens are given as examples [54]. The effects of ploidy-level variation on host adaptability under diverse environmental conditions (biotic and abiotic stress) could indirectly influence parasite resistance.
8. Challenges of Polyploidization
8.1. Changes in Cellular Architecture
Due to an increase in an organism’s genomic content, cell volume usually increases. It has the consequent change in the relationship between the cell’s tridimensional and bidimensional components [196,197]. Doubling the genome is expected to double the amount of chromatin, but only causes a 1.6-fold increase in the nuclear envelope surface. Cell size expansion can contribute to anatomical imbalances and deleterious effects, such as gene redundancy shields polyploids from the mutations’ prejudicial effect, infertility, brittle wood, and watery fruits [198]. Moreover, polyploidization can cause albinism [199].
8.2. Mitotic and Meiotic Abnormalities
Normal mitosis and meiosis are frequently disrupted in polyploids. Due to additional sets of chromosomes present in an induced polyploid, faces various challenges during mitotic chromosome segregation. As we observed that the homozygosity or heterozygosity level significantly differs in polyploids. It depends on the formation pathway like autopolyploid or allopolyploid that affect the performance of the polyploids in fertility, growth and even yield [200,201]. Autotetraploid yeast demonstrates increased mitotic loss of chromosomes, producing aneuploidy cells [202]. Spindle abnormalities usually cause difficulties in mitosis. Chaotic mitotic chromosomal segregation also occurs in wild yeast [203]. However, there is little knowledge regarding the mitotic stability of polyploid plant cells. Meiosis requires three or more chromosome sets in which the frequency and manner of development of aneuploidy depend on the type of polyploidy. Triploidy and aneuploidy, which may arise from meiotically unpaired DNA, are more unstable states than tetraploidy. These frequently lead to or result from the more stable polyploidy states. Both conditions may have potentially detrimental effects on genome regulation [52]. Several experiments resulted in 30–40% aneuploidy of autotetraploid maize [204,205]. Another problem occurs in triploids and pentaploids; trivalent cannot be solved into balanced products in triploids, and a spontaneous division of multiple forms of chromosomes produces mainly aneuploid gametes. In addition, normal chromosomal segregation is another challenge in auto and allopolyploid where the multivalent complex structure is often associated. Our study found that the multivalent has an important role in abnormal chromosomal segregation reducing fertility [200,206].
8.3. Epigenetic Instability
Aneuploidy can cause epigenetic and genomic instability [207,208]. In autopolyploids, instability can directly or indirectly contribute to genome duplication. The proof was demonstrated when diploid and tetraploid Arabidopsis thaliana compared epigenetic effects at a transgenic locus [158,209,210]. Epigenetic changes in the gene have also been found in allopolyploids. Theoretically, A. thaliana has also demonstrated regulatory improvements in autopolyploid strains in parents. Such changes involved silencing or activating genes, including activating a Spa-CACTA family DNA transposon [201,210]. Therefore, mismatches in gene expression and gene product regulatory controls can decrease fitness.
9. Conclusions and Future Perspective
In plant breeding, polyploidization is a successful technique for developing novel traits. Polyploidy causes significant transcriptomic and regulatory changes that bring physiological and morphological changes. Disrupted regulatory factor stoichiometries, small RNAs, and other genome interactions could potentially set these in motion, cascading through entire networks of transformed regulatory modules from single-gene expression modification. Plants with duplicate whole chromosome sets have more distinctive features, such as a different phytochemical profile, higher content of desired pharmaceutical molecules, plant shape, flower color, size and style, fragrance, vase life, and extended flowering time. However, they do not always act in the same way. Furthermore, polyploid clones of Eucalyptus grandis, E. urophylla recently produced a fiber with higher length and thickness, resulting in improved paper formation and strength, suggesting that polyploids could be used in pulp and paper production. Stable C35 citrange tetraploids are becoming popular in high-density orchards.
The latest polyploidization trend involves polyploid characterization in their protocol regarding polyploid ultrastructure, bioactive compounds, photosynthetic capabilities, and metabolomics studies. Despite progress, we still lack a thorough understanding of polyploidization. Conflicting results have been reported for different polyploid species, and a single hypothesis cannot be proposed to explain plant polyploid evolution. Nevertheless, advances in sequencing technology, improved experimental analysis, multi-omics data quality, and more efficient analytical methods are likely to enhance our understanding of polyploidization in the near future significantly. In abiotic and biotic stress management cases, revealing the underlying mechanism is the most important research prospect of polyploidy. Polyploidy-based breeding combines the advantages of heterosis and apomixes, which can be a viable option for crop improvement in the future. A molecular approach to understanding the effects of polyploid plants on insects is necessary concerning polyploid-insect interaction.
