Abstract
Drought generates a complex scenario worldwide in which agriculture should urgently be reframed from an integrative point of view. It includes the search for new water resources and the use of tolerant crops and genotypes, improved irrigation systems, and other less explored alternatives that are very important, such as biotechnological tools that may increase the water use efficiency. Currently, a large body of evidence highlights the role of specific strains in the main microbial rhizosphere groups (arbuscular mycorrhizal fungi, yeasts, and bacteria) on increasing the drought tolerance of their host plants through diverse plant growth-promoting (PGP) characteristics. With this background, it is possible to suggest that the joint use of distinct PGP microbes could produce positive interactions or additive beneficial effects on their host plants if their co-inoculation does not generate antagonistic responses. To date, such effects have only been partially analyzed by using single omics tools, such as genomics, metabolomics, or proteomics. However, there is a gap of information in the use of multi-omics approaches to detect interactions between PGP and host plants. This approach must be the next scale-jump in the study of the interaction of soil–plant–microorganism. In this review, we analyzed the constraints posed by drought in the framework of an increasing global demand for plant production, integrating the important role played by the rhizosphere biota as a PGP agent. Using multi-omics approaches to understand in depth the processes that occur in plants in the presence of microorganisms can allow us to modulate their combined use and drive it to increase crop yields, improving production processes to attend the growing global demand for food.
1. Introduction
Projections of agroclimatic models indicate a strong impact of global climate change (GCC), represented by both temperature increases of 0.5 to 2 °C by 2100 and a significant decrease in rainfall [1,2]. This change will undoubtedly promote a reconversion of agronomic practices to produce the necessary food for a growing world population, both in volume and requirements of high-quality products [3]. Regardless of the plant species cropped, agricultural production depends on a significant proportion of water being supplied mainly by rainfall, which has had a strong impact on the production of the last few seasons due to a marked mega-drought in many places around the world [1].
Under this complex scenario, the availability of water sources for irrigation is also a major structural problem, since the availability of adequate infrastructure to allow water storage is insufficient to ensure access to water during periods of higher demand [4]. Such constraints require the implementation of innovative agricultural approaches to increase resilience to climate variability, such as the incorporation of new species to be cropped. Additionally, lands where new crop species are chosen to be incorporated in must be evaluated in terms of alternatives to increase the “water use efficiency”, together with an increased plant tolerance to drought [5,6,7,8]. The above is a major challenge for global agricultural activity given the extent of the predicted climate change effects on agriculture [9].
Consequently, one of the major questions to be answered under this complex paradigm is not how to access more water in the short–medium term (that could only be answered at the infrastructural level and in the long term) but how it would be possible to keep the current yields of plant production with the scarce water availability, or even how to increase them based on the projected demand. An alternative that has generated much interest in recent years is the use of plant growth-promoting (PGP) microorganisms as inoculants (biofertilizers) in agricultural plants [6,7,10,11]. The above is based on the numerous microbial strains that have developed tolerance to the environmental stresses in which they commonly are exposed. This, together with the microbial fast growth and multiplication rate, can generate a high abundance of some desirable traits in a very short time.
Among the multiple PGP microbial groups, the arbuscular mycorrhizal (AM) fungi stand out because they establish strict mutualist symbiosis with most of the agricultural plant species, mainly characterized by providing mineral nutrition (as P) and water transport to the host plants [5,12,13]. Moreover, the development of biotechnological tools using AM fungi constitutes one of the most environmentally friendly alternatives to address the above-described constraints in a context of resilient agriculture [12,14,15,16,17]. Additionally, other free-living microorganisms such as yeast and bacteria present diverse PGP traits, which also support their use as biofertilizers. While their beneficial effects on plant growth have been extensively described, they also produce effects in plants that can be based on (or can promote) molecular, biochemical, and physiological changes that are lesser known.
Despite the increasing use of microbial inoculants from both monospecific isolates and consortia of yeast and bacteria (for instance, [7,18]), some points need to be addressed, such as: (a) the basis by which such microorganisms generate beneficial responses in plants; and (b) the multiple microbial or “ecological” interactions that may occur in the rhizosphere [19,20]. In the last case, it may not necessarily produce positive effects, being in some cases even negative [21]. Therefore, as a basis for the design of optimized bioinoculants, it is necessary to understand their degree of compatibility. This can be achieved by avoiding negative interactions such as competition or predation and promoting commensalism and cooperation [22]. Finally, the microbial functionality in generating desirable responses in the host plants will be registered as yield and food quality increases.
There are previous experiences that link, at the molecular level, the responses of plants to inoculation with certain PGP microorganisms [11,23,24]. Meanwhile, to the best of our knowledge, there are no systematized works to develop microbial consortia engineered to different hosts, including microbe–microbe interactions + PGP traits + shifts in plant mechanisms, to develop optimized biofertilizers for a specific plant species. In contrast, it is commonly concluded that the effects observed by the inoculation with a PGP microorganism in a model plant could easily be transferred to other species without a deep understanding of the physiological, biochemical, or molecular changes in the host plant (regarding the uninoculated plant) [7,10].
The above scenario generates double uncertainty: (i) whether the inoculant can be beneficial to more than one host plant species; (ii) whether the plant responses can be mechanistically equivalent between different host plants using the same inoculant. Currently, the multiple soil–microorganism–plant interactions represent an unexplored “black box”. Much less effort has been put into the validation at the field level of an inoculant’s effectiveness beyond the argued socially friendly decrease in the use of chemical inputs, as commonly advertised by marketing agricultural companies.
Against this scenario, the current “omics” tools emerge as an attainable alternative to clarify “what is happening in this black box”, allowing us to describe and predict behaviors in plants based on powerful genomic, transcriptomic, proteomic and metabolomic tools. Therefore, with this focused review, we hoped to summarize the current and updated knowledge regarding the role of microbial tools used as bioinoculants to improve the plant production. In addition, we highlight the projections based on the use of single-omics platforms and multi-omics approaches. With this, our aim was to elucidate the mechanisms that explain the improvements in yield and food quality under drought stress, as one the main sensible effects of climate change in agriculture.
2. The Drought as Main Constraint for Plant Production in a Global Climate Change Scenario
Agriculture meets our food demands (food security), being the main practice that contributes to the economy in many countries worldwide. However, the intensification of agriculture has also led to the degradation and exhaustion of soils; moreover, about 38% of suitable agricultural lands around the world have been degraded by inadequate management practices [25,26]. Supplying the increasing world population with sufficient food is mandatory. Because of this, food security is the most important challenge in the 21st century. However, nowadays, food security is strongly threatened by global warming [27].
Global warming and associated climate change not only affect air temperature but also influence the amount and distribution of rainfall [28]. Predictive models of GCC have shown that the frequency of precipitation events and net volumes have drastically changed during the past one hundred years. It has caused frequent and severe periods of drought in large areas around the world [29].
The agricultural sector is responsible for about 75% of the total global consumption of water [30]. However, in the wake of GCC, drought has emerged as one of the major abiotic stresses and is considered the strongest environmental stress that limits the plant growth, reducing crop productivity [31,32]. Due to the droughts, yield reductions have been reported in the order of 21 to 40%, from 1980 to 2015, for wheat (Triticum aestivum) and maize (Zea mays) productions, two of the most important crops worldwide [33]. Moreover, considering that among 80–95% of the fresh weight of plants consists of water, it is evident that drought stress leads to changes on a multidimensional level, modifying different physiological, biochemical, molecular, and morphological processes in plants [34,35,36].
The responses of plants to water stress depend on the length and severity of the water deficiency [37]. Many plants have developed mechanisms to tolerate water stress, but these mechanisms are varied and depend on the plant species. Included among them are developmental, physiological, morphological, ecological, biochemical and molecular mechanisms [38]. Mainly, the mechanisms involved in plant tolerance to drought are based on maintaining cell water homeostasis under drought conditions, allowing diminished water loss and increasing the water inlet to the plants [39].
To improve plant responses against the water stress, different approaches are currently being studied, such as traditional breeding methods, transgenic technology, and priming methods, among others. However, because of the complexity of drought effects on plants and the specific responses of plants to the water stress, each method has some problems and limitations [40,41,42].
In addition, the role of root-associated microbial communities able to improve plant drought tolerance has only been explored in recent years [43]. In this way, different studies have reported that beneficial soil microorganisms improve plant tolerance to abiotic stresses by producing a root—soil interface that directly or indirectly enhances the absorption of water and nutrients. The most important groups of microorganisms related to increased tolerance to water scarcity include the PGP rhizobacteria and AM fungi [5].
