All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes
Abstract
1. Introduction
2. Results
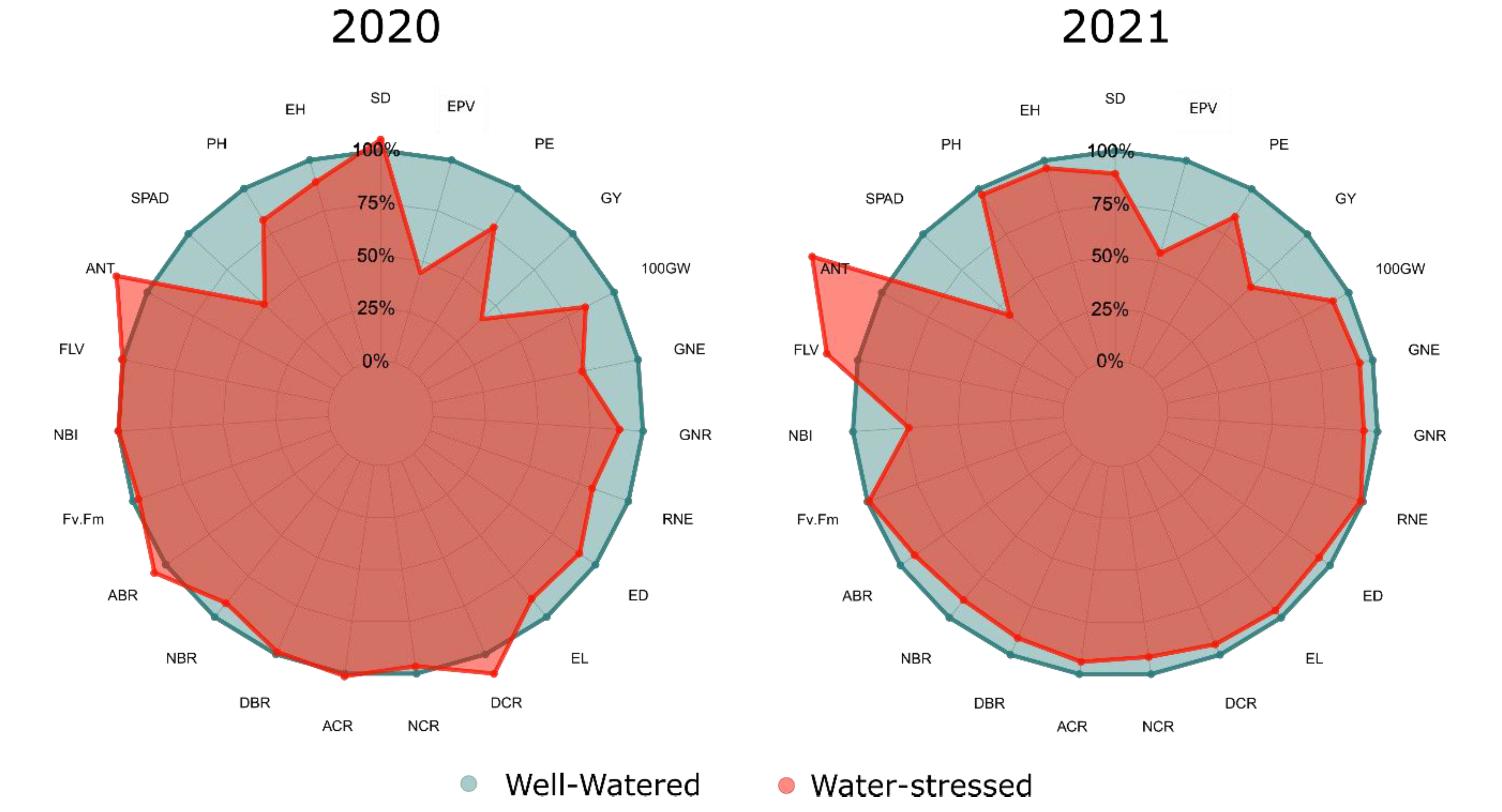
2.1. Analysis of Variance, Estimates of Means, and Impact of Water Limitation on the Morphoagronomic, Physiological, and Morphological Traits of the Root System
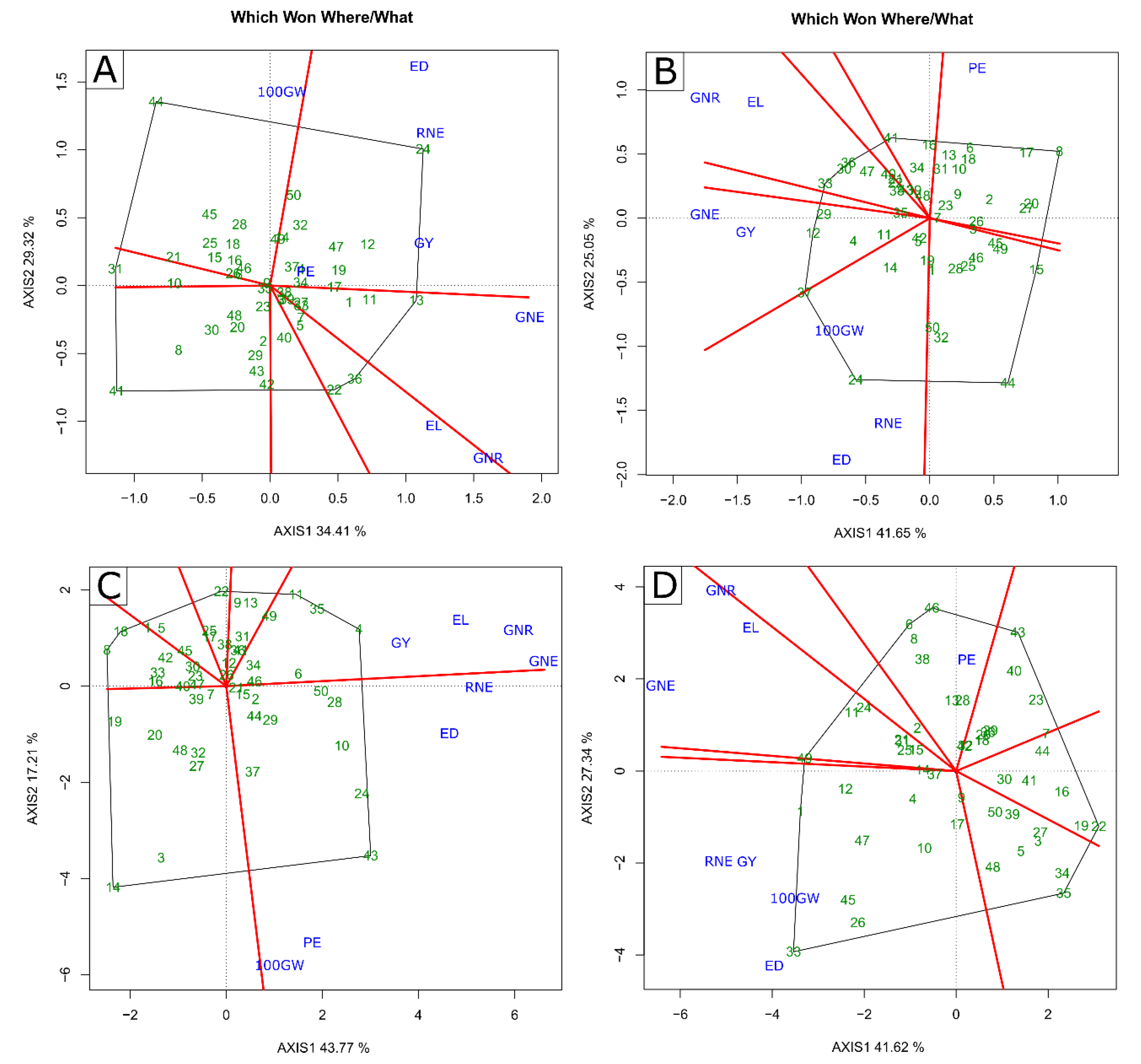
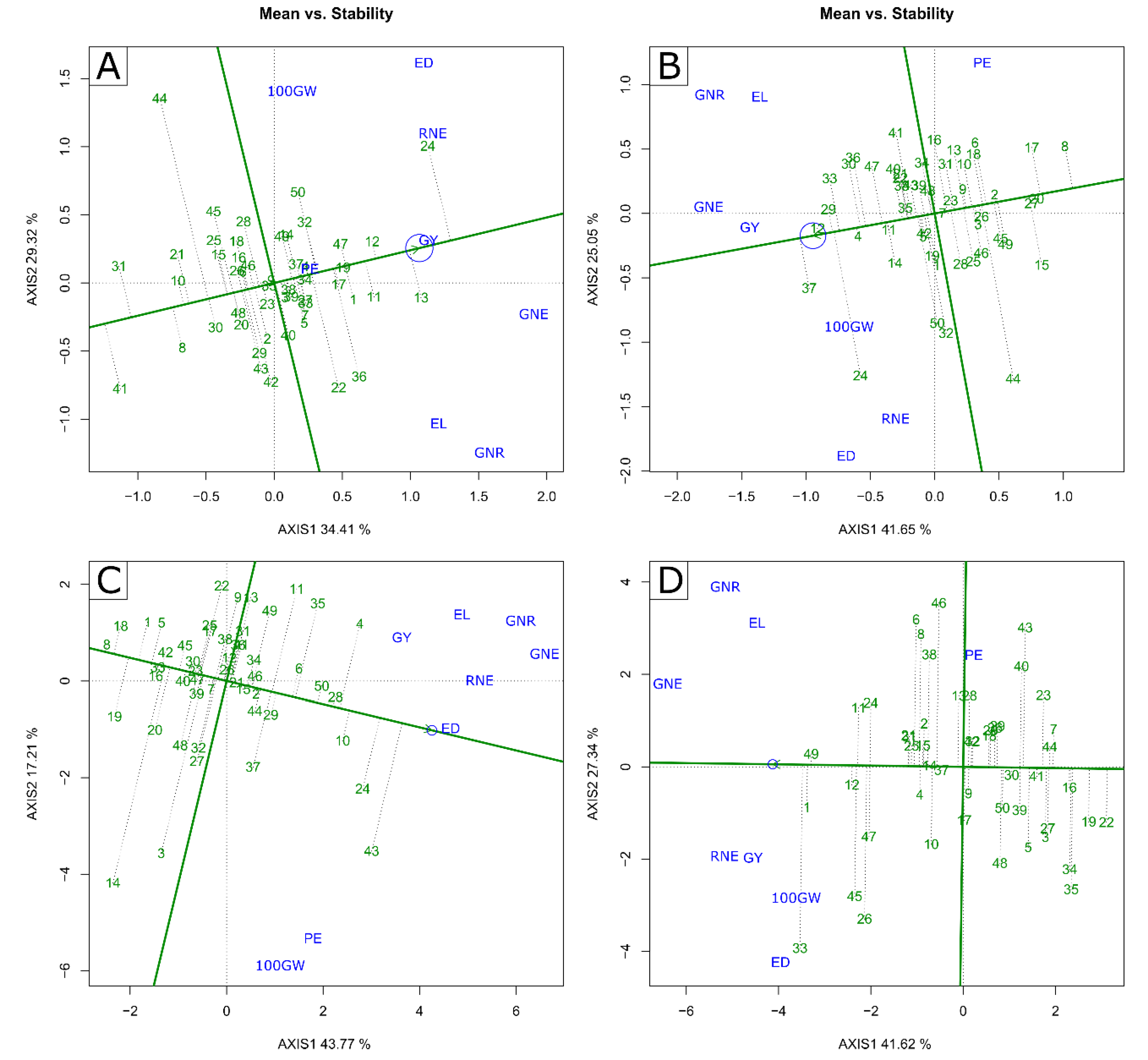
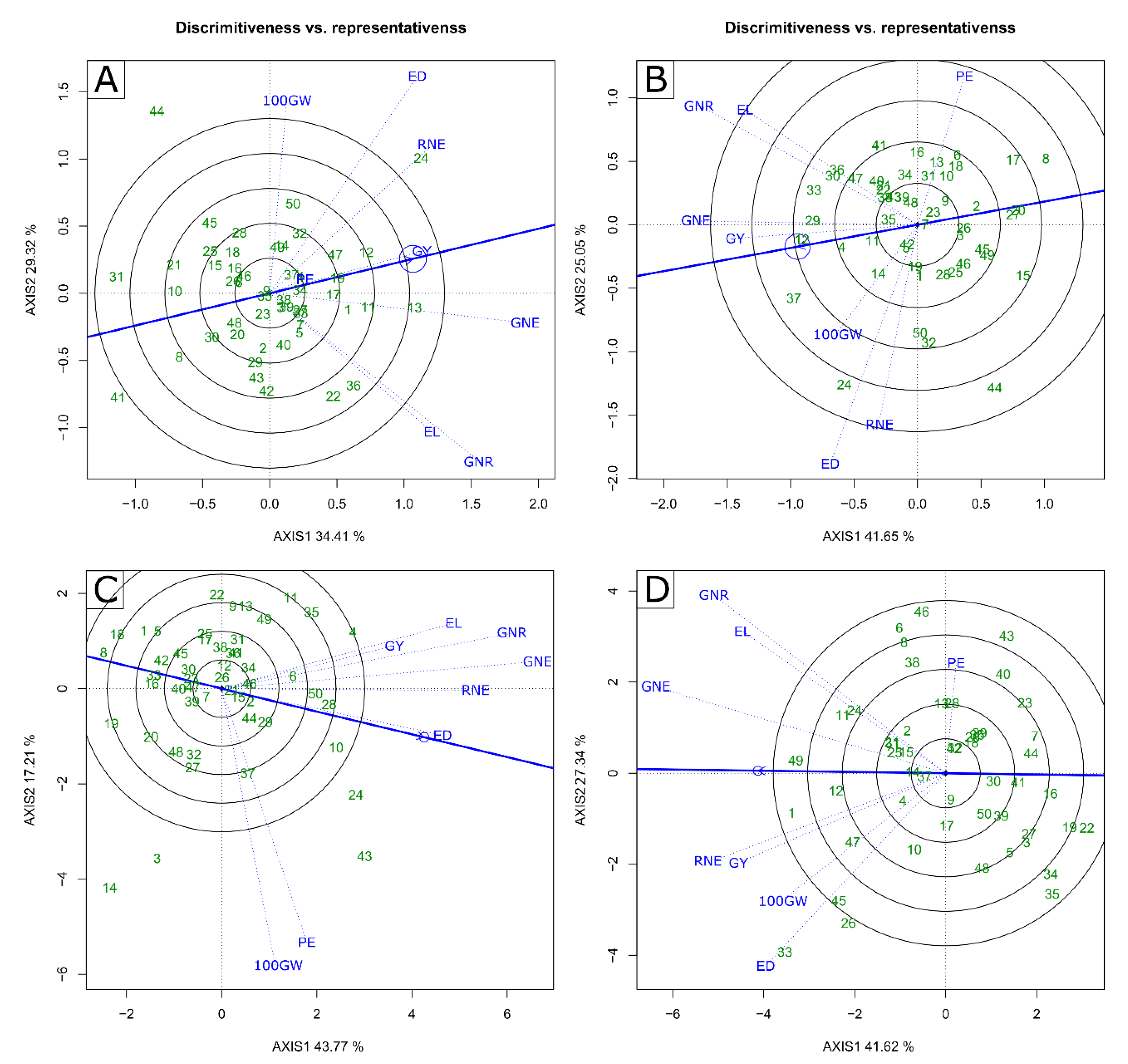
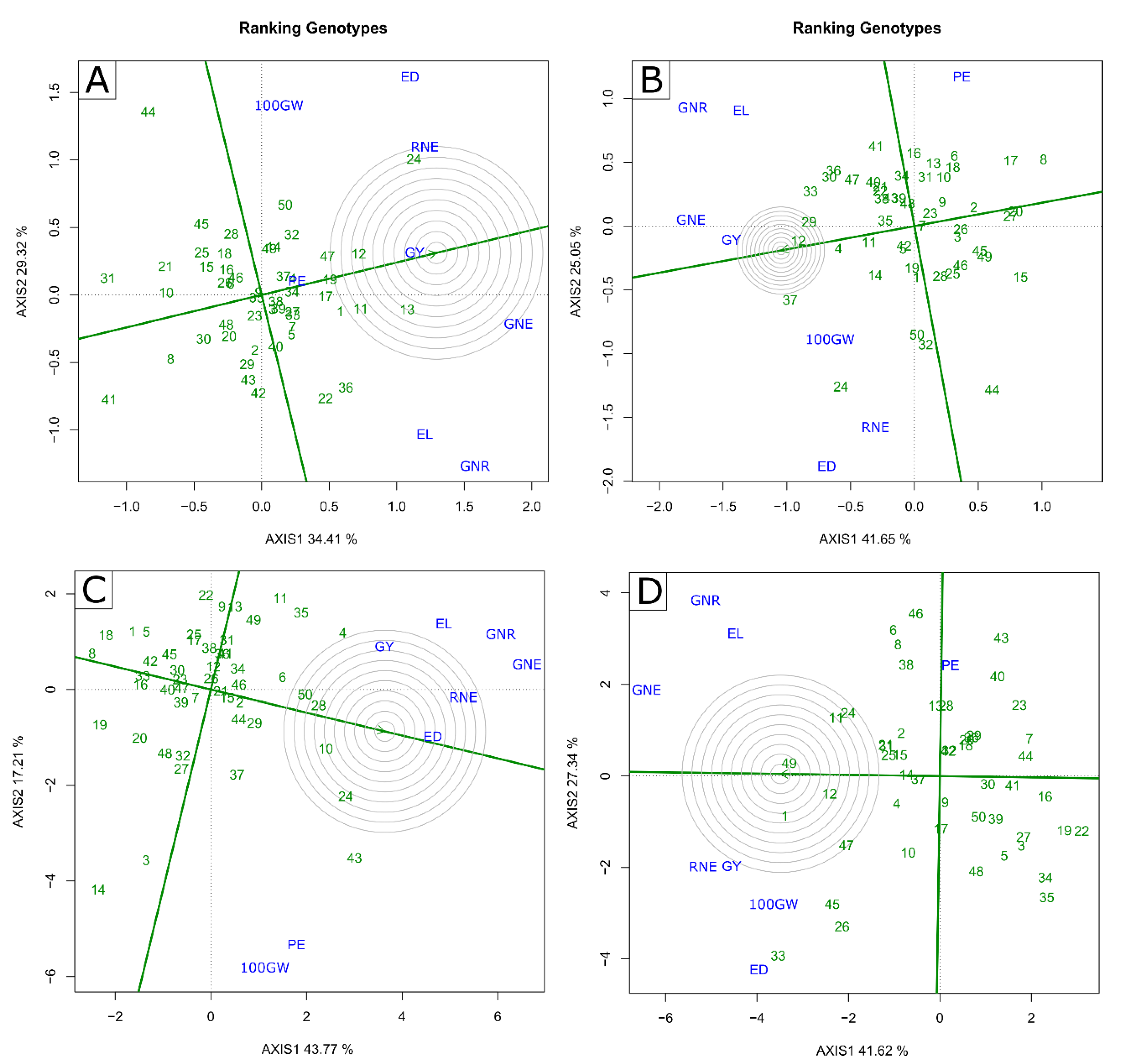
2.2. Multivariate Analysis—GT Biplot
3. Discussion
3.1. Genetic Variability in Different WC and CS
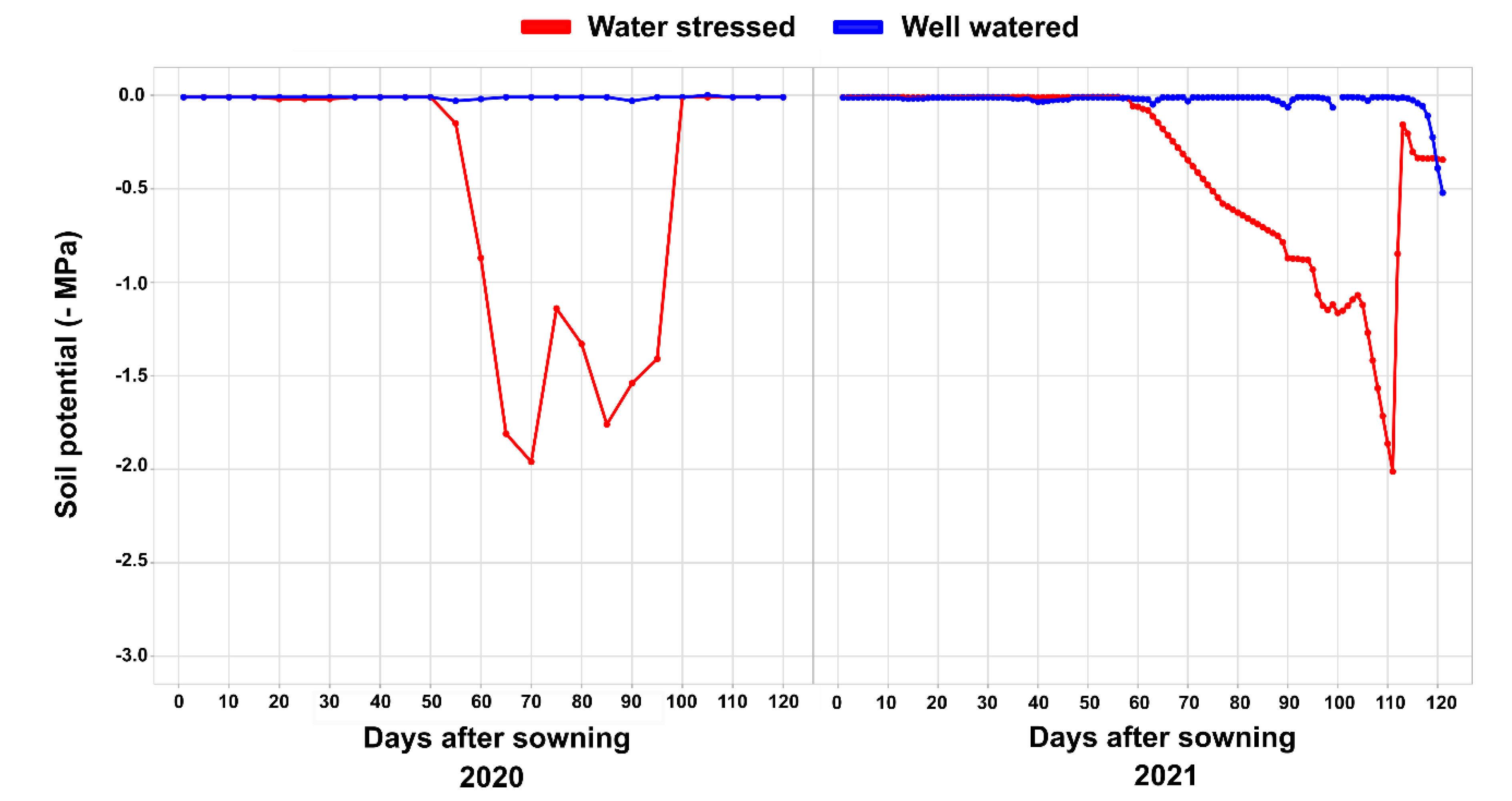
3.2. Impact of Water Limitation
3.3. Identification of Superior Genotypes and Traits of Interest for Indirect Selection
4. Materials and Methods
4.1. Genotypes
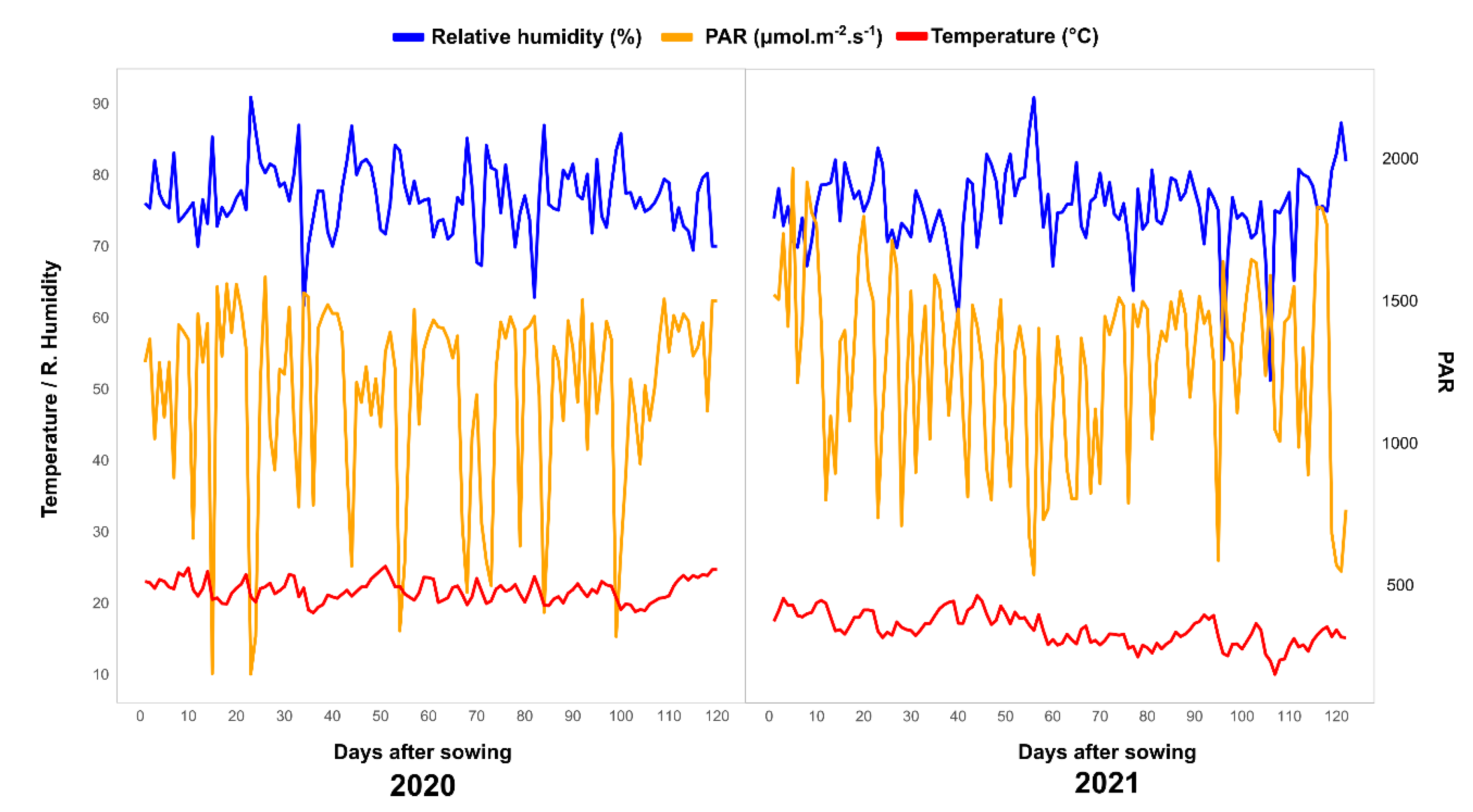
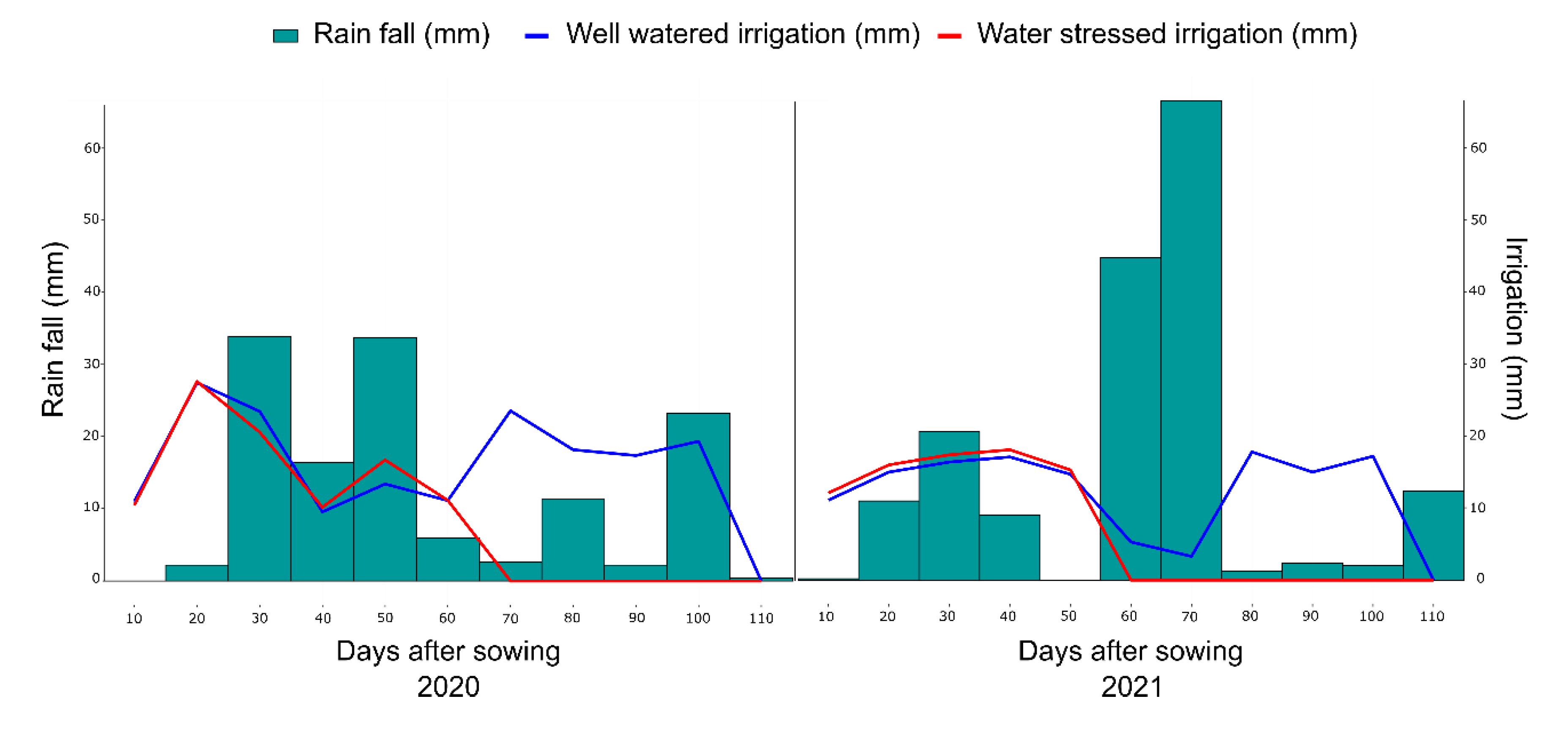
4.2. Experimental Conditions and Crop Management
4.3. Agronomic, Physiological, and Morphological Traits of the Root System Evaluated
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Inbred Lines | Pedigree | Origen | Donor Institution | Climate Adaptation |
|---|---|---|---|---|
| L217 | IAC 125 | Brazil | IAC | Tropical |
| L381 | SAM | USA | EUA | Temperate |
| L326 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L502 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L292 | URUG 298 | Uruguay | CIMMYT | Temperate |
| L503 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L76 | Viçosa-Viçosa | Brazil | UFV | Tropical |
| L291 | URUG 298 | Uruguay | CIMMYT | Temperate |
| L273 | PARA 172 | Paraguay | CIMMYT | Temperate |
| L328 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L61 | BRS Angela | Brazil | EMBRAPA | Tropical |
| L625 | PA 091 | Brazil | UEM | Tropical |
| L688 | UENF 14 | Brazil | UENF | Tropical |
| L213 | IAC 125 | Brazil | IAC | Tropical |
| L322 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L481 | SE 013 | Brazil | UEM | Tropical |
| L477 | SE 013 | Brazil | UEM | Tropical |
| L684 | UENF 14 | Brazil | UENF | Tropical |
| L509 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L501 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L358 | PR 023 | Brazil | UEM | Tropical |
| L476 | SE 013 | Brazil | UEM | Tropical |
| L472 | SE 013 | Brazil | UEM | Tropical |
| L221 | IAC 125 | Brazil | IAC | Tropical |
| L204 | IAC 125 | Brazil | IAC | Tropical |
| L386 | SAM | USA | EUA | Temperate |
| L222 | IAC 125 | Brazil | IAC | Tropical |
| L691 | UENF 14 | Brazil | UENF | Tropical |
| L594 | RS 20 | Brazil | IPAGRO/AGROESTE | Temperate |
