Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review
Abstract
1. Introduction
2. Botanical Aspects
2.1. Morphology
2.2. Taxonomy and the Geographical Distribution
2.3. Traditional Use
3. Phytochemistry of Anacyclus pyrethrum
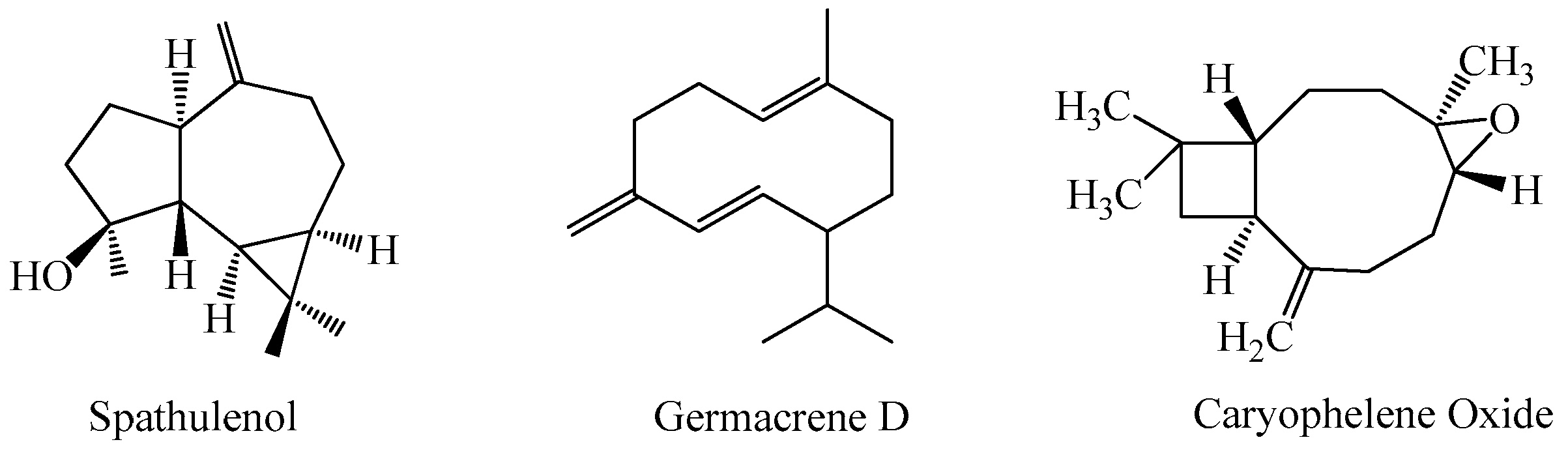
3.1. Chemical Compounds of the Essential Oils
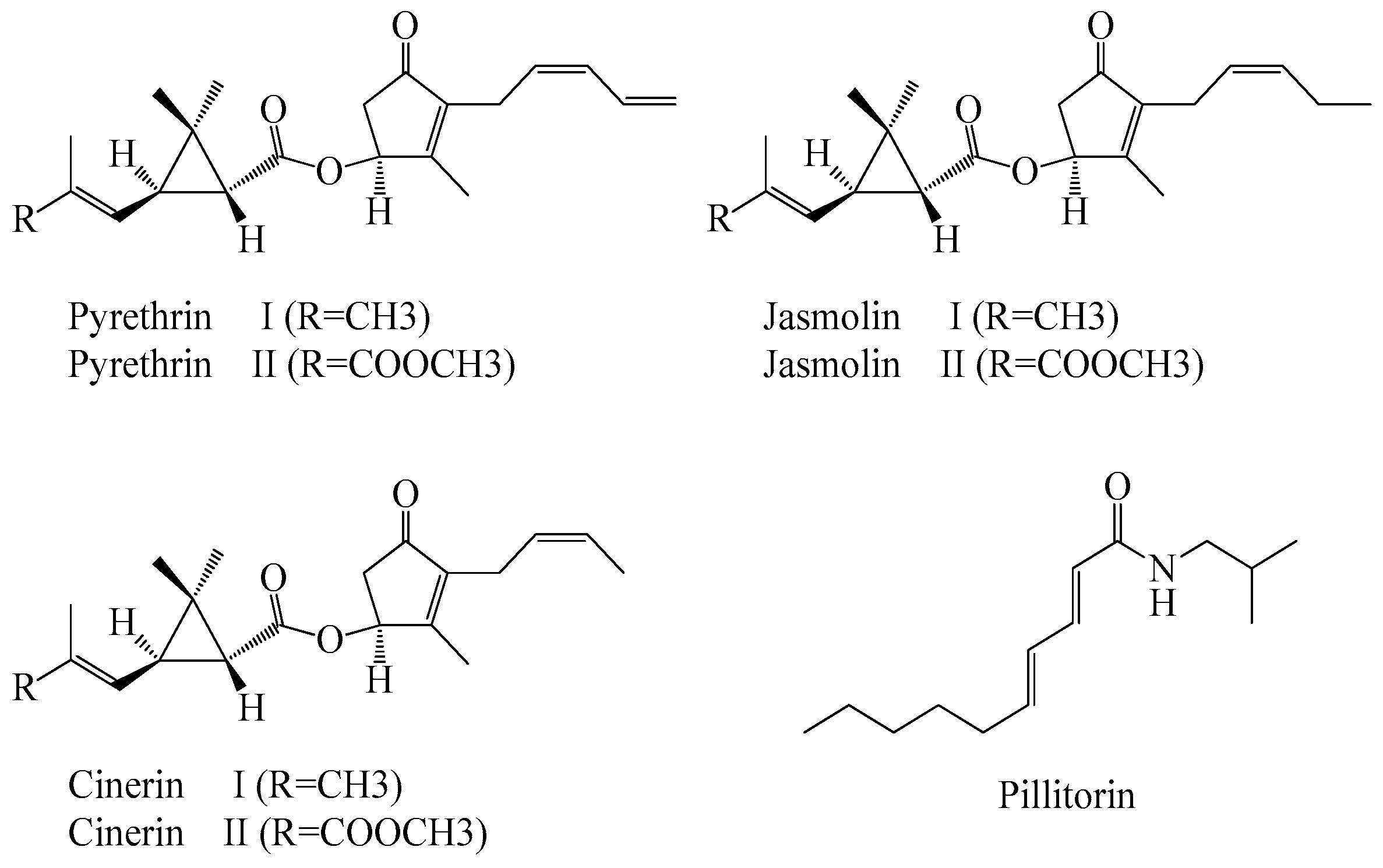
3.2. Non-Volatile Compounds
4. Biological and Pharmacological Actions
4.1. Antioxidant Activity
4.2. Antidiabetic Activity
4.3. Anesthetic Activity
4.4. Insecticidal Activity
4.5. Antidepressant Activity
4.6. Antimicrobial Activity
| Used Part | Extracts | Method | Tested Strains | Results (Inhibition Diameter in mm) | References |
|---|---|---|---|---|---|
| Roots | EO | Method of disk diffusion | S. aureus | 7 | [27] |
| Aqu. mac. ext. | E. coli sensible | 9 | |||
| E. coli resistant | 9 | ||||
| S. aureus | 9 | ||||
| P. aeruginosa sensible | 6 | ||||
| P. aeruginosa resistant | 7 | ||||
| K. pneumoniae | 7 | ||||
| MeOH ext. | E. coli resistant | 7 | |||
| S. aureus | 7 | ||||
| P. aeruginosa resistant | 7 | ||||
| Aqu. ext. | E. coli sensible | 8 | |||
| Roots | MeOH ext. | E. coli | 23 | [66] | |
| Stems/Leaves | EtOH ext. | Disc-diffusionmethod | L. monocytogenes | 14 | [46] |
| S. aureus | 20 | ||||
| Bacilluscereus | 16 | ||||
| C. albicans | 18 | ||||
| Aqu. ext. | L. monocytogenes | 10 | |||
| S. aureus | 16 | ||||
| Bacilluscereus | 12 | ||||
| C. albicans | 15 | ||||
| Chl. ext. | L. monocytogenes | 8 | |||
| S. aureus | 11 | ||||
| E. coli | 10 | ||||
| C. albicans | 10 | ||||
| Leaves | Aqu. ext. | Disc-diffusion method | C. albicans | 6 7 8 | [67] |
4.7. Anti-Inflammatory Activity
4.8. Aphrodisiac Activity
4.9. Anticonvulsant Activity
4.10. Other Activities
5. Toxicological Evidence
6. Methodology
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOs: | Essential Oils |
| MeOH ext.: | Hydromethanolic extract |
| EtOH ext.: | Hydroethanolic extract |
| Aqu. ext.: | Aqueous extract |
| Chl.. ext.: | Chloroform extract |
| BuOH fra.: | Butanol fraction |