3. Rhizosphere Microbial Groups and Ecological Roles for Plant Production under Drought Conditions
Currently, due to the importance of the functions that soil provides, it is widely recognized that soil represents one of the main frontiers of science. The functions that soil provides strongly determine the productivity of terrestrial ecosystems, both natural and agricultural-modified ones [44,45]. The sustainable development of agriculture is mediated by biotic and abiotic factors, the rhizosphere being an environment that can be considered the common point shared by both factors. The health of the rhizosphere matrix is closely related to the state of its present PGP microbial communities [46].
In this ecological niche, factors such as the availability of oxygen, nutrients (C:N:P ratio) and water modulate a significant fraction of the total interactions in the rhizosphere [47]. In addition, in this volume of soil, it is possible to find exudates from plant roots (rhizodeposits), which are considered an essential component of the rhizosphere [48,49]. The presence or absence of rhizodeposits can significantly affect rhizosphere interactions since they fulfill signaling functions to give way to the symbiotic association between the plant and PGP microorganisms [50].
Another fundamental characteristic in the ecology of the rhizosphere is the restriction interactions of microorganisms. It has the characteristic of restricting other microorganisms from interacting with the plant (e.g., plant pathogen microorganisms). This converts the rhizosphere into a bridge of chemical communication between the plant and the PGP microorganisms for the formation of protective microbial biofilms, acting as biocontrol agents in both the defense and resistance process of the host plant [51].
The rhizosphere comprises a complex and dynamic microenvironment, with mediating characteristics in the selection of organisms that interact with the host plant. The rhizosphere can be understood as a holobiont assemblage, which is formed by the association of a host and its microbial communities [52,53]. The holobiont impacts the stability, adaptation and evolution of the organisms involved in the assemblage [54].
Under stress conditions, such as drought, salt, and poor nutrient availability, the proper functioning of the holobiont is essential for the development and growth of plants, and thus, for improving their tolerance and nutrient-obtaining capacities [55]. In summary, the ecological relationship between the rhizosphere and the characteristics of PGP microorganisms is essential for the growth, nutrition, and quality of crops, both under favorable and stress conditions. This importance is reflected even more in a context of climate change, where interactions at the rhizosphere level play a fundamental role in the health of crops [56], carbon sequestration, nutrient cycling, and rhizosphere ecosystem functioning [57,58].
Plants are closely associated with soil microorganisms both externally and internally in various ways [59]. Numerous works have demonstrated the important role played by rhizosphere microorganisms in plant growth, highlighting those with PGP capacity (Figure 1). These PGP traits have been evidenced in several studies using different rhizosphere microorganisms (Table 1 and Table 2). PGP microorganisms, in addition to benefiting plant growth, are also characterized by having the ability to produce hormones and lytic enzymes, showing a promising use as bioinoculants [60]. Some microorganisms are also involved in the production of secondary metabolites, thus promoting carbon sequestration, nitrogen fixation, and phosphorus solubilization in the rhizosphere [61,62].
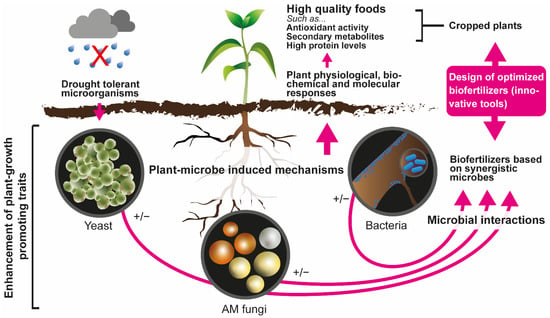
Figure 1.
Generalized representation of the main effects of plant growth-promoting (PGP) traits on cropped plants under drought conditions as the basis for the design of optimized biofertilizers. Possible effects of PGP microorganisms on plants are shown with +/− since such effects might be positive or negative.
PGP microorganisms (fungi, yeasts, and bacteria) are present in the soil and can contribute to maintaining or enhancing soil health, improving plant growth, inducing systemic resistance in plants, and increasing stress tolerance against different unfavorable environmental conditions, both biotic and abiotic [63]. Several works have described these PGP attributes, emphasizing mainly their ability to: (i) ensure greater nutrient availability for plants [64], (ii) stimulate changes in their root structure [13], (iii) promote the establishment and growth of plants under conditions of abiotic stress (e.g., potentially toxic elements, salinity, and drought) [5,12,65,66,67], or (iv) help with the control of phytopathogens [68].
Studies looking for beneficial microorganisms have typically used various characteristics to assess their PGP capacity, such as the microbial production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase, siderophores, indole-3-acetic acid (IAA), and enzymes for the solubilization of nutrients (mainly phosphates) [6,7,14,69,70]. In addition, certain microbes (e.g., some bacteria and yeast) can potentially mitigate the phytotoxic effects by producing organic acids and extracellular polymeric substances, such as exopolysaccharides (EPSs). Additionally, AM fungi contribute with this through the production of glomalin, which is a fungal glycoprotein [18,71,72,73,74,75].
All the above beneficial traits are normally observed at rhizosphere level, with scarce reports that include the effects on host plants. The use of PGP microbes has been analyzed through effects on plants such as: (i) improvements in photosynthetic variables [7,8,76,77], (ii) biofortification of plants with essential [78,79] and beneficial nutrients [19,20], (iii) modification of antioxidant responses [66,80], and (iv) changes in secondary metabolite profiles [81,82,83], among others.
However, it should be noticed that the most recent reports highlight the increased tolerance of plants against water stress, such as those based in salinity and drought, because of direct and indirect effects by the above-described traits. In this sense, microorganisms with PGP capabilities have been shown to promote plant growth under water deficit stress conditions, suggesting that they are naturally acclimatized to the stressed environment [84].
Bacteria with PGP attributes, for instance, are key soil components able to establish beneficial associations with plants [46]. Additionally, bacteria are the most studied group of microorganisms under drought stress conditions, including the genera Acinetobacter, Azospirillum, Azotobacter, Arthrobacter, Bacillus, Beijerinckia, Brevundimonas, Burkholderia, Clostridium, Delftia, Duganella, Erwinia, Enterobacter, Flavobacterium, Hydrogenophaga, Methylobacterium, Paenibacillus, Pantoea, Proteus, Providencia, Pseudomonas, Psychrobacter, Rhizobium, Serratia, Stenotrophomonas, Streptoccoccus, and Streptomyces [85,86,87,88,89].
In order to enhance plant growth, the most common effect of bacteria on plants exposed to drought conditions is the improvement in the antioxidant plant responses, reducing the cell damage (mainly in membranes) and alleviating their status of stress (Table 1).

Table 1.
Recent research (2020–2022) of plant growth-promoting (PGP) bacteria and their effects on plants growing under drought stress.
Table 1.
Recent research (2020–2022) of plant growth-promoting (PGP) bacteria and their effects on plants growing under drought stress.