| L655 | ARZM 13 050 | Argentina | CIMMYT | Temperate |
| L391 | SAM | USA | EUA | Temperate |
| L332 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L325 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L507 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L263 | PARA 172 | Paraguay | CIMMYT | Temperate |
| L219 | IAC 125 | Brazil | IAC | Tropical |
| L366 | PR 023 | Brazil | UEM | Tropical |
| L394 | SAM | USA | EUA | Temperate |
| L383 | SAM | USA | EUA | Temperate |
| L382 | SAM | USA | EUA | Temperate |
| L330 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L652 | ARZM 13 050 | Argentina | CIMMYT | Temperate |
| L693 | UENF 14 | Brazil | UENF | Tropical |
| L510 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L321 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L324 | UFV M-2 Barão de Viçosa | Brazil | UFV | Tropical |
| L513 | PA 170 Roxo | Paraguay | CIMMYT | Temperate |
| L294 | URUG 298 | Uruguay | CIMMYT | Temperate |
| L384 | SAM | USA | EUA | Temperate |
| L220 | IAC 125 | Brazil | IAC | Tropical |
References
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-Generation Breeding Strategies for Climate-Ready Crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, B. Global Role of Plant Breeding in Tackling Climate Change. Int. J. Agric. Sci. Food Technol. 2021, 7, 223–229. [Google Scholar] [CrossRef]
- Snowdon, R.J.; Wittkop, B.; Chen, T.-W.; Stahl, A. Crop Adaptation to Climate Change as a Consequence of Long-Term Breeding. Theor. Appl. Genet. 2021, 134, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Tao, F.; Luo, Y.; Cao, J.; Li, Z.; Xie, R.; Li, S. Planning Maize Hybrids Adaptation to Future Climate Change by Integrating Crop Modelling with Machine Learning. Environ. Res. Lett. 2021, 16, 124043. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Junior, A.T.; de Lima, V.J.; Carena, M.J.; Azeredo, V.C.; Mafra, G.S.; Santos, P.H.A.D.; Leite, J.T.; Schmitt, K.F.M.; dos Santos Junior, D.R.; et al. Driving Sustainable Popcorn Breeding for Drought Tolerance in Brazil. Front. Plant Sci. 2021, 12, 1942. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Wu, X.; Wang, W. Identification of Drought Tolerant Mechanisms in a Drought-Tolerant Maize Mutant Based on Physiological, Biochemical and Transcriptomic Analyses. BMC Plant Biol. 2020, 20, 315. [Google Scholar] [CrossRef]
