A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research
Abstract
1. Introduction
2. Results
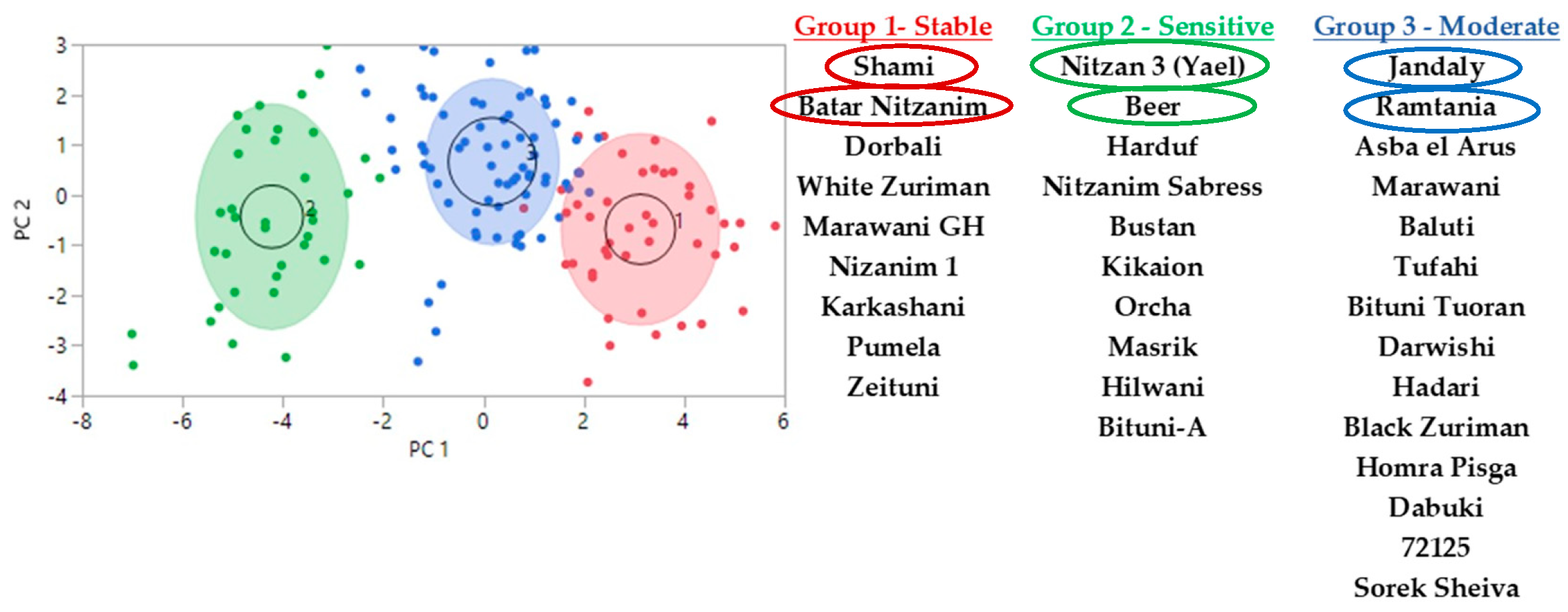
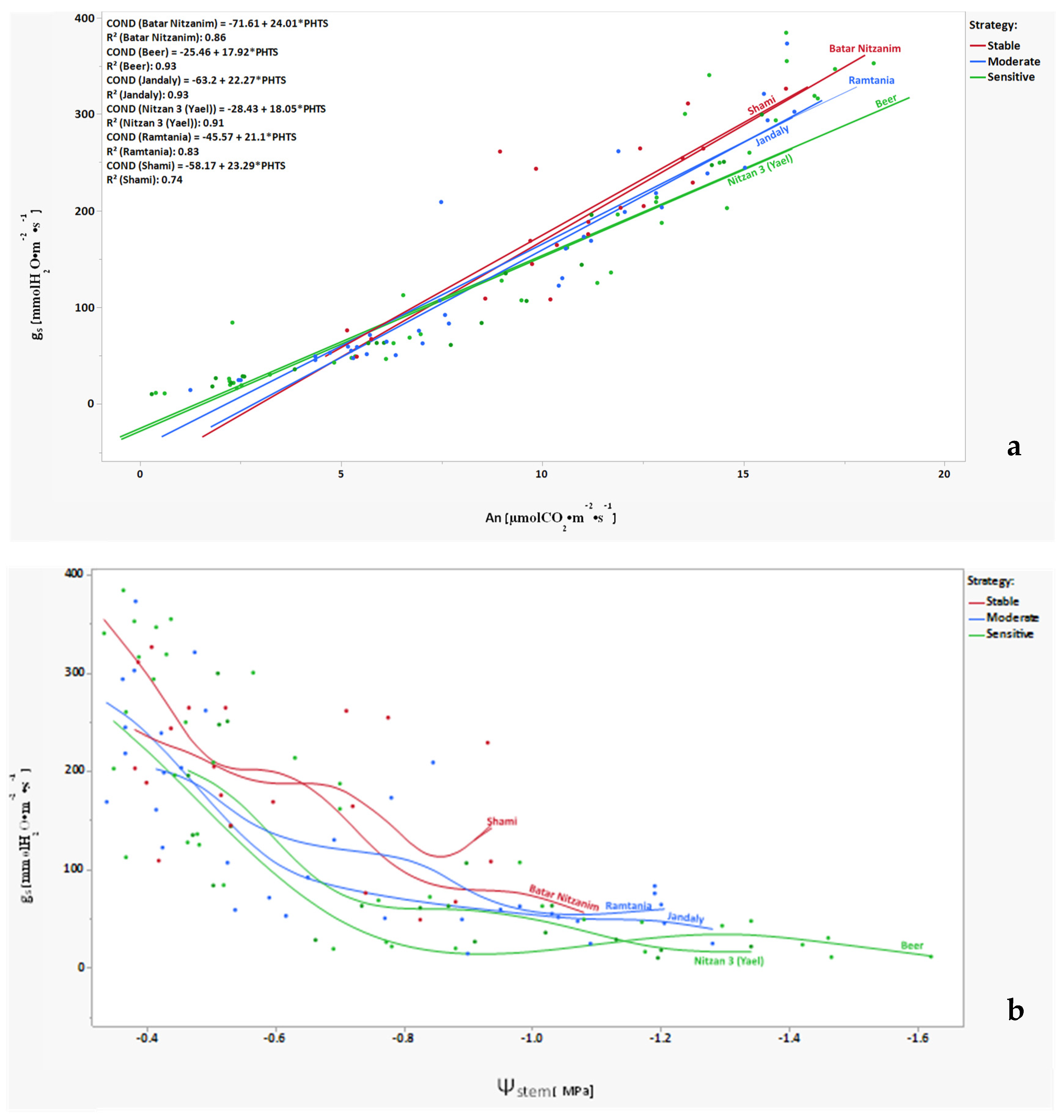
2.1. Drought Tolerance Characterization of Varieties in the Collection
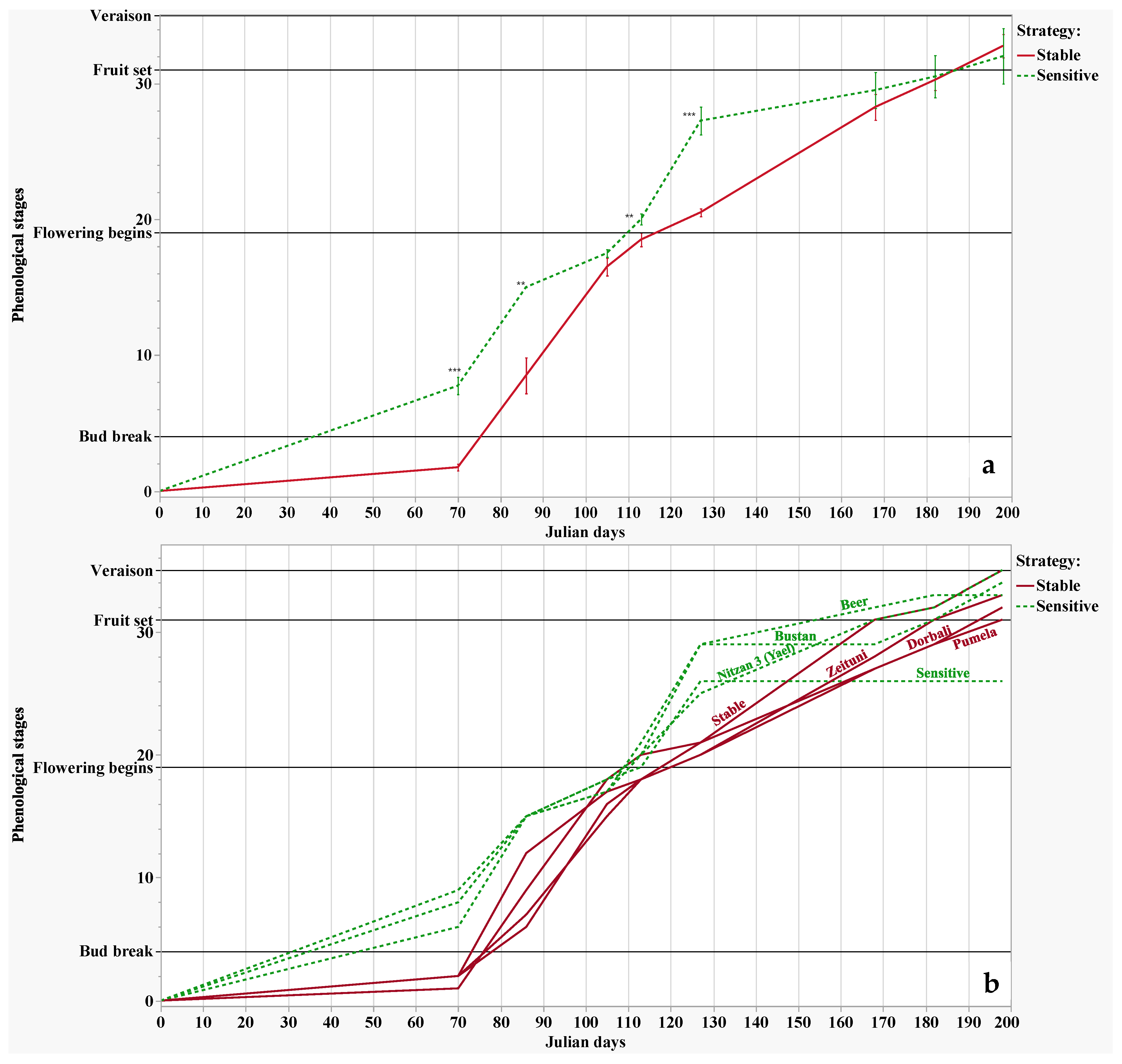
2.2. Phenological Observations
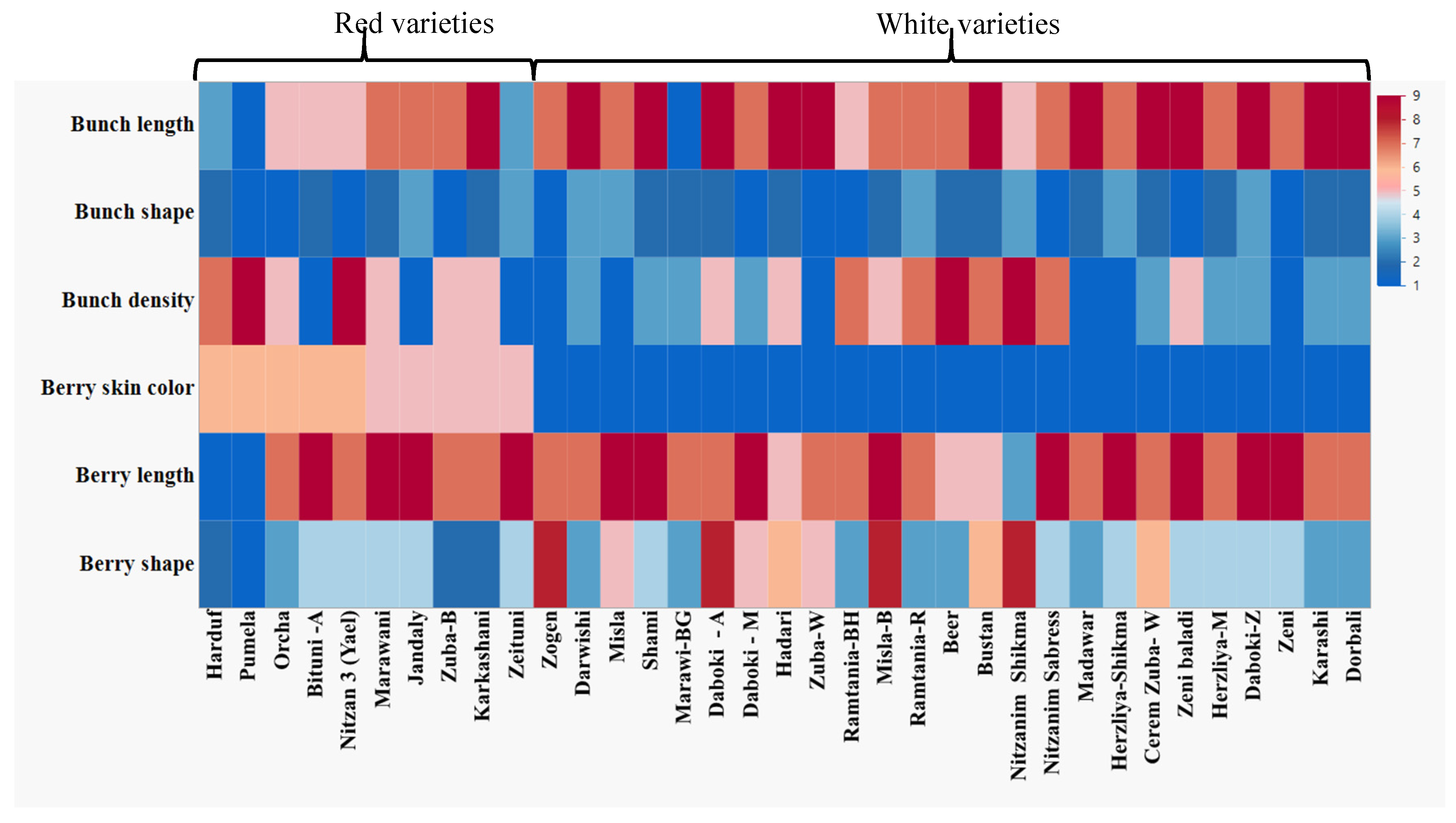
2.3. Phenotyping for Various Viticultural Traits
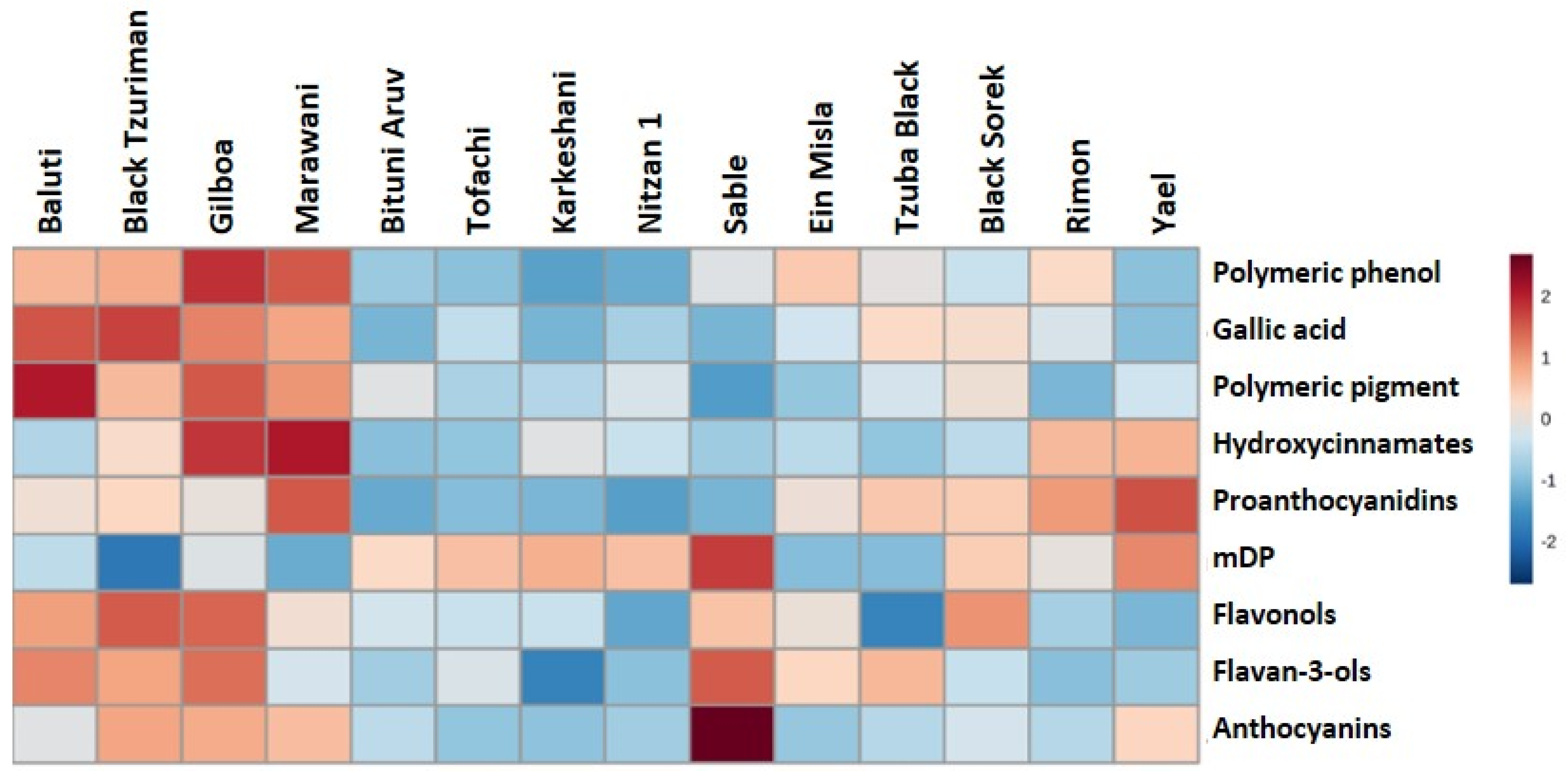
2.4. Metabolic Profiling—Phenolics
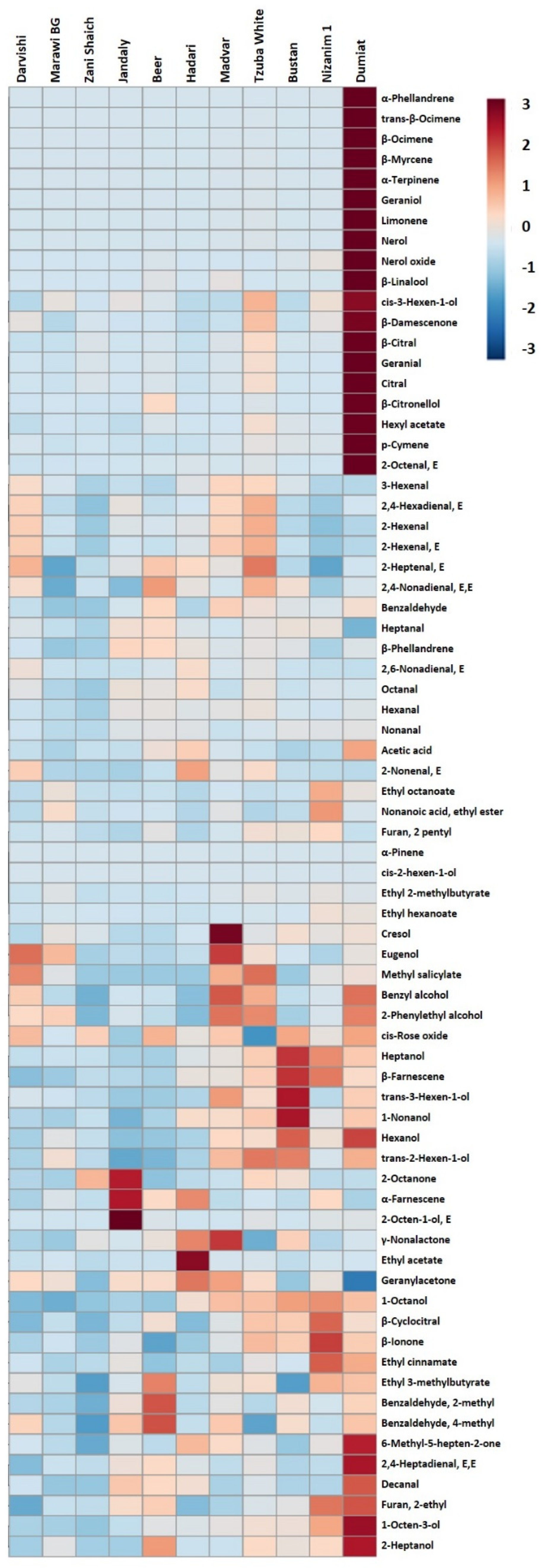
2.5. Volatile Aroma Profiles
3. Discussion
4. Materials and Methods
4.1. In-Field Conditions
4.2. Experimental Conditions and Physiological Parameters for the Drought Stress Trial
4.2.1. Midday Stem Water Potential (Ψstem)
4.2.2. Gas Exchange
4.2.3. Statistical Analysis of Physiological Parameters
4.3. Phenological Stages
4.4. Ampelographic Characterization of Grapevine Varieties
4.5. Volatile Aroma Compounds
4.6. Phenolics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Imazio, S.; Maghradze, D.; de Lorenzis, G.; Bacilieri, R.; Laucou, V.; This, P.; Scienza, A.; Failla, O. From the Cradle of Grapevine Domestication: Molecular Overview and Description of Georgian Grapevine (Vitis vinifera L.) Germplasm. Tree Genet. Genomes 2013, 9, 641–658. [Google Scholar] [CrossRef]
- Santiago, J.L.; Boso, S.; Gago, P.; Alonso-Villaverde, V.; Martínez, M.C. A Contribution to the Maintenance of Grapevine Diversity: The Rescue of Tinta Castañal (Vitis vinifera L.), a Variety on the Edge of Extinction. Sci. Hortic. 2008, 116, 199–204. [Google Scholar] [CrossRef]