| Crop | Microorganism | PGP Traits Evidenced | Specific Effects | References |
|---|---|---|---|---|
| Sorghum bicolor L. | Streptomyces laurentii and Penicillium sp. | P and Zn-solubilization. Siderophores, hydrogen cyanide, NH3 and IAA production | + Plant growth + Chlorophyll content + Production of osmolytes − Lipid peroxidation | [89] |
| Poncirus trifoliata | Ochrobacetrum sp., Microbacterium sp., Enterobacter sp., and Enterobacter cloacae | N-fixation, P-solubilization, ACC deaminase activity, siderophore and IAA production | + Proline accumulation in leaves + Relative water content + Cell membrane stability index + Genes like sbP5CS2 and sbP5CS1 | [59] |
| Zea mays L. | Arthrobacter arilaitensis and Streptomyces pseudovenezuelae | P-solubilization, ACC deaminase activity, IAA, siderophore, and ammonia production | + Shoot and root lengths + Dry shoot and root weights + Chlorophyll content + Numbers of leaves | [90] |
| Triticum aestivum | Pseudomonas azotoformans | P-solubilization, ACC deaminase activity, EPS and IAA production. Expression of biofilm genes AdnA and FliC | + Plant growth + Dry weight of root and shoot + Photosynthetic pigments content − CAT, SOD, and GR activity | [91] |
| Glycine Max L. | Bacillus cereus, Pseudomonas otitidis, and Pseudomonas sp. | P-solubilization, ACC deaminase activity, IAA and ammonia production | + Plant growth + Stomatal density + Relative water content + Chlorophyll pigments + Sugar, protein, and proline content − MDA and H2O2 | [92] |
| Oryza sativa L. | Bacillus megaterium, Bacillus altitudinis, and Bacillus endophyticus | ACC deaminase activity, IAA, EPS, and GA production under stress conditions | + Plant growth + Carotenoids + Total proteins + Sugar content | [93] |
| Zea mayz L. | Bacillus subtilis strains (DHK and B1N1) | P-solubilization, ACC deaminase activity, IAA and siderophore production. Antagonism with Fusarium oxysporum and Rhizoctonia solani | + Plant growth + SOD, POD, and CAT activity + Chlorophyll content + Amino acid content − ROS species | [94] |
| Solanum lycopersicum | Streptomyces strains (IT25 and C-2012) | P-solubilization, siderophore production, ACC deaminase activity. Salinity tolerance (NaCl 13%) | + Plant growth + Leaf relative water content + Proline, MDA, H2O2, and total sugar content + Gene expression of ERF1 and WRKY70 + APX activity − CAT and GPX activity | [95] |
| Triticum aestivum L. | Pseudomonas sp. and Serratia marcescens | P-solubilization, Zn-solubilization, ACC deaminase activity, IAA, EPS, siderophore, and ammonia production | + Osmolyte accumulation + Chlorophyll and carotenoids + Zn and Fe content in grains | [96] |
| Triticum aestivum L. | Pseudomona helmanticensis and Pseudomona baetica | P-solubilization and IAA production in presence of salinity (NaCl 4%) in different drought stress | + Soil p-available + Shoot and root dry weight + Grain yield + P uptake by shoot | [97] |
| Oryza Sativa L. | Diverse PGP microorganism | P-solubilization, siderophore, EPS, N-fixation, expression of nifH and polR genes. Drought tolerance | + Rice seedling + Shoot length + Shoot and root fresh weight + Antioxidant capability + Proline and soluble sugar content | [98] |
| Zea mays L. | Bacillus subtilis and Bacillus safensis | P-solubilization, ACC deaminase activity, IAA, EPS, biofilm, and alginate production | + Total chlorophyll, carotenoid, and soluble sugar − Proline accumulation − Antioxidant enzymes − ACC accumulation, ACC oxidase and ACC synthase under salt stress | [14] |
| Eleusine coracana L. | Variovorax paradoxus, Ochrobactrum anthropi, Pseudomonas palleroniana, Pseudomonas fluorescens, and Pseudomonas palleroniana | P-solubilization, ACC deaminase activity, IAA and siderophore production, N-fixation | + Overall growth parameters and nutrient concentration + SOD, GPX, CAT, and APX activity + Proline, phenol, and chlorophyll − H2O2 and MDA | [99] |
| Sorghum bicolor | Streptomyces sp. and Nocardiopsis sp. | P-solubilization, ACC deaminase activity, IAA and siderophore production under drought, heat, and Cd stress | + Plant growth and photosynthetic pigments + Translocation of Cd from root to shoot + SOD, APX, and CAT activity − MDA concentration | [100] |
(+): Increase; (−): Decrease; CAT: Catalase; SOD: Superoxide dismutase; POD: Peroxidase; APX: Ascorbate perosidase; GPX: Guaiacol peroxidase; GR: Glutation reductase; GTS: Glutation transferase; PPO: polyphenoloxidase; MDA: Malondialdehyde; AsA: Ascorbic Acid; AMF: Arbuscular mycorrhizal fungi; EPS: exopolysaccharide IAA: Indole acetic acid; ABA: abscisic acid.
AM fungi are another important microbial group studied by its PGP effects. AM fungi are an obligate biotroph that depend on living root tissue for carbohydrate supply, which allows them to complete their life cycle [101]. AM fungal colonization on plant roots occurs when its hyphae penetrate the epidermis and grow extensively between and within living cortical cells, forming a very large and dynamic interface between both symbionts. It enhances plant growth and yield and also decreases the effects of several abiotic stresses [102].
This obligate symbiosis can promote the formation of stable aggregates and improve water storage in the soil through the production of glomalin, which is released in large amounts into the soil [5,75,103]. Moreover, the formation of AM symbiosis can change the efficiency of water uptake by modifying the ionic balances (Na/K) in the host plant, as well as modifying the relative expression of PIP aquaporin and ionic NHX antiporter genes under osmotic stress [5,104]. New reports using AM fungi in association with other PGP microorganisms have also shown a beneficial (synergic or additive) effect on plant growth and development when established in water-scarce environments (Table 2).

Table 2.
Recent research (2020–2022) regarding the use of arbuscular mycorrhizal fungi (AMF) with or without complements and their effects on plant under drought stress.
Table 2.
Recent research (2020–2022) regarding the use of arbuscular mycorrhizal fungi (AMF) with or without complements and their effects on plant under drought stress.
| TCrop | AMF + Complement | Specific Effects | Reference |
|---|---|---|---|
| Glycine max L. | Glomus clarum, Glomus mosseae, and Gigaspora margarita + Bradyrhizobium japonicum | + Number of nodules + Grain yield and growth + CAT and POD in seeds + Proline content + Gene expression of CAT and POD − Gene expression of P5CS, P5CR, PDH, and P5CDH | [105] |
| Poncirus trifoliata | Funneliformis mosseae | + Leaf gas exchange + Soil pH, and ammonium content + H+-ATPase activity on shoot and roots + Regulation of H+-ATPase gene PtAHA2 | [106] |
| Poncirus trifoliata | Funneliformis mosseae | + Growth traits and leaf water potential + Gene expression of two aquaporin protein + Chlorophyll concentration | [107] |
| Glycine max L. | Acaulospora laevis, Septoglomus deserticola, and Rhizophagus irregularis + Bacillus amyloliquefaciens | + Plant biomass + Phenol, flavonoid, glycine betaine content and GTS + GA, trans-zeatin-riboside, and IAA in seeds + ATP content and hydrolytic activities of plasma membrane − ABA | [108] |
| Ephedra foliate | Claroideoglomus etunicatum, Rhizophagus intraradices, and Funneliformis mosseae | + Plant growth, chlorophyll content, nitrate and nitrite reductase activity, antioxidant activity, and ascorbic acid content + Content of proline, glucose, and total soluble protein + Sucrose phosphate synthetase activity, IAA, IBA, GA, and ABA − Glutathione level | [109] |
| Nicotiana tabacum | Glomus versiforme + Phosphorus supplementation | + Osmolytes content, proline, sugars, and free amino acids + Antioxidant activities of SOD, CAT, APX, POD, and GR, and AsA and GSH content. + IAA, ABA concentrations in roots and leaves. − ROS accumulation and lipid peroxidation | [110] |
| Solanum lycopersicum | Glomus sp., Sclerocystis sp. and Acaulospora sp. + Acinetobacter sp., and Rahnella aquatilis + compost | + Biomass, fruit number per plant, and fruit yield + Sugar content on shoot − PPO activity and increase of POD activity | [111] |
| Camellia sinensis | Claroideoglomus etunicatum | + Plant growth and leaf water content + Antioxidant activity as SOD, CAT, GPX, and APX + Regulation of CsSODin and CsCAT genes − O2− and MDA content | [112] |
| Vaccinium corymbosum | Funneliformis mosseae | + Proteins involved in amino acid metabolism, antioxidant system, signal transduction, and photosynthesis + Carotenoid biosynthesis + Photosynthetic capacity | [113] |
| Malus hupehensis | Rhizophagus irregularis | + Plant growth + Total chlorophyll content, net photosynthetic rate, stomatal conductance, and transpiration + SOD, POD, and CAT + Proline and total sugar content − Accumulation of MDA, H2O2 and O2− | [114] |
| Populus cathayana | Rhizophagus intraradices | + Plant biomass, root-to-root radio, photosynthetic rate, stomatal conductance + Intercellular CO2 concentration and transpiration rate. + SOD, POD, soluble sugar content especially on shoot + Gene expression of PcGRF10 and PcGRF11 genes induced by AMF | [115] |
| Pheonix dactylifera | AMF consortium + plant growth-promoting rhizobacteria (PGPR) consortium | + Plant biomass, rise of phosphorus uptake, and boosted plant-water relationship + Total soluble sugar and protein content. + Soil organic matter, phosphorus, and glomalin content − H2O2 and MDA accumulation | [116] |
| Solanum lycopersicum | Funneliformis mosseae, Rhizophagus irregularis and Funneliformis coronatum | − H2O2 and MDA content (especially F. mosseae) | [117] |
| Thymus daenensis and Thymus bulgaris | Funneliformis moseae and Rhizophagus intraradices | + Root and shoot dry weight, relative water content, photosynthetic pigments, gas change, and nutritional parameters + Essential oil production + Root colonization and soil spore density − Proline, MDA, electrolyte leakage, and stomatal resistance | [118] |
| Trifolium repens L. | Funneliformis mosseae and Paraglomus occultum | + Root total length, surface area, and volume + Leaf relative water content + SOD, CAT, POD, and ABA levels in root − MDA content | [119] |
(+): Increase; (−): Decrease; CAT: Catalase; SOD: Superoxide dismutase; POD: Peroxidase; APX: Ascorbate perosidase; GPX: Guaiacol peroxidase; GR: Glutation reductase; GTS: Glutation transferase; PPO: polyphenoloxidase; MDA: Malondialdehyde; AsA: Ascorbic Acid; AMF: Arbuscular mycorrhizal fungi; IAA: Indole acetic acid; ABA: abscisic acid.