- Barbosa, P.A.M.; Fritsche-Neto, R.; Andrade, M.C.; Petroli, C.D.; Burgueño, J.; Galli, G.; Willcox, M.C.; Sonder, K.; Vidal-Martínez, V.A.; Sifuentes-Ibarra, E.; et al. Introgression of Maize Diversity for Drought Tolerance: Subtropical Maize Landraces as Source of New Positive Variants. Front. Plant Sci. 2021, 12, 2023. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, L.; Djemel, A.; Malvar, R.A.; Gutièrrez, J.; Reyes, R.; Pedrol, N.; Revilla, P. Variability and Mechanisms of Drought Tolerance in Maize Populations from Honduras. Maydica 2018, 63, 11. [Google Scholar]
- de Oliveira Santos, T.; do Amaral Junior, A.T.; Bispo, R.B.; de Lima, V.J.; Kamphorst, S.H.; Leite, J.T.; dos Santos Júnior, D.R.; Santos, P.H.A.D.; de Oliveira, U.A.; Schmitt, K.F.M.; et al. Phenotyping Latin American Open-Pollinated Varieties of Popcorn for Environments with Low Water Availability. Plants 2021, 10, 1211. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Gonçalves, G.M.B.; Do Amaral, A.T.; De Lima, V.J.; Leite, J.T.; Schmitt, K.F.M.; Dos Santos, D.R.; Santos, J.S.; De Oliveira, F.T.; Corrêa, C.C.G.; et al. Screening of Popcorn Genotypes for Drought Tolerance Using Canonical Correlations. Agronomy 2020, 10, 1519. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Gonçalves, G.M.B.; do Amaral Júnior, A.T.; de Lima, V.J.; Schmitt, K.F.M.; Leite, J.T.; Azeredo, V.C.; Gomes, L.P.; de Souza Silva, J.G.; Carvalho, C.M.; et al. Supporting Physiological Trait for Indirect Selection for Grain Yield in Drought-Stressed Popcorn. Plants 2021, 10, 1510. [Google Scholar] [CrossRef]
- Leite, J.T.; do Amaral Junior, A.T.; Kamphorst, S.H.; de Lima, V.J.; dos Santos Junior, D.R.; Schmitt, K.F.M.; de Souza, Y.P.; de Oliveira Santos, T.; Bispo, R.B.; Mafra, G.S.; et al. Water Use Efficiency in Popcorn (Zea mays L. Var. Everta): Which Physiological Traits Would Be Useful for Breeding? Plants 2021, 10, 1450. [Google Scholar] [CrossRef]
- Avramova, V.; Nagel, K.A.; Abdelgawad, H.; Bustos, D.; Duplessis, M.; Fiorani, F.; Beemster, G.T.S. Screening for Drought Tolerance of Maize Hybrids by Multi-Scale Analysis of Root and Shoot Traits at the Seedling Stage. J. Exp. Bot. 2016, 67, 2453–2466. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Durães, F.O.M.; dos Santos, M.X.; Gama, E.E.G.E.; Magalhães, P.C.; Albuquerque, P.E.P.; Guimarães, C.T. Fenotipagem Associada a Tolerância a Seca Em Milho; Circular técnica 39; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2004; 17p, pp. 1–15. [Google Scholar]
