The Pattern of Rare Earth Elements Like a Possible Helpful Tool in Traceability and Geographical Characterization of the Soil-Olive System (Olea europaea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Layout, Plant Material, and Sampling
2.3. Sample Preparation
2.4. Elemental Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Soil
3.2. Olive Pulp
3.3. Plant/Soil Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryła, P. The impact of obtaining a European quality sign on origin food producers. Qual. Assur. Saf. Crops Foods 2018, 10, 155–164. [Google Scholar] [CrossRef]
- Aceto, M.; Robotti, E.; Oddone, M.; Baldizzone, M.; Bonifacino, G.; Bezzo, G.; Di Stefano, R.; Gosetti, F.; Mazzucco, E.; Manfredi, M.; et al. A traceability study on the Moscato wine chain. Food Chem. 2013, 138, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A preliminary study on the authentication and traceability of extra virgin olive oil made from Taggiasca olives by means of trace and ultra-trace elements distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef] [PubMed]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Nicolini, G.; Versini, G. Characterisation of authentic Italian extra-virgin olive oils by stable isotope ratios of C, O and H and mineral composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Chiocchini, F.; Portarena, S.; Ciolfi, M.; Brugnoli, E.; Lauteri, M. Isoscapes of carbon and oxygen stable isotope compositions in tracing authenticity and geographical origin of Italian extra-virgin olive oils. Food Chem. 2016, 202, 291–301. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food authentication: State of the art and prospects. Curr. Opin. Food Sci. 2016, 10, 22–31. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope ratio analysis to determine the geographical origin of foods in the European Union. Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Armenta, S.; de la Guardia, M. Trace-element composition and stable-isotope ratio for discrimination of foods with Protected Designation of Origin. Trends Anal. Chem. 2009, 28, 1295–1311. [Google Scholar] [CrossRef]