On the other hand, the yeast microbial group has also been studied for their PGP capacity. However, there are fewer studies compared to the groups described above, mainly including some works exploring its PGP attributes under water stress conditions. For instance, Silambarasan et al. [7,18] demonstrated the ability of EPS produced by yeasts to promote the formation of stable aggregates and improve the storage of water in soil.
Furthermore, recent evidence showed that yeast application can upregulate soil enzymes under drought stress conditions, which increased the nutrient content in the soil, also improving the osmotic state of roots and the activity of antioxidant enzymes in the plants treated with the strains [63]. PGP microorganisms are undoubtedly a key element for the adaptation of plants to new unfavorable environmental scenarios. Deepening studies oriented to evaluate the interspecies synergistic potential of PGP microorganisms in the current framework of climate change are required.
4. The “Omics” Approaches and the Development of Optimized Bioinoculants
As stated above, the use of microorganism for improved drought tolerance has shown promising results in different crops [120,121]. In this sense, rhizosphere engineering (modification in the microbial rhizosphere community by known PGP microorganisms) represents a faster and more advantageous alternative to improve drought tolerance in crops than other tools, such as genetic engineering or genetic improvement [122].
The interaction of the newly added microorganisms with the rhizosphere is the first barrier to overpass to establish a relation with the already naturally present microbiome, and in the better situation, form a mutualistic and cooperative relationship [22,123]. Then, the newly formed microbiome interacts with the plant root and may produce an increase in the physiological traits that allow the plant to confront the drought stress.
This promotes, for instance, the formation of lateral roots [124], generates increases in hormone levels (ABA, cytokinin, and gibberellin) associated with increases in the water content and the hydric status of plant organs [125,126,127], improves the expression of osmotic adjustment systems (proline), and promotes the production of antioxidant enzymes [128], among others.
However, a negative effect on the plant growth and development is also a possibility [129]. The way how this microbial community increases the tolerance to abiotic stress is not completely understood yet. This gap in information is due to the complex media where microbial communities are developing, which is the most complex compartment on the Earth’s surface [24]. In this sense, understanding the interaction among microbial communities, plants, and the biotic and abiotic factors is an essential step to improve the design of bioinoculants [130,131].
A modern approach to study this interaction is the use of “Omics” technologies, which are also being applied in microbial science (Table 3). This set of new techniques allows to integrate the information of genome, proteome, transcriptome, and metabolome and gives information about the biological changes that underlie the drought tolerance produced by advanced bioinoculants [24,132].

Table 3.
Recent research regarding the use of PGP microorganisms and their effects on plant under drought stress through omics approaches.
Metabolomics is based on tools that allow the identification of the complete metabolites synthesized by an organism and can be used for the determination of how this metabolic profile changes in different conditions, such as drought stress [139]. The usual workflow to determine a metabolic profile starts with sample acquisition, sample preparation, data acquisition, bioinformatic analysis, and biochemical interpretation [140].
The widely used techniques for separating and determining the metabolic profile from different microorganisms are based on thin-layer chromatography (TLC), column chromatography (CC), flash chromatography (FC), gas chromatography coupled to mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), liquid chromatography coupled to mass spectrometry (LC-MS and LC-MS/MS), liquid chromatography with ultraviolet, visible, fluorescence, or diode array detection (LC-UV-VIS, LC-FD, or LC-DAD), gas–liquid chromatography, and Fourier transform infrared (FTIR), near-infrared (NIR), and nuclear magnetic resonance (NMR) spectroscopies [140,141].
For example, the application of the metabolomics approach in the study of drought condition in the root of the trifoliate orange and the interaction with the AM fungus Rhizophagus intraradices showed a total of 88 and 17 metabolites upregulated and downregulated, respectively, also showing an improvement in the physiological status of the mycorrhized plants [142]. In the same way, the use of metabolomics tools in the application of a consortium of Bacillus subtilis, B. thuringiensis, and B. megaterium in chickpea showed an accumulation of riboflavin, L-asparagine, aspartate, glycerol, nicotinamide, and 3-hydroxy-3-methyglutarate under drought condition, together with the reduction in the deleterious impact on the plant status [143].
On the other hand, proteomics is the use of different technics that allows to determine the complete contents of the different proteins present in an organism under specific circumstances [144]. Proteomics analysis can be used in certain ways, such as for translational proteomics, protein–protein interaction, post-translational modification, and proteomics studies at a comparative level for the comparison of protein profiles, including drought stress [141].
The typical workflow of proteomics analysis starts with the extraction of proteins in a plant tissue of interest, digestion of proteins with specific enzymes into peptides and identification of resulting peptides by mass spectrometry, quantification of protein expression, and determination of post-translational modifications [145]. The widely used techniques used for the study of proteomes include 1D and 2D gel electrophoresis, followed by mass spectrometry with different ionization sources. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF MS/MS) and electrospray ionization (ESI) are the main mass spectrometry tools used in proteomics approaches [141].
With the use of proteomics approaches, it was possible to find in pepper plants inoculated with B. licheniformis K11 a total of 15 differential expressed proteins that confer tolerance to drought stress [145]. Similar results were observed for the inoculation of Rhizobium leguminosarum and Pseudomonas putida to Vicia faba, which produced changes in the proteomic profile with an improvement in the tolerance to drought stress [145].
Genomics is the study of all genes present in an organism with the respective identification of sequences, intragenic sequences, and genes structures [145]; meanwhile, transcriptomics is focused on the determination of the RNA present in the specific organs, which is highly dependent on the specific environmental condition that makes the transcriptomic highly variable [146]. In this sense, the study of genomics allows to know the potential of microorganisms to produce secondary metabolites or proteins to enhance the growth and development of drought tolerance with plant interaction.
Furthermore, transcriptomics allows to determine how this genomic potential is expressed under specific circumstances. In both cases, the starting point is the extraction of the nucleic acid, but for RNA, it is necessary to synthesize the cDNA and then sequence it in a next-generation sequencing platform [141]. The use of the integrated genomic and transcriptomic approach showed that some colonization genes such as FixL/FixK/FixJ and NodD were upregulated in the presence of beneficial microorganisms [147]. Moreover, there are several types of plant growth-promoting rhizobacteria (PGPR) with the full genome assembly, which previously have shown an improvement in the plant tolerance to drought stress, such as B. amyloliquefaciens [148], Serratia plymuthica [149], Hartmannibacter diazotrophicus [150], and Rhizophagus irregularis [151].
The omics approaches still have important bottlenecks, such as the need of different, focused, and specialized researchers; and the gap in both information and integration of data available in the worldwide databases (e.g., one-stop shop) [152]. Currently, data integration can be performed post-analysis by performing “networking” after individual analyses [153] or carrying out an integrated data analysis in parallel. This last one requires specialized tools to merge data from different platforms prior to the final interpretations of the results [132,154]. For instance, MetaboAnalyst allows for multiple integration of the metabolome, transcriptome, proteome, and genome into a wide spectrum of biological samples, including plants [155], as well as data processing and statistical analyses based on the R platform [132].
Undoubtedly, the research possibilities with the incorporation of multi-omics approaches will be a strong basis for the functional interpretation of the effects that advanced biofertilizers have on their host plants. Based on the strong progress that these approaches have had in other sciences (mainly medicine and human health), it is feasible to state that they may represent the starting point for the design of optimized biofertilizers in a wide range of plant species of agricultural and environmental interest.
5. Perspectives and Conclusions
As stated above, a large body of evidence highlights the role of specific microbial strains within the main rhizosphere microbial groups in conferring drought tolerance to plants. However, much less known are the physiological, molecular, and biochemical mechanisms and responses displayed by plants as a consequence of the presence and action of these PGP microorganisms.
In the case of yeasts, these microorganisms have very interesting PGP characteristics that have not been widely reported, especially regarding water stress. Nevertheless, recent studies have demonstrated their great potential as coadjutants in plant growth to cope with other abiotic stresses. Such evidence makes us presume that yeasts can be a key element with a great biotechnological value that needs to be explored as a tool to face the food shortage that promises to be accentuated with the advance of GCC.
Therefore, the next steps for the designing of biofertilizers supported by the use of multi-omics approaches could represent a significant leap in the research regarding the role of rhizosphere microbial communities in plant production under drought conditions. In this sense, the integration of omics platforms will strongly support the mechanistic understanding that underlies the use of beneficial microorganisms in plant production through rhizosphere engineering.