- Maraš, V.; Tello, J.; Gazivoda, A.; Mugoša, M.; Perišić, M.; Raičević, J.; Štajner, N.; Ocete, R.; Božović, V.; Popović, T.; et al. Population Genetic Analysis in Old Montenegrin Vineyards Reveals Ancient Ways Currently Active to Generate Diversity in Vitis vinifera. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Harst-Langenbucher, M.; Alleweldt, G. Conservation of the Genetic Resources of Vitis. Vitis 1990, 29, 446–454. [Google Scholar]
- Kulus, D.; Zalewska, M. Cryopreservation as a Tool Used in Long-Term Storage of Ornamental Species—A Review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Razdan, M.K. Plant Tissue Culture Theory and Practice, a Revised Edition; Bhojwani, S.S., Razdan, M.K., Eds.; Elsevier, North Holland: Amsterdam, The Netherland, 1996. [Google Scholar]
- Bi, W.-L.; Pan, C.; Hao, X.-Y.; Cui, Z.-H.; Kher, M.M.; Marković, Z.; Wang, Q.-C.; Teixeira da Silva, J.A. Cryopreservation of Grapevine (Vitis Spp.)—A Review. Vitr. Cell. Dev. Biol.—Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef]
- Karlsson, J.O.M.; Toner, M. Long-Term Storage of Tissues by Cryopreservation: Critical Issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Carra, A.; Carimi, F.; Bettoni, J.C.; Pathirana, R. Progress and Challenges in the Application of Synthetic Seed Technology for Ex situ Germplasm Conservation in Grapevine (Vitis spp.). In Synthetic Seeds; Faisal, M., Alatar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 439–467. ISBN 9783030246310. [Google Scholar]