- Medini, M.; Janin, M.; Verdoux, P.; Techer, I. Methodological development for 87Sr/86Sr measurement in olive oil and preliminary discussion of its use for geographical traceability of PDO Nîmes (France). Food Chem. 2015, 171, 78–83. [Google Scholar] [CrossRef]
- Tescione, I.; Marchionni, S.; Casalini, M.; Vignozzi, N.; Mattei, M.; Conticelli, S. 87Sr/86Sr isotopes in grapes of different cultivars: A geochemical tool for geographic traceability of agriculture products. Food Chem. 2018, 258, 374–380. [Google Scholar] [CrossRef]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Chammem, N. Phenols, flavors, and the mediterranean diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
- Barbera, M. Reuse of food waste and wastewater as a source of polyphenolic compounds to use as food additives. J. AOAC Int. 2020, 103, 906–914. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Hidalgo, M.J.; Pozzi, M.T.; Furlong, O.J.; Marchevsky, E.J.; Pellerano, R.G. Classification of organic olives based on chemometric analysis of elemental data. Microchem. J. 2018, 142, 30–35. [Google Scholar] [CrossRef]
- Kang, X.; Zhao, Y.; Shang, D.; Zhai, Y.; Ning, J.; Sheng, X. Elemental analysis of sea cucumber from five major production sites in China: A chemometric approach. Food Control 2018, 94, 361–367. [Google Scholar] [CrossRef]
- Luykx, D.M.A.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Sayago, A.; González-Domínguez, R.; Beltrán, R.; Fernández-Recamales, A. Combination of complementary data mining methods for geographical characterization of extra virgin olive oils based on mineral composition. Food Chem. 2018, 261, 42–50. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Farmaki, E.G.; Thomaidis, N.S.; Minioti, K.S.; Ioannou, E.; Georgiou, C.A.; Efstathiou, C.E. Geographical characterization of greek olive oils using rare earth elements content and supervised chemometric techniques. Anal. Lett. 2012, 45, 920–932. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Haroutounian, S.A.; Georgiou, C.A. Rare earth elements minimal harvest year variation facilitates robust geographical origin discrimination: The case of PDO “Fava Santorinis”. Food Chem. 2016, 213, 238–245. [Google Scholar] [CrossRef]