Author Contributions
M.M.I., D.M.D. designed and organized the ideas. S.O.N., A.B.S. (Abu Bakar Siddique 1), A.B.S. (Abu Bakar Siddique 2), and O.H. worked on the acquisition of data, analysis, and interpretation. N.C.P. gave his valuable time for critical revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Research and Development Department (R & D), Quality Feeds Limited, Dhaka, Bangladesh, for their technical help to complete this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Adams, K.L.; Wendel, J.F. Exploring the genomic mysteries of polyploidy in cotton. Biol. J. Linn. Soc. 2004, 82, 573–581. [Google Scholar] [CrossRef]
- Lee, J.; Grant, D.; Vallejos, C.E.; Shoemaker, R. Genome organization in dicots. II. Arabidopsis as a ‘bridging species’ to resolve genome evolution events among legumes. Theor. Appl. Genet. 2001, 103, 765–773. [Google Scholar] [CrossRef]
- Udall, J.A.; Wendel, J.F. Polyploidy and crop improvement. Crop Sci. 2006, 46, S-3–S-14. [Google Scholar] [CrossRef]
- Henry, I.M.; Dilkes, B.P.; Miller, E.S.; Burkart-Waco, D.; Comai, L. Phenotypic consequences of aneuploidy in Arabidopsis thaliana. Genetics 2010, 186, 1231–1245. [Google Scholar] [CrossRef]
- Pavlíková, Z.; Paštová, L.; Münzbergová, Z. Synthetic polyploids in Vicia cracca: Methodology, effects on plant performance and aneuploidy. Plant Syst. Evol. 2017, 303, 827–839. [Google Scholar] [CrossRef]
- Shoemaker, R.; Polzin, K.; Labate, J.; Specht, J.; Brummer, E.; Olson, T.; Young, N.; Concibido, V.; Wilcox, J.; Tamulonis, J.P.; et al. Genome duplication in soybean (Glycine subgenus soja). Genetics 1996, 144, 329–338. [Google Scholar] [CrossRef]
- Chai, J.; Su, Y.; Huang, F.; Liu, S.; Tao, M.; Murphy, R.W. The gap in research on polyploidization between plants and vertebrates: Model systems and strategic challenges. Sci. Bull. 2015, 60, 1471–1478. [Google Scholar] [CrossRef]
- Islam, M.M.; Yesmin, R.; Jung, M.J.; Kim, H.Y.; Kim, C.K.; Lim, K.B. Investigation of the morphological and cytogenetic variations of an intraspecific Asiatic lily hybrid using 5S and 18S rDNA probes. Hortic. Environ. Biotechnol. 2020, 61, 339–346. [Google Scholar] [CrossRef]
- Nei, M.; Nozawa, M. Roles of mutation and selection in speciation: From Hugo de Vries to the modern genomic era. Genome Biol. Evol. 2011, 3, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Davis, D.; Birchler, J.A. Dosage effects on gene expression in a maize ploidy series. Genetics 1996, 142, 1349–1355. [Google Scholar] [CrossRef]
- Paterson, A.H. Polyploidy, evolutionary opportunity, and crop adaptation. Genetica 2005, 123, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Stuessy, T.; Weiss-Schneeweiss, H. What drives polyploidization in plants? New Phytol. 2019, 223, 1690–1692. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Haerum, B.K.; Clark, A.G. Evolutionary changes in cis and trans gene regulation. Nature 2004, 430, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Wagler, T.N.; Quijada, P.; Doebley, J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat. Genet. 2006, 38, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi, J.; Turcotte, E.; Kohel, R. Genetics, cytology, and evolution of Gossypium. Adv. Genet. 1985, 23, 271–375. [Google Scholar]
- Wendel, J.F.; Cronn, R.C. Polyploidy and the evolutionary history of cotton. Adv. Agron. 2003, 78, 139–186. [Google Scholar]
- Bae, S.J.; Islam, M.M.; Kim, H.Y.; Lim, K.B. Induction of Tetraploidy in Watermelon with Oryzalin Treatments. Hortic. Sci. Technol. 2020, 38, 385–393. [Google Scholar]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.B.; Miller, C.; Ramanna, M.; van Tuyl, J. Nitrous oxide N2O incudes 2n gametes in sterile F1 hybrids of Oriental × Asiatic lilies (Lilium) and leads to intergenomic recombination. Euphytica 2006, 148, 303–309. [Google Scholar] [CrossRef]
- Jo, Y.K.; Mazharul, I.M.; Kim, C.K.; Kim, H.Y.; Lim, K.B. Morphological Characteristics and FISH Analysis of Hibiscus F1 Hybrids and Parental Lines. Hortic. Sci. Technol. 2019, 37, 630–639. [Google Scholar]
- Lim, K.B.; Barba-Gonzalez, R.; Zhou, S.; Ramanna, M.; Van Tuyl, J.M. Interspecific hybridization in lily (Lilium): Taxonomic and commercial aspects of using species hybrids in breeding. Floric. Ornam. Plant Biotechnol. 2008, 5, 138–145. [Google Scholar]
- Mazharul, I.; Reshma, Y.; Jung, J.; Mohammad, D.; Lim, K. Cytogenetic assessment of Lilium longiflorum × L. hansonii revealed by genomic in situ hybridization (GISH). Acta Hortic. 2019, 1237, 79–86. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Arens, P. Lilium: Breeding history of the modern cultivar assortment. Acta Hortic. 2010, 900, 223–230. [Google Scholar] [CrossRef]
- Ahmad, P.; Sharma, S. Salt stress and phyto-biochemical responses of plants. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar]