- Butler, D. Grapevine Gene Bank under Threat. Nature 2014, 506, 18. [Google Scholar] [CrossRef]
- Meng, B.; Martelli, G.P.; Golino, D.A.; Fuchs, M. Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-57704-3. [Google Scholar]
- Burr, T.J.; Otten, L. Crown Gall of Grape: Biology and Disease Management. Annu. Rev. Phytopathol. 1999, 37, 53–80. [Google Scholar] [CrossRef]
- Molitor, D.; Beyer, M. Epidemiology, Identification and Disease Management of Grape Black Rot and Potentially Useful Metabolites of Black Rot Pathogens for Industrial Applications—A Review. Ann. Appl. Biol. 2014, 165, 305–317. [Google Scholar] [CrossRef]
- Boso, S.; Alonso-Villaverde, V.; Gago, P.; Santiago, J.L.; Martínez, M.C. Susceptibility to Downy Mildew (Plasmopara viticola) of Different Vitis Varieties. Crop Prot. 2014, 63, 26–35. [Google Scholar] [CrossRef]
- Li, Z.; dos Santos, R.F.; Gao, L.; Chang, P.; Wang, X. Current Status and Future Prospects of Grapevine Anthracnose Caused by Elsinoe ampelina: An Important Disease in Humid Grape-Growing Regions. Mol. Plant Pathol. 2021, 22, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Pettenuzzo, S.; Cappellin, L.; Grando, M.S.; Costantini, L. Phenotyping Methods to Assess Heat Stress Resilience in Grapevine. J. Exp. Bot. 2022, 73, 5128–5148. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The Physiology of Drought Stress in Grapevine: Towards an Integrative Definition of Drought Tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Miras-Avalos, J.M.; Araujo, E.S. Optimization of Vineyard Water Management: Challenges, Strategies, and Perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Sivan, A.; Rahimi, O.; Lavi, B.; Salmon-Divon, M.; Weiss, E.; Drori, E.; Hübner, S. Genomic Evidence Supports an Independent History of Levantine and Eurasian Grapevines. Plants People Planet 2021, 3, 414–427. [Google Scholar] [CrossRef]
- Klein, L.L.; Miller, A.J.; Ciotir, C.; Hyma, K.; Uribe-Convers, S.; Londo, J. High-Throughput Sequencing Data Clarify Evolutionary Relationships among North American Vitis Species and Improve Identification in USDA Vitis Germplasm Collections. Am. J. Bot. 2018, 105, 215–226. [Google Scholar] [CrossRef]
- Cretazzo, E.; Moreno Sanz, P.; Lorenzi, S.; Benítez, M.L.; Velasco, L.; Emanuelli, F. Genetic Characterization by SSR Markers of a Comprehensive Wine Grape Collection Conserved at Rancho de La Merced (Andalusia, Spain). Plants 2022, 11, 1088. [Google Scholar] [CrossRef]
- Community of Madrid Collection of Vine Varieties “Grapevine Collection of “El Encín”. Available online: https://www.comunidad.madrid/servicios/medio-rural/coleccion-variedades-vid (accessed on 24 May 2022).
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic Diversity and Population Structure Assessed by SSR and SNP Markers in a Large Germplasm Collection of Grape. BMC Plant Biol. 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- VIVC Vitis International Variety Catalogue. Available online: https://www.vivc.de/index.php?r=cultivarname%2Findex (accessed on 4 May 2022).