While it is necessary to realistically recognize that the tangible results could primarily be framed at the local level, considering particular soil conditions, rhizospheres, and crop plants, the research at the pilot scale will establish the basis for the generation and massification of optimized biofertilizers. However, some considerations must be addressed, as the development of local biological collections for the ex situ maintenance of PGP microbes able to enhance drought tolerance represents a valuable resource for testing their applicability in different crops, in line with the global advice for the maintenance of genetic resources oriented to agriculture and food production [156].
Moreover, the projection of the fundamental and mechanistic bases studied in the plant, considering different efficient rhizosphere modifications, can also be focused on different types of environmental stress, such as the low availability of nutrients, salinity, heavy metals, extreme pH values, and many other environmental and soil constraints that currently affect enormous areas of arable land surfaces worldwide.
Author Contributions
Conceptualization, C.V. and P.C.; investigation, C.V., C.S. (Christian Santander), R.P., V.G. and F.G.; writing—original draft preparation, C.V., C.S. (Christian Santander), R.P., V.G., H.A. and F.G.; writing—review and editing, A.R., C.S. (Cledir Santos), H.A. and P.C.; visualization, C.V., H.A. and P.C.; supervision, A.R., C.S. (Cledir Santos) and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work in the current research line was funded by ANID (Agencia Nacional de Investigación y Desarrollo, Chile) through the grants ANID/FONDECYT/1210964, ANID/FONDECYT/3210588, ANID/FONDECYT/3210752, ANID/FONDECYT/1221024, and ANID/FONDAP/15130015. The authors also acknowledge the grant InES19-FRO19001 from the Ministerio de Educación, Chile, executed by Universidad de La Frontera.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors want to thank to the anonymous reviewers for their careful read.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A Climate Dynamics Perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Gerten, D.; Heck, V.; Jägermeyr, J.; Bodirsky, B.L.; Fetzer, I.; Jalava, M.; Kummu, M.; Lucht, W.; Rockström, J.; Schaphoff, S.; et al. Feeding Ten Billion People Is Possible within Four Terrestrial Planetary Boundaries. Nat. Sustain. 2020, 3, 200–208. [Google Scholar] [CrossRef]
- ODEPA. Reflexiones y Desafíos Al 2030; Apey, A., Barrera, D., Rivas, T., Eds.; ODEPA: Santiago, Chile, 2018; ISBN 9789567244300. [Google Scholar]
- Santander, C.; Aroca, R.; Ruiz-Lozano, J.M.; Olave, J.; Cartes, P.; Borie, F.; Cornejo, P. Arbuscular Mycorrhiza Effects on Plant Performance under Osmotic Stress. Mycorrhiza 2017, 27, 639–657. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Abraham, J.; Valentine, A. Simultaneous Mitigation of Aluminum, Salinity and Drought Stress in Lactuca Sativa Growth via Formulated Plant Growth Promoting Rhodotorula mucilaginosa CAM4. Ecotoxicol. Environ. Saf. 2019, 180, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Role of Plant Growth–promoting Rhizobacterial Consortium in Improving the Vigna radiata Growth and Alleviation of Aluminum and Drought Stresses. Environ. Sci. Pollut. Res. 2019, 26, 27647–27659. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P. Role of Curtobacterium herbarum Strain CAH5 on Aluminum Bioaccumulation and Enhancement of Lactuca sativa Growth under Aluminum and Drought Stresses. Ecotoxicol. Environ. Saf. 2019, 183, 109573. [Google Scholar] [CrossRef]
- Rojas, M.; Lambert, F.; Ramirez-Villegas, J.; Challinor, A.J. Emergence of Robust Precipitation Changes across Crop Production Areas in the 21st Century. Proc. Natl. Acad. Sci. USA 2019, 116, 6673–6678. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Cornejo, P.; Seguel, A.; Aguilera, P.; Meier, S.; Larsen, J.; Borie, F. Arbuscular Mycorrhizal Fungi Improve Tolerance of Agricultural Plants to Cope Abiotic Stress Conditions BT. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Microbial Interactions and Agro-Ecological Impacts; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; Volume 2, pp. 55–80. ISBN 978-981-10-6593-4. [Google Scholar]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus Acquisition Efficiency Related to Root Traits: Is Mycorrhizal Symbiosis a Key Factor to Wheat and Barley Cropping? Front. Plant Sci. 2018, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Borie, F.; Aguilera, P.; Castillo, C.; Valentine, A.; Seguel, A.; Barea, J.M.; Cornejo, P. Revisiting the Nature of Phosphorus Pools in Chilean Volcanic Soils as a Basis for Arbuscular Mycorrhizal Management in Plant P Acquisition. J. Soil Sci. Plant Nutr. 2019, 19, 390–401. [Google Scholar] [CrossRef]
- Barea, J.M.; Palenzuela, J.; Cornejo, P.; Sánchez-Castro, I.; Navarro-Fernández, C.; Lopéz-García, A.; Estrada, B.; Azcón, R.; Ferrol, N.; Azcón-Aguilar, C. Ecological and Functional Roles of Mycorrhizas in Semi-Arid Ecosystems of Southeast Spain. J. Arid Environ. 2011, 75, 1292–1301. [Google Scholar] [CrossRef]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The Role of Arbuscular Mycorrhizas in Decreasing Aluminium Phytotoxicity in Acidic Soils: A Review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef]
- Seguel, A.; Meier, F.; Azcón, R.; Valentine, A.; Meriño-Gergichevich, C.; Cornejo, P.; Aguilera, P.; Borie, F. Showing Their Mettle: Extraradical Mycelia of Arbuscular Mycorrhizae Form a Metal Filter to Improve Host Al Tolerance and P Nutrition. J. Sci. Food Agric. 2020, 100, 803–810. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Evaluation of the Production of Exopolysaccharide by Plant Growth Promoting Yeast Rhodotorula sp. Strain CAH2 under Abiotic Stress Conditions. Int. J. Biol. Macromol. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Borie, F.; Cornejo, P.; Mora, M.L. Enhanced Selenium Content in Wheat Grain by Co-Inoculation of Selenobacteria and Arbuscular Mycorrhizal Fungi: A Preliminary Study as a Potential Se Biofortification Strategy. J. Cereal Sci. 2013, 57, 275–280. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Armada, E.; López-Castillo, O.M.; Cornejo, P.; Mora, M.L.; Azcón, R. Inoculation with Selenobacteria and Arbuscular Mycorrhizal Fungi to Enhance Selenium Content in Lettuce Plants and Improve Tolerance against Drought Stress. J. Soil Sci. Plant Nutr. 2016, 16, 201–225. [Google Scholar] [CrossRef]
- Larsen, J.; Cornejo, P.; Barea, J.M. Interactions between the Arbuscular Mycorrhizal Fungus Glomus intraradices and the Plant Growth Promoting Rhizobacteria Paenibacillus polymyxa and P. macerans in the Mycorrhizosphere of Cucumis sativus. Soil Biol. Biochem. 2009, 41, 286–292. [Google Scholar] [CrossRef]
- Großkopf, T.; Soyer, O.S. Synthetic Microbial Communities. Curr. Opin. Microbiol. 2014, 18, 72–77. [Google Scholar] [CrossRef]
- Guerrero-Molina, M.F.; Lovaisa, N.C.; Salazar, S.M.; Martínez-Zamora, M.G.; Díaz-Ricci, J.C.; Pedraza, R.O. Physiological, Structural and Molecular Traits Activated in Strawberry Plants after Inoculation with the Plant Growth-Promoting Bacterium Azospirillum brasilense REC3. Plant Biol. 2015, 17, 766–773. [Google Scholar] [CrossRef] [PubMed]
- White, R.A.; Rivas-Ubach, A.; Borkum, M.I.; Köberl, M.; Bilbao, A.; Colby, S.M.; Hoyt, D.W.; Bingol, K.; Kim, Y.M.; Wendler, J.P.; et al. The State of Rhizospheric Science in the Era of Multi-Omics: A Practical Guide to Omics Technologies. Rhizosphere 2017, 3, 212–221. [Google Scholar] [CrossRef]
- Reddy, P.P. Plant Growth Promoting Rhizobacteria for Horticultural Crop Protection; Springer India: New Delhi, India, 2014; pp. 978–981. [Google Scholar]
- Gomiero, T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Muir, J.F.; Nisbett, N.; Lawrence, D.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The Future of the Global Food System. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2769–2777. [Google Scholar] [CrossRef]
- Watts, G.; Battarbee, R.W.; Bloomfield, J.P.; Crossman, J.; Daccache, A.; Durance, I.; Elliott, J.A.; Garner, G.; Hannaford, J.; Hannah, D.M.; et al. Climate Change and Water in the UK—Past Changes and Future Prospects. Prog. Phys. Geogr. 2015, 39, 6–28. [Google Scholar] [CrossRef]
- Mansfield, L.A.; Nowack, P.J.; Kasoar, M.; Everitt, R.G.; Collins, W.J.; Voulgarakis, A. Predicting Global Patterns of Long-Term Climate Change from Short-Term Simulations Using Machine Learning. Npj Clim. Atmospheric Sci. 2020, 3, 44–52. [Google Scholar] [CrossRef]
- Molden, D. Water for Food Water for Life: A Comprehensive Assessment of Water Management in Agriculture; International Water Management Institute: London, UK, 2007; 664p, ISBN 9781844073962. [Google Scholar]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Drought Effects on Root and Tuber Production: A Meta-Analysis. Agric. Water Manag. 2016, 176, 122–131. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-Deficit Stress-Induced Anatomical Changes in Higher Plants. Comptes Rendus. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Sawant, K. Drought Stress Adaptation: Metabolic Adjustment and Regulation of Gene Expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Lisar, S.Y.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012; pp. 1–14. ISBN 978-953-307-963-9. [Google Scholar]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Khan, H.A.; Ziaf, K.; Amjad, M.; Iqbal, Q. Exogenous Application of Polyamines Improves Germination and Early Seedling Growth of Hot Pepper. Chil. J. Agric. Res. 2012, 72, 429–433. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The Transcriptional Regulatory Network in the Drought Response and Its Crosstalk in Abiotic Stress Responses Including Drought, Cold, and Heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Ballesteros, G.I.; Castro-Nallar, E.; Meneses, C.; Gallardo-Cerda, J.; Torres-Díaz, C. A First Insight into the Structure and Function of Rhizosphere Microbiota in Antarctic Plants Using Shotgun Metagenomic. Polar Biol. 2019, 42, 1825–1835. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O.; Prigent-Combaret, C.; Cruz, C.; Stefan, M.; Kutu, F.; Glick, B.R. The Application of Plant Growth-Promoting Rhizobacteria in Solanum lycopersicum Production in the Agricultural System: A Review. PeerJ 2022, 10, e13405. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant–Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The Chemistry of Plant–microbe Interac-tions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 871, 1473. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Marchesi, J.R.; Mougel, C.; Selosse, M. Host-Microbiota Interactions: From Holobiont Theory to Analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Li, E.; de Jonge, R.; Liu, C.; Jiang, H.; Friman, V.P.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Jousset, A. Rapid Evolution of Bacte-rial Mutualism in the Plant Rhizosphere. Nat. Commun. 2021, 12, 3829. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant Growth Promoting Rhizobacteria (PGPR) Confer Drought Resistance and Stimulate Biosynthesis of Secondary Metabolites in Pennyroyal (Mentha pulegium L.) under Water Shortage Condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil Microbiomes and Climate Change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Bettenfeld, P.; Cadena i Canals, J.; Jacquens, L.; Fernandez, O.; Fontaine, F.; van Schaik, E.; Courty, P.E.; Trouvelot, S. The Microbiota of the Grapevine Holobiont: A Key Component of Plant Health. J. Adv. Res. 2022, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, S.F.M.; Tanunchai, B.; Wu, Y.T.; Sansupa, C.; Schädler, M.; Dawoud, T.M.; Buscot, F.; Purahong, W. Deciphering Trifolium Pratense, L. Holobiont Reveals a Microbiome Resilient to Future Climate Changes. MicrobiologyOpen 2021, 10, e1217. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; George, P.; Kumar, M.; Aher, L.; Raina, S.K.; Rane, J.; Annapurna, K.; Minhas, P.S. Multi-Trait PGP Rhi-zobacterial Endophytes Alleviate Drought Stress in a Senescent Genotype of Sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as Promising Complement of Chem-ical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Jansson, C.; Faiola, C.; Wingler, A.; Zhu, X.G.; Kravchenko, A.; de Graaff, M.A.; Ogden, A.J.; Handakumbura, P.P.; Werner, C.; Beckles, D.M. Crops for Carbon Farming. Front. Plant Sci. 2021, 12, 636709. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phos-phate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Naguib, D.M. Effect of Yeast Application on Soil Health and Root Metabolic Status of Corn Seedlings under Drought Stress. Arch. Microbiol. 2022, 204, 233. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Cornejo, P.; Pérez-Tienda, J.; Meier, S.; Valderas, A.; Borie, F.; Azcón-Aguilar, C.; Ferrol, N. Copper Compartmentalization in Spores as a Survival Strategy of Arbuscular Mycorrhizal Fungi in Cu-Polluted Environments. Soil Biol. Biochem. 2013, 57, 925–928. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of Two Arbuscular Mycorrhizal Fungal Inocula to Improve Saline Stress Tolerance in Lettuce Plants by Changes of Antioxidant Defense Mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Aponte, H.; Herrera, W.; Cameron, C.; Black, H.; Meier, S.; Paolini, J.; Tapia, Y.; Cornejo, P. Alteration of Enzyme Activities and Functional Diversity of a Soil Contaminated with Copper and Arsenic. Ecotoxicol. Environ. Saf. 2020, 192, 110264. [Google Scholar] [CrossRef] [PubMed]
- Durán, P.; Viscardi, S.; Acuña, J.J.; Cornejo, P.; Azcón, R.; de la Luz Mora, M. Endophytic Selenobacteria and Arbuscular Mycorrhizal Fungus for Selenium Biofortification and Gaeumannomyces graminis Biocontrol. J. Soil Sci. Plant Nutr. 2018, 18, 1021–1035. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica Oxyrrhina with Plant Growth Promoting Bacteria for the Improvement of Heavy Metal Phytoremediation under Drought Conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Banik, A.; Pandya, P.; Patel, B.; Rathod, C.; Dangar, M. Characterization of Halotolerant, Pigmented, Plant Growth Promot-ing Bacteria of Groundnut Rhizosphere and Its in-Vitro Evaluation of Plant-Microbe Protocooperation to Withstand Salinity and Metal Stress. Sci. Total Environ. 2018, 630, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Ginocchio, R.; Cornejo, P. Influence of Copper on Root Exudate Patterns in Some Metallophytes and Agricultural Plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Demanet, R.; Acuña, J.J.; Viscardi, S.; Jorquera, M.; Rengel, Z.; Durán, P. Aluminum-Tolerant Bacteria Improve the Plant Growth and Phosphorus Content in Ryegrass Grown in a Volcanic Soil Amended with Cattle Dung Manure. Appl. Soil Ecol. 2017, 115, 19–26. [Google Scholar] [CrossRef]
- Sarabia, M.; Cornejo, P.; Azcón, R.; Carreón-Abud, Y.; Larsen, J. Mineral Phosphorus Fertilization Modulates Interactions between Maize, Rhizosphere Yeasts and Arbuscular Mycorrhizal Fungi. Rhizosphere 2017, 4, 89–93. [Google Scholar] [CrossRef]
- Aguilera, P.; Larsen, J.; Borie, F.; Berríos, D.; Tapia, C.; Cornejo, P. New Evidences on the Contribution of Arbuscular My-corrhizal Fungi Inducing Al Tolerance in Wheat. Rhizosphere 2018, 5, 43–50. [Google Scholar] [CrossRef]
- Chávez, D.; Machuca, Á.; Fuentes-Ramirez, A.; Fernandez, N.; Cornejo, P. Shifts in Soil Traits and Arbuscular Mycorrhizal Symbiosis Represent the Conservation Status of Araucaria araucana Forests and the Effects after Fire Events. For. Ecol. Manag. 2020, 458, 117806. [Google Scholar] [CrossRef]
- Ademar Avelar Ferreira, P.; Ceretta, C.A.; Hildebrand Soriani, H.; Luiz Tiecher, T.; Fonsêca Sousa Soares, C.R.; Rossato, L.V.; Nicoloso, F.T.; Brunetto, G.; Paranhos, J.T.; Cornejo, P. Rhizophagus clarus and Phosphate Alter the Physiological Re-sponses of Crotalaria juncea Cultivated in Soil with a High Cu Level. Appl. Soil Ecol. 2015, 91, 37–47. [Google Scholar] [CrossRef]
- Griebenow, S.; Zuniga-Feest, A.; Muñoz, G.; Cornejo, P.; Kleinert, A.; Valentine, A. Photosynthetic Metabolism during Phos-phate Limitation in a Legume from the Mediterranean-Type Fynbos Ecosystem. J. Plant Physiol. 2019, 243, 153051. [Google Scholar] [CrossRef]
- Cornejo, P.; Rubio, R.; Castillo, C.; Azcón, R.; Borie, F. Mycorrhizal Effectiveness on Wheat Nutrient Acquisition in an Acidic Soil from Southern Chile as Affected by Nitrogen Sources. J. Plant Nutr. 2008, 31, 1555–1569. [Google Scholar] [CrossRef]
- Seguel, A.; Cornejo, P.; Ramos, A.; Von Baer, E.; Cumming, J.; Borie, F. Phosphorus Acquisition by Three Wheat Cultivars Contrasting in Aluminium Tolerance Growing in an Aluminium-Rich Volcanic Soil. Crop Pasture Sci. 2017, 68, 305–316. [Google Scholar] [CrossRef]
- Meier, S.; Azcón, R.; Cartes, P.; Borie, F.; Cornejo, P. Alleviation of Cu Toxicity in Oenothera picensis by Copper-Adapted Arbuscular Mycorrhizal Fungi and Treated Agrowaste Residue. Appl. Soil Ecol. 2011, 48, 117–124. [Google Scholar] [CrossRef]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of Fertilization and Arbuscular Mycorrhizal Fungal Inoculation on Antioxidant Profiles and Activities in Fragaria ananassa Fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanhueza, M.; Gómez, F.; Tereucán, G.; Valenzuela, T.; García, S.; Cornejo, P.; Hermosín-Gutiérrez, I. Changes in the Content of Anthocyanins, Flavonols, and Antioxidant Activity in Fragaria ananassa Var. Camarosa Fruits under Tradi-tional and Organic Fertilization. J. Sci. Food Agric. 2019, 99, 2404–2410. [Google Scholar] [CrossRef]
- Aguilera, A.; Tereucán, G.; Ercoli, S.; Cornejo, P.; Gomez, M.R.; Uhlmann, L.; Guigas, C.; Esatbeyoglu, T.; Ruiz, A. Influence of Organic and Chemical Fertilisation on Antioxidant Compounds Profiles and Activities in Fruits of Fragaria ananassa Var. Camarosa. J. Soil Sci. Plant Nutr. 2020, 20, 715–724. [Google Scholar] [CrossRef]
- Bandeppa; Paul, S.; Aggarwal, C.; Manjunatha, B.S.; Rathi, M.S. Characterization of Osmotolerant Rhizobacteria for Plant Growth Promoting Activities in Vitro and during Plant-Microbe Association under Osmotic Stress. Indian J. Exp. Biol 2018, 56, 582–589. [Google Scholar]
- Singh, N.P.; Singh, R.K.; Meena, V.S.; Meena, R.K. Can We Use Maize (Zea mays) Rhizobacteria as Plant Growth Promoter? Vegetos 2015, 28, 86–99. [Google Scholar] [CrossRef]
- Yadav, A.N. Agriculturally Important Micro Biomes: Biodiversity and Multifarious PGP Attributes for Amelioration of Di-verse Abiotic Stresses in Crops for Sustainable Agriculture. Biomed. J. Sci. Tech. Res. 2017, 1, 861–864. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, R.; Kumar, S.; Kumar, V.; Sugitha, T.C.K.; Singh, B.; Chauahan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Beneficial Microbiomes: Biodiversity and Potential Biotechnological Applications for Sustainable Agriculture and Human Health. J. Appl. Biol. Biotechnol. 2017, 5, 45–57. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in Crops: Diversity, Distribution, and Poten-tial Role in Crop Improvement. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–332. ISBN 9780444639882. [Google Scholar]
- Kour, D.; Rana, K.L.; Kaur, T.; Sheikh, I.; Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Microbe-Mediated Allevia-tion of Drought Stress and Acquisition of Phosphorus in Great Millet (Sorghum bicolor L.) by Drought-Adaptive and Phos-phorus-Solubilizing Microbes. Biocatal. Agric. Biotechnol. 2020, 23, 101501. [Google Scholar] [CrossRef]
- Chukwuneme, C.F.; Babalola, O.O.; Kutu, F.R.; Ojuederie, O.B. Characterization of Actinomycetes Isolates for Plant Growth Promoting Traits and Their Effects on Drought Tolerance in Maize. J. Plant Interact. 2020, 15, 93–105. [Google Scholar] [CrossRef]
- Ansari, F.A.; Jabeen, M.; Ahmad, I. Pseudomonas Azotoformans FAP5, a Novel Biofilm-Forming PGPR Strain, Alleviates Drought Stress in Wheat Plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Dubey, A.; Saiyam, D.; Kumar, A.; Hashem, A.; Abduallah, E.F.; Khan, M.L. Bacterial Root Endophytes: Characterization of Their Competence and Plant Growth Promotion in Soybean (Glycine max (L.) Merr.) under Drought Stress. Int. J. Environ. Res. Public Health 2021, 18, 931. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, A.K.; Muthukrishanan, G.; Truu, J.; Truu, M.; Ostonen, I.; Subramanian Kizhaeral, S.; Panneerselvam, P.; Go-palasubramanian, S.K. The Foliar Application of Rice Phyllosphere Bacteria Induces Drought-Stress Tolerance in Oryza sativa (L.). Plants 2021, 10, 387. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Sharma, M. Alleviation of Drought Stress in Maize (Zea mays L.) by Using Endogenous Endophyte Bacillus subtilis in North West Himalayas. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 361–370. [Google Scholar] [CrossRef]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces Alleviate Drought Stress in Tomato Plants and Modulate the Expression of Transcription Factors ERF1 and WRKY70 Genes. Sci. Hortic. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V. Multifarious Effect of ACC Deaminase and EPS Producing Pseudomonas sp. and Serratia marcescens to Augment Drought Stress Tolerance and Nutrient Status of Wheat. World J. Microbiol. Biotechnol. 2021, 37, 198. [Google Scholar] [CrossRef]
- Karimzadeh, J.; Alikhani, H.A.; Etesami, H.; Pourbabaei, A.A. Improved Phosphorus Uptake by Wheat Plant (Triticum aes-tivum L.) with Rhizosphere Fluorescent Pseudomonads Strains Under Water-Deficit Stress. J. Plant Growth Regul. 2021, 40, 162–178. [Google Scholar] [CrossRef]
- Pang, Z.; Zhao, Y.; Xu, P.; Yu, D. Microbial Diversity of Upland Rice Roots and Their Influence on Rice Growth and Drought Tolerance. Microorganisms 2020, 8, 1329. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Rhizobacteria Producing ACC Deaminase Mitigate Water-Stress Re-sponse in Finger Millet (Eleusine coracana (L.) Gaertn.). 3 Biotech 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant Growth-Promoting Actinobacterial Inoculant Assisted Phytoremediation Increases Cadmium Uptake in Sorghum bicolor under Drought and Heat Stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal Fungi (AMF) Protects Photosynthetic Apparatus of Wheat under Drought Stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved Photosynthetic Efficacy of Maize (Zea mays) Plants with Arbuscular Mycor-rhizal Fungi (AMF) under High Temperature Stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-Related Soil Protein in a Mediterranean Ecosystem Affected by a Copper Smelter and Its Contribution to Cu and Zn Sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and Biochemical Responses of Soybean Plants Inoculated with Arbuscular Mycorrhizal Fungi and Bradyrhizobium under Drought Stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in Trifoliate Orange by Regulating H+-ATPase Activity and Gene Expression. Front. Plant Sci. 2021, 12, 659694. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular Mycorrhizas Modulate Root Polyamine Metabolism to Enhance Drought Tolerance of Trifoliate Orange. Environ. Exp. Bot. 2020, 171, 103926. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Abd Elgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Alhaj Hamoud, Y.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and Mycorrhiza Confers Tolerance to Drought Stress and Improve Seed Yield and Quality of Soybean Plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Al-Arjani, A.B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular Mycorrhizal Fungi Modulates Dynamics Tolerance Expression to Mitigate Drought Stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF Inoculation and Phosphorus Supplementation Alleviates Drought Induced Growth and Photosynthetic Decline in Nicotiana tabacum by Up-Regulating Antioxidant Metabolism and Osmolyte Accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Tahiri, A.-I.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the Potential Role of Compost, PGPR, and AMF in Improving Tomato Plant Growth, Yield, Fruit Quality, and Water Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, Y.J.; Wu, Q.S.; Yang, T.Y.; Kuča, K. Arbuscular Mycorrhizal Fungi Improve the Antioxidant Capacity of Tea (Camellia sinensis) Seedlings under Drought Stress. Not. Bot. Horti. Agrobot. Cluj-Napoca 2020, 48, 1993–2005. [Google Scholar] [CrossRef]
- Gui, L.X.; Lu, S.S.; Chen, Q.; Yang, L.; Xiao, J.X. ITRAQ-Based Proteomic Analysis Reveals Positive Impacts of Arbuscular Mycorrhizal Fungi Inoculation on Photosynthesis and Drought Tolerance in Blueberry. Trees—Struct. Funct. 2021, 35, 81–92. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular Mycorrhizal Fungi Enhanced Drought Re-sistance in Apple by Regulating Genes in the MAPK Pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Han, Y.; Lou, X.; Zhang, W.; Xu, T.; Tang, M. Arbuscular Mycorrhizal Fungi Enhanced Drought Resistance of Populus cathayana by Regulating the 14-3-3 Family Protein Genes. Microbiol. Spectr. 2022, 10, e02456-21. [Google Scholar] [CrossRef]
- Akensous, F.Z.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-Rahou, Y.; Ben Ahmed, H.; Nasri, N.; Hafidi, M.; Meddich, A. Boosting Date Palm (Phoenix dactylifera L.) Growth under Drought Stress: Effects of Innovative Biostimulants. Gesunde Pflanz. 2022; in press. [Google Scholar] [CrossRef]
- Haddidi, I.; Duc, N.H.; Tonk, S.; Rápó, E.; Posta, K. Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy 2020, 10, 1657. [Google Scholar] [CrossRef]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular Mycorrhizal Fungi Inoculation Improve Essen-tial Oil and Physiological Parameters and Nutritional Values of Thymus daenensis Celak and Thymus vulgaris L. under Normal and Drought Stress Conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Liang, S.M.; Jiang, D.J.; Xie, M.M.; Zou, Y.N.; Wu·, Q.S.; Kuca, K. Physiological Responses of Mycorrhizal Symbiosis to Drought Stress in White Clover. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 1–10. [Google Scholar] [CrossRef]
- Gupta, A.; Bano, A.; Rai, S.; Mishra, R.; Singh, M.; Sharma, S.; Pathak, N. Mechanistic Insights of Plant-Microbe Interaction towards Drought and Salinity Stress in Plants for Enhancing the Agriculture Productivity. Plant Stress 2022, 4, 100073. [Google Scholar] [CrossRef]
- Kour, D.; Khan, S.S.; Kaur, T.; Kour, H.; Singh, G.; Yadav, A.; Yadav, A.N. Drought Adaptive Microbes as Bioinoculants for the Horticultural Crops. Heliyon 2022, 8, e09493. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Allen White, R.; Handakumbura, P.P.; Jansson, C. Rhizosphere Engineering: Enhancing Sustainable Plant Ecosystem Productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Foster, K.R.; Bell, T. Competition, Not Cooperation, Dominates Interactions among Culturable Microbial Species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) Growth En-hancement by Azospirillum Sp. under Drought Stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR Strain Phyllobacterium brassicacearum STM196 Induc-es a Reproductive Delay and Physiological Changes That Result in Improved Drought Tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-Producing, Plant Growth-Promoting Rhizobacteria That Confer Resistance to Drought Stress in Platycladus orientalis Container Seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin Secreting Rhizo-bacterium, Pseudomonas putida H-2-3 Modulates the Hormonal and Stress Physiology of Soybean to Improve the Plant Growth under Saline and Drought Conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia natronolimnaea Modulates the Expression of Stress Responsive Genes Providing Protection of Wheat from Salinity Stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The Role of Microbial Signals in Plant Growth and Development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Dangi, A.K.; Sharma, B.; Khangwal, I.; Shukla, P. Combinatorial Interactions of Biotic and Abiotic Stresses in Plants and Their Molecular Mechanisms: Systems Biology Approach. Mol. Biotechnol. 2018, 60, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Imam, J.; Singh, P.K.; Shukla, P. Plant Microbe Interactions in Post Genomic Era: Perspectives and Applications. Front. Microbiol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef]
- Llorens, E.; Sharon, O.; Camañes, G.; García-Agustín, P.; Sharon, A. Endophytes from Wild Cereals Protect Wheat Plants from Drought by Alteration of Physiological Responses of the Plants to Water Stress. Environ. Microbiol. 2019, 21, 3299–3312. [Google Scholar] [CrossRef]
- Omar, S.A.; Fetyan, N.A.H.; Eldenary, M.E.; Abdelfattah, M.H.; Abd-Elhalim, H.M.; Wrobel, J.; Kalaji, H.M. Alteration in Expression Level of Some Growth and Stress-Related Genes after Rhizobacteria Inoculation to Alleviate Drought Tolerance in Sensitive Rice Genotype. Chem. Biol. Technol. Agric. 2021, 8, 41. [Google Scholar] [CrossRef]
- Jayakumar, A.; Padmakumar, P.; Nair, I.C.; Radhakrishnan, E.K. Drought Tolerant Bacterial Endophytes with Potential Plant Probiotic Effects from Ananas Comosus. Biologia 2020, 75, 1769–1778. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Nephali, L.; Moodley, V.; Piater, L.; Steenkamp, P.; Buthelezi, N.; Dubery, I.; Burgess, K.; Huyser, J.; Tugizimana, F. A Metabolomic Landscape of Maize Plants Treated with a Microbial Biostimulant Under Well-Watered and Drought Conditions. Front. Plant Sci. 2021, 12, 676632. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Opperman, H.; Brand, M.; Huyser, J.; Tugizimana, F. Molecular Mechanisms Associated with Microbial Biostimulant-Mediated Growth Enhancement, Priming and Drought Stress Tolerance in Maize Plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Meta-Omics of Endophytic Microbes in Agricultural Biotechnology. Biocatal. Agric. Biotechnol. 2022, 42, 102332. [Google Scholar] [CrossRef]
- Chen, X.L.; Sun, M.C.; Chong, S.L.; Si, J.P.; Wu, L.S. Transcriptomic and Metabolomic Approaches Deepen Our Knowledge of Plant–Endophyte Interactions. Front. Plant Sci. 2022, 12, 700200. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, T.; Shukla, P. Bioinoculant Capability Enhancement through Metabolomics and Systems Biology Approaches. Brief. Funct. Genom. 2018, 18, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.M.; Zhang, F.; Zou, Y.N.; Kuča, K.; Wu, Q.S. Metabolomics Analysis Reveals Drought Responses of Trifoliate Orange by Arbuscular Mycorrhizal Fungi with a Focus on Terpenoid Profile. Front. Plant Sci. 2021, 12, 102332. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer arietinum L.) Induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Tyers, M.; Mann, M. From Genomics to Proteomics: One Approach. Am. Lab. 2003, 34, 28. [Google Scholar]
- Alberton, D.; Valdameri, G.; Moure, V.R.; Monteiro, R.A.; de Pedrosa, F.O.; Müller-Santos, M.; de Souza, E.M. What Did We Learn from Plant Growth-Promoting Rhizobacteria (PGPR)-Grass Associations Studies Through Proteomic and Metabolomic Approaches? Front. Sustain. Food Syst. 2020, 4, 607343. [Google Scholar] [CrossRef]
- De Cremer, K.; Mathys, J.; Vos, C.; Froenicke, L.; Michelmore, R.W.; Cammue, B.P.A.; De Coninck, B. RNAseq-Based Tran-scriptome Analysis of Lactuca sativa Infected by the Fungal Necrotroph Botrytis cinerea. Plant Cell Environ. 2013, 36, 1992–2007. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From Saprophytes to Endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Belbahri, L.; Bouket, A.C.; Rekik, I.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Petrovova, E.; Oszako, T.; Cherrad, S.; Vacher, S.; et al. Comparative Genomics of Bacillus amyloliquefaciens Strains Reveals a Core Genome with Traits for Habitat Adapta-tion and a Secondary Metabolites Rich Accessory Genome. Front. Microbiol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Jones, M.L. Genomic Analysis of Serratia plymuthica MBSA-MJ1: A Plant Growth Promoting Rhizobacteria That Improves Water Stress Tolerance in Greenhouse Ornamentals. Front. Microbiol. 2021, 12, 653556. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Ratering, S.; Hain, T.; Fritzenwanker, M.; Goesmann, A.; Blom, J.; Chakraborty, T.; Bunk, B.; Spröer, C.; Overmann, J.; et al. Complete Genome Sequence of the Plant Growth-Promoting Bacterium Hartmannibacter diazotrophicus Strain E19T. Int. J. Genomics 2019, 2019, 7586430. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Broadhurst, D.I.; Kell, D.B. Statistical Strategies for Avoiding False Discoveries in Metabolomics and Related Experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-Omics of Permafrost, Active Layer and Thermokarst Bog Soil Microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Kuo, T.C.; Tian, T.F.; Tseng, Y.J. 3Omics: A Web-Based Systems Biology Tool for Analysis, Integration and Visualization of Human Transcriptomic, Proteomic and Metabolomic Data. BMC Syst. Biol. 2013, 7, 64. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Trans-parent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Biodiversity for Food and Agriculture; Bélanger, J., Pilling, D., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2019; 572p, Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 17 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).