Genetic Containment for Molecular Farming
Abstract
1. Introduction
| Type of Expression System | Species | Product | Application(s) | Reference |
|---|---|---|---|---|
| Whole plant—stable Food crops | Z. mays (corn) | expansin (enzyme) | Biofuel production | [10] |
| Z. mays (corn) | Avidin | Diagnostics | [17] | |
| Spinacia oleracea (spinach) | Rabies glycoprotein | Vaccine | [21] | |
| O. sativa (rice) | Lysozyme | Numerous | [22] | |
| Whole plant—stable Non-food crops | N. tabacum (tobacco) | Polyhydroxybuterate | Plastic production | [6] |
| L. minor L. (duckweed) | Peptide Me2 | Avian flu vaccine | [7] | |
| N. tabacum | 185-kD antigen I/II (antibody) | Anti-cavity | [23] | |
| N. tabacum | Insulin | Diabetes treatment | [24] | |
| Whole plant—transient | N. benthamiana | ZMappTM | Therapeutic | [11] |
| Pisum sativum (pea) | Human growth hormone | Therapeutic | [25] | |
| Lactuca sativa L. (lettuce) | hOAT (antibodies) | Therapeutic | [26] | |
| N. benthamiana | aprotinin (serine protease inhibitor) | Therapeutic | [27] | |
| Culture of hairy roots | N. tabacum | M12 (antibody) | Therapeutic | [28] |
| N. tabacum | interleukin-2 | Therapeutic | [29] | |
| Solanum tuberosum (potato) | hepatitis-B surface antigen | Vaccine | [30] | |
| Artemisia | Artemisinin | Malaria treatment | [3] | |
| Cell suspension | Daucus carota (carrot) | Elelyso (Enzyme) | Gaucher’s disease treatment | [19] |
| N. tabacum | Human growth hormone | Therapeutic | [5] | |
| P. patens (moss) | Factor H | Therapeutic | [31] | |
| Taxus ssp. | Taxol | Cancer treatment | [4] |
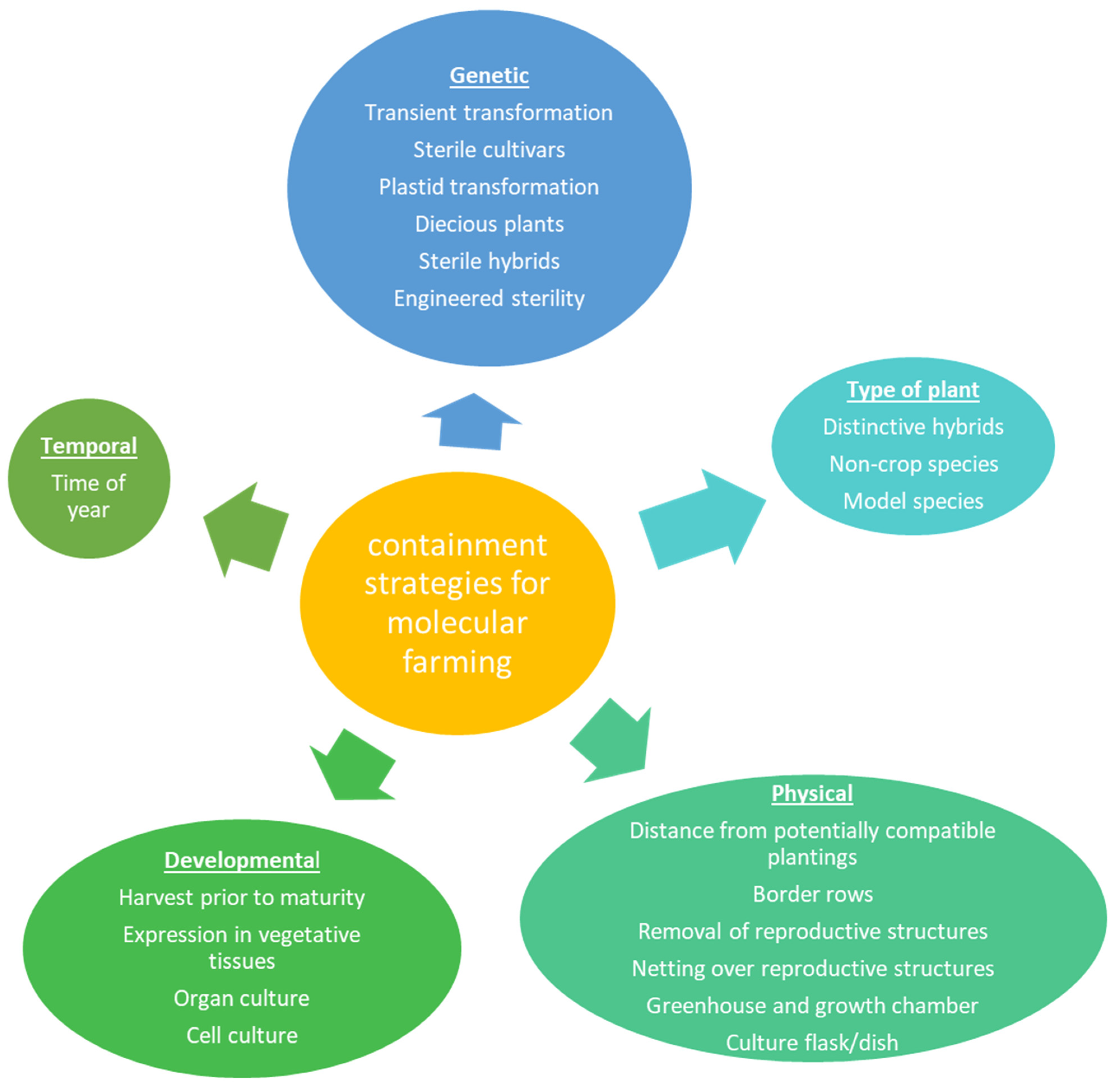
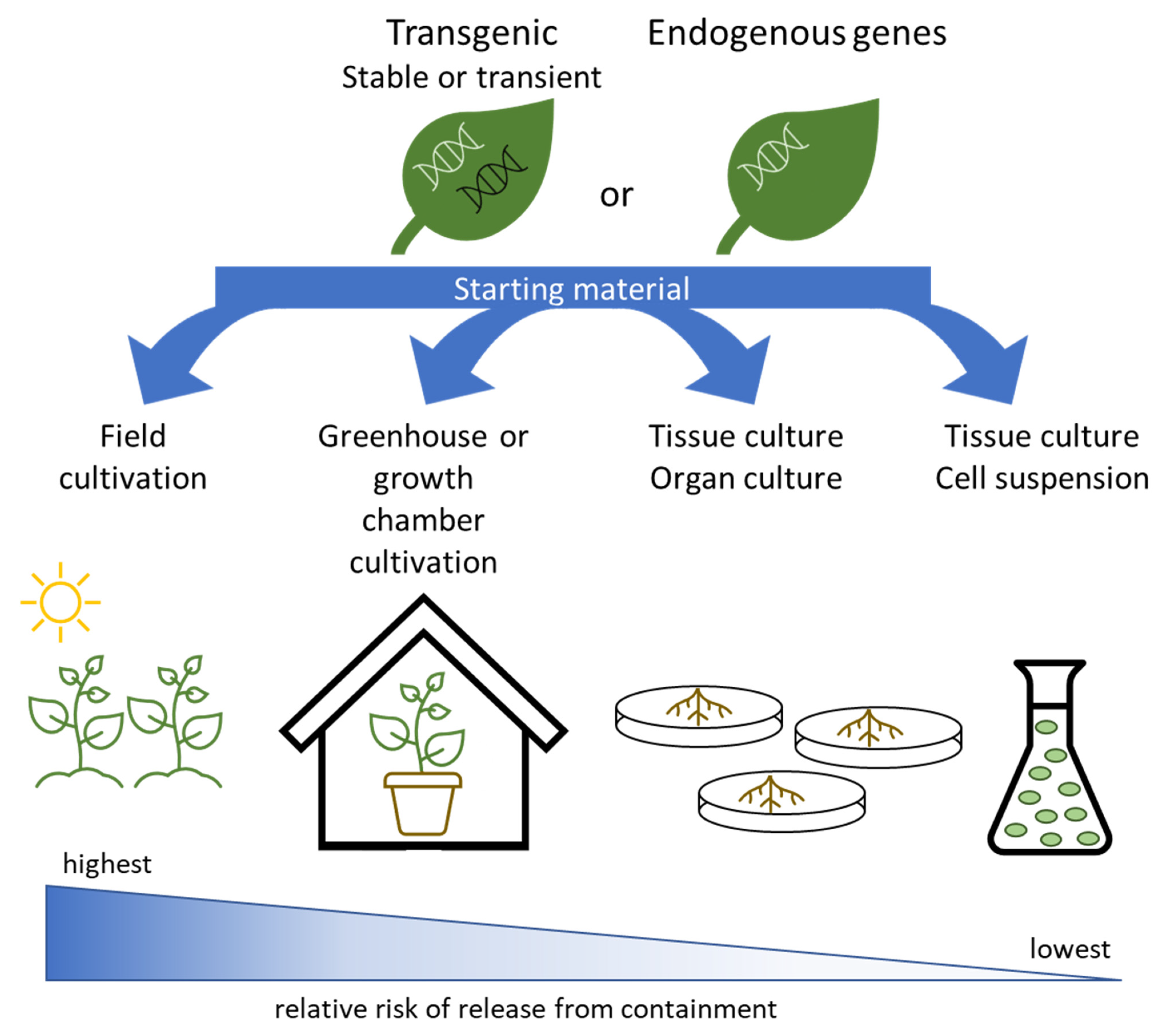
2. Plant Containment
Systems for Expression and Growth
3. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J. The expression of a nopaline synthase-human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Haq, T.A.; Mason, H.S.; Clements, J.D.; Arntzen, C.T. Oral Immunization with a Recombinant Bacterial Antigen Produced in Transgenic Plants. Science 1995, 268, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Kayani, W.K.; Kiani, B.H.; Dilshad, E.; Mirza, B. Biotechnological approaches for artemisinin production in Artemisia. World J. Microbiol. Biotechnol. 2018, 34, 54. [Google Scholar] [CrossRef]
- Liu, W.C.; Gong, T.; Zhu, P. Advances in exploring alternative Taxol sources. R. Soc. Chem. Adv. 2016, 6, 48800–48809. [Google Scholar] [CrossRef]
- Xu, J.; Okada, S.; Tan, L.; Goodrum, K.J.; Kopchick, J.J.; Kieliszewski, M.J. Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life. Transgenic Res. 2010, 19, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Bohmert-Tatarev, K.; McAvoy, S.; Daughtry, S.; Peoples, O.P.; Snell, K.D. High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol. 2011, 155, 1690–1708. [Google Scholar] [CrossRef]