- Taylor, A.; Barlow, N.; Day, M.P.; Hill, S.; Martine, N.; Patriarca, M. Atomic Spectrometry Update: Review of advances in the analysis of clinical and biological materials foods and beverages. J. Anal. At. Spectrom. 2018, 33, 338–382. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R. Research in olive oil: Challenges for the near future. J. Agric. Food Chem. 2010, 58, 12569–12577. [Google Scholar] [CrossRef]
- Otero, N.; Vitòria, L.; Soler, A.; Canals, A. Fertiliser characterisation: Major, trace and rare earth elements. Appl. Geochem. 2005, 20, 1473–1488. [Google Scholar] [CrossRef]
- Joebstl, D.; Bandoniene, D.; Meisel, T.; Chatzistathis, S. Identification of the geographical origin of pumpkin seed oil by the use of rare earth elements and discriminant analysis. Food Chem. 2010, 123, 1303–1309. [Google Scholar] [CrossRef]
- Durante, C.; Bertacchini, L.; Bontempo, L.; Camin, F.; Manzini, D.; Lambertini, P.; Marchetti, A.; Paolini, M. From soil to grape and wine: Variation of light and heavy elements isotope ratios. Food Chem. 2016, 210, 648–659. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements. An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Liang, T.; Ding, S.; Song, W.; Chong, Z.; Zhang, C.; Li, H. A review of fractionations of rare earth elements in plant. J. Rare Earths 2008, 26, 7–15. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Markert, B. Instrumental Multielement Analysis of Plant Samples; Osnabrueck University: Osnabrueck, Germany, 1993; ISBN 3-527-28297-1. [Google Scholar]
- Barbera, M.; Zuddas, P.; Palazzolo, E.; Saiano, F. The distribution of Rare Earth Elements discriminates the growth substrate of Vitis vinifera L. Chemosphere 2021, 266, 128993. [Google Scholar] [CrossRef]
- Censi, P.; Saiano, F.; Pisciotta, A.; Tuzzolino, N. Geochemical behaviour of rare earths in Vitis vinifera grafted onto different rootstocks and growing on several soils. Sci. Total Environ. 2014, 473, 597–608. [Google Scholar] [CrossRef]
- Pisciotta, A.; Tutone, L.; Saiano, F. Distribution of YLOID in soil-grapevine system (Vitis vinifera L.) as tool for geographical characterization of agro-food products. A two years case study on different grafting combinations. Food Chem. 2017, 221, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Crescimanno, G.; Garofalo, P. Management of irrigation with saline water incracking clay soils. Soil Sci. Soc. Am. J. 2006, 70, 1774–1787. [Google Scholar] [CrossRef]