- Castillo, E.; To Phuc, T.; Ismail, A.M.; Inubushi, K. Response to salinity in rice: Comparative effects of osmotic and ionic stresses. Plant Prod. Sci. 2007, 10, 159–170. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Raja, S.; Ravikrishna, R.; Kommalapati, R.; Valsaraj, K. Monitoring of fogwater chemistry in the gulf coast urban industrial corridor: Baton Rouge (Louisiana). Environ. Monit. Assess. 2005, 110, 99–120. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Asanuma, K.I.; Kusutani, A.; Toyota, T.M. Leaf gas exchange properties of potato under different temperature and soil moisture at different growth stages. Environ. Control. Biol. 2000, 38, 229–239. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Vollenweider, P.; Günthardt-Goergv, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137, 455–465. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G. Climate-driven change in plant–insect interactions along elevation gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Tobin, P.C.; Nagarkatt, S.; Loeb, G.; Saunders, M.C. Historical and projected interactions between climate change and insect voltinism in a multivoltine species. Glob. Chang. Biol. 2008, 14, 951–957. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Crow, J.F. Hitoshi Kihara, Japan’s pioneer geneticist. Genetics 1994, 137, 891. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef]
- Forrester, N.J.; Rebolleda-Gómez, M.; Sachs, J.L.; Ashman, T.L. Polyploid plants obtain greater fitness benefits from a nutrient acquisition mutualism. New Phytol. 2020, 227, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Katepa-Mupondwa, F.M.; Christie, B.R.; Michaels, T.E. An improved breeding strategy for autotetraploid alfalfa (Medicago sativa L.). Euphytica 2002, 123, 139–146. [Google Scholar] [CrossRef]
- Barton, N.H. The role of hybridization in evolution. Mol. Ecol. 2008, 10, 551–568. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Plant Genetics and Breeding; Wiley-Blackwell: Malden, UK, 2007. [Google Scholar]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, Y.X. Plant polyploidy and evolution. J. Integr. Plant Biol. 2019, 61, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Salman Minkov, A.; Sabath, N.; Mayrose, I. Whole-genome duplication as a key factor in crop domestication. Nat. Plants 2016, 2, 16115. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyl, J.; Lim, K.B.; Ramanna, M. Interspecific hybridization and introgression. In Breeding for Ornamentals: Classical and Molecular Approaches; Vainstain, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 85–103. [Google Scholar]
- Gaul, H. Present aspects of induced mutations in plant breeding. Euphytica 1958, 7, 275–289. [Google Scholar] [CrossRef]
- Winterfeld, G.; Ley, A.; Hoffmann, M.H.; Paule, J.; Röser, M.J. Dysploidy and polyploidy trigger strong variation of chromosome numbers in the prayer-plant family (Marantaceae). Plant Syst. Evol. 2020, 306, 36. [Google Scholar] [CrossRef]
- Simioni, C.; Schifino Wittmann, M.T.; Dall’Agnol, M. Sexual polyploidization in red clover. Sci. Agric. 2006, 63, 26–31. [Google Scholar] [CrossRef][Green Version]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X.; Cheng, F. Plant Polyploidy: Origin, Evolution, and Its Influence on Crop Domestication. Hortic. Plant J. 2019, 5, 231–239. [Google Scholar] [CrossRef]
- Otto, S.P.; Whitton, J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000, 34, 401–437. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosomes in plants: By treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Chauvin, J.E.; Souchet, C.; Dantec, J.; Ellissèche, D. Chromosome doubling of 2x Solanum species by oryzalin: Method development and comparison with spontaneous chromosome doubling in vitro. Plant Cell Tissue Organ Cult. 2003, 73, 65–73. [Google Scholar] [CrossRef]
- Song, P.; Kang, W.; Peffley, E.B. Chromosome doubling of Allium fistulosum × A. cepa interspecific F1 hybrids through colchicine treatment of regenerating callus. Euphytica 1997, 93, 257–262. [Google Scholar] [CrossRef]
- Omran, S.; Guerra Sanz, J.; Cardenas, J.G. Methodology of tetraploid induction and expression of microsatellite alleles in triploid watermelon. In Proceedings of the IXth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008; pp. 381–384. [Google Scholar]
- Awoleye, F.; Van Duren, M.; Dolezel, J.; Novak, F. Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 1994, 76, 195–202. [Google Scholar] [CrossRef]
- Dunn, B.L.; Lindstrom, J.T. Oryzalin-induced chromosome doubling in Buddleja to facilitate interspecific hybridization. HortScience 2007, 42, 1326–1328. [Google Scholar] [CrossRef]
- Henny, R.J.; Holm, J.R.; Chen, J.; Scheiber, M. In vitro induction of tetraploids in Dieffenbachia × ‘Star Bright M-1′ by colchicine. HortScience 2009, 44, 646–650. [Google Scholar] [CrossRef]
- Teng, E.; Leonhardt, K. In vitro and in vivo polyploidization of Dracaena with oryzalin. Acta Hortic 2007, 813, 509–516. [Google Scholar] [CrossRef]
- Meyer, E.M.; Touchell, D.H.; Ranney, T.G. In vitro shoot regeneration and polyploid induction from leaves of Hypericum species. HortScience 2009, 44, 1957–1961. [Google Scholar] [CrossRef]
- Reshma, Y.; Mazharul, I.M.; Kim, H.Y.; Kim, C.K.; Lim, K.B. Role of Growth Regulators in the Somatic Organogenesis of Haworthia Inflorescences in Vitro. Hortic. Sci. Technol. 2020, 38, 394–404. [Google Scholar]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Kermani, M.; Sarasan, V.; Roberts, A.; Yokoya, K.; Wentworth, J.; Sieber, V. Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor. Appl. Genet. 2003, 107, 1195–1200. [Google Scholar] [CrossRef]
- Allum, J.; Bringloe, D.; Roberts, A. Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: The effects of node length, oryzalin concentration and exposure time. Plant Cell Rep. 2007, 26, 1977–1984. [Google Scholar] [CrossRef]