| AcEth fra.: | Ethyl acetate fraction |
| Res pha.: | Residual phase |
| Aqu. mac. ext.: | Aqueous macerate extract |
| EtOH mac. ext.: | Hydroethanolic macerate extract |
References
- Ouarghidi, A.; Abbad, A. Etude ethnobotanique, ethno-taxonomique et ethnoécologique de Anacyclus pyrethrum var. pyrethrum (L.) Link. (Asteraceae) dans la vallée d’Ait Mhamed (Région d’Azilal, Maroc). Rev. D’ethnoécologie 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Schauenberg, P.; Paris, F. Guides Des Plantes Médicinales Analyse, Description Et Utilisation De 400 Plantes; Delachaux et Niestlé: Paris, France, 2006; pp. 33–34. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle: Médecine Arabe Ancienne Et Savoirs Populaires-Saint–Etienne; Ibis Press: Saint–Etienne, Paris, 1997. [Google Scholar]
- Hmamouchi, M. Les Plantes Médicinales Et Aromatiques Marocaines; Fedala: Rabat, Morocco, 1999; p. 389. [Google Scholar]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. Anacyclus pyrethrum (L): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- El mokhtari, K.; El kouali, M.; Talbi, M.; Hajji, L.; El Brouzi, A. Chemical composition and insecticidal activity of Anacyclus pyrethrum essential oil from the Bensliman area against Culex pipiens. Mediterr. J. Chem. 2020, 10, 13–21. [Google Scholar] [CrossRef]
- Elazzouzi, H.; Zekri, N.; Zair, T.; El Belghiti, M.A. Volatiles profiling and antioxidant activity of moroccan Artemisia ifranensis J. didier and Anacyclus pyrethrum link essential oils. Egypt. J. Chem. 2020, 63, 3937–3947. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn-Tattou, M.; El oualidi, J. Flore Pratique du Maroc. Volume 3. Dicotyledones, Monocotyledones; Institut Scientifique, Université Mohammed V de Rabat: Rabat, Morocco, 2014; p. 755. [Google Scholar]
- Cazin, F.-J. Traité pratique et raisonné des plantes médicinales indigènes. Textes. Boulogne, l’auteur; Labé. 1er vol., XI-661 p. Paris 1850. Available online: https://uses.plantnet-project.org/fr/Pyr%C3%A8thre_(Cazin_1868) (accessed on 25 September 2022).
- Humphries, C.J. A revision of the genus Anacyclus L. (Compositae: Anthemideae). Bulletin of British Museum (Natural History). Botany 1979, 7, 142. [Google Scholar]
- Cherrat, A.; Elazzouzi, H.; Bouzoubae, A.; El amrani, M.; Oulhaj, H.; Boukil, A.; Zair, T. Ethno botanical study and socio-economic role of Anacyclus pyrethrum L. and Thymus zygis subsp. gracilis (Boiss.), in the Timahdite town, province of Ifrane-Morocco. J. Chem. Pharm. Res. 2015, 7, 385–398. [Google Scholar]
- Jalayer, N.N.; Niakan, M.; Khodadadi, E. Determination of antibacterial activity of Anacyclus pyrethrum extract against some of the oral bacteria: An in vitro study. J. Dent. 2012, 13, 59–63. [Google Scholar]
- Boulos, L. Medicinal plants of North Africa; Reference Publications Inc.: Algonac Michigan, MI, USA, 1983. [Google Scholar]
- Annalakshmi, R.; Uma, R.; Subash Chandran, G.; Muneeswaran, A. Commonplants used in the treatment of jaundice in southern India as a natural remefier—a review. Indian J. Drugs Dis. 2012, 1, 47–50. [Google Scholar]
- Kumar, V.K.; Lalitha, K.G. Pharmacognostical studies on the root of Anacyclus pyrethrum DC. Indian J. Nat. Pro. Resour. 2012, 3, 518–526. [Google Scholar]
- Najem, M.; Nassiri, L.; Ibijbijen, J. Appellations vernaculaires des plantes toxiques à usage médicinal dans le Moyen Atlas central-Maroc Vernacular names of toxic plants used as medicine in the central Middle Atlas-Morocco. Ethnobot. Res. Appl. 2020, 20, 1–30. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tatou, M. Catalogue Des Plantes Vasculaires Rares, Menacées ou Endémiques du Maroc; Raimondo, F.M., Valdés, B., Eds.; Herbarium Mediterraneum: Rabat, Morocco, 1999; pp. 5–243. [Google Scholar]
- Ouarghidi, A.; Powell, B.; Martin, G.J.; Abbad, A. Traditional Sustainable Harvesting Knowledge and Distribution of a Vulnerable Wild Medicinal Root (A. pyrethrum var. pyrethrum) in Ait M’hamed Valley, Morocco. Econ. Bot. 2017, 71, 83–95. [Google Scholar] [CrossRef]
- El hassani, M.; Douiri, E.M.; Bammi, J.; Zidane, L.; Badoc, Q.; Douira, A. Plantes médicinales de la Moyenne Moulouya (Nord-Est du Maroc). Ethnopharmacologia 2013, 50, 39–53. [Google Scholar]
- Benlamdini, N.; Elhafian, M.; Rochdi, A.; Zidane, L. Etude floristique et ethnobotanique de la flore médicinale du Haut Atlas oriental (Haute Moulouya). J. Appl. Biosci. 2014, 78, 67–71. [Google Scholar] [CrossRef]
- Daoudi, A.; Nassiri, L.; Ibijbijen, J.; Et Boukil, A. Etude Ethnobotanique Du Pyrèthre D’afrique « Anacyclus Pyrethrum L.» Dans Le Cercle Meknès, El Hajeb, Khénifra Azrou Et Ifrane–Maroc-. Sci. Ed. Mersenne 2014, 6, 140504. [Google Scholar]
- Hachi, M.; Hachi, T.; Belahbib, N.; Dahmani, J.; Zidane, L. Contribution a l’etude floristique et ethnobotanique de la flore medicinaleutilisee au niveau de la ville de khenifra (maroc). Int. J. Innov. Appl. Stud. 2015, 11, 754–770. [Google Scholar]
- Bammou, M.; Daoudi, A.; Sellam, K.; El Rhaffari, L.; Ibijbijen, J.; Nassiri, L. Etude Ethnobotanique des Astéracées dans la Région Meknès-Tafilalet (Maroc). Int. J. Innov. Appl. Stud. 2015, 13, 789–815. [Google Scholar]
- El Hilah, F.; Ben Akka, F.; Dahmani, J.; Belahbib, N.; Zidane, L. Etude ethnobotanique des plantes médicinales utilisées dans le traitement des infections du système respiratoire dans le plateau central marocain. J. Anim. Plant Sci. 2015, 25, 3886–3897. [Google Scholar]
- Fadil, M.; Farah, A.; Haloui, T.; Rachiq, S. Etude ethnobotanique des plantes exploitées par les coopératives et les associations de la région Meknès-Tafilalet au Maroc. Phytothérapie 2015, 13, 19–30. [Google Scholar] [CrossRef]
- Elazzouzi, H.; Soro, A.; Elhilali, F.; Bentayeb, A.; Alaoui, M.; Belghiti, E. Phytochemical study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in vitro study of antibacterial activity of pyrethrum. Adv. Nat. Appl. Sci. 2014, 8, 131–140. [Google Scholar]
- Selles, C.; Dib, M.E.A.; Djabou, N.; Beddou, F.; Muselli, A.; Tabti, B.; Costa, J.; Hammouti, B. Antimicrobial activity and evolution of the composition of essential oil from Algerian Anacyclus pyrethrum L. through the vegetative cycle. Nat. Prod. Res. 2013, 27, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Khannouchi, S.; Elhilali, F.; Zair, T. Biocidal effects of aqueous extract of the roots of Anacyclus pyrethrum (Asteraceae) on Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). Mediterr. J. Chem. 2012, 1, 316–325. [Google Scholar] [CrossRef]
- Selles, C. Valorisation D’une Plante Médicinale à Activité Antidiabétique de la région de Tlemcen: Anacyclus pyrethrum L. Application de L’extrait Aqueux à L’inhibition de Corrosion D’un Acier Doux Dans H2SO4 0.5M. Doctoral Thesis, Universite Abou Bekr Belkaid, Tlemcen, Algerie, 2012; 175p. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, A.; Amalich, S.; Regragui, M.; Bouzoubae, A.; Elamrani, M.; Mahjoubi, M.; Bourakhouadar, M.; Zair, T. Polyphenols content and evaluation of antioxidant activity of Anacyclus pyrethrum (L.) Lag. From Timahdite a Moroccan Middle Atlas region. Int. J. Adv. Res. 2017, 5, 569–577. [Google Scholar] [CrossRef]
- Subasri, G.; John, A.S. Screening of phytochemical compounds, trace metals and Antimicrobial activity of Anacyclus pyrethrum. Int. J. Adv. Sci. Res. 2016, 2, 32–37. [Google Scholar] [CrossRef]
- Boonen, J.; Sharma, V.; Dixit, V.; De Spiegeleer, B. New N-alkylamides from Anacyclus pyrethrum. Planta Med. 2011, 77, PG94. [Google Scholar] [CrossRef]
- Usmani, A.; Khushtar, M.; Arif, M.; Siddiqui, M.A.; Sing, S.P.; Mujahid, M.d. Pharmacognostic and phytopharmacology study of Anacyclus pyrethrum: An insight. J. Appl. Pharm. Sci. 2016, 6, 144–150. [Google Scholar] [CrossRef]
- Annalakshmi, R.; Uma, R.; Subash, C.G.; Muneeswaran, A. A treasure of Medicinal herb Anacyclus pyrethrum A review. Indian J. Drugs Dis. 2012, 1, 59–67. [Google Scholar]
- Crombie, L. Isolation and Structure of an N-iso Butyldienediynamide from Pellitory (Anacyclus pyrethrum DC.). Nature 1954, 174, 832–833. [Google Scholar] [CrossRef]
- Kushwaha, M.N.; Vijay, S.J.; Swatantra, P. Plant Anacyclus pyrethrum-A Review. Res. J. Pharmacogn. Phytochem. 2012, 4, 164–170. [Google Scholar]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Sharma, V.; Dixit, V.K.; Burvenich, C.; De Spiegeleer, B. LC-MS N-alkylamide profiling of an ethanolic Anacycluspyrethrumroot extract. Planta Med. 2012, 78, 1787–1795. [Google Scholar] [PubMed]
- Canli, K.; Yetgin, A.; Akata, I.; Altuner, E.M. Antimicrobial Activity and Chemical Composition Screening of Anacyclus pyrethrum Root. Indian J. Pharma. Edu. Res. 2017, 51, S244–S248. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Sujarajini, V. Bioactivities Of Anacyclus pyrethrum (L.) Lag. extracts and natural products. Turk. J. Anal. Chem. 2020, 2, 55–61. [Google Scholar]
- Althaus, J.B.; Malyszek, C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Alkamides from Anacyclus pyrethrum L. and their in vitro antiprotozoalactivity. Molecules 2017, 22, 796. [Google Scholar] [CrossRef]
- Ji, R.; Quan, Q.; Guo, X.; Zhang, J.; Song, Y.; Zhu, M.; Tan, P.; Han, J.; Liu, Y. Simultaneous determination of five N-alkylamides in the root of Anacyclus pyrethrum by HPLC and profiling of components in its methanolic root extract by UPLC/Q-TOF-MS. Rev. Bras. Farmacogn. 2019, 29, 152–161. [Google Scholar] [CrossRef]
- Elazzouzi, H.; Zekri, N.; Zair, T.; Alaoui El Belghiti, M. Total phenolic and flavonoid contents of Anacyclus pyrethrum Link plant extracts and their Antioxidant activity. Karbala Int. J. Mod. Sci. 2019, 5, 280–287. [Google Scholar] [CrossRef]
- Selles, C.; Dib, M.E.A.; Allali, H.; Tabti, B. Evaluation of Antimicrobial and Antioxidant Activities of Solvent Extracts of Anacyclus Pyrethrum L., from Algeria. Mediterr. J. Chem. 2012, 2, 408–415. [Google Scholar] [CrossRef]
- Ardalani, H.; Amiri, F.H.; Hadipanah, A.; Kongstad, K.T. Potential antidiabetic phytochemicals in plant roots: A review of in vivo studies. J. Diabetes Metab. Disord. 2021, 20, 1837–1854. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Abdelaali, B.; Omar, B.; Najoua, S.; Hamada, I.; Hanae, N.M.; Mohamed, E.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar]
- Hachi, M.; Ouafae, B.; Hachi, T.; Mohamed, E.B.; Imane, B.; Atmane, R.; Zidane, L. Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco). Lazaroa 2016, 37, 135–144. [Google Scholar] [CrossRef]
- Shahraki, M.R.; Dehvari, J.; Shahrakipoo, M.; Shahreki, E.; Sharaki, R.A.; Dashipour, A.R. The Effects of Anacyclus pyrethrum Alcoholic Root Extract on FSH, LH, Testosterone and Sperm Count in Diabetic Male Rats. Zahedan J. Res. Med. Sci. 2019, 21, e88515. [Google Scholar] [CrossRef]
- Tyagi Satyanand, M.; Hashim, M.; Narendra Kumar, S.; Manoj Kumar, S.; Poonam, B.; Rahul Kumar, S. Antidiabetic Effect of Anacyclus pyrethrum DC in Alloxan Induced Diabetic Rats. Eur. J. Biol. Sci. 2011, 3, 117–120. [Google Scholar]
- Selles, C.; Medjdoub, H.; Dib, M.E.A.; Zerriouh, M.; Tabti, B. Anti-diabetic activity of aqueous root extract of Anacyclus pyrethrum L. In streptozotocin-induceddiabetic rats. J. Med. Plants Res. 2012, 6, 3193–3198. [Google Scholar] [CrossRef]
- Kumar, V.K.; Lalitha, K.G. In vitro study on α-amylase inhibitory activity of an Ayurvedic medicinal plant, Anacyclus pyrethrum DC root. Indian J. Pharm. 2014, 46, 350–351. [Google Scholar]
- Muralikrishnan, K.; Asokan, S.; Geetha, P.P.R.; Ahmed, K.S.; Ayyappadasan, G. Comparative evaluation of the local anesthetic activity of root extract of Anacyclus pyrethrum and its interaction at the site of injection in guinea pigs. Anesth. Essays Res. 2017, 11, 444–448. [Google Scholar] [CrossRef]
- Bhatt, H.V.; Panchal, G.; Devasankariah, G.; Gopalakrishna, G.; Patel, V.K. Local anaesthetic activity of Anacyclus pyrethrum in laboratory animals. Asian J. Microbiol. Biotechnol. Environ. Sci. Pap. 2001, 3, 83–85. [Google Scholar]
- Patel, V.K.; Patel, R.V.; Bhatt, H.; Gopalakrishna, G.; Devasankariah, G. A clinical appraisal of Anacyclus pyrethrum root extract in dental patients. Phytother. Res. 1992, 6, 158–159. [Google Scholar] [CrossRef]
- Burgess, I.F.; Brown, C.M.; Burgess, N.A. Synergized pyrethrin mouses a new approach to head lice eradication. Pyrethrum Post 1994, 19, 41–46. [Google Scholar]
- Elazzouzi, H.; Khennouchi, S.; Bentayeb, A.; Elhilali, F.; Zair, T. Biocidal effects of alkaloids extracted from Anacyclus pyrethrum L. (Asteraceae) on Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). Int. J. Innov. Appl. Stud. 2015, 13, 756–774. [Google Scholar]
- Atia-tul-Wahab, M.S.; Uzma, N.; Shakil, A.; Choudharyand Atta-ur-Rahman, M.I. Study of Anacyclus pyrethrum L. Root Extract againstAedesaegypti Linn. Larvae: Potential Vector Control for Dengue Viral Fever. Rec. Nat. Prod. 2021, 15, 476–486. [Google Scholar] [CrossRef]
- Rechelson, E. Pharmacology of Antidepressants. Mayo. Clin. Proc. 2001, 76, 516–527. [Google Scholar] [CrossRef]
- Badhe, S.R.; Badhe, R.V.; Ghaisas, M.; Chopade, V.V.; Deshpande, A.D. Evaluations of antidepressant activity of Anacyclus pyrethrum root extract. International. J. Green Pharm. 2010, 4, 79. [Google Scholar] [CrossRef]
- Sujith, K.; Suba, V.; Ronald Darwin, C. Neuropharmacological Profile of Ethanolic Extract of Anacyclus Pyrethrum in Albino Wistar Rats. Int. J. Pharm. Sci. Res. 2011, 2, 2109–2114. [Google Scholar]
- Billing, J.; Sherman, P.W. Antimicrobial Functions of Spices: Why Some Like It Hot. Q. Rev. Biol. 1998, 73, 3–49. [Google Scholar] [CrossRef]
- Sqalli, H.; EL Ouarti, A.; Ennabili, A.; Ibnsouda, S.; Farah, A.; Haggoud, A.; Houari, A.; Iraqui, M. Evaluation de l’effet antimycobactérien de plantes du centre-nord du maroc. Bull. Soc. Pharm. Bordx. 2007, 146, 271–288. [Google Scholar]
- Jalayer, N.N.; Niakan, M.; Khodadadi, E.; Mohamadi-Motlagh, A. The Antibacterial Activity of Methanolic Anacyclus Pyrethrum and Pistacia Lentiscus L. Extract on Escherichia Coli. Iran. J. Microbiol. 2016, 8, 372–376. [Google Scholar]
- Chavan, P.A. Evaluation of Antimicrobial Activity of Various Medicinal Plants Extracts of Latur Zone against Pathogens. Int. J. Life Sci. Sci. Res. 2016, 2, 612–618. [Google Scholar] [CrossRef]
- Rimbau, V.; Risco, E.; Canigueral, S.; Iglesias, J. Anti-inflammatory Activity of Some Extracts from Plants used in the Traditional Medicine of North-African Countries. Phytother. Res. 1996, 10, 421–423. [Google Scholar] [CrossRef]
- Shahrakipour, M.; Arab, M.R. The Effects of Aqueous Extract of Anacyclus Pyrethrum on Sperm Count and Reproductive Organs in Adult Male Rats. J. Res. Med. Sci. 2013, 17, 42–46. [Google Scholar]
- Sharma, V.; Boonen, J.; Spiegeleer, B.D.; Dixit, V.K. Androgenic and Spermatogenic Activity of Alkylamide-Rich Ethanol Solution Extract of Anacyclus Pyrethrum DC. Phytother. Res. 2013, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Evaluation of the Anabolic, Aphrodisiac and Reproductive Activity of Anacyclus pyrethrum DC in Male Rats. Sci. Pharm. 2009, 77, 97–110. [Google Scholar] [CrossRef]
- Adloo, M.; Bahadori, M.; Shojaeifard, M.B. The impact of hydroalcoholic extract of Anacyclus pyrethrum plant on epileptic seizure induced by pentylenetetrazole in male rat. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 72. [Google Scholar] [CrossRef]
- Yousef, B.A.; Awad, Z.; Adam, S.; Abdelgadir, S.; Mergani, A. Assessment of Anticonvulsant Activities of Petroleum Ether Extract of Anacyclus pyrethrum Roots on Experimental Rats. Pharm. Biomed. Res. 2021, 7, 47–54. [Google Scholar] [CrossRef]
- Bezza, K.; El Gabbas, Z.; Laadraoui, J.; Laaradia, M.A.; Oufquir, S.; Chait, A. Ameliorative potential of Anacyclus pyrethrum extract in generalized seizures in rat: Possible cholinergic mediated mechanism. Bangladesh J. Pharm. 2019, 14, 188–195. [Google Scholar] [CrossRef]
- Kalam, M.A.; Shafat, K.M.; Ghulamuddin, S.; Ghufran, A. Evaluation of Anticonvulsant Activity of Aqer Qerha (Anacyclus pyrethrum DC.) Root in Experimental Animals. Hippocrat. J. Unani. Med. 2015, 10, 1–12. [Google Scholar]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Tripathi, M.; Gupta, Y.K. Effect of Anacyclus pyrethrum on pentylenetetrazole-induced kindling, spatial memory, oxidative stress and rho-kinase II expression in mice. Neurochem. Res. 2013, 38, 547–556. [Google Scholar] [CrossRef]
- Zaidi, S.M.A.; Pathan, S.; Jain, G.; Ahmad, F.; Jamil, S.; Singh, S.; Khar, R. Anticonvulsant and neuropharmacological studies of Ana- cyclus pyrethrum root extract. Neurosci. Res. 2009, 65, S249–S250. [Google Scholar] [CrossRef]
- Usmani, A.; Mujahid; Khushtar, M.; Siddiqui, H.H.; Rahman, A. Hepatoprotective effect of Anacyclus pyrethrum Linn. against antitubercular drug-induced hepatotoxicity in SD rats. J. Complement. Integr. Med. 2016, 13, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mansoori, B.; Baradaran, P.C.; Baradaran, S.C.; Baradaran, B. Anacyclus Pyrethrum Extract Exerts Anticancer Activities on the Human Colorectal Cancer Cell Line (HCT) by Targeting Apoptosis, Metastasis and Cell Cycle Arrest’. J. Gastrointest. Cancer 2017, 48, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Yousaf, F.; Shahid, M.; Riaz, M.; Atta, A.; Fatima, H. Immunomodulatory potential of Anacyclus pyrethrum (L.) and Mucuna pruriens (L.) in male albino rats. J. Biol. Regul. Homeost. Agents 2017, 31, 425–429. [Google Scholar]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Immunomodulatory activity of petroleum ether extract of Anacyclus pyrethrum. Pharm. Bio. 2010, 48, 1247–1254. [Google Scholar] [CrossRef]
- Najem, M.; Belaidi, R.; Harouak, H.; Bouiamrine, E.; Ibijbijen, J.; Nassiri, L. Occurrence de plantes toxiques en phytothérapie traditionnelle dans la région du Moyen Atlas central Maroc. J. Anim. Plant Sci. 2018, 35, 5651–5673. [Google Scholar]
- Chaachouay, N.; Benkhnigue, O.; Douira, A.; Zidane, L. Poisonous medicinal plants used in the popular pharmacopoeia of the Rif, northern Morocco. Toxicon 2020, 189, 24–32. [Google Scholar] [CrossRef]
- Kumar, K.V.; Lalitha, K.G. Acute Oral Toxicity Studies of Anacyclus Pyrethrum Dc Root in Albino Rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 675–678. [Google Scholar]
- Najem, M.; Harouak, H.; Ibijbijen, J.; Nassiri, L. Oral disorders and ethnobotanical treatments: A field study in the central Middle Atlas (Morocco). Heliyon 2020, 6, e04707. [Google Scholar] [CrossRef]
- Hmamouchi, M. Les Plantes Medicinals et Aromatiques Marocains, Utilisation Traditionnelles, Marché, Biologie, écologie, Chimie, Pharmacologie, Toxicologie, 2nd ed.; Fedala: Rabat, Morocco, 2001; p. 389. [Google Scholar]
- Daoudi, A.; Bammou, M.; Ibijbijen, J.; Nassiri, L. Antibacterial Activity of Aqueous Extracts of Anacyclus Pyrethrum (L) Link and Corrigiola Telephiifolia-Pourr. From the Middle Atlas Region-Morocco. Eur. Sci. J. 2017, 13, 1857–7881. [Google Scholar]
- Hemalal, K.P. Isolation, Characterization and Some Synthesis Studies on Insecticidal Natural Products from Sri Lankan Plants. Ph.D. Thesis, The Open University, London, UK, 1998. [Google Scholar]
- Jawhari, F.Z.; El Moussaoui, A.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Bouhrim, M.; Kharchoufa, L.; Miry, A.; Bousta, D.; Bari, A. Evaluation of the acute toxicity of the extracts of Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus Maire in Swiss mice. Vet. World 2021, 14, 457–467. [Google Scholar] [PubMed]
- Lodhi, M.; Memon, Z.; Shaheen, S.; Kamran, F. The herbal cure for epilepsy: An overview pakistan journal of medicine and dentistry. Phytochemistry 2017, 6, 48–53. [Google Scholar]
- Ramchandra, A.M.; Chacko, B.; Victor, P.J. Pyrethroid Poisoning. Indian J. Crit. Care Med. 2019, 23 (Suppl. S4), S267–S271. [Google Scholar] [CrossRef] [PubMed]
- Sujith, K.; Darwin, R.; Suba, V. Toxicological evaluation of ethanolic extract of Anacyclus pyrethrum in albino wistar rats. Asian Paicfic J. Trop. Dis. 2012, 2, 437–441. [Google Scholar] [CrossRef]
- Bezza, K.; Laadraoui, J.; El Gabbas, Z.; Laaradia, M.A.; Oufquir, S.; Aboufatima, R. Effects of Anacyclus pyrethrum on affective behaviors and memory during withdrawal from cigarette smoke exposure in rats. Pharmacogn. Mag. 2020, 16, S123–S132. [Google Scholar] [CrossRef]
- Gautam, O.P.; Savita, V.; Jain, S.K. Anticonvulsant and myorelaxation activity of anacyclus pyrethrum dc. (Akarkara) root extract. Pharmacologyonline 2011, 1, 121–125. [Google Scholar]
- Shahrajabian, M.H.; Wenli, S.; Qi, C. Spanish chamomile (Anacyclus pyrethrum) and pyrethrum (Tanacetum cineraiifolium): Organic and natural pesticides and treasure of medicinal herbs. Not. Sci. Biol. 2021, 13, 10816. [Google Scholar] [CrossRef]
- Manouze, H.; Ghestem, A.; Bennis, M.; Zahra, S.; Benoliel, J.-J. Antiseizure effects of Anacyclus pyrethrum in socially isolated rats with and without a positive handling strategy. Epilepsia Wiley 2021, 62, 2551–2564. [Google Scholar] [CrossRef]
- Anonymous. Ecological & Human Health Assessment Report Integrated Mosquito and Vector Management Programs. Appendix B; Cardno entrix: Portland, OR, USA, 2013; Available online: https://www.mosquitoes.org/files/b28d1b22e/MVCAC-DPEIR_APP-B_Risk-Assessment_JUN2013_R7.pdf (accessed on 25 September 2022).
- Casida, J.E. Pyrethrum Flowers and Pyrethroid Insecticides. Environ. Health Perspect. 1980, 34, 189–202. [Google Scholar] [CrossRef]
- Hamimed, S.; Boulebda, N.; Laouer, H.; Belkhiri, A. Bioactivity-guided isolation of alkamides from a cytotoxic fraction of the ethyl acetate extract of Anacyclus pyrethrum (L.) DC. roots. Curr. Issues Pharm. Med. Sci. 2018, 31, 180–185. [Google Scholar] [CrossRef]
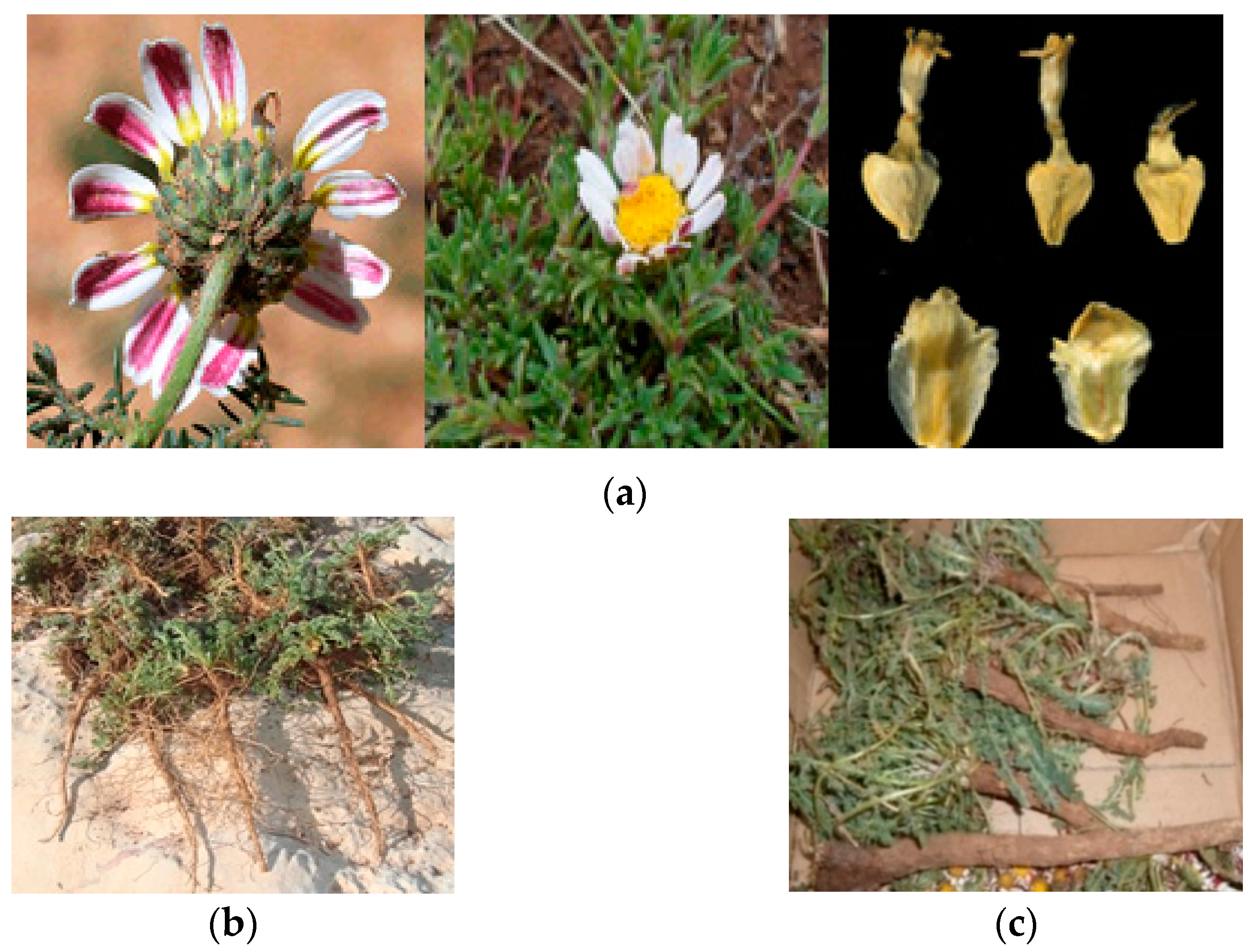
| Used Part | Mode of Preparation | Medicinal Use | References |
|---|---|---|---|
| Root | Decoction | Stomach diseases and stomatitis | [20] |
| Stem | Powder | Cysts of the reproductive system | [21] |
| Root | Powder | Rheumatic, gastrointestinal, oral diseases, respiratory, genitourinary, skin and dermatitis diseases | [22,23] |
| Root | Decoction/Powder | Osteoarthritis disorders, stomatitis, inflammation of the urinary and genital organs, and respiratory diseases | [24,25] |
| Root | Infusion/Decoction | sore throats, toothache and skin revitalization | [26] |
| Root | Powder/Decoction | Articular rheumatism, dental pain, intestinal pain and colic | [1] |
| Variety | Used Part | Extract | Method | Result (in IC50 or Absorbance (A)) | References |
|---|---|---|---|---|---|
| var. Pyrethrum | Roots Flowers Seeds Leaves | EtOH | DPPH˙ | 0.18 mg/mL 0.16 mg/mL 0.01 mg/mL 0.04 mg/mL | [6] |
| Roots Flowers Seeds Leaves | ABTS | 0.14 mg/mL 0.07 mg/mL 0.05 mg/mL 0.03 mg/mL | |||
| Roots Flowers Seeds Leaves | FRAP | 1.19 mg/mL 1.08 mg/mL 0.49 mg/mL 0.38 mg/mL | |||
| var. Depressus | Roots Flowers Seeds Leaves | DPPH˙ | 0.07 mg/mL 0.08 mg/mL 0.04 mg/mL 0.03 mg/mL | ||
| Roots Flowers Seeds Leaves | ABTS | 0.05 mg/mL 0.05 mg/mL 0.05 mg/mL 0.03 mg/mL | |||
| Roots Flowers Seeds Leaves | FRAP | 0.38 mg/mL 0.59 mg/mL 0.25 mg/mL 0.23 mg/mL | |||
| var. Pyrethrum | Stems/leaves | MeOH ext. | DPPH˙ | 0.056 mg/mL | [46] |
| Aqu. ext. | 0.114 mg/mL | ||||
| Chl. ext. | 0.154 mg/mL | ||||
| Root | MeOH ext. | DPPH˙ | 12.38 µg/mL | [39] | |
| FRAP | 50.89 µg/mL | ||||
| BCB | 107.07 µg/mL | ||||
| Aqu. ext. | DPPH˙ | 13.41 µg/mL | |||
| FRAP | 60.17 µg/mL | ||||
| BCB | 120.66 µg/mL | ||||
| MeOH ext. | DPPH˙ | 0.15 mg/mL | [45] | ||
| AcEth ext. | 0.14 µg/mL | ||||
| BuOH ext. | 0.15 mg/mL | [8] | |||
| HE | DPPH˙ | 30.50 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elazzouzi, H.; Fadili, K.; Cherrat, A.; Amalich, S.; Zekri, N.; Zerkani, H.; Tagnaout, I.; Hano, C.; Lorenzo, J.M.; Zair, T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants 2022, 11, 2578. https://doi.org/10.3390/plants11192578
Elazzouzi H, Fadili K, Cherrat A, Amalich S, Zekri N, Zerkani H, Tagnaout I, Hano C, Lorenzo JM, Zair T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants. 2022; 11(19):2578. https://doi.org/10.3390/plants11192578
Chicago/Turabian StyleElazzouzi, Hanane, Kamal Fadili, Ali Cherrat, Smail Amalich, Nadia Zekri, Hannou Zerkani, Imane Tagnaout, Christophe Hano, Jose M. Lorenzo, and Touria Zair. 2022. "Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review" Plants 11, no. 19: 2578. https://doi.org/10.3390/plants11192578
APA StyleElazzouzi, H., Fadili, K., Cherrat, A., Amalich, S., Zekri, N., Zerkani, H., Tagnaout, I., Hano, C., Lorenzo, J. M., & Zair, T. (2022). Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants, 11(19), 2578. https://doi.org/10.3390/plants11192578