- Samczynski, Z.; Dybczynski, R.S.; Polkowska-Motrenko, H.; Chajduk, E.; Pyszynska, M.; Danko, B.; Czerska, E.; Kulisa, K.; Doner, K.; Kalbarczyk, P. Two new reference materials based on tobacco leaves: Certification for over a dozen of toxic and essential elements. Sci. World J. 2012, 2012, 216380. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, L.; Steinmann, M.; Lucot, E.; Pierret, M.C.; Stille, P.; Prunier, J.; Badot, P.M. Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 2013, 366, 143–163. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Byrne, R.H.; Li, B. Comparative complexation behavior of the rare earths. Geochim. Cosmochim. Acta 1995, 59, 4575–4589. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 2. [Google Scholar] [CrossRef]
- Beltrán, B.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef]
- Pošćić, F.; Žanetić, M.; Fiket, Z.; Furdek Turk, M.; Mikac, N.; Bačić, N.; Lučić, M.; Romić, M.; Bakić, H.; Jukić Špika, M.; et al. Accumulation and partitioning of rare earth elements in olive trees and extra virgin olive oil from Adriatic coastal region. Plant Soil 2020, 448, 133–151. [Google Scholar] [CrossRef]
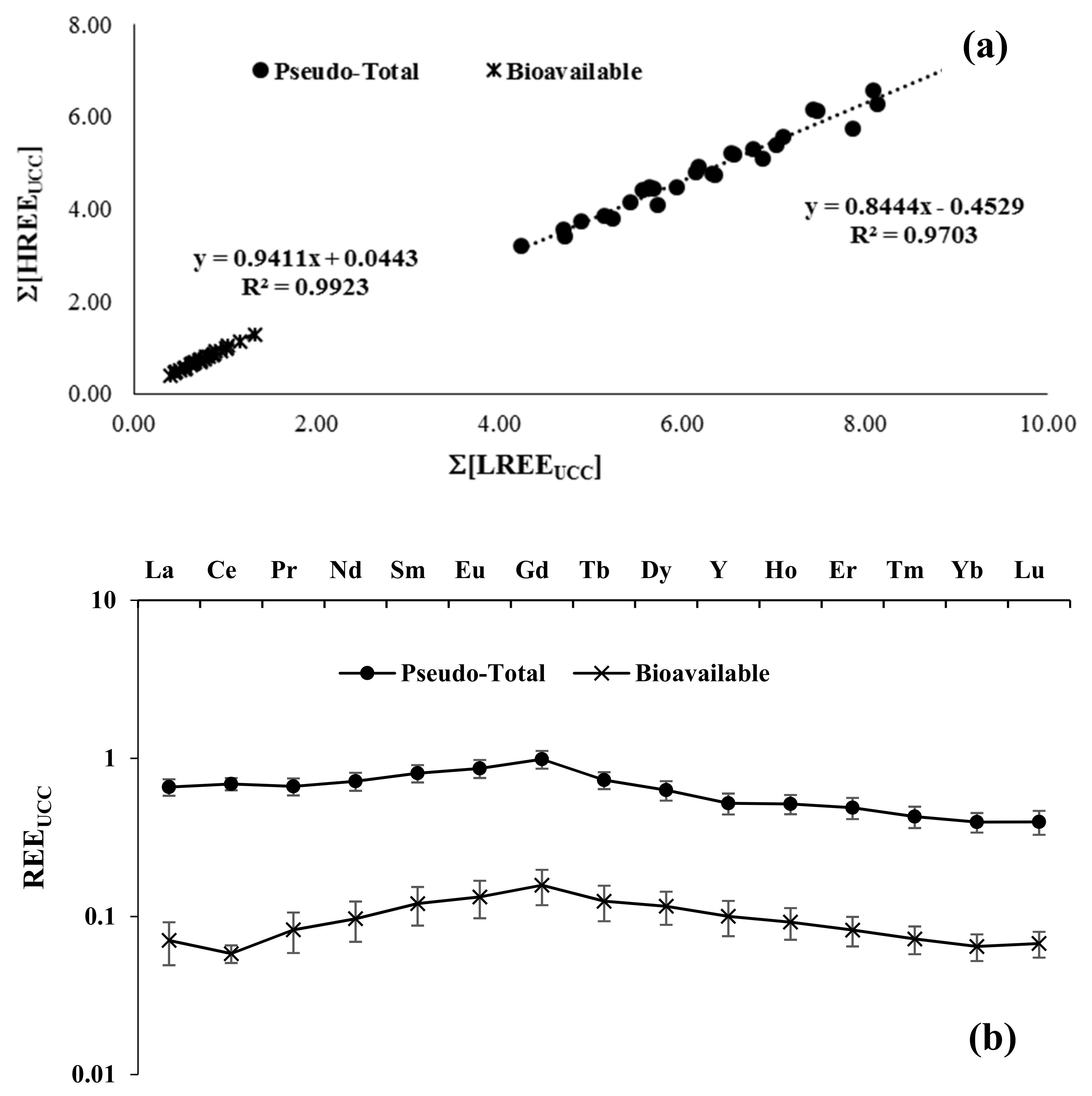
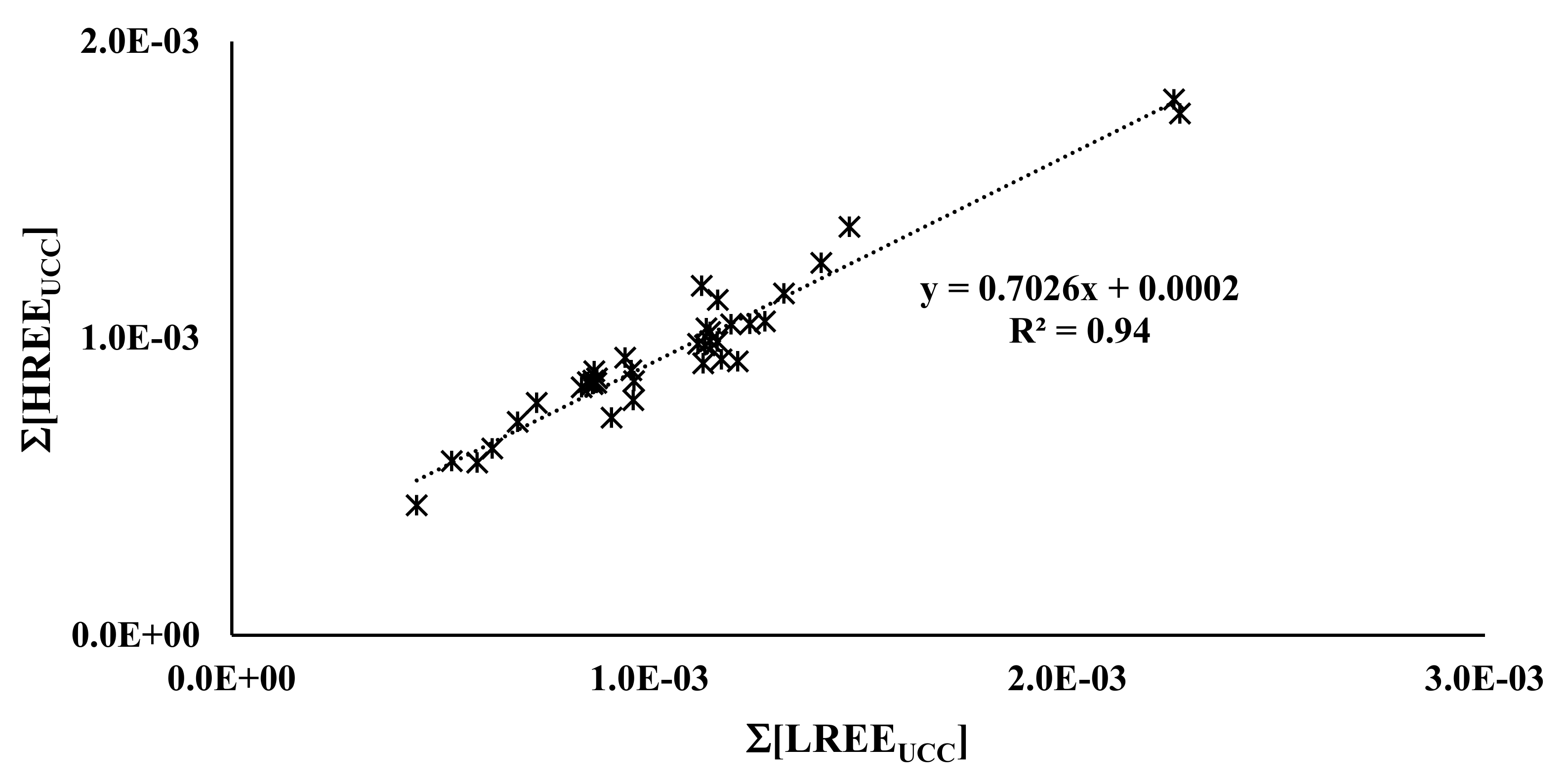
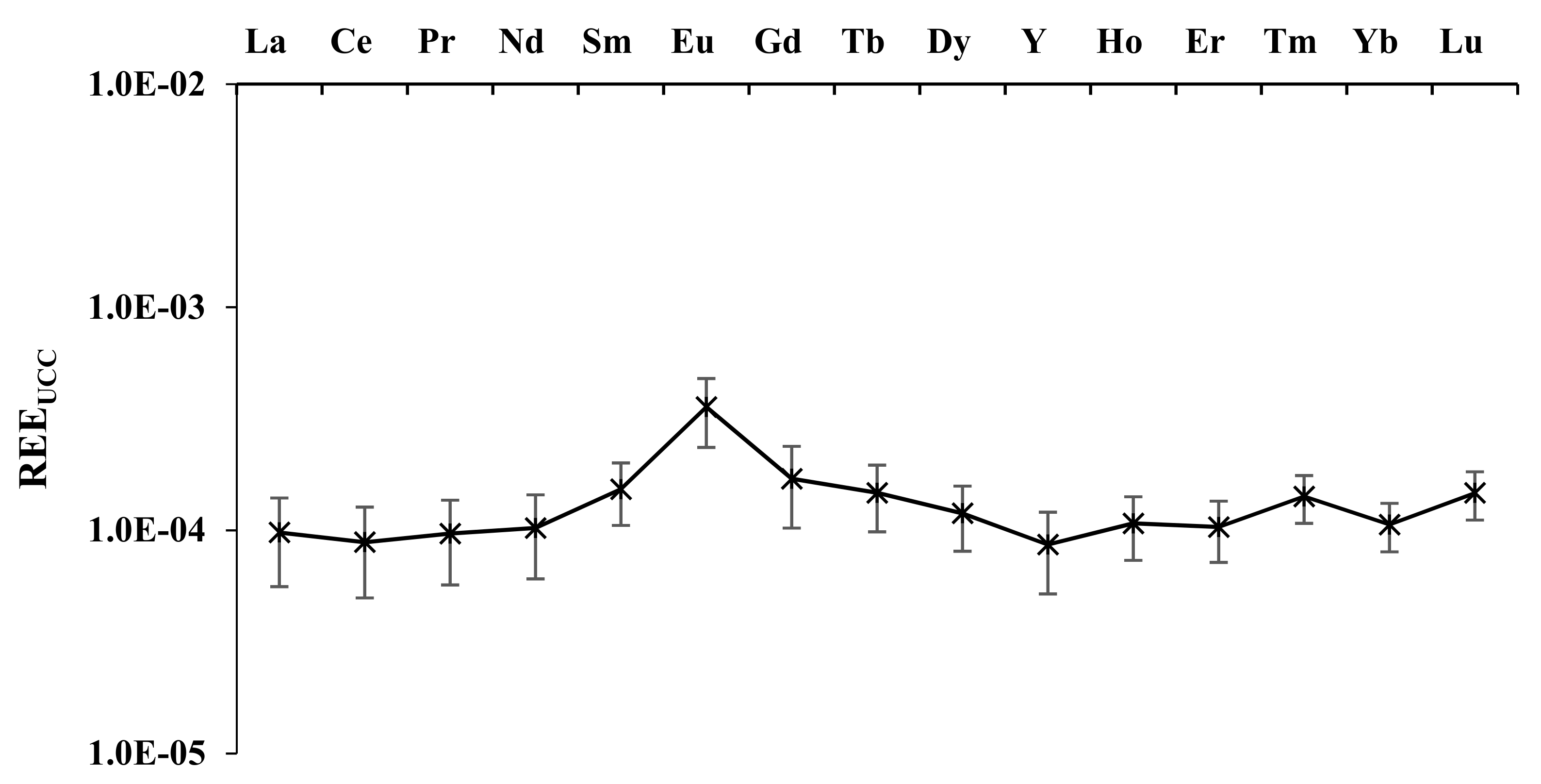
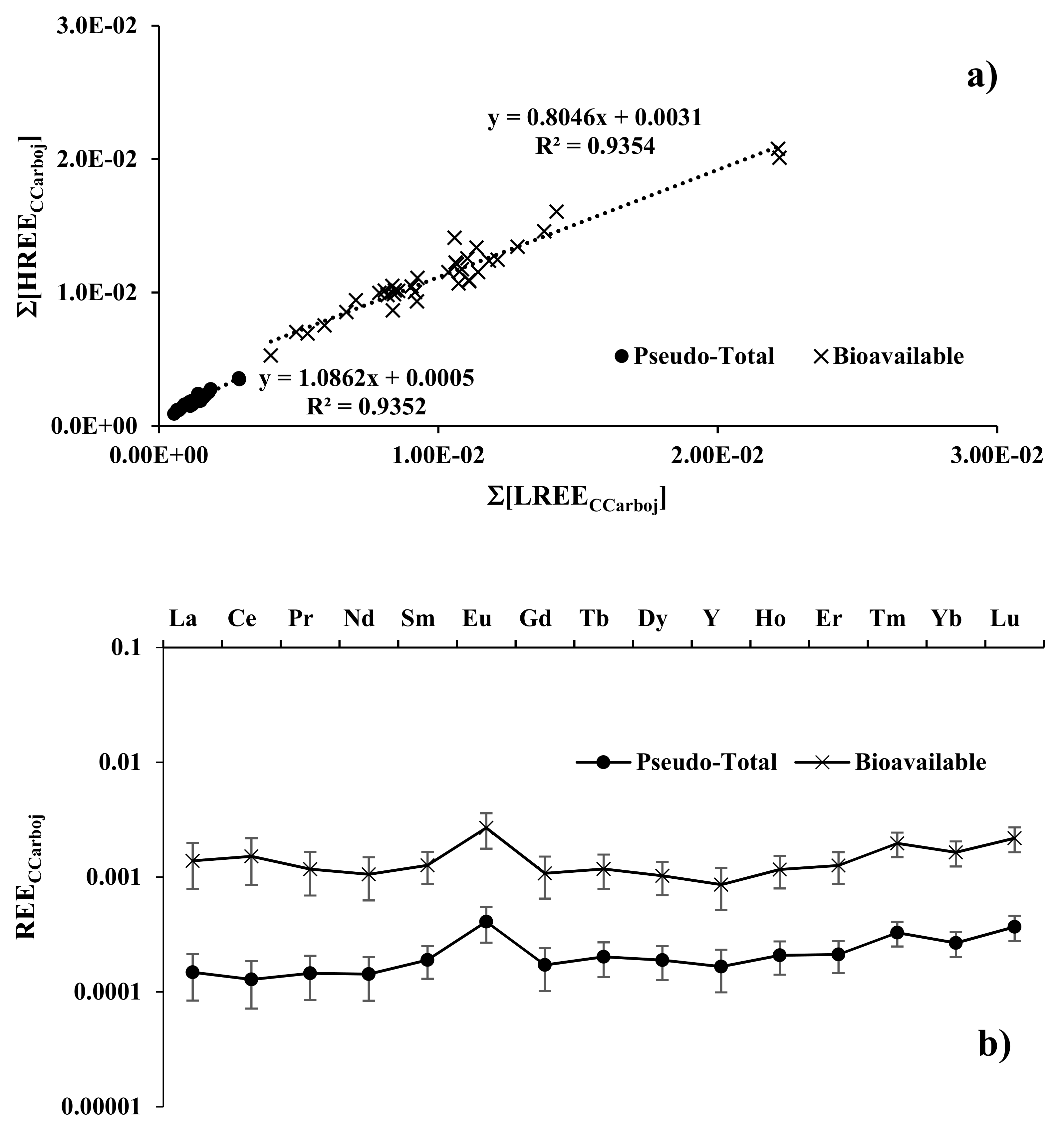
| N | Cultivar |
|---|---|
| 1 | Pizzutella |
| 2 | Nuciddara |
| 3 | Verdella |
| 4 | Rotondella |
| 5 | Selvatico |
| 6 | Ogliara |
| 7 | Tonda Iblea 1 |
| 8 | Zaituna |
| 9 | Nocellara del Belice |
| 10 | Minuta |
| 11 | Cerasuola 1 |
| 12 | Pizzo di Corvo |
| 13 | Tonda Iblea 2 |
| 14 | Passulunara |
| 15 | Moresca 1 |
| 16 | Opera Pia Castelnuovo |
| 17 | Nocellara Belice |
| 18 | Olivo di Castiglione |
| 19 | Bariddara o Barilara |
| 20 | Moresca 2 |
| 21 | Nocellara Belice SO |
| 22 | Iacona |
| 23 | Nocellara Messinese |
| 24 | Monaca |
| 25 | Cacaridduna |
| 26 | Cirasuola |
| 27 | Tonda Iblea 3 |
| 28 | Ebano Ita |
| 29 | Cerasuola 2 |
| 30 | Tunnulidda |
| 31 | Moresca 3 |
| 32 | Vetrana |
| 33 | Fastucara (Aragona) |
| 34 | Lunga del Vassallo |
| 35 | Ebano |
| 36 | Cerasuola 3 |
| 37 | Bottone di gallo |