- Rose, J.; Kubba, J.; Tobutt, K. Induction of tetraploidy in Buddleia globosa. Plant Cell Tissue Organ Cult. 2000, 63, 121–125. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Ureshino, K.; Miyajima, I.; Ozaki, Y.; Okubo, H. Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell Tissue Organ Cult. 2003, 72, 19–25. [Google Scholar] [CrossRef]
- Lu, C.; Bridgen, M.P. Chromosome doubling and fertility study of Alstroemeria aurea × A. caryophyllaea. Euphytica 1997, 94, 75–81. [Google Scholar] [CrossRef]
- Xavier de Mello e Silva, P.A.K.; Callegari-Jacques, S.; Bodanese-Zanettini, M.H. Induction and identification of polyploids in Cattleya intermedia Lindl. (Orchidaceae) by in vitro techniques. Cienc. Rural 2000, 30, 105–111. [Google Scholar] [CrossRef]
- Takamura, T.; Lim, K.B.; Van Tuyl, J. Effect of a new compound on the mitotic polyploidization of Lilium longiflorum and Oriental hybrid lilies. Acta Hortic. 2001, 572, 37–42. [Google Scholar] [CrossRef]
- Takamura, T. Cyclamen. Flower Breed. Genet. 2007, 2007, 459–478. [Google Scholar]
- Chauvin, J.; Label, A.; Kermarrec, M. In vitro chromosome-doubling in tulip (Tulipa gesneriana L.). J. Hortic. Sci. Biotechnol 2005, 80, 693–698. [Google Scholar] [CrossRef]
- Ascough, G.D.; Van Staden, J.; Erwin, J.E. Effectiveness of colchicine and oryzalin at inducing polyploidy in Watsonia lepida NE Brown. HortScience 2008, 43, 2248–2251. [Google Scholar] [CrossRef]
- Cohen, D.; Yao, J.L. In vitro chromosome doubling of nine Zantedeschia cultivars. Plant Cell Tissue Organ Cult. 1996, 47, 43–49. [Google Scholar] [CrossRef]
- Chen, L.L.; Gao, S.L. In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci. Hortic 2007, 112, 339–344. [Google Scholar] [CrossRef]
- de Carvalho, J.F.R.P.; de Carvalho, C.R.d.P.; Otoni, W.C. In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Organ Cult. 2005, 80, 69–75. [Google Scholar] [CrossRef]
- Rubuluza, T.; Nikolova, R.; Smith, M.; Hannweg, K. In vitro induction of tetraploids in Colophospermum mopane by colchicine. S. Afr. J. Bot. 2007, 73, 259–261. [Google Scholar] [CrossRef]
- Roy, A.; Leggett, G.; Koutoulis, A. In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep. 2001, 20, 489–495. [Google Scholar] [CrossRef]
- Adaniya, S.; Shirai, D. In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci. Hortic. 2001, 88, 277–287. [Google Scholar] [CrossRef]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J. Polyploidy and interspecific hybridization: Partners for adaptation, speciation and evolution in plants. Ann. Bot 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.R. Some applications and misapplications of induced polyploidy to plant breeding. Polyploidy 1980, 13, 445–470. [Google Scholar]
- Van Tuyl, J.M.; Lim, K.B. Interspecific hybridisation and polyploidisation as tools in ornamental plant breeding. Acta Hortic. 2003, 612, 13–22. [Google Scholar] [CrossRef]
- Kamstra, S.A.; De Jeu, M.J.; Kuipers, A.G.; Jacobsen, E. Homoeologous chromosome pairing in the distant hybrid Alstroemeria aurea × A. inodora and the genome composition of its backcross derivatives determined by fluorescence in situ hybridization with species-specific probes. Heredity 1999, 82, 69–78. [Google Scholar] [CrossRef]
- Lim, K.B.; Wennekes, J.; Jong, J.H.; Jacobsen, E.; Van Tuyl, J.M. Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 2001, 44, 911–918. [Google Scholar] [CrossRef]
- Takahashi, C.; Leitch, I.; Ryan, A.; Bennett, M.; Brandham, P. The use of genomic in situ hybridization (GISH) to show transmission of recombinant chromosomes by a partially fertile bigeneric hybrid, Gasteria lutzii × Aloe aristata (Aloaceae), to its progeny. Chromosoma 1997, 105, 342–348. [Google Scholar]
- Veilleux, R. Diploid and polyploid gametes in crop plants: Mechanisms of formation and utilization in plant breeding. Plant Breed Rev. 1985, 3, 253–288. [Google Scholar]
- Brownfield, L.; Köhler, C. Unreduced gamete formation in plants: Mechanisms and prospects. J. Exp. Bot 2011, 62, 1659–1668. [Google Scholar] [CrossRef]
- Bretagnolle, F.A.; Thompson, J.D. Gametes with the somatic chromosome number: Mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995, 129, 1–22. [Google Scholar] [CrossRef]
- Lim, K.B.; Barba Gonzalez, R.; Zhou, S.; Ramanna, M.; Van Tuyl, J.M. Meiotic polyploidization with homoeologous recombination induced by caffeine treatment in interspecific lily hybrids. Korean J. Genet. 2005, 27, 219. [Google Scholar]
- Mohammad, D.D.; Mazharul, I.M.; Ann, T.C.; Kim, H.Y.; Lim, K.B. Phenotypic Characteristics and Karyotype Analysis of Hibiscus sabdariffa var. sabdariffa by Fluorescence in Situ Hybridization (FISH). Hortic. Sci. Technol. 2020, 38, 695–704. [Google Scholar]
- Duque, R.E.; Phan, S.; Hudson, J.L.; Till, G.; Ward, P. Functional defects in phagocytic cells following thermal injury. Application of flow cytometric analysis. Am. J. Pathol 1985, 118, 116. [Google Scholar] [PubMed]
- Sabharwal, P.; Doležel, J. Interspecific hybridization in Brassica: Application of flow cytometry for analysis of ploidy and genome composition in hybrid plants. Biol. Plant 1993, 35, 169–177. [Google Scholar] [CrossRef]
- Suda, J.; Krahulcová, A.; Trávníek, P.; Krahulec, F. Ploidy level versus DNA ploidy level: An appeal for consistent terminology. Taxon 2006, 55, 447–450. [Google Scholar] [CrossRef]