- Lynch, J.P. Steep, Cheap and Deep: An Ideotype to Optimize Water and N Acqu isition by Maize Root Systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Zia, S.; Romano, G.; Spreer, W.; Sanchez, C.; Cairns, J.; Araus, J.L.; Müller, J. Infrared Thermal Imaging as a Rapid Tool for Identifying Water-Stress Tolerant Maize Genotypes of Different Phenology. J. Agron. Crop Sci. 2013, 199, 75–84. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Araus, J.L.; Cairns, J.E.; Yousfi, S.; Fernie, A.R. Metabolite Profiles of Maize Leaves in Drought, Heat and Combined Stress Field Trials Reveal the Relationship between Metabolism and Grain Yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Oliveira, G.H.F.; Amaral, C.B.; Revolti, L.T.M.; Buzinaro, R.; Moro, G.V. Genetic Variability in Popcorn Synthetic Population. Acta Sci.—Agron. 2019, 41, 1–9. [Google Scholar] [CrossRef]
- Steve, I.O.; Babatunde, O.I. Chemical Compositions and Nutritional Properties of Popcorn-Based Complementary Foods Supplemented with Moringa Oleifera Leaves Flour. J. Food Res. 2013, 2, 117. [Google Scholar] [CrossRef]
- de Jesus Freitas, I.L.; do Amaral Junior, A.T.; Viana, A.P.; Pena, G.F.; da Silva Cabral, P.; Vittorazzi, C.; da Conceição Silva, T.R. Ganho Genético Avaliado Com Índices de Seleção e Com REML/Blup Em Milho-Pipoca. Pesqui. Agropecu. Bras. 2013, 48, 1464–1471. [Google Scholar] [CrossRef]
- Cheim, L.M.G.; Costa, F.M.; de Almeida Silva, N.C.; Caneppele, C.; de Souza Cesar, A.L.T.M.; Rossignoli, P.A.; de Melo Faria, A.M. Caracterização de Sementes de Uma Variedade Crioula de Milho Pipoca (Zea mays L. Subsp. Mays) Cultivada Em Sistema Orgânico Pela Agricultura Familiar. Res. Soc. Dev. 2021, 10, e7110817141. [Google Scholar] [CrossRef]
- Faria Junior, A.C.; Sérgio Lourenço de Freitas, P.; Dallacort, R.; Scabora Mioto, L.; Danilo Barbieri, J. Zoneamento Agrícola Da Cultura Do Milho Pipoca Para o Estado de Mato Grosso Agricultural Zoning of Popcorn Corn for the State of Mato Grosso, Brazil. Brazilian J. Appl. Technol. Agric. Sci. 2020, 13, 2020. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; De Lima, V.J.; Leite, J.T.; Carvalho, C.M.; Xavier, K.B.; Campostrini, E. Popcorn Breeding for Water-Stress Tolerance or for Agronomic Water-Use Efficiency? Genet. Mol. Res. 2018, 17, 1–18. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral, A.T.; de Lima, V.J.; Moreira Guimarães, L.J.; Medeiros Schmitt, K.F.; Leite, J.T.; Diniz Santos, P.H.A.; Chaves, M.M.; Mafra, G.S.; dos Santos, D.R.; et al. Can Genetic Progress for Drought Tolerance in Popcorn Be Achieved by Indirect Selection? Agronomy 2019, 9, 792. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; Vergara-Diaz, O.; Gracia-Romero, A.; Fernandez-Gallego, J.A.; Chang-Espino, M.C.; Buchaillot, M.L.; Rezzouk, F.Z.; de Lima, V.J.; Serret, M.D.; et al. Heterosis and Reciprocal Effects for Physiological and Morphological Traits of Popcorn Plants under Different Water Conditions. Agric. Water Manag. 2022, 261, 107371. [Google Scholar] [CrossRef]