- Lubin, B.C.R.; Inbar, N.; Pinkus, A.; Stanevsky, M.; Cohen, J.; Rahimi, O.; Anker, Y.; Shoseyov, O.; Drori, E. Ecogeographic Conditions Dramatically Affect Trans-Resveratrol and Other Major Phenolics’ Levels in Wine at a Semi-Arid Area. Plants 2022, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Wolkovich, E.M.; García De Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the World’s Future Wine-Growing Regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Jones, G.V.; E, D.R. Climate Influences on Grapevine Phenology, Grape Composition, and Wine Production and Quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Pszczólkowski, P. Resurgence of Minority and Autochthonous Grapevine Varieties in South America: A Review of Their Oenological Potential. J. Sci. Food Agric. 2020, 100, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Migicovsky, Z.; Warschefsky, E.; Klein, L.L.; Miller, A.J. Using Living Germplasm Collections to Characterize, Improve, and Conserve Woody Perennials. Crop Sci. 2019, 59, 2365–2380. [Google Scholar] [CrossRef]
- Kovaleski, A.P.; Reisch, B.I.; Londo, J.P. Deacclimation Kinetics as a Quantitative Phenotype for Delineating the Dormancy Transition and Thermal Efficiency for Budbreak in Vitis Species. AoB PLANTS 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Londo, J.P.; Kovaleski, A.P. Deconstructing Cold Hardiness: Variation in Supercooling Ability and Chilling Requirements in the Wild Grapevine Vitis riparia. Aust. J. Grape Wine Res. 2019, 25, 276–285. [Google Scholar] [CrossRef]
- Gottschalk, C.; Van Nocker, S. Diversity in Seasonal Bloom Time and Floral Development among Apple Species and Hybrids. J. Am. Soc. Hortic. Sci. 2013, 138, 367–374. [Google Scholar] [CrossRef]
- Drori, E.; Munitz, S.; Pinkus, A.; Stanevsky, M.; Netzer, Y. The Effect of Irrigation-Initiation Timing on the Phenolic Composition and Overall Quality of Cabernet Sauvignon Wines Grown in a Semi-Arid Climate. Foods 2022, 11, 770. [Google Scholar] [CrossRef]
- Sorek, Y.; Greenstein, S.; Netzer, Y.; Shtein, I.; Jansen, S.; Hochberg, U. An Increase in Xylem Embolism Resistance of Grapevine Leaves during the Growing Season Is Coordinated with Stomatal Regulation, Turgor Loss Point and Intervessel Pit Membranes. New Phytol. 2021, 229, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhou-Tsang, A.; Wu, Y.; Henderson, S.W.; Walker, A.R.; Borneman, A.R.; Walker, R.R.; Gilliham, M. Grapevine Salt Tolerance. Aust. J. Grape Wine Res. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Drori, E.; Rahimi, O.; Marrano, A.; Henig, Y.; Brauner, H.; Salmon-Divon, M.; Netzer, Y.; Prazzoli, M.L.; Stanevsky, M.; Failla, O.; et al. Collection and Characterization of Grapevine Genetic Resources (Vitis vinifera) in the Holy Land, towards the Renewal of Ancient Winemaking Practices. Sci. Rep. 2017, 7, 44463. [Google Scholar] [CrossRef]
- Drori, E.; Rahimi, O.; Henig, Y.; Lorenzi, S.; Brauner, H.; Marrano, A.; Amar, Z.; Netzer, Y.; Failla, O.; Grando, M.S. Ampelographic and Genetic Characterization of an Initial Israeli Grapevine Germplasm Collection. Vitis—J. Grapevine Res. 2015, 54, 107–110. [Google Scholar]
- Muñoz, G.; Gaforio, L.; Muñoz, S.; Cabello, F. Manual for Standadization of OIV Vitis Descriptors; Instituto Nacional de Investigacion y Technologia Agraria y Alimentaria: Madrid, Spain, 2011. [Google Scholar]
- Negrul, A.M. Origin of Cultivated Grapevine and Its Classification. In Ampelography of the Soviet Union; Baranov, A., Kai, Y.F., Lazarevski, M.A., Palibin, T.V., Prosmoserdov, N.N., Eds.; Pischepromizdat: Moscow, Russia, 1946. [Google Scholar]
- Mateo, J..; Jiménez, M. Monoterpenes in Grape Juice and Wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Riaz, S.; De Lorenzis, G.; Velasco, D.; Koehmstedt, A.; Maghradze, D.; Bobokashvili, Z.; Musayev, M.; Zdunic, G.; Laucou, V.; Andrew Walker, M.; et al. Genetic Diversity Analysis of Cultivated and Wild Grapevine (Vitis vinifera L.) Accessions around the Mediterranean Basin and Central Asia. BMC Plant Biol. 2018, 18, 137. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Rustioni, L.; Cola, G.; Ricciardi, V.; Bianco, P.A.; Maghradze, D.; Failla, O.; Quaglino, F.; Toffolatti, S.L.; De Lorenzis, G. Georgian Grapevine Cultivars: Ancient Biodiversity for Future Viticulture. Front. Plant Sci. 2021, 12, 630122. [Google Scholar] [CrossRef]
- Vršič, S. An Overwiew of Ampelographic Research and Modifications of Grapevine Assortment. Agricultura 2012, 9, 11–20. [Google Scholar]
- Organisation Internationale de la Vigne et du Vin (OIV) Second Edition of the OIV Descriptor List for Grape Varieties and Vitis Species. Available online: https://www.oiv.int/public/medias/2274/code-2e-edition-finale.pdf (accessed on 25 August 2022).