- He, Y.; Sun, Y.; Zheng, R.; Ai, Y.; Cao, Z.; Bao, M. Induction of tetraploid male sterile Tagetes erecta by colchicine treatment and its application for interspecific hybridization. Hortic. Plant J. 2016, 2, 284–292. [Google Scholar]
- Younis, A.; Ramzan, F.; Hwang, Y.J.; Lim, K.B. FISH and GISH: Molecular cytogenetic tools and their applications in ornamental plants. Plant Cell Rep. 2015, 34, 1477–1488. [Google Scholar] [CrossRef]
- Andres, R.J.; Kuraparthy, V. Development of an improved method of mitotic metaphase chromosome preparation compatible for fluorescence in situ hybridization in cotton. J. Cotton Sci. 2013, 17, 149–156. [Google Scholar]
- Lim, K.B.; De Jong, H.; Yang, T.J.; Park, J.Y.; Kwon, S.J.; Kim, J.S. Characterization of rDNAs and tandem repeats in the heterochromatin of Brassica rapa. Mol. Cells 2005, 19, 436–444. [Google Scholar]
- Gan, Y.; Liu, F.; Chen, D.; Wu, Q.; Qin, Q.; Wang, C. Chromosomal Locations of 5S and 45S rDNA in Gossypium genus and its phylogenetic implications revealed by FISH. PLoS ONE 2013, 8, e68207. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Chen, Q.; Zhang, L.; Tang, H.; Luo, Y. Phylogenetic insight into subgenera Idaeobatus and Malachobatus (Rubus, Rosaceae) inferring from ISH analysis. Mol. Cytogenet. 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Budylin, M.; Kan, L.Y.; Romanov, V.; Khrustaleva, L. GISH study of advanced generation of the interspecific hybrids between Allium cepa L. and Allium fistulosum L. with relative resistance to downy mildew. Russ. J. Genet. 2014, 50, 387–394. [Google Scholar] [CrossRef]
- Książczyk, T.; Taciak, M.; Zwierzykowski, Z. Variability of ribosomal DNA sites in Festuca pratensis, Lolium perenne, and their intergeneric hybrids, revealed by FISH and GISH. J. Appl. Genet. 2010, 51, 449–460. [Google Scholar] [CrossRef]
- Kwon, M.J.; Ramzan, F.; Ahn, Y.J.; Hwang, Y.J.; Kang, Y.I.; Kim, C.K. Chromosomal analysis of Lilium longiflorum × Asiatic hybrids using GISH (genomic in situ hybridization). Hortic. Environ. Biotechnol. 2017, 58, 591–600. [Google Scholar] [CrossRef]
- Ramzan, F.; Younis, A.; Lim, K.B. Application of genomic in situ hybridization in horticultural science. Int. J. Genom. 2017, 129, 7561909. [Google Scholar] [CrossRef] [PubMed]
- Allario, T.; Brumos, J.; Colmenero-flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sun, H.; Li, L.; Bell, R.L. In vitro colchicine-induced polyploid plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar, ‘Fertility’. J. Hortic. Sci. Biotechnol. 2009, 84, 548–552. [Google Scholar] [CrossRef]
- Levin, D.A. The role of chromosomal change in plant evolution. Syst. Bot. 2004, 29, 460–461. [Google Scholar]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N. Induction and identification of colchicine induced polyploidy in Gladiolus grandiflorus ‘White Prosperity’. Folia Hortic. 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Vichiato, M.R.; Vichiato, M.; Pasqual, M.; Rodrigues, F.A.; Castro, D.M. Morphological effects of induced polyploidy in Dendrobium nobile Lindl.(Orchidaceae). Crop Breed. Appl. Biotechnol. 2014, 14, 154–159. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S. Better salinity tolerance in tetraploid vs diploivolkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Ari, E.; Djapo, H.; Mutlu, N.; Gurbuz, E.; Karaguzel, O. Creation of variation through gamma irradiation and polyploidization in Vitex agnus-castus L. Sci. Hortic 2015, 195, 74–81. [Google Scholar] [CrossRef]
- Tan, F.Q.; Tu, H.; Liang, W.J.; Long, J.M.; Wu, X.M.; Zhang, H.Y. Comparative metabolic and transcriptional analysis of a doubled diploid and its diploid citrus rootstock (C. junos cv. Ziyang xiangcheng) suggests its potential value for stress resistance improvement. BMC Plant Biol. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Amah, D.; Van Biljon, A.; Maziya-Dixon, B.; Labuschagne, M.T.; Swennen, R. Effects of in vitro polyploidization on agronomic characteristics and fruit carotenoid content; implications for banana genetic improvement. Front. Plant Sci. 2019, 10, 1450. [Google Scholar] [CrossRef]
- Prabhukumar, K.; Thomas, V.; Sabu, M.; Prasanth, M.; Mohanan, K. Induced mutation in ornamental gingers (Zingiberaceae) using chemical mutagens viz. colchicine, acridine and ethyl methane sulphonate. J. Hortic. For. Biotechnol. 2015, 19, 18–27. [Google Scholar]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Van Laere, K.; França, S.C.; Vansteenkiste, H.; Van Huylenbroeck, J.; Steppe, K.; Van Labeke, M.C. Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol. Plant. 2011, 33, 1149–1156. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, X. Cytological characteristics of numerically unreduced pollen production in Populus tomentosa Carr. Euphytica 2010, 173, 151–159. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Guo, X.; Xu, L.; Lei, P.; Luo, Q.; Liu, J.; Li, W.; Tao, L.; Meng, F. The lectin gene TRpL1 of tetraploid Robinia pseudoacacia L. response to salt stress. J. For. Res. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yamashita, S.; Ohta, K.; Hosoki, T. Morphological characteristics and their inheritance in colchicine-induced Salvia polyploids. J. Jpn. Soc. Hortic. Sci. 2008, 77, 186–191. [Google Scholar] [CrossRef]
- Ranney, T.G. Polyploidy: From evolution to new plant development. Comb. Proc. Int. Plant Propagators’ Soc. 2006, 2006, 137–142. [Google Scholar]