- Araus, J.L.; Sánchez, C.; Cabrera-Bosquet, L. Is Heterosis in Maize Mediated through Better Water Use? New Phytol. 2010, 187, 392–406. [Google Scholar] [CrossRef]
- Romano, G.; Zia, S.; Spreer, W.; Sanchez, C.; Cairns, J.; Luis, J.; Müller, J. Use of Thermography for High Throughput Phenotyping of Tropical Maize Adaptation in Water Stress. Comput. Electron. Agric. 2011, 79, 67–74. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting Maize Productivity: Ideotypes Associated with Grain Yield under Drought Stress and Well-Watered Conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef]
- Viana, F.N.; Chaves, M.M.; Kamphorst, S.H.; do Amaral Junior, A.T.; de Lima, V.J.; Leite, J.T.; Schmidt, K.F.M.; de Oliveira, U.A.; Lamego, D.L.; Pereira, J.L.; et al. Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes. Agronomy 2022, 12, 1517. [Google Scholar] [CrossRef]
- Zinselmeier, S.A.; Lauer, M.J.; Boyer, J.S. Reversing Drought-Induced Losses in Grain Yield: Sucrose Maintains Embryo Growth in Maize. Crop Sci. 1995, 35, 1390. [Google Scholar] [CrossRef]
- Parsons, L.; Ren, Y.; Yobi, A.; Hurst, P.; Angelovici, R.; Rodriguez, O.; Holding, D.R. Production and Selection of Quality Protein Popcorn Hybrids Using a Novel Ranking System and Combining Ability Estimates. Front. Plant Sci. 2020, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Virot, E.; Ponomarenko, A. Popcorn: Critical Temperature, Jump and Sound. J. R. Soc. Interface 2015, 12, 20141247. [Google Scholar] [CrossRef] [PubMed]
- Coan, M.M.D.; Pinto, R.J.B.; Kuki, M.C.; Amaral Júnior, A.T.; Figueiredo, A.S.T.; Scapim, C.A.; Warburton, M. Inheritance Study for Popping Expansion in Popcorn vs. Flint Corn Genotypes. Agron. J. 2019, 111, 2174–2183. [Google Scholar] [CrossRef]
- Freire, A.I.; de Melo Castro, E.; Pereira, A.M.; Cruz, R.R.P.; de Souza, F.B.M.; Chagas, W.F.T.; de Souza, J.C. Amylose Content and Micromorphology of Popcorn Progenies with Different Popping Expansion Volumes. Ciência Rural 2020, 50, e20180962. [Google Scholar] [CrossRef]
- De Lima, V.J.; Do Amaral Júnior, A.T.; Kamphorst, S.H.; Bispo, R.B.; Leite, J.T.; De Oliveira Santos, T.; Medeiros Schmitt, K.F.; Chaves, M.M.; De Oliveira, U.A.; Araújo Diniz Santos, P.H.; et al. Combined Dominance and Additive Gene Effects in Trait Inheritance of Drought-Stressed and Full Irrigated Popcorn. Agronomy 2019, 9, 782. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of Water Deficit Stress in Maize: Phenology and Yield Components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef]