| 38 | Passulunara (Naro) |
| Cv | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | av | ±σ | |
| 1 | 31.5 | 0.5 | 60.5 | 1.0 | 6.8 | 0.2 | 25.6 | 0.6 | 6.0 | 0.4 | 2.5 | 0.3 | 5.3 | 0.3 | 0.83 | 0.05 | 3.5 | 0.1 | 30.3 | 1.4 | 0.73 | 0.03 | 2.1 | 0.1 | 0.33 | 0.02 | 1.6 | 0.1 | 0.35 | 0.01 |
| 2 | 15.0 | 0.5 | 28.0 | 1.2 | 3.5 | 0.2 | 12.8 | 0.7 | 3.7 | 0.2 | 2.3 | 0.1 | 2.9 | 0.1 | 0.42 | 0.03 | 2.0 | 0.1 | 16.5 | 0.7 | 0.38 | 0.01 | 1.5 | 0.1 | 0.21 | 0.03 | 1.0 | 0.1 | 0.22 | 0.02 |
| 3 | 26.7 | 0.9 | 50.7 | 0.8 | 6.0 | 0.2 | 21.0 | 0.4 | 5.0 | 0.5 | 2.0 | 0.1 | 4.6 | 0.2 | 0.64 | 0.04 | 2.8 | 0.2 | 24.0 | 0.7 | 0.52 | 0.02 | 1.6 | 0.2 | 0.25 | 0.02 | 1.3 | 0.1 | 0.28 | 0.02 |
| 4 | 18.4 | 0.2 | 35.3 | 1.2 | 4.5 | 0.1 | 16.6 | 0.4 | 4.3 | 0.2 | 1.9 | 0.1 | 3.6 | 0.1 | 0.54 | 0.02 | 2.4 | 0.2 | 19.2 | 0.7 | 0.46 | 0.05 | 1.6 | 0.1 | 0.23 | 0.02 | 1.2 | 0.1 | 0.26 | 0.02 |
| 5 | 22.3 | 0.2 | 42.4 | 0.9 | 5.1 | 0.1 | 19.8 | 0.2 | 4.6 | 0.2 | 2.6 | 0.1 | 4.1 | 0.1 | 0.59 | 0.06 | 2.6 | 0.2 | 22.3 | 0.1 | 0.51 | 0.05 | 1.8 | 0.6 | 0.25 | 0.02 | 1.2 | 0.1 | 0.27 | 0.01 |
| 6 | 21.4 | 1.1 | 41.6 | 1.8 | 5.2 | 0.3 | 19.4 | 1.0 | 4.5 | 0.3 | 2.4 | 0.2 | 4.1 | 0.4 | 0.56 | 0.01 | 2.6 | 0.1 | 22.6 | 1.7 | 0.54 | 0.03 | 1.7 | 0.2 | 0.23 | 0.01 | 1.3 | 0.1 | 0.24 | 0.04 |
| 7 | 20.4 | 0.3 | 39.4 | 0.9 | 4.9 | 0.4 | 17.9 | 0.6 | 4.8 | 0.7 | 2.4 | 0.3 | 4.1 | 0.4 | 0.74 | 0.26 | 2.7 | 0.4 | 20.6 | 0.5 | 0.59 | 0.16 | 1.9 | 0.4 | 0.39 | 0.23 | 1.4 | 0.2 | 0.43 | 0.23 |
| 8 | 12.0 | 0.1 | 22.9 | 0.3 | 2.8 | 0.1 | 10.3 | 0.3 | 3.0 | 0.3 | 1.2 | 0.1 | 2.3 | 0.1 | 0.36 | 0.02 | 1.5 | 0.1 | 12.2 | 0.5 | 0.33 | 0.03 | 1.2 | 0.1 | 0.20 | 0.01 | 0.9 | 0.1 | 0.20 | 0.01 |
| 9 | 18.7 | 0.2 | 36.2 | 0.7 | 4.0 | 0.1 | 15.3 | 0.4 | 4.0 | 0.3 | 1.3 | 0.1 | 3.5 | 0.1 | 0.53 | 0.04 | 2.2 | 0.2 | 19.1 | 0.4 | 0.45 | 0.03 | 1.6 | 0.2 | 0.22 | 0.02 | 1.2 | 0.1 | 0.24 | 0.02 |
| 10 | 49.7 | 1.7 | 96.9 | 5.9 | 11.4 | 0.3 | 43.4 | 1.6 | 9.1 | 0.4 | 4.1 | 0.1 | 8.4 | 0.2 | 1.19 | 0.05 | 5.3 | 0.2 | 52.8 | 1.3 | 0.98 | 0.04 | 3.0 | 0.2 | 0.41 | 0.02 | 2.3 | 0.1 | 0.40 | 0.02 |
| 11 | 11.4 | 0.1 | 25.0 | 0.4 | 3.2 | 0.1 | 13.8 | 0.2 | 4.0 | 0.2 | 2.0 | 0.1 | 3.4 | 0.1 | 0.50 | 0.05 | 2.3 | 0.2 | 21.7 | 0.4 | 0.47 | 0.02 | 1.4 | 0.1 | 0.26 | 0.01 | 1.1 | 0.1 | 0.22 | 0.03 |