- Faria, R.T.D.; Takahashi, L.S.; Lone, A.B. UEL 6: Nova cultivar de Dendrobium. Hortic. Bras. 2009, 27, 114–115. [Google Scholar] [CrossRef][Green Version]
- Schepper, S.D.; Leus, L.; Mertens, M.; Debergh, P.; Bockstaele, E.V.; Loose, M.D. Somatic polyploidy and its consequences for flower coloration and flower morphology in azalea. Plant Cell Rep. 2001, 20, 583–590. [Google Scholar] [CrossRef]
- Obute, G.C.; Ndukwu, B.; Chukwu, O.F. Targeted mutagenesis in Vigna unguiculata (L.) Walp. and Cucumeropsis mannii (NAUD) in Nigeria. Afr. J. Biotechnol. 2007, 6, 2467–2472. [Google Scholar] [CrossRef]
- Wu, J.H.; Ferguson, A.R.; Murray, B.G.; Jia, Y.; Datson, P.M.; Zhang, J. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Ann. Bot. 2012, 109, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Samatadze, T.E.; Yurkevich, O.Y.; Khazieva, F.M.; Basalaeva, I.V.; Konyaeva, E.A.; Burova, A.E.; Zoshchuk, S.A.; Morozov, A.I.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological and Cytogenetic Characterization of Colchicine-Induced Tetraploid Plants of Polemonium caeruleum L. (Polemoniaceae). Plants 2022, 11, 2585. [Google Scholar] [CrossRef]
- Khaing, T.; Perera, A.; Sumanasinghe, V.; Wijesundara, D. Improvement of Gymnostachyum species by induced mutation. Trop. Agric. Res. 2007, 19, 265–272. [Google Scholar]
- Majdi, M.; Karimzadeh, G.; Malboobi, M.A.; Omidbaigi, R.; Mirzaghaderi, G. Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. Hort. Sci. 2010, 45, 16–21. [Google Scholar] [CrossRef]
- Serrano-Fuentes, M.K.; Gómez-Merino, F.C.; Cruz-Izquierdo, S.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Gamma Radiation (60Co) Induces Mutation during In Vitro Multiplication of Vanilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae 2022, 8, 503. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Nalousi, A.M. Artificial polyploidy induction for improvement of ornamental and medicinal plants. Plant Cell Tissue Organ Cult. 2020, 107, 447–469. [Google Scholar] [CrossRef]
- Rathod, A.; Patil, S.; Taksande, P.; Karad, G.; Kalamkar, V.; Jayade, V. Effect of colchicine on morphological and biometrical traits in African marigold. J. Soils Crops 2018, 28, 72–80. [Google Scholar]
- Jones, K.D.; Reed, S.M.; Rinehart, T.A. Analysis of ploidy level and its effects on guard cell length, pollen diameter, and fertility in Hydrangea macrophylla. HortScience 2007, 42, 483–488. [Google Scholar] [CrossRef]
- Alexander, L. Ploidy level influences pollen tube growth and seed viability in interploidy crosses of Hydrangea macrophylla. Front. Plant Sci. 2020, 11, 100. [Google Scholar] [CrossRef]
- Cheniclet, C.; Rong, W.Y.; Causse, M.; Frangne, N.; Bolling, L.; Carde, J.P. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 2005, 139, 1984–1994. [Google Scholar] [CrossRef]
- Cohen, H.; Fait, A.; Tel Zur, N. Morphological, cytological and metabolic consequences of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biol. 2013, 13, 173. [Google Scholar] [CrossRef]
- Jokari, S.; Shekafandeh, A.; Jowkar, A. In vitro tetraploidy induction in Mexican lime and sour orange and evaluation of their morphological and physiological characteristics. Plant Cell Tiss Org. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Hassan, J.; Miyajima, I.; Ozaki, Y.; Mizunoe, Y.; Sakai, K.; Zaland, W.J.P. Tetraploid induction by colchicine treatment and crossing with a diploid reveals less-seeded fruit production in Pointed Gourd (Trichosanthes dioica Roxb). Plants 2020, 9, 370. [Google Scholar] [CrossRef]
- Zlesak, D.C.; Thill, C.A.; Anderson, N.O. Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 2005, 141, 281–290. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Xu, S.; Deng, Z. Induction, regeneration and characterization of tetraploids and variants in Tapestry caladium. Plant Cell Tissue Organ Cult. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Cornish, K. Colchicine induced polyploidy has the potential to improve rubber yield in Taraxacum koksaghyz. Ind. Crops Prod. 2018, 112, 75–81. [Google Scholar] [CrossRef]
- Pan-pan, H.; Wei Xu, L.; Hui Hui, L.; Xu, X.Z. In vitro induction and identification of autotetraploid of Bletilla striata (Thunb.) Reichb. f. by colchicine treatment. Plant Cell Tissue Organ Cult. 2018, 132, 425–432. [Google Scholar] [CrossRef]
- Ye, Y.; Tong, J.; Shi, X.; Yuan, W.; Li, G. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic. 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Thatayaone, M.; Saji, G.; Meagle, J.; Kuruvila, B. Biochemical and nutritional characteristics of some commercial banana (Musa spp.) cultivars of Kerala. Plant Sci. Today 2022, 9, 681–686. [Google Scholar] [CrossRef]
- Do Amaral, C.M.; Dos Santos-Serejo, J.D.A.; Silva, S.D.O.E.; Da Silva Ledo, C.A.; Amorim, E.P. Agronomic characterization of autotetraploid banana plants derived from ‘Pisang Lilin’(AA) obtained through chromosome doubling. Euphytica 2015, 202, 435–443. [Google Scholar] [CrossRef]