- Maletić, E.; Pejić, I.; Karoglan Kontić, J.; Zdunić, G.; Preiner, D.; Šimon, S.; Andabaka, Ž.; Žuljmihaljević, M.; Bubola, M.; Marković, Z.; et al. Ampelographic and Genetic Characterization of Croatian Grapevine Varieties. Vitis—J. Grapevine Res. 2015, 54, 93–98. [Google Scholar]
- Rustioni, L.; Cola, G.; Maghradze, D.; Abashidze, E.; Argiriou, A.; Aroutiounian, R.; Brazão, J.; Chipashvili, R.; Cocco, M.; Cornea, V.; et al. Description of the Vitis vinifera L. Phenotypic Variability in Eno-Carpological Traits by a Euro-Asiatic Collaborative Network among Ampelographic Collections. Vitis—J. Grapevine Res. 2019, 58, 37–46. [Google Scholar] [CrossRef]
- Rustioni, L.; Maghradze, D.; Popescu, C.F.; Cola, G.; Abashidze, E.; Aroutiounian, R.; Brazão, J.; Coletti, S.; Cornea, V.; Dejeu, L.; et al. First Results of the European Grapevine Collections’ Collaborative Network: Validation of a Standard Eno-Carpological Phenotyping Method. Vitis—J. Grapevine Res. 2014, 53, 219–226. [Google Scholar]
- Drori, E.; Levy, D.; Smirin-Yosef, P.; Rahimi, O.; Salmon-Divon, M. CircosVCF: Circos Visualization of Whole-Genome Sequence Variations Stored in VCF Files. Bioinformatics 2017, 33, 1392–1393. [Google Scholar] [CrossRef] [PubMed]
- Karasik, A.; Rahimi, O.; David, M.; Weiss, E.; Drori, E. Development of a 3D Seed Morphological Tool for Grapevine Variety Identification, and Its Comparison with SSR Analysis. Sci. Rep. 2018, 8, 6545. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.M.; Ware, D.; et al. Genetic Structure and Domestication History of the Grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Foster, T.M.; Aranzana, M.J. Attention Sports Fans! The Far-Reaching Contributions of Bud Sport Mutants to Horticulture and Plant Biology. Hortic. Res. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Li, R.; Xie, X.; Ma, F.; Wang, D.; Wang, L.; Zhang, J.; Xu, Y.; Wang, X.; Zhang, C.; Wang, Y. Resveratrol Accumulation and Its Involvement in Stilbene Synthetic Pathway of Chinese Wild Grapes during Berry Development Using Quantitative Proteome Analysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Y.; Yu, X.; Liu, J.; Yang, L.; Gao, Y.; Ke, G.; Zhou, M.; Mu, B.; Xiao, S.; et al. Overexpression of Two CDPKs from Wild Chinese Grapevine Enhances Powdery Mildew Resistance in Vitis vinifera and Arabidopsis. New Phytol. 2021, 230, 2029–2046. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Sun, X.Y.; Fang, Y. Oenological Potential and Health Benefits of Chinese Non-Vitis vinifera Species: An Opportunity to the Revalorization and to Breed New Varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef]
- Lin, J.; Massonnet, M.; Cantu, D. The Genetic Basis of Grape and Wine Aroma. Hortic. Res. 2019, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Cao, X.Y.; Wei, X.F.; Fang, Y.L. First Study on the Fatty Acids and Their Derived Volatile Profiles from Six Chinese Wild Spine Grape Clones (Vitis davidii Foex). Sci. Hortic. 2021, 275, 109709. [Google Scholar] [CrossRef]
- Shtein, I.; Wolberg, S.; Munitz, S.; Zait, Y.; Rosenzweig, T.; Grünzweig, J.M.; Ohana-Levi, N.; Netzer, Y. Multi-Seasonal Water-Stress Memory versus Temperature-Driven Dynamic Structural Changes in Grapevine. Tree Physiol. 2021, 41, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Bock, H.-H. Clustering Methods: A History of k-Means Algorithms. In Selected Contributions in Data Analysis and Classification. Studies in Classification, Data Analysis, and Knowledge Organization; Brito, P., Cucumel, G., Bertrand, P., de Carvalho, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 161–172. [Google Scholar]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The K-Means Algorithm: A Comprehensive Survey and Performance Evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Hendrickson, D.A.; Lerno, L.A.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Hopfer, H.; Block, K.L.; Brenneman, C.A.; Oberholster, A. Impact of Mechanical Harvesting and Optical Berry Sorting on Grape and Wine Composition. Am. J. Enol. Vitic. 2016, 67, 385–397. [Google Scholar] [CrossRef]
- Canuti, V.; Conversano, M.; Calzi, M.L.; Heymann, H.; Matthews, M.A.; Ebeler, S.E. Headspace Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry for Profiling Free Volatile Compounds in Cabernet Sauvignon Grapes and Wines. J. Chromatogr. A 2009, 1216, 3012–3022. [Google Scholar] [CrossRef]
- Tyagi, K.; Lerno, L.; De Rosso, M.; Maoz, I.; Lichter, A.; Ebeler, S.E.; Flamini, R. Extraction and Analysis of Phenolic Compounds from Grape Berries. In Plant Secondary Metabolism Engineering. Methods in Molecular Biology, Vol. 2469; Fett-Neto, A.G., Ed.; Humana: New York, NY, USA, 2022; pp. 1–17. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shecori, S.; Kher, M.M.; Tyagi, K.; Lerno, L.; Netzer, Y.; Lichter, A.; Ebeler, S.E.; Drori, E. A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research. Plants 2022, 11, 2563. https://doi.org/10.3390/plants11192563
Shecori S, Kher MM, Tyagi K, Lerno L, Netzer Y, Lichter A, Ebeler SE, Drori E. A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research. Plants. 2022; 11(19):2563. https://doi.org/10.3390/plants11192563
Chicago/Turabian StyleShecori, Shani, Mafatlal M. Kher, Kamal Tyagi, Larry Lerno, Yishai Netzer, Amnon Lichter, Susan E. Ebeler, and Elyashiv Drori. 2022. "A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research" Plants 11, no. 19: 2563. https://doi.org/10.3390/plants11192563
APA StyleShecori, S., Kher, M. M., Tyagi, K., Lerno, L., Netzer, Y., Lichter, A., Ebeler, S. E., & Drori, E. (2022). A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research. Plants, 11(19), 2563. https://doi.org/10.3390/plants11192563