| 12 | 8.2 | 0.2 | 16.2 | 0.1 | 2.2 | 0.1 | 8.7 | 0.4 | 2.6 | 0.4 | 1.4 | 0.2 | 2.1 | 0.1 | 0.36 | 0.03 | 1.4 | 0.1 | 10.0 | 0.1 | 0.30 | 0.04 | 1.0 | 0.1 | 0.18 | 0.02 | 0.9 | 0.1 | 0.16 | 0.02 |
| 13 | 20.6 | 0.4 | 40.8 | 0.4 | 4.6 | 0.1 | 18.5 | 0.9 | 4.6 | 0.2 | 2.5 | 0.1 | 4.3 | 0.3 | 0.65 | 0.04 | 2.7 | 0.1 | 24.6 | 0.7 | 0.61 | 0.04 | 1.7 | 0.2 | 0.29 | 0.03 | 1.5 | 0.1 | 0.27 | 0.03 |
| 14 | 90.7 | 0.4 | 186.1 | 0.5 | 31.1 | 0.1 | 134.7 | 0.5 | 32.1 | 0.3 | 7.5 | 0.1 | 30.9 | 0.3 | 4.40 | 0.10 | 22.8 | 0.4 | 189.1 | 0.4 | 4.51 | 0.02 | 12.4 | 0.2 | 1.73 | 0.05 | 10.4 | 0.1 | 1.59 | 0.05 |
| 15 | 17.6 | 0.2 | 34.5 | 0.1 | 4.3 | 0.1 | 16.0 | 0.5 | 4.1 | 0.3 | 1.9 | 0.2 | 3.7 | 0.1 | 0.55 | 0.02 | 2.5 | 0.1 | 21.3 | 0.1 | 0.51 | 0.03 | 1.6 | 0.2 | 0.24 | 0.02 | 1.2 | 0.1 | 0.25 | 0.03 |
| 16 | 17.9 | 0.4 | 33.5 | 0.4 | 4.4 | 0.1 | 16.4 | 0.4 | 4.5 | 0.6 | 1.4 | 0.1 | 3.6 | 0.2 | 0.56 | 0.01 | 2.4 | 0.2 | 18.5 | 0.1 | 0.44 | 0.01 | 1.4 | 0.1 | 0.26 | 0.02 | 1.2 | 0.1 | 0.23 | 0.02 |
| 17 | 9.0 | 0.3 | 17.4 | 0.5 | 2.2 | 0.1 | 8.6 | 0.2 | 2.6 | 0.1 | 1.1 | 0.1 | 2.0 | 0.1 | 0.33 | 0.02 | 1.5 | 0.2 | 10.2 | 0.1 | 0.31 | 0.02 | 1.0 | 0.1 | 0.19 | 0.01 | 0.9 | 0.1 | 0.19 | 0.05 |
| 18 | 6.5 | 0.2 | 11.9 | 0.1 | 1.6 | 0.1 | 5.8 | 0.1 | 2.3 | 0.2 | 1.0 | 0.1 | 1.5 | 0.2 | 0.25 | 0.01 | 1.1 | 0.1 | 8.1 | 0.1 | 0.20 | 0.02 | 0.8 | 0.1 | 0.14 | 0.01 | 0.7 | 0.1 | 0.15 | 0.03 |
| 19 | 17.5 | 1.0 | 34.0 | 0.6 | 4.2 | 0.2 | 16.2 | 0.6 | 4.3 | 0.2 | 1.4 | 0.1 | 3.8 | 0.2 | 0.55 | 0.04 | 2.4 | 0.1 | 19.3 | 0.3 | 0.47 | 0.03 | 1.6 | 0.1 | 0.25 | 0.01 | 1.3 | 0.1 | 0.26 | 0.02 |
| 20 | 18.1 | 0.1 | 36.2 | 0.4 | 4.4 | 0.1 | 17.2 | 0.5 | 3.9 | 0.3 | 1.4 | 0.1 | 3.6 | 0.1 | 0.56 | 0.03 | 2.2 | 0.1 | 21.3 | 0.1 | 0.44 | 0.03 | 1.7 | 0.2 | 0.23 | 0.01 | 1.1 | 0.1 | 0.23 | 0.02 |
| 21 | 16.1 | 0.3 | 31.1 | 1.1 | 3.8 | 0.1 | 14.4 | 0.1 | 4.1 | 0.1 | 1.6 | 0.1 | 3.3 | 0.1 | 0.50 | 0.06 | 2.2 | 0.1 | 17.9 | 0.5 | 0.44 | 0.03 | 1.5 | 0.1 | 0.27 | 0.02 | 1.3 | 0.1 | 0.25 | 0.04 |
| 22 | 15.0 | 0.2 | 28.7 | 0.8 | 3.6 | 0.2 | 13.6 | 0.7 | 3.2 | 1.0 | 1.1 | 0.5 | 2.7 | 0.6 | 0.42 | 0.07 | 2.0 | 0.2 | 14.7 | 0.7 | 0.38 | 0.01 | 1.2 | 0.1 | 0.20 | 0.03 | 1.2 | 0.2 | 0.20 | 0.04 |