- Jadrná, P.; Plavcová, O.; Kobza, F. Morphological changes in colchicine--treated Pelargonium× hortorum LH Bailey greenhouse plants. Hortic. Sci. 2011, 37, 27–33. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Riaz, A.; Zafar, T.; Hassan, M.; Umar, H.M.; Hassan, J.; Alam, W.; Muhammad, S.; Mahmood, M.; Sohail, H.; et al. Improving growth performance of jatropha curcas by inducing polyploidy through colchicine treatment. Am. J. Plant Sci. 2016, 7, 769–772. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Ruter, J.M. Development and Evaluation of diploid and polyploid Hibiscus moscheutos. HortScience 2017, 52, 676–681. [Google Scholar] [CrossRef]
- Padoan, D.; Mossad, A.; Chiancone, B.; Germana, M.A.; Khan, P.S. Ploidy levels in Citrus clementine affects leaf morphology, stomatal density and water content. Theor. Exp. Plant Physiol. 2013, 25, 83–290. [Google Scholar] [CrossRef]
- Wong, C.; Murray, B.G. Variable changes in genome size associated with different polyploid events in Plantago (Plantaginaceae). J. Hered. 2012, 103, 711–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lattier, J.; Chen, H.; Contreras, R.N. Variation in genome size, ploidy, stomata, and rDNA signals in Althea. J. Am. Soc. Hortic. Sci. 2019, 144, 130–140. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Sun, K.; Chang, D.; Bai, S.; Shen, Y. Ploidy level and DNA content of Erianthus arundinaceus as determined by flow cytometry and the association with biological characteristics. PLoS ONE 2016, 11, e0151948. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lei, T.; Meng, F.; Wei, C.; Li, X.; Guo, H. Polyploidy index and its implications for the evolution of polyploids. Front. Genet. 2019, 10, 807. [Google Scholar] [CrossRef]
- Nair, N.V.; Praneetha, M. Cyto-morphological studies on three Erianthus arundinaceus (Retz.) Jeswiet accessions from the Andaman-Nicobar Islands, India. Cytologia 2006, 71, 107–109. [Google Scholar] [CrossRef][Green Version]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.; Wani, A.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Tu, Y.; Jiang, A.; Gan, L.; Hossain, M.; Zhang, J.; Peng, B. Genome duplication improves rice root resistance to salt stress. Rice 2014, 7, 15. [Google Scholar] [CrossRef]
- Jiang, A.; Gan, L.; Tu, Y.; Ma, H.; Zhang, J.; Song, Z.; He, Y.; Cai, D.; Xue, X. The effect of genome duplication on seed germination and seedling growth of rice under salt stress. Aust. J. Crop Sci. 2013, 7, 1814. [Google Scholar]
- Jiang, J. Fluorescence in situ hybridization in plants: Recent developments and future applications. Chromosome Res. 2019, 27, 153–165. [Google Scholar] [CrossRef]
- Meng, H.B.; Jiang, S.S.; Hua, S.J.; Lin, X.Y.; Li, Y.L.; Guo, W.L. Comparison between a tetraploid turnip and its diploid progenitor (Brassica rapa L.): The adaptation to salinity stress. Agr. Sci. China 2011, 10, 363–375. [Google Scholar] [CrossRef]
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. C. R. Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Bomblies, K. Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr. Opin. Plant Biol. 2016, 30, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Abdolinejad, R.; Shekafandeh, A. Tetraploidy Confers Superior in vitro Water-Stress Tolerance to the Fig Tree (Ficus carica) by Reinforcing Hormonal, Physiological, and Biochemical Defensive Systems. Front. Plant Sci. 2022, 12, 796215. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 40. [Google Scholar] [CrossRef]
- Del Pozo, J.C.; Ramirez-parra, E. Deciphering the molecular bases for drought tolerance in A rabidopsis autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Akinroluyo, O.K.; Jasukune, K.; Kemesyte, V.; Statkeviciute, G. Drought stress response of Westerwolths ryegrass (Lolium multiflorum ssp. multiflorum) cultivars differing in their ploidy level. Zemdirbyste 2020, 107, 161–170. [Google Scholar] [CrossRef]
- Li, W.D.; Biswas, D.K.; Xu, H.; Xu, C.Q.; Wang, X.Z.; Liu, J.K.; Jiang, G.M. Photosynthetic responses to chromosome doubling in relation to leaf anatomy in Lonicera japonica subjected to water stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef]
- Godfree, R.C.; Marshall, D.J.; Young, A.G.; Miller, C.H.; Mathews, S. Empirical evidence of fixed and homeostatic patterns of polyploid advantage in a keystone grass exposed to drought and heat stress. R. Soc. Open Sci. 2017, 4, 170934. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, S.; Chen, Y.; Guan, Z.; Yin, D.; Chen, F. In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Xu, X.; Lu, J.; Bradley, F. Applications of polyploids in muscadine grape (Vitis Rotundifolia Michx.) breeding. Acta Hortic. 2010, 1046, 411–417. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Z.; Wang, J.; Chen, T.; Gao, J.; Zheng, J. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci. Hortic. 2020, 264, 109168. [Google Scholar] [CrossRef]
- Yin, C.; Li, P.; Li, H.; Xu, L.; Zhao, J.; Shan, T. Enhancement of diosgenin production in Dioscorea zingiberensis seedling and cell cultures by beauvericin from the endophytic fungus Fusarium redolens Dzf2. J. Med. Plant Res. 2011, 5, 6550–6554. [Google Scholar]
- Arvanitis, L.; Wiklund, C.; Münzbergova, Z.; Dahlgren, J.P.; Ehrlén, J. Novel antagonistic interactions associated with plant polyploidization influence trait selection and habitat preference. Ecol. Lett. 2010, 13, 330–337. [Google Scholar] [CrossRef]
- Edger, P.P.; Heidel Fischer, H.M.; Bekaert, M.; Rota, J.; Glöckner, G.; Platts, A.E. The butterfly plant arms-race escalated by gene and genome duplications. Proc. Natl. Acad. Sci. USA 2015, 112, 8362–8366. [Google Scholar] [CrossRef]