- Firsov, A.; Tarasenko, I.; Mitiouchkina, T.; Shaloiko, L.; Kozlov, O.; Vinokurov, L.; Rasskazova, E.; Murashev, A.; Vainstein, A.; Dolgov, S. Expression and Immunogenicity of M2e Peptide of Avian Influenza Virus H5N1 Fused to Ricin Toxin B Chain Produced in Duckweed Plants. Front. Chem. 2018, 6, 22. [Google Scholar] [CrossRef]
- Fox, J.L. Turning plants into protein factories. Nat. Biotechnol. 2006, 24, 1191–1193. [Google Scholar] [CrossRef]
- Weichert, N.; Hauptmann, V.; Helmold, C.; Conrad, U. Seed-Specific Expression of Spider Silk Protein Multimers Causes Long-Term Stability. Front. Plant Sci. 2016, 7, 6. [Google Scholar] [CrossRef]
- Yoon, S.; Devaiah, S.P.; Choi, S.E.; Bray, J.; Love, R.; Lane, J.; Drees, C.; Howard, J.H.; Hood, E.E. Over-expression of the cucumber expansin gene (Cs-EXPA1) in transgenic maize seed for cellulose deconstruction. Transgenic Res. 2016, 25, 173–186. [Google Scholar] [CrossRef]
- Chen, Q.; Davis, K.R. The potential of plants as a system for the development and production of human biologics. F1000Research 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2021, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Streatfield, S.J.; Rybicki, E.P. Advances in molecular farming: Key technologies, scaled up production and lead targets. Plant Biotechnol. J. 2015, 13, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular farming-The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Kurup, V.M.; Thomas, J. Edible Vaccines: Promises and Challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; I. Bulaon, C.J.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Hood, E.E.; Witcher, D.R.; Maddock, S.; Meyer, T.; Baszczynski, C.; Bailey, M.; Flynn, P.; Register, J.; Marshall, L.; Bond, D.; et al. Commercial production of avidin from transgenic maize: Characterization of transformation, production, processing, extraction and purification. Mol. Breed. 1997, 3, 291–306. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins-Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472. [Google Scholar] [CrossRef]
- Ma, J.K.; Barros, E.; Bock, R.; Christou, P.; Dale, P.J.; Dix, P.J.; Fischer, R.; Irwin, J.; Mahoney, R.; Pezzotti, M.; et al. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005, 6, 593–599. [Google Scholar] [CrossRef]
- Yusibov, V.; Hooper, D.C.; Spitsin, S.V.; Fleysh, N.; Kean, R.B.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J.; et al. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef]
- Yang, D.; Wu, L.; Hwang, Y.S.; Chen, L.; Huang, N. Expression of the REB transcriptional activator in rice grains improves the yield of recombinant proteins whose genes are controlled by a Reb-responsive promoter. Proc. Natl. Acad. Sci. USA 2001, 98, 11438–11443. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Hunjan, M.; Smith, R.; Lehner, T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin. Exp. Immunol. 1989, 77, 331–337. [Google Scholar]
- Li, D.; O’Leary, J.; Huang, Y.; Huner, N.P.; Jevnikar, A.M.; Ma, S. Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Fujiki, M.; Mett, V.; Kaczmarczyk, J.; Shamloul, M.; Musiychuk, K.; Underkoffler, S.; Yusibov, V.; Mett, V. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol. J. 2009, 4, 230–237. [Google Scholar] [CrossRef]
- Negrouk, V.; Eisner, G.; Lee, H.; Han, K.; Taylor, D.; Wong, H.C. Highly efficient transient expression of functional recombinant antibodies in lettuce. Plant Sci. 2005, 169, 433–438. [Google Scholar] [CrossRef]
- Pogue, G.P.; Vojdani, F.; Palmer, K.E.; Hiatt, E.; Hume, S.; Phelps, J.; Long, L.; Bohorova, N.; Kim, D.; Pauly, M.; et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010, 8, 638–654. [Google Scholar] [CrossRef]
- Hakkinen, S.T.; Raven, N.; Henquet, M.; Laukkanen, M.L.; Anderlei, T.; Pitkanen, J.P.; Twyman, R.M.; Bosch, D.; Oksman-Caldentey, K.M.; Schillberg, S.; et al. Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol. Bioeng. 2014, 111, 336–346. [Google Scholar] [CrossRef]
- Liu, C.; Towler, M.J.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Production of Mouse Interleukin-12 Is Greater in Tobacco Hairy Roots Grown in a Mist Reactor Than in an Airlift Reactor. Biotechnol. Bioeng. 2008, 102, 1074–1086. [Google Scholar] [CrossRef]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Srinivas, L.; Revathi, C.J.; Bapat, V.A. Expression of hepatitis B surface antigen in potato hairy roots. Plant Sci. 2005, 170, 918–925. [Google Scholar] [CrossRef]
- Buttner-Mainik, A.; Parsons, J.; Jerome, H.; Hartmann, A.; Lamer, S.; Schaaf, A.; Schlosser, A.; Zipfel, P.F.; Reski, R.; Decker, E.L. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol. J. 2011, 9, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, H.; Stoger, E.; Twyman, R.M.; Buyel, J.F. Current Status and Perspectives of the Molecular Farming Landscape. In Molecular Pharming: Applications, Challenges, and Emerging Areas; Kermode, A.R., Jiang, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 3–24. [Google Scholar]
- Menary, J.; Hobbs, M.; Mesquita de Albuquerque, S.; Pacho, A.; Drake, P.M.W.; Prendiville, A.; Ma, J.K.; Fuller, S.S. Shotguns vs Lasers: Identifying barriers and facilitators to scaling-up plant molecular farming for high-value health products. PLoS ONE 2020, 15, e0229952. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.E. Egg-based vaccines. Pediatr. Rev. 2006, 27, 118–119. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef]
- Ritala, A.; Hakkinen, S.T.; Schillberg, S. Molecular pharming in plants and plant cell cultures: A great future ahead? Pharm. Bioprocess. 2014, 2, 223–226. [Google Scholar] [CrossRef]
- Husken, A.; Prescher, S.; Schiemann, J. Evaluating biological containment strategies for pollen-mediated gene flow. Environ. Biosaf. Res. 2010, 9, 67–73. [Google Scholar] [CrossRef]
- Murphy, D.J. Improving containment strategies in biopharming. Plant Biotechnol. J. 2007, 5, 555–569. [Google Scholar] [CrossRef]
- Daniell, H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002, 20, 581–586. [Google Scholar] [CrossRef]
- Klocko, A.L. Strategies to facilitate containment of genetically engineered crops. CAB Rev. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Kausch, A.; Hauge, J.; Deresienski, A.; Tilelli, M.; Nelson, K. Male Sterility and Hybrid Plant Systems for Gene Confinement. In Plant Gene Containment; Oliver, M., Li, Y., Eds.; Wiley-Blackwell John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Merlin, M.; Pezzotti, M.; Avesani, L. Edible plants for oral delivery of biopharmaceuticals. Br. J. Clin. Pharmacol. 2017, 83, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.; Presa, S.; Espi, J.; Pineda, B.; Anton, M.T.; Moreno, V.; Buesa, J.; Granell, A.; Orzaez, D. Neutralizing antibodies against rotavirus produced in transgenically labelled purple tomatoes. Plant Biotechnol. J. 2012, 10, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Vleeshouwers, V.G.A.A.; Jacobsen, E.; Van Der Vossen, E.; VIsser, R.G.F. Molecular breeding for resistance to Phytophthora infestans (Mont.) de Bary in potato (Solanum tuberosum L.): A perspective of cisgenesis. Plant Breed. 2009, 128, 109–117. [Google Scholar] [CrossRef]
- Vanblaere, T.; Flachowsky, H.; Gessler, C.; Broggini, G.A. Molecular characterization of cisgenic lines of apple ‘Gala’ carrying the Rvi6 scab resistance gene. Plant Biotechnol. J. 2014, 12, 2–9. [Google Scholar] [CrossRef]
- Van Hove, L.; Gillund, F. Is it only the regulatory status? Broadening the debate on cisgenic plants. Environ. Sci. Eur. 2017, 29, 22. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as Factories for Human Pharmaceuticals: Applications and Challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef]
- Mirzaee, M.; Osmani, Z.; Frebortova, J.; Frebort, I. Recent advances in molecular farming using monocot plants. Biotechnol. Adv. 2022, 58, 107913. [Google Scholar] [CrossRef]
- Zhu, Q.; Tan, J.; Liu, Y.G. Molecular farming using transgenic rice endosperm. Trends Biotechnol. 2022, 40, 1248–1260. [Google Scholar] [CrossRef]
- Ou, J.; Guo, Z.; Shi, J.; Wang, X.; Liu, J.; Shi, B.; Guo, F.; Zhang, C.; Yang, D. Transgenic rice endosperm as a bioreactor for molecular pharming. Plant Cell Rep. 2014, 33, 585–594. [Google Scholar] [CrossRef]
- Ma, J.K.; Drake, P.M.; Christou, P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Maselko, M. Transgene Biocontainment Strategies for Molecular Farming. Front. Plant Sci. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Puzzling industry response to ProdiGene fiasco. Nat. Biotechnol. 2003, 21, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Drugs in crops—The unpalatable truth. Nat. Biotechnol. 2004, 22, 133. [Google Scholar] [CrossRef]
- Tremblay, R.; Wang, D.; Jevnikar, A.M.; Ma, S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010, 28, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O., 3rd. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Canada, G.O. Health Canada Authorizes Medicago COVID-19 Vaccine for Adults 18 to 64 Years of Age. Available online: https://www.canada.ca/en/health-canada/news/2022/02/health-canada-authorizes-medicago-covid-19-vaccine-for-adults-18-to-64-years-of-age.html (accessed on 31 August 2022).
- Menassa, R.; Nguyen, V.; Jevnikar, A.; Brandle, J. A self-contained system for the field production of plant recombinant interleukin-10. Mol. Breed. 2001, 8, 177–185. [Google Scholar] [CrossRef]
- Besumbes, O.; Sauret-Gueto, S.; Phillips, M.A.; Imperial, S.; Rodriguez-Concepcion, M.; Boronat, A. Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of Taxol. Biotechnol. Bioeng. 2004, 88, 168–175. [Google Scholar] [CrossRef]
- Gasdaska, J.R.; Spencer, D.; Dickey, L. Advantages of Therapeutic Protein Production in the Aquatic Plant Lemna. BioProcess J. 2003, 2, 49–56. [Google Scholar] [CrossRef]
- Pulice, G.; Pelaz, S.; Matias-Hernandez, L. Molecular Farming in Artemisia annua, a Promising Approach to Improve Anti-malarial Drug Production. Front. Plant Sci. 2016, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Motolinia-Alcantara, E.A.; Castillo-Araiza, C.O.; Rodriguez-Monroy, M.; Roman-Guerrero, A.; Cruz-Sosa, F. Engineering Considerations to Produce Bioactive Compounds from Plant Cell Suspension Culture in Bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klocko, A.L. Genetic Containment for Molecular Farming. Plants 2022, 11, 2436. https://doi.org/10.3390/plants11182436
Klocko AL. Genetic Containment for Molecular Farming. Plants. 2022; 11(18):2436. https://doi.org/10.3390/plants11182436
Chicago/Turabian StyleKlocko, Amy L. 2022. "Genetic Containment for Molecular Farming" Plants 11, no. 18: 2436. https://doi.org/10.3390/plants11182436
APA StyleKlocko, A. L. (2022). Genetic Containment for Molecular Farming. Plants, 11(18), 2436. https://doi.org/10.3390/plants11182436