| 23 | 19.6 | 1.1 | 37.7 | 1.8 | 4.4 | 0.2 | 16.5 | 0.5 | 4.2 | 0.3 | 1.9 | 0.2 | 3.3 | 0.2 | 0.49 | 0.02 | 2.1 | 0.2 | 17.2 | 0.9 | 0.45 | 0.03 | 1.3 | 0.1 | 0.24 | 0.03 | 1.3 | 0.1 | 0.23 | 0.04 |
| 24 | 20.2 | 0.5 | 38.2 | 1.7 | 4.5 | 0.2 | 16.7 | 1.1 | 4.4 | 0.3 | 1.3 | 0.1 | 3.4 | 0.2 | 0.52 | 0.02 | 2.3 | 0.1 | 18.4 | 1.2 | 0.45 | 0.04 | 1.6 | 0.1 | 0.26 | 0.01 | 1.7 | 0.4 | 0.31 | 0.05 |
| 25 | 25.0 | 2.4 | 49.2 | 4.4 | 5.8 | 0.6 | 22.2 | 2.6 | 5.3 | 0.6 | 2.0 | 0.2 | 4.4 | 0.6 | 0.67 | 0.01 | 2.9 | 0.3 | 27.1 | 1.9 | 0.58 | 0.05 | 1.9 | 0.2 | 0.34 | 0.06 | 1.4 | 0.2 | 0.27 | 0.03 |
| 26 | 20.4 | 2.1 | 40.7 | 5.0 | 4.8 | 0.6 | 18.8 | 2.3 | 4.6 | 0.5 | 1.5 | 0.2 | 3.5 | 0.4 | 0.53 | 0.03 | 2.5 | 0.2 | 21.2 | 2.1 | 0.50 | 0.05 | 1.6 | 0.3 | 0.26 | 0.05 | 1.5 | 0.2 | 0.32 | 0.03 |
| 27 | 19.7 | 1.1 | 36.7 | 2.5 | 4.5 | 0.3 | 17.3 | 0.6 | 4.8 | 0.3 | 2.7 | 0.4 | 3.8 | 0.2 | 0.57 | 0.06 | 2.5 | 0.2 | 20.1 | 2.0 | 0.53 | 0.05 | 2.0 | 0.3 | 0.33 | 0.03 | 1.3 | 0.1 | 0.23 | 0.02 |
| 28 | 15.4 | 0.2 | 29.3 | 0.4 | 3.4 | 0.1 | 13.0 | 0.3 | 3.3 | 0.2 | 1.3 | 0.1 | 2.8 | 0.1 | 0.44 | 0.01 | 1.9 | 0.1 | 14.6 | 0.1 | 0.38 | 0.01 | 1.4 | 0.1 | 0.27 | 0.01 | 1.4 | 0.2 | 0.31 | 0.02 |
| 29 | 21.9 | 1.9 | 41.7 | 3.6 | 4.9 | 0.4 | 18.4 | 1.8 | 4.7 | 0.3 | 2.5 | 0.2 | 3.9 | 0.2 | 0.63 | 0.06 | 2.5 | 0.1 | 20.9 | 1.8 | 0.51 | 0.05 | 1.9 | 0.1 | 0.30 | 0.05 | 1.4 | 0.2 | 0.31 | 0.01 |
| 30 | 25.0 | 0.8 | 46.9 | 1.3 | 5.5 | 0.1 | 21.0 | 0.6 | 4.9 | 0.6 | 2.9 | 0.1 | 4.2 | 0.1 | 0.65 | 0.02 | 2.8 | 0.1 | 25.0 | 0.3 | 0.55 | 0.02 | 1.9 | 0.1 | 0.30 | 0.03 | 1.5 | 0.1 | 0.26 | 0.04 |
| 31 | 22.4 | 0.6 | 40.9 | 1.1 | 5.0 | 0.2 | 18.3 | 0.5 | 4.7 | 0.3 | 2.4 | 0.1 | 3.9 | 0.2 | 0.58 | 0.01 | 2.6 | 0.1 | 19.7 | 0.9 | 0.52 | 0.02 | 1.8 | 0.1 | 0.31 | 0.03 | 1.4 | 0.1 | 0.31 | 0.03 |
| 32 | 29.1 | 1.1 | 54.7 | 1.2 | 6.4 | 0.1 | 23.7 | 0.2 | 5.7 | 0.2 | 2.3 | 0.1 | 5.5 | 0.2 | 0.74 | 0.02 | 3.1 | 0.1 | 23.4 | 0.7 | 0.77 | 0.04 | 1.9 | 0.1 | 0.35 | 0.02 | 1.2 | 0.1 | 0.27 | 0.01 |
| 33 | 21.5 | 0.8 | 34.9 | 1.3 | 4.6 | 0.2 | 17.6 | 0.8 | 4.1 | 0.1 | 3.0 | 0.4 | 4.6 | 0.2 | 0.55 | 0.05 | 2.5 | 0.2 | 17.4 | 0.6 | 0.53 | 0.02 | 1.7 | 0.2 | 0.30 | 0.05 | 1.2 | 0.1 | 0.24 | 0.02 |