- Thompson, J.N.; Cunningham, B.M.; Segraves, K.A.; Althoff, D.M.; Wagner, D. Plant polyploidy and insect/plant interactions. Am. Nat. 1997, 150, 730–743. [Google Scholar] [CrossRef]
- Kao, R.H. Implications of polyploidy in the host plant of a dipteran seed parasite. West. N. Am. Nat. 2008, 68, 225–230. [Google Scholar] [CrossRef][Green Version]
- Nuismer, S.L.; Thompson, J.N. Plant polyploidy and non-uniform effects on insect herbivores. Proc. Royal Soc. B 2001, 268, 1937–1940. [Google Scholar] [CrossRef]
- Gross, K.; Schiestl, F.P. Are tetraploids more successful? Floral signals, reproductive success and floral isolation in mixed-ploidy populations of a terrestrial orchid. Ann. Bot. 2015, 115, 263–273. [Google Scholar] [CrossRef]
- Segraves, K.A.; Anneberg, T.J. Species interactions and plant polyploidy. Am. J. Bot. 2016, 103, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, Z.; Zhao, S.; Gu, Q.; Li, L. Study on Resistance to Fusarium Wilt in Different Polyploidy of Watermelons. J. Changjiang Veg. 2009, 18. [Google Scholar]
- Gonda, I.; Milavski, R.; Adler, C.; Abu-Abied, M.; Tal, O.; Faigenboim, A.; Chaimovitsh, D.; Dudai, N. Genome-based high-resolution mapping of fusarium wilt resistance in sweet basil. Plant Sci. 2022, 11, 111316. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, Z.; Fu, J.; Ma, Y. Characterization of fungi resistance in two autotetraploid apple cultivars. Sci. Hortic 2017, 220, 27–35. [Google Scholar] [CrossRef]
- Gottula, J.; Lewis, R.; Saito, S.; Fuchs, M. Allopolyploidy and the evolution of plant virus resistance. BMC Evol. Biol. 2014, 14, 149. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Wang, F.; Liu, T.; Chen, W. Expression and suppression of leaf rust resistance genes in amphidiploids from crosses of diploids and tetraploids. Acta Phytopathol. Sin. 2007, 5, 8. [Google Scholar]
- Zhu, Z.; Zhou, R.; Dong, Y.C.; Jia, J. Analysis of powdery mildew resistance genes in some tetraploid wheat-Aegilops amphidiploids and their parents. Plant Genet. Resour. 2003, 2, 137–143. [Google Scholar]
- Coate, J.E.; Doyle, J.J. Quantifying whole transcriptome size, a prerequisite for understanding transcriptome evolution across species: An example from a plant allopolyploid. Genome Biol. Evol. 2010, 2, 534–546. [Google Scholar] [CrossRef] [PubMed]
- De Godoy, L.M.; Olsen, J.V.; Cox, J.; Nielsen, M.L.; Hubner, N.C.; Fröhlich, F. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 2008, 455, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Mitchell, S.E.; Rogers, E.S.; Little, T.J.; Read, A.F. Host-parasite and genotype-by-environment interactions: Temperature modifies potential for selection by a sterilizing pathogen. Evolution 2005, 59, 70–80. [Google Scholar] [CrossRef]
- Melaragno, J.E.; Mehrotra, B.; Coleman, A.W. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 1993, 5, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Olmo, E. Nucleotype and cell size in vertebrates: A review. Basic Appl. Histochem 1983, 27, 227. [Google Scholar]
- Pacey, E.K.; Maherali, H.; Husband, B.C. Polyploidy increases storage but decreases structural stability in Arabidopsis thaliana. Current Biology 2022. [CrossRef] [PubMed]
- Petersen, K.K.; Hagberg, P.; Kristiansen, K.; Forkmann, G. In vitro chromosome doubling of Miscanthus sinensis. Plant Breed 2002, 121, 445–450. [Google Scholar] [CrossRef]
- Pelé, A.; Rousseau-Gueutin, M.; Chèvre, A.M. Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front. Plant Sci. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Bharadwa, J.; Dinesh, N. Polyploidy in crop improvement and evolution. In Plant Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 619–638. [Google Scholar]
- Mayer, V.W.; Aguilera, A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutat. Res.-Fund. Mol. M. 1990, 231, 177–186. [Google Scholar] [CrossRef]
- Klinner, U.; Böttcher, F. Mitotically unstable polyploids in the yeast Pichia guilliermondii. J. Basic MicroBiol. 1992, 32, 331–338. [Google Scholar] [CrossRef]
- Doyle, G. Aneuploidy and inbreeding depression in random mating and self-fertilizing autotetraploid populations. Theor. Appl. Genet. 1986, 72, 799–806. [Google Scholar] [CrossRef]
- Müntzing, A. Cyto-genetic properties and practical value of tetraploid rye. Hereditas 2010, 37, 17–84. [Google Scholar] [CrossRef]
- Bomblies, K.; Madlung, A. Polyploidy in the Arabidopsis genus. Chromosome Res. 2014, 22, 117–134. [Google Scholar] [CrossRef]
- Papp, I.; Iglesias, V.; Moscone, E.; Michalowski, S.; Spiker, S.; Park, Y.D. Structural instability of a transgene locus in tobacco is associated with aneuploidy. Plant J. 1996, 10, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, C.; Yang, H.; Hou, J.; Ji, T.; Cheng, J.; Veitia, R.A.; Birchler, J.A. The Gene Balance Hypothesis: Epigenetics and Dosage Effects in Plants. Plant Epigenetics Epigenomics 2020, 2093, 161–171. [Google Scholar]
- Scheid, O.M.; Afsar, K.; Paszkowski, J. Formation of stable epialleles and their paramutation-like interaction in tetraploid Arabidopsis thaliana. Nat. Genet. 2003, 34, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Scheid, O.M.; Jakovleva, L.; Afsar, K.; Maluszynska, J.; Paszkowski, J. A change of ploidy can modify epigenetic silencing. Proc. Natl. Acad. Sci. USA 1996, 93, 7114–7119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).