- Benchikh-Lehocine, M.; Revilla, P.; Malvar, R.A.; Djemel, A. Response to Selection for Reduced Anthesis-Silking Interval in Four Algerian Maize Populations. Agronomy 2021, 11, 382. [Google Scholar] [CrossRef]
- Ali, M.L.; Luetchens, J.; Singh, A.; Shaver, T.M.; Kruger, G.R.; Lorenz, A.J. Greenhouse Screening of Maize Genotypes for Deep Root Mass and Related Root Traits and Their Association with Grain Yield under Water-Deficit Conditions in the Field. Euphytica 2016, 207, 79–94. [Google Scholar] [CrossRef]
- de Araujo Rufino, C.; Fernandes-Vieira, J.; Martín-Gil, J.; de Souza Abreu Júnior, J.; Tavares, L.C.; Fernandes-Correa, M.; Martín-Ramos, P. Water Stress Influence on the Vegetative Period Yield Components of Different Maize Genotypes. Agronomy 2018, 8, 151. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Zhao, C.; Wang, Y.; Zhang, R. Physiological and Proteomic Analyses Revealed the Response Mechanisms of Two Different Drought-Resistant Maize Varieties. BMC Plant Biol. 2021, 21, 513. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ali, A.; Waseem, M.; Muzaffar, A.; Ahmed, S.; Ali, S.; Bajwa, K.S.; Awan, M.F.; Samiullah, T.R.; Nasir, I.A.; et al. Correlation Analysis for Morpho-Physiological Traits of Maize (Zea mays L.). Life Sci. J. 2014, 11, 9–13. [Google Scholar]
- Qin, L.; Sun, L.; Wei, L.; Yuan, J.; Kong, F.; Zhang, Y.; Miao, X.; Xia, G.; Liu, S. Maize SRO1e Represses Anthocyanin Synthesis through Regulating the MBW Complex in Response to Abiotic Stress. Plant J. 2021, 105, 1010–1025. [Google Scholar] [CrossRef]
- Saad-allah, K.M.; Nessem, A.A.; Ebrahim, M.K.H.; Gad, D. Evaluation of Drought Tolerance of Five Maize Genotypes by Virtue of Physiological and Molecular Responses. Agronomy 2022, 12, 59. [Google Scholar] [CrossRef]
- Havaux, M. Stress Tolerance of Photosystem II in Vivo. Plant Physiol. 1992, 100, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Mickelbart, M.V. Drought, Leaf Gas Exchange, and Chlorophyll Fluorescence of Field-Grown Papaya. J. Am. Soc. Hortic. Sci. 1998, 123, 714–718. [Google Scholar] [CrossRef]
- Ruas, K.F.; Baroni, D.F.; de Souza, G.A.R.; de Paula Bernado, W.; Paixão, J.S.; dos Santos, G.M.; Filho, J.A.M.; de Abreu, D.P.; de Sousa, E.F.; Rakocevic, M.; et al. A Carica papaya L. Genotype with Low Leaf Chlorophyll Concentration Copes Successfully with Soil Water Stress in the Field. Sci. Hortic. 2022, 293, 110722. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High Throughput Phenotyping of Maize (Zea mays L.) Root Architecture in the Field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Hauck, A.L.; Novais, J.; Grift, T.E.; Bohn, M.O. Characterization of Mature Maize (Zea mays L.) Root System Architecture and Complexity in a Diverse Set of Ex-PVP Inbreds and Hybrids. Springerplus 2015, 4, 424. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Phenes That Reduce the Metabolic Costs of Soil Exploration: Opportunities for 21st Century Agriculture. Plant, Cell Environ. 2015, 38, 1775–1784. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Klein, S.P.; Schneider, H.M.; Perkins, A.C.; Brown, K.M.; Lynch, J.P. Multiple Integrated Root Phenotypes Are Associated with Improved Drought Tolerance. Plant Physiol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced Crown Root Number Improves Water Acquisition under Water Deficit Stress in Maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; de Lima, V.J.; Schimitt, K.F.M.; Leite, J.T.; Azeredo, V.C.; Pena, G.F.; Santos, P.H.A.D.; Júnior, D.R.S.; da Silva Júnior, S.B.; Bispo, R.B.; et al. Water Stress Adaptation of Popcorn Roots and Association with Agronomic Traits. Genet. Mol. Res. 2018, 17, gmr18078. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of Root System Architecture by DEEPER ROOTING 1 Increases Rice Yield under Drought Conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Guo, S.; Cheng, S.; Guan, Y.; Cai, H.; Mi, G.; Yuan, L.; Chen, F. Enhanced Crown Root Number and Length Confers Potential for Yield Improvement and Fertilizer Reduction in Nitrogen-Efficient Maize Cultivars. F. Crop. Res. 2019, 241, 107562. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; de Lima, V.J.; Santos, P.H.A.D.; Rodrigues, W.P.; Vivas, J.M.S.; Gonçalves, G.M.B.; Schmitt, K.F.M.; Leite, J.T.; Vivas, M.; et al. Comparison of Selection Traits for Effective Popcorn (Zea mays L. Var. Everta) Breeding Under Water Limiting Conditions. Front. Plant Sci. 2020, 11, 1289. [Google Scholar] [CrossRef]
- Dao, A.; Sanou, J.; Traor, E.V.S.; Gracen, V.; Danquah, E.Y. Selection of Drought Tolerant Maize Hybrids Using Path Coefficient Analysis and Selection Index. Pakistan J. Biol. Sci. 2017, 20, 132–139. [Google Scholar] [CrossRef]
- Mason, S.; Kmail, Z.; Galusha, T.; Jukić, Ž. Path Analysis of Drought Tolerant Maize Hybrid Yield and Yield Components across Planting Dates. J. Cent. Eur. Agric. 2019, 20, 194–207. [Google Scholar] [CrossRef]