| 34 | 24.9 | 0.7 | 48.1 | 0.7 | 5.3 | 0.1 | 20.4 | 0.5 | 4.8 | 0.2 | 2.6 | 0.1 | 4.7 | 0.1 | 0.65 | 0.03 | 2.8 | 0.2 | 21.8 | 1.5 | 0.58 | 0.05 | 1.8 | 0.1 | 0.31 | 0.04 | 1.9 | 0.1 | 0.36 | 0.03 |
| 35 | 31.4 | 1.3 | 58.8 | 5.2 | 7.2 | 0.2 | 27.3 | 1.1 | 6.3 | 0.4 | 2.3 | 0.2 | 7.2 | 0.3 | 0.89 | 0.06 | 3.8 | 0.2 | 32.0 | 1.6 | 0.78 | 0.03 | 2.3 | 0.3 | 0.37 | 0.02 | 1.4 | 0.1 | 0.23 | 0.01 |
| 36 | 19.8 | 1.7 | 37.3 | 3.3 | 4.6 | 0.3 | 18.8 | 1.8 | 4.6 | 0.2 | 2.2 | 0.3 | 5.4 | 0.4 | 0.62 | 0.03 | 2.7 | 0.3 | 18.1 | 1.8 | 0.56 | 0.08 | 1.7 | 0.1 | 0.33 | 0.04 | 2.3 | 0.4 | 0.44 | 0.09 |
| 37 | 50.5 | 3.0 | 100.3 | 6.7 | 11.7 | 1.1 | 44.0 | 5.3 | 9.5 | 0.5 | 3.4 | 0.5 | 10.0 | 3.4 | 1.22 | 0.20 | 5.3 | 0.4 | 46.6 | 2.3 | 1.01 | 0.14 | 3.1 | 0.3 | 0.44 | 0.09 | 1.6 | 0.1 | 0.31 | 0.04 |
| 38 | 21.0 | 1.4 | 37.5 | 4.7 | 4.8 | 0.4 | 18.0 | 1.1 | 4.4 | 0.2 | 2.8 | 0.1 | 4.7 | 0.3 | 0.59 | 0.02 | 2.6 | 0.2 | 19.6 | 1.5 | 0.56 | 0.04 | 1.8 | 0.1 | 0.36 | 0.03 | 1.0 | 0.1 | 0.20 | 0.01 |
| Sign. | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbera, M.; Saiano, F.; Tutone, L.; Massenti, R.; Pisciotta, A. The Pattern of Rare Earth Elements Like a Possible Helpful Tool in Traceability and Geographical Characterization of the Soil-Olive System (Olea europaea L.). Plants 2022, 11, 2579. https://doi.org/10.3390/plants11192579
Barbera M, Saiano F, Tutone L, Massenti R, Pisciotta A. The Pattern of Rare Earth Elements Like a Possible Helpful Tool in Traceability and Geographical Characterization of the Soil-Olive System (Olea europaea L.). Plants. 2022; 11(19):2579. https://doi.org/10.3390/plants11192579
Chicago/Turabian StyleBarbera, Marcella, Filippo Saiano, Livia Tutone, Roberto Massenti, and Antonino Pisciotta. 2022. "The Pattern of Rare Earth Elements Like a Possible Helpful Tool in Traceability and Geographical Characterization of the Soil-Olive System (Olea europaea L.)" Plants 11, no. 19: 2579. https://doi.org/10.3390/plants11192579
APA StyleBarbera, M., Saiano, F., Tutone, L., Massenti, R., & Pisciotta, A. (2022). The Pattern of Rare Earth Elements Like a Possible Helpful Tool in Traceability and Geographical Characterization of the Soil-Olive System (Olea europaea L.). Plants, 11(19), 2579. https://doi.org/10.3390/plants11192579








