The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop
Abstract
1. Introduction
2. Results
2.1. Dry Matter Yield
2.2. Qualitative Characteristics of Above-Ground Biomass
3. Discussion
4. Materials and Methods
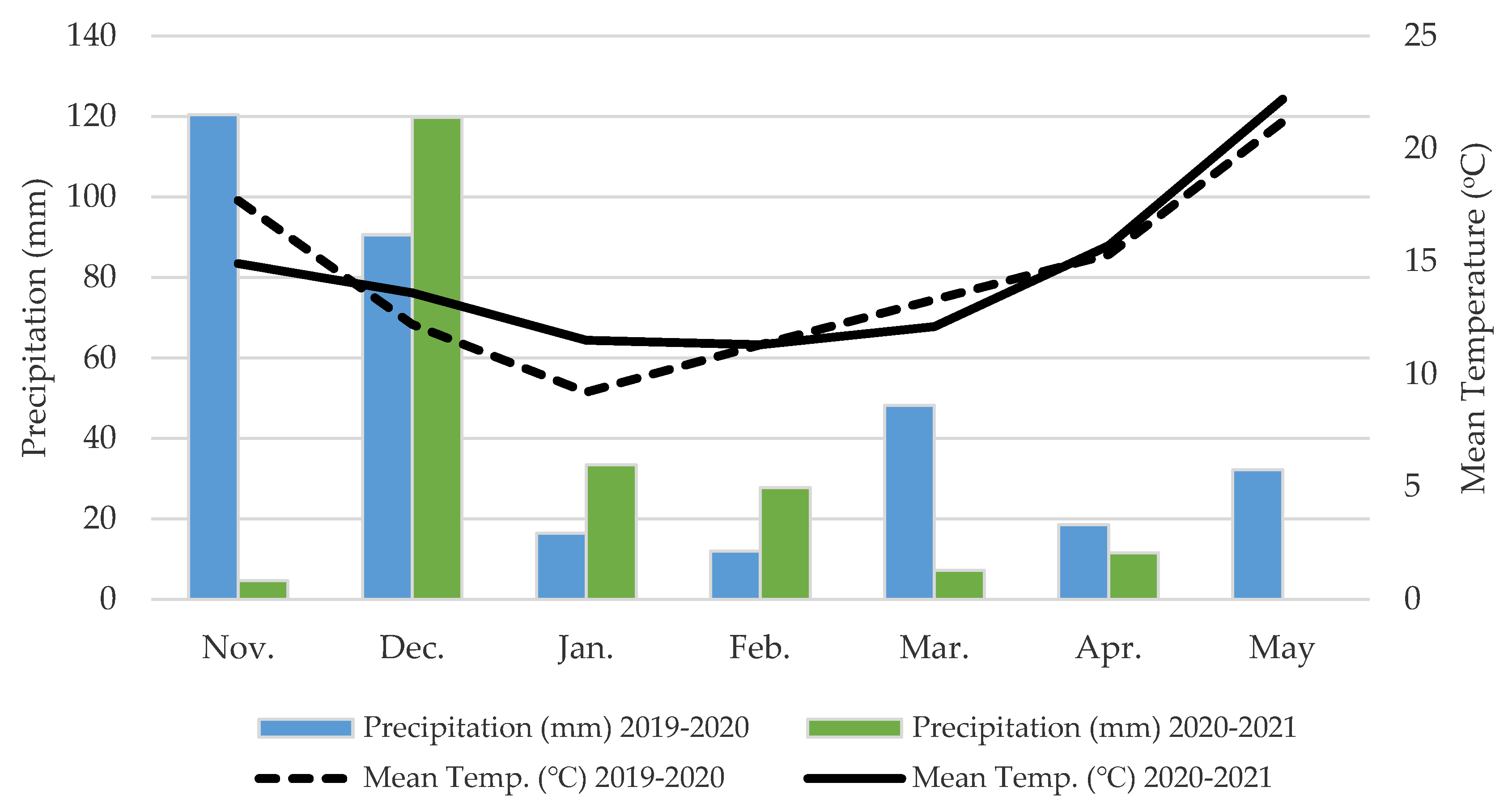
4.1. Site Description and Experimental Design
4.2. Sampling Procedures, Measurements and Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations (UN). World Population Projected to Reach 9.8 billion in 2050, and 11.2 billion in 2100. United Nations Department of Economic and Social Affairs. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 23 September 2022).
- Food and Agriculture Organization of the United Nations (FAO). World Livestock 2011—Livestock in Food Security; FAO: Rome, Italy, 2011. [Google Scholar]
- Ponnampalam, E.N.; Holman, B.W.B.; Kerry, J.P. Impact of animal nutrition on muscle composition and meat quality. In Meat Quality: Genetic and Environmental Factors; Przybylski, W., Hopkins, D., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 101–146. [Google Scholar]
- Demeyer, D.; Doreau, M. Targets and procedures for altering ruminant meat and milk lipids. Proc. Nutr. Soc. 1999, 58, 593–607. [Google Scholar] [CrossRef]
- Kaasschieter, G.A.; de Jong, R.; Schiere, J.B.; Zwart, D. Towards a sustainable livestock production in developing countries and the importance of animal health strategy therein. Vet. Q. 1992, 14, 66–75. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S.; Polyorach, S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J. Anim. Sci. Biotechnol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Tona, G.O. Current and Future Improvements in Livestock Nutrition and Feed Resources. In Animal Husbandry and Nutrition; Yücel, B., Taşkin, T., Eds.; Intercopen: London, UK, 2018. [Google Scholar] [CrossRef]
- Thomas, J.; Kuruvilla, M.K.; Hrideek, K.T. Mustard. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 1, pp. 388–398. [Google Scholar]
- Bagchi, G.D.; Srivastava, N.G. Spices and flavoring crops: Fruits and seeds. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 5465–5477. [Google Scholar]
- Kakabouki, I.; Karydogianni, S.; Roussis, I.; Bilalis, D. Effect of organic and inorganic fertilization on weed flora and seed yield in black mustard (Brassica nigra (L.) Koch) crops. Int. J. Agric. Nat. Resour. 2020, 47, 79–89. [Google Scholar] [CrossRef]
- Sahay, S.; Inam, A.; Inam, A.; Iqbal, S. Modulation in growth, photosynthesis and yield attributes of black mustard (B. nigra cv. IC247) by interactive effect of wastewater and fly ash under different NPK levels. Cogent Food Agric. 2015, 1, 1087632. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, K. Bioaccumulation and distribution of heavy metals in black mustard (Brassica nigra Koch). Environ. Monit. Assess. 2009, 153, 449–459. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef]
- Wanasundara, J.P. Proteins of Brassicaceae oilseeds and their potential as a plant protein source. Food Sci. Nutr. 2011, 51, 635–677. [Google Scholar]
- Čolović, D.; Banjac, V.; Rakita, S.; Čolović, R.; Marjanović-Jeromela, A.; Vidosavljević, S.; Kokić, B. By-products of black (Brassica nigra) and white (Sinapis slba) mustard seed production as animal feed: Possibilities and hazards. J. Process. Energy Agric. 2018, 22, 188–191. [Google Scholar] [CrossRef]
- Montemurro, F.; Diacono, M. Towards a better understanding of agronomic efficiency of nitrogen: Assessment and improvement strategies. Agronomy 2016, 6, 31. [Google Scholar] [CrossRef]
- Delogu, G.; Cattivelli, L.; Pecchioni, N.; De Falcis, D.; Maggiore, T.; Stanca, A.M. Uptake and agronomic efficiency of nitrogen in winter barley and winter wheat. Eur. J. Agron. 1998, 9, 11–20. [Google Scholar] [CrossRef]
- Sinclair, T.R.; de Wit, C.T. Photosynthate and nitrogen requirements for seed production by various crops. Science 1975, 18, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Bakken, L.R.; Frostegard, A. Sources and sinks for N2O, can microbiologist help to mitigate N2O emissions? Environ. Microbiol. 2017, 19, 4801–4805. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M. Problems in the use of urea as a nitrogen fertilizer. Soil Use Manag. 2007, 6, 70–71. [Google Scholar] [CrossRef]
- Suter, H.C.; Sultana, H.; Davies, R.; Walker, C.; Chen, D. Influence of enhanced efficiency fertilisation techniques on nitrous oxide emissions and productivity response from urea in a temperate Australian ryegrass pasture. Soil Res. 2016, 54, 523–532. [Google Scholar] [CrossRef]
- Wang, H.; Köbke, S.; Dittert, K. Use of urease and nitrification inhibitors to reduce gaseous nitrogen emissions from fertilizers containing ammonium nitrate and urea. Glob. Ecol. Conserv. 2020, 22, e00933. [Google Scholar] [CrossRef]
- Li, Y.; Mingfang, H.; Tenuta, M.; Ma, Z.; Gui, D.; Li, X.; Zeng, F.; Gao, X. Agronomic evaluation of polymercoated urea and urease and nitrification inhibitors for cotton production under drip-fertigation in a dry climate. Sci. Rep. 2020, 10, 1472. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils: A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Franzen, D.W. Nitrogen Extenders and Additives for Field Crops (SF1581); NCERA-103 Committee; North Dakota State University: Fargo, ND, USA, 2022; pp. 1–11. [Google Scholar]
- Karydogianni, S.; Darawsheh, M.K.; Kakabouki, I.; Zisi, C.; Folina, A.E.; Roussis, I.; Tselia, Z.; Bilalis, D. Effect of nitrogen fertilizations, with and without Inhibitors, on cotton growth and fiber quality. Agron. Res. 2020, 18, 432–449. [Google Scholar]
- Krol, D.J.; Forrestal, J.P.; Wall, D.; Lanigan, J.G.; Sanz-Gomez, J.; Richards, G.K. Nitrogen fertilizers with urease inhibitors reduce nitrous oxide and ammonia losses, while retaining yield in temperate grassland. Sci. Total Environ. 2020, 725, 138329. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cow. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Orskov, E.R.; Ryle, M. Energy Nutrition in Ruminants; Elsevier Press: New York, NY, USA, 1990. [Google Scholar]
- Bilalis, D.J.; Roussis, I.; Cheimona, N.; Kakabouki, I.; Travlos, I.S. Organic Agriculture and Innovative Feed Crops. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; Volume 23, pp. 55–100. [Google Scholar]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Roussis, I.; Katsenios, N.; Efthimiadou, A.; Papastylianou, P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021, 41, 71. [Google Scholar] [CrossRef]
- Sharratt, B.S.; McWilliams, D.A. Microclimatic and rooting characteristics of narrow-row versus conventional-row corn. Agron. J. 2005, 97, 1129–1135. [Google Scholar] [CrossRef]
- Edwards, J.T.; Purcell, L.C.; Vories, E.D. Light interception and yield potential of short-season maize (Zea mays L.) hybrids in the Midsouth. Agron. J. 2005, 97, 225–234. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Pedersen, P. Effect of row spacing and seeding rate on soybean yield. Agron. J. 2008, 100, 704–710. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zuo, Q.; Huang, H.; Liao, Q.; Wu, C.; Lu, J.; Wu, J.; Zhou, G. The yield of mechanically harvested rapeseed (Brassica napus L.) can be increased by optimum plant density and row spacing. Sci. Rep. 2015, 5, 18835. [Google Scholar] [CrossRef]
- Smith, R.; Slater, F.M. The effects of organic and inorganic fertilizer applications to Miscanthus × giganteus, Arundo donax and Phalaris arundinacea, when grown as energy crops in Wales, UK. Glob. Chang. Biol. Bioenergy 2010, 2, 169–179. [Google Scholar] [CrossRef]
- Baghdadi, A.; Halim, R.A.; Ghasemzadeh, A.; Ramlan, M.F.; Sakimin, S.Z. Impact of organic and inorganic fertilizers on the yield and quality of silage corn intercropped with soybean. PeerJ 2018, 6, e5280. [Google Scholar] [CrossRef]
- Sandrakirana, R.; Arifin, Z. Effect of organic and chemical fertilizers on the growth and production of soybean (Glycine max) in dry land. Rev. Fac. Nac. Agron. Medellín. 2021, 74, 9643–9653. [Google Scholar] [CrossRef]
- Stickler, F.C.; Laude, H.H. Effect of row spacing and plant population on performance of corn, grain sorghum and forage sorghum. Agron. J. 1960, 52, 275–277. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Schroth, G.; Mandarino, J.M.G. Effect of nitrogen, row spacing, and plant density on yield, yield components, and plant physiology in soybean-wheat intercropping. Agron. J. 2015, 107, 2162–2170. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Blennerhassett, J.D.; Quin, B.F. Reducing NH3, N2O and NO3−–N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils. 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A. Combining urease and nitrification inhibitors with incorporation reduces ammonia and nitrous oxide emissions and increases corn yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Drulis, P.; Kriauciuniene, Z.; Liakas, V. The effect of combining N-fertilization with urease inhibitors and biological preparations on maize biological productivity. Agronomy 2022, 12, 2264. [Google Scholar] [CrossRef]
- Rayburn, E.B. Matching plant species to your environment, weather, and climate. In Horse Pasture Management; Sharpe, P., Ed.; Academic Press: New York, NY, USA, 2019; pp. 233–243. [Google Scholar]
- Horrocks, R.D.; Valentine, J.F. Harvested Forages; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Xie, Z.L.; Zhang, T.F.; Chen, X.Z.; Li, G.D.; Zhang, J.G. Effects of maturity stages on the nutritive composition and silage quality of whole crop wheat. Asian-Australas. J. Anim. Sci. 2012, 25, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Krawutschke, M.; Kleen, J.; Weiher, N.; Loges, R.; Taube, F.; Gierus, M. Changes in crude protein fractions of forage legumes during the spring growth and summer regrowth period. J. Agric. Sci. 2013, 151, 72–90. [Google Scholar] [CrossRef]
- Throop, H.L. Nitrogen deposition and herbivory affect biomass production and allocation in an annual plant. OIKOS 2005, 111, 91–100. [Google Scholar] [CrossRef]
- Widdicombe, W.D.; Thelen, K.D. Row width and plant density effect on corn forage hybrids. Agron. J. 2002, 94, 326–330. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L. Approaches for increasing nitrogen and water use efficiency simultaneously. Glob. Food Sec. 2016, 9, 29–35. [Google Scholar] [CrossRef]
- Allende-Montalbán, R.; Martín-Lammerding, D.; Delgado, M.d.M.; Porcel, M.A.; Gabriel, J.L. Urease inhibitors effects on the nitrogen use efficiency in a maize–wheat rotation with or without water deficit. Agriculture 2021, 11, 684. [Google Scholar] [CrossRef]
- Coleman, S.W.; Henry, D.A. Nutritive Value of Herbage. In Sheep Nutrition; Freer, M., Dove, H., Eds.; CABI Pub. in Association with CSIRO Pub.: Wallingford, UK, 2002; pp. 1–26. [Google Scholar]
- Dewhurst, R.J.; Scollan, N.D.; Younell, S.J.; Tweed, J.K.S.; Humphreys, M.O. Influence of species, cutting date and cutting interval on the fatty acid composition of grasses. Grass Forage Sci. 2001, 56, 68–74. [Google Scholar] [CrossRef]
- Boufaïed, H.; Chouinard, P.Y.; Tremblay, G.F.; Petit, H.V.; Michaud, R.; Bélanger, G. Fatty acids in forages. I. Factors affecting concentrations. Can. J. Anim. Sci. 2003, 83, 501–511. [Google Scholar] [CrossRef]
- Dasci, M.; Comakli, B. Effects of fertilization on forage yield and quality in range sites with different topographic structure. Turkish J. Field Crop. 2011, 16, 15–22. [Google Scholar]
- Kakabouki, I.; Bilalis, D.; Karkanis, A.; Zervas, G.; Tsiplakou, E.; Hela, D. Effects of fertilization and tillage system on growth and crude protein content of quinoa (Chenopodium quinoa Willd.): An alternative forage crop. Emir. J. Food Agric. 2014, 26, 18–24. [Google Scholar] [CrossRef]
- Roussis, I.; Kakabouki, I.; Tsiplakou, E.; Bilalis, D. Influence of plant density and fertilization on yield and crude protein of Nigella sativa L.: An alternative forage and feed source. In Nigella sativa: Properties, Uses and Effects; Berghuis, S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; pp. 145–180. [Google Scholar]
- Reddy, B.V.S.; Reddy, P.S.; Bidinger, F.R.; Blümmel, M. Crop management factors influencing yield and quality of crop residues. Field Crops Res. 2003, 84, 57–77. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Angelini, L.G.; Bonari, E. Influence of fertilisation and harvest time on fuel quality of giant reed (Arundo donax L.) in Central Italy. Eur. J. Agron. 2010, 32, 219–227. [Google Scholar] [CrossRef]
- Krachunov, I. Estimation of energy feeding value of forages for ruminants II. Energy prediction through crude fiber content. J. Mt. Agric. Balk. 2007, 10, 122–134. [Google Scholar]
- Beauchemin, K.A. Using ADF and NDF in dairy cattle diet formulation-a western Canadian perspective. Anim. Feed Sci. Technol. 1996, 58, 101–111. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Duodu, K.G.; Dowell, F.E. Sorghum and Millets: Quality Management Systems. In Sorghum and Millets: Chemistry, Technology and Nutritional Attributes, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 421–442. [Google Scholar]
- Han, F.; Ullrich, S.E.; Romagosa, I.; Clancy, J.A.; Froseth, J.A.; Wesenberg, D.M. Quantitative genetic analysis of acid detergent fibre content in barley grain. J. Cereal Sci. 2003, 38, 167–172. [Google Scholar] [CrossRef]
- Messman, M.A.; Weiss, W.P.; Erickson, D.O. Effects of nitrogen fertilization and maturity of bromegrass on in situ ruminal digestion kinetics of fiber. J. Anim. Sci. 1991, 69, 1151–1161. [Google Scholar] [CrossRef]
- Benett, C.G.S.; Buzetti, S.; Silva, K.S.; Bergamaschine, A.F.; Fabricio, J.A. Yield and bromatologic composition of Marandu grass as function of sources and doses of nitrogen. Ciênc. Agrotec. 2008, 32, 1629–1636. [Google Scholar] [CrossRef]
- Sbrissia, A.F.; da Silva, S.C. Tiller size/density compensation in Marandu palisadegrass swards. Rev. Brasi. Zootec. 2008, 37, 35–47. [Google Scholar] [CrossRef]
- Kering, M.K.; Guretzky, J.A.; Funderburg, E.; Mosali, J. Effect of nitrogen fertilizer rate and harvest season on forage yield, quality, and macronutrient concentrations in Midland Bermuda grass. Commun. Soil Sci. Plant Anal. 2011, 42, 1958–1971. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Martin, N.P.; Lamb, J.F.S.; Cuomo, G.R.; Jewett, J.G.; Quering, S.R. Leaf and stem properties of alfalfa entries. Agron. J. 2000, 92, 733–739. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant, 2nd ed.; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Pereira, J.R.; Neres, M.A.; Sandini, I.E.; Fluck, A.C.; Costa, O.A.D.; Sartor, L.R. Chemical compounds and gas production kinetics of annual ryegrass hay in distinct nitrogen levels and cutting heights. Turk. J. Vet. Anim. Sci. 2020, 44, 1243–1249. [Google Scholar] [CrossRef]
- Leite, R.G.; Cardoso, A.D.S.; Fonseca, N.V.B.; Silva, M.L.C.; Tedeschi, L.O.; Delevatti, L.M.; Ruggieri, A.C.; Reis, R.A. Effects of nitrogen fertilization on protein and carbohydrate fractions of Marandu palisadegrass. Sci Rep. 2021, 11, 14786. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, Association of Official Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]

| Source of Variance | Df | DM Yield | DM Content | CP Content | CP Yield | CF |
|---|---|---|---|---|---|---|
| Year (Y) | 1 | 0.0248 ns | 0.0040 ns | 0.9768 ns | 0.0524 ns | 1.6580 ns |
| Plant Density (PD) | 1 | 9.9495 ** | 10.2966 ** | 11.1732 ** | 2.5311 ns | 3.4333 ns |
| Fertilization (F) | 3 | 37.3676 *** | 37.5041 *** | 22.9083 *** | 58.1784 *** | 0.7898 ns |
| Y × PD | 1 | 0.0134 ns | 0.0110 ns | 0.0575 ns | 0.0859 ns | 0.3267 ns |
| Y × F | 3 | 0.4005 ns | 0.6889 ns | 0.1593 ns | 0.7508 ns | 0.1106 ns |
| PD × F | 3 | 0.5494 ns | 0.4754 ns | 4.9791 ns | 2.3535 ns | 2.2104 ns |
| Y × PD × F | 3 | 0.0318 ns | 0.0176 ns | 0.0769 ns | 0.0072 ns | 0.1207 ns |
| Source of Variance | Df | CA | NDF | ADF | CHO | NFC |
| Year (Y) | 1 | 0.3525 ns | 0.8023 ns | 3.5077 ns | 0.2103 ns | 1.4313 ns |
| Plant Density (PD) | 1 | 0.6374 ns | 3.8650 ns | 3.5698 ns | 3.6233 ns | 2.1571 ns |
| Fertilization (F) | 3 | 1.1141 ns | 7.2726 *** | 9.4025 *** | 11.8053 *** | 19.0120 *** |
| Y × PD | 1 | 0.0723 ns | 0.2196 ns | 0.0711 ns | 0.1302 ns | 0.0173 ns |
| Y × F | 3 | 0.0477 ns | 0.3641 ns | 0.5735 ns | 0.0901 ns | 0.5744 ns |
| PD × F | 3 | 2.9494 * | 1.6596 ns | 1.5429 ns | 3.2970 * | 4.5739 * |
| Y × PD × F | 3 | 0.0214 ns | 0.1033 ns | 0.0650 ns | 0.0452 ns | 0.1005 ns |
| Plant Density (Plants m−2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertilization | 46 | 76 | 46 | 76 | 46 | 76 | 46 | 76 | ||||
| DM Yield | DM Content (%) | CP Content | CP Yield | |||||||||
| 2019–2020 | (tn ha−1) | Mean | Mean | (% of DM) | Mean | (tn ha−1) | Mean | |||||
| Control | 7.08 | 7.82 | 7.45 c | 17.57 | 16.85 | 17.21 c | 18.15 | 12.98 | 15.57 c | 1.29 | 0.99 | 1.14 c |
| Urea | 14.99 | 17.48 | 16.23 a | 25.89 | 23.99 | 24.94 a | 22.03 | 20.79 | 21.41 a | 3.31 | 3.63 | 3.47 a |
| Urea + NI + UI | 15.68 | 18.34 | 17.01 a | 28.43 | 25.76 | 27.09 a | 22.09 | 23.51 | 22.80 a | 3.45 | 4.31 | 3.88 a |
| Compost | 9.42 | 12.24 | 10.83 b | 22.45 | 19.12 | 20.79 b | 20.21 | 17.58 | 18.89 b | 1.90 | 2.15 | 2.02 b |
| Mean | 11.79 B | 13.97 A | 23.58 A | 21.43 B | 20.62 A | 18.71 B | 2.49 A | 2.77 A | ||||
| FPlant Density | 5.2173 * (Tukey = 1.707) | 4.7546 * (Tukey = 1.664) | 5.6664 * (Tukey = 1.101) | 1.7033 ns | ||||||||
| FFertilization | 22.7083 *** (Tukey = 2.176) | 19.8362 *** (Tukey = 3.035) | 15.7790 *** (Tukey = 1.529) | 34.1834 *** (Tukey = 0.662) | ||||||||
| FPlant Density × Fertilization | 0.2566 ns | 0.3231 ns | 2.9523 ns | 1.1902 ns | ||||||||
| 2020–2021 | ||||||||||||
| Control | 7.99 | 8.84 | 8.42 c | 18.51 | 17.69 | 18.10 c | 19.82 | 13.81 | 16.82 c | 1.60 | 1.21 | 1.40 c |
| Urea | 14.18 | 16.09 | 15.14 a | 26.05 | 24.14 | 25.09 a | 22.20 | 21.07 | 21.63 ab | 3.15 | 3.38 | 3.27 a |
| Urea + NI + UI | 14.73 | 17.55 | 16.14 a | 27.43 | 24.63 | 26.03 a | 22.77 | 23.29 | 23.03 a | 3.35 | 4.06 | 3.70 a |
| Compost | 10.36 | 12.95 | 11.65 b | 22.45 | 19.98 | 21.21 b | 20.71 | 18.53 | 19.62 b | 2.17 | 2.40 | 2.29 b |
| Mean | 11.82 B | 13.86 A | 23.61 A | 21.60 B | 21.37 A | 19.17 B | 2.57 A | 2.76 A | ||||
| FPlant Density | 5.0832 * (Tukey = 1.110) | 5.2728 * (Tukey = 1.216) | 5.5777 * (Tukey = 0.648) | 0.8791 ns | ||||||||
| FFertilization | 15.0869 *** (Tukey = 2.788) | 17.661 *** (Tukey = 1.759) | 8.3990 ** (Tukey = 2.321) | 24.3321 *** (Tukey = 0.621) | ||||||||
| FPlant Density × Fertilization | 0.2353 ns | 0.2488 ns | 2.2147 ns | 1.1696 ns | ||||||||
| Plant Density (Plants m−2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertilization | 46 | 76 | 46 | 76 | 46 | 76 | 46 | 76 | ||||
| CF (% of DM) | CA (% of DM) | NDF (% of DM) | ADF (% of DM) | |||||||||
| 2019–2020 | Mean | Mean | Mean | Mean | ||||||||
| Control | 2.64 | 2.53 | 2.59 a | 13.99 | 13.22 | 13.61 a | 43.51 | 38.88 | 41.20 c | 33.37 | 31.94 | 32.66 c |
| Urea | 2.67 | 2.53 | 2.60 a | 14.48 | 13.57 | 14.03 a | 43.71 | 43.79 | 43.75 bc | 36.20 | 34.57 | 35.39 bc |
| Urea + NI + UI | 2.76 | 2.50 | 2.63 a | 13.61 | 15.03 | 14.32 a | 47.63 | 44.53 | 46.08 ab | 38.02 | 35.66 | 36.84 ab |
| Compost | 2.59 | 2.76 | 2.68 a | 14.12 | 13.73 | 13.93 a | 47.28 | 46.94 | 47.11 a | 37.77 | 38.92 | 38.35 a |
| Mean | 2.66 A | 2.58 A | 14.05 A | 13.89 A | 45.53 A | 43.54 A | 36.34 A | 35.28 A | ||||
| FPlant Density | 0.7386 ns | 0.1178 ns | 3.7800 ns | 1.2396 ns | ||||||||
| FFertilization | 0.1596 ns | 0.3880 ns | 6.5442 ** (Tukey = 2.632) | 6.4133 ** (Tukey = 2.762) | ||||||||
| FPlant Density × Fertilization | 0.8459 ns | 1.3120 ns | 1.1939 ns | 0.6393 ns | ||||||||
| 2020–2021 | ||||||||||||
| Control | 2.58 | 2.35 | 2.46 a | 13.88 | 13.05 | 13.47 a | 44.54 | 41.78 | 43.17 b | 36.77 | 33.99 | 35.38 b |
| Urea | 2.63 | 2.31 | 2.47 a | 14.31 | 13.41 | 13.86 a | 44.72 | 44.66 | 44.69 ab | 36.92 | 35.27 | 36.10 b |
| Urea + NI + UI | 2.71 | 2.45 | 2.58 a | 13.66 | 14.87 | 14.27 a | 47.29 | 44.05 | 45.67 ab | 38.77 | 36.37 | 37.57 ab |
| Compost | 2.54 | 2.71 | 2.63 a | 13.95 | 13.17 | 13.56 a | 46.98 | 48.12 | 47.55 a | 38.51 | 39.69 | 39.10 a |
| Mean | 2.62 A | 2.46 A | 13.95 A | 13.63 A | 45.89 A | 44.66 A | 37.74 A | 36.33 A | ||||
| FPlant Density | 3.3081 ns | 0.7034 ns | 1.9219 ns | 2.4782 ns | ||||||||
| FFertilization | 0.8138 ns | 0.8646 ns | 4.0608 * (Tukey = 2.979) | 3.3738 * (Tukey = 2.291) | ||||||||
| FPlant Density × Fertilization | 1.5655 ns | 1.7403 ns | 0.6901 ns | 0.9904 ns | ||||||||
| Plant Density (Plants m−2) | ||||||
|---|---|---|---|---|---|---|
| Fertilization | 46 | 76 | 46 | 76 | ||
| CHO (% of DM) | NFC (% of DM) | |||||
| 2019–2020 | Mean | Mean | ||||
| Control | 65.22 | 71.27 | 68.25 a | 21.71 | 24.39 | 23.05 a |
| Urea | 60.73 | 63.14 | 61.94 bc | 17.02 | 19.35 | 18.18 b |
| Urea + NI + UI | 61.72 | 58.70 | 60.21 c | 14.08 | 14.18 | 14.13 c |
| Compost | 62.99 | 66.16 | 64.58 b | 15.72 | 19.22 | 17.47 b |
| Mean | 62.67 A | 64.82 A | 17.13 A | 19.28 A | ||
| FPlant Density | 1.9679 ns | 1.6417 ns | ||||
| FFertilization | 7.1572 *** (Tukey = 3.627) | 10.5627 *** (Tukey = 2.303) | ||||
| FPlant Density × Fertilization | 2.8491 ns | 3.5275 ns | ||||
| 2020–2021 | ||||||
| Control | 63.72 | 70.79 | 67.25 a | 19.17 | 21.01 | 20.09 a |
| Urea | 60.79 | 63.07 | 61.93 bc | 16.06 | 18.41 | 17.24 b |
| Urea + NI + UI | 61.03 | 59.13 | 60.08 c | 13.74 | 15.08 | 14.41 c |
| Compost | 62.71 | 65.99 | 64.35 ab | 15.73 | 17.87 | 16.80 b |
| Mean | 62.06 A | 64.75 A | 16.18 A | 18.09 A | ||
| FPlant Density | 5.6762 ns | 4.7703 ns | ||||
| FFertilization | 7.5967 ** (Tukey = 4.050) | 9.9054 *** (Tukey = 1.897) | ||||
| FPlant Density × Fertilization | 2.6744 ns | 2.2524 ns | ||||
| Trait | Coefficient of Correlaiton (r) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM Content | DM Yield | CP Content | CP Yield | CF | CA | NDF | ADF | CHO | NFC | ||
| DM Content | 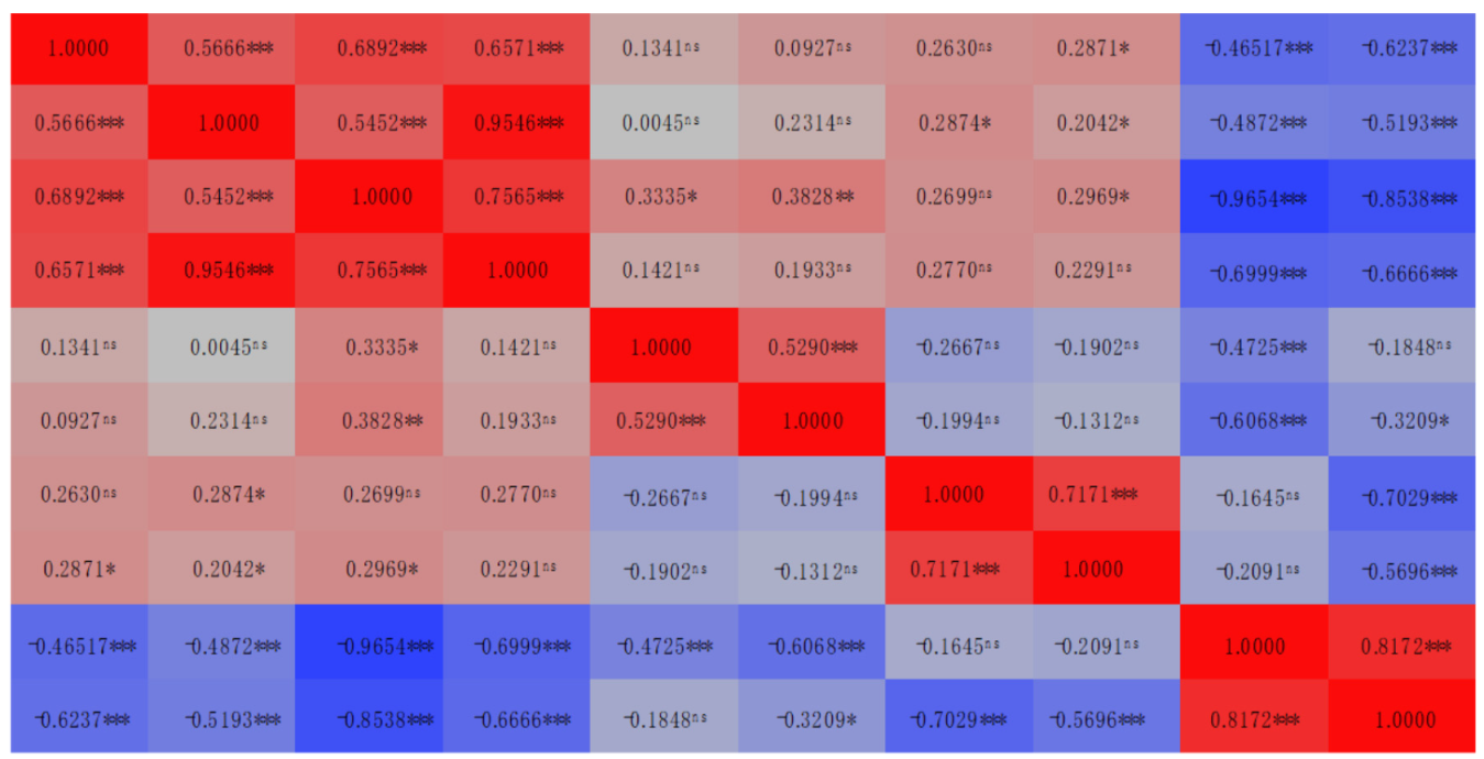 |  | |||||||||
| DM Yield | |||||||||||
| CP Content | |||||||||||
| CP Yield | |||||||||||
| CF | |||||||||||
| CA | |||||||||||
| NDF | |||||||||||
| ADF | |||||||||||
| CHO | |||||||||||
| NFC | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karydogianni, S.; Roussis, I.; Mavroeidis, A.; Kakabouki, I.; Tigka, E.; Beslemes, D.; Stavropoulos, P.; Katsenios, N.; Tsiplakou, E.; Bilalis, D. The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop. Plants 2022, 11, 2683. https://doi.org/10.3390/plants11202683
Karydogianni S, Roussis I, Mavroeidis A, Kakabouki I, Tigka E, Beslemes D, Stavropoulos P, Katsenios N, Tsiplakou E, Bilalis D. The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop. Plants. 2022; 11(20):2683. https://doi.org/10.3390/plants11202683
Chicago/Turabian StyleKarydogianni, Stella, Ioannis Roussis, Antonios Mavroeidis, Ioanna Kakabouki, Evangelia Tigka, Dimitrios Beslemes, Panteleimon Stavropoulos, Nikolaos Katsenios, Eleni Tsiplakou, and Dimitrios Bilalis. 2022. "The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop" Plants 11, no. 20: 2683. https://doi.org/10.3390/plants11202683
APA StyleKarydogianni, S., Roussis, I., Mavroeidis, A., Kakabouki, I., Tigka, E., Beslemes, D., Stavropoulos, P., Katsenios, N., Tsiplakou, E., & Bilalis, D. (2022). The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop. Plants, 11(20), 2683. https://doi.org/10.3390/plants11202683