- Gazal, A.; Ahmed Dar, Z.; Ahmad Lone, A.; Yousuf, N.; Gulzar, S. Studies on Maize Yield under Drought Using Correlation and Path Coefficient Analysis. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 516–521. [Google Scholar] [CrossRef][Green Version]
- Fadhli, N.; Farid, M.; Rafiuddin; Efendi, R.; Azrai, M.; Anshori, M.F. Multivariate Analysis to Determine Secondary Characters in Selecting Adaptive Hybrid Corn Lines under Drought Stress. Biodiversitas 2020, 21, 3617–3624. [Google Scholar] [CrossRef]
| Traits | Joint Analysis | WS | WW | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | WC | G | CS*WC | CS*G | WC*G | CS*WC*G | 2020 | 2021 | 2020 | 2021 | ||||||
| Agronomic | 100-grain weight (g) | *** | *** | *** | *** | *** | *** | *** | 9.22 ± 1.39 | ** | 9.15 ± 1.25 | ** | 10.91 ± 1.60 | ** | 10.00 ± 1.56 | ** |
| Popping expansion (g·mL−1) | *** | *** | *** | *** | *** | *** | *** | 17.44 ± 3.90 | ** | 18.76 ± 3.18 | ** | 22.20 ± 3.87 | ** | 22.16 ± 3.47 | ** | |
| Grain yield (kg·ha−1) | *** | *** | *** | *** | *** | *** | *** | 461.02 ± 200.62 | ** | 812.08 ± 303.25 | ** | 1519.64 ± 504.04 | ** | 1285.05 ± 419.59 | ** | |
| Expanded popcorn volume (m3·ha−1) | *** | *** | *** | *** | *** | *** | *** | 8.07 ± 3.91 | ** | 15.34 ± 6.47 | ** | 33.46 ± 11.63 | ** | 28.25 ± 9.62 | ** | |
| Row number per ear (unit.) | *** | *** | *** | *** | *** | *** | *** | 18.71 ± 2.86 | ** | 22.54 ± 4.23 | ** | 22.90 ± 3.21 | ** | 22.79 ± 3.01 | ** | |
| Grain number per row (unit.) | ** | *** | *** | *** | *** | *** | *** | 11.64 ± 1.34 | ** | 11.79 ± 1.30 | ** | 13.11 ± 1.07 | ** | 12.61 ± 1.22 | ** | |
| Grain number per ear (unit.) | ** | *** | *** | *** | *** | *** | *** | 220.07 ± 40.78 | ** | 270.77 ± 70.05 | ** | 299.63 ± 45.02 | ** | 288.98 ± 54.43 | ** | |
| Ear diameter (mm) | *** | *** | *** | ** | *** | *** | *** | 25.18 ± 2.07 | ** | 26.07 ± 2.13 | ** | 27.79 ± 1.79 | ** | 27.85 ± 2.37 | ** | |
| Ear length (cm) | *** | *** | *** | *** | *** | *** | *** | 9.72 ± 1.51 | ** | 12.08 ± 1.39 | ** | 10.96 ± 1.34 | ** | 12.63 ± 1.33 | ** | |
| Ear height (cm) | *** | *** | *** | *** | *** | *** | *** | 81.08 ± 12.24 | ** | 90.31 ± 11.52 | ** | 90.90 ± 13.55 | ** | 93.84 ± 10.08 | ** | |
| Plant height (cm) | *** | *** | *** | *** | *** | *** | *** | 128.94 ± 14.80 | ** | 155.00 ± 16.68 | ** | 156.38 ± 14.05 | ** | 160.07 ± 15.47 | ** | |
| Stem diameter (mm) | *** | *** | *** | *** | *** | *** | *** | 12.91 ± 0.96 | ** | 12.80 ± 1.43 | ** | 12.28 ± 0.89 | ** | 14.37 ± 1.33 | ** | |
| Physiologic | SPAD index | *** | *** | *** | *** | *** | *** | *** | 15.14 ± 6.35 | ** | 14.17 ± 4.06 | ** | 29.75 ± 5.60 | ** | 32.45 ± 5.54 | ** |
| Anthocyanin index | *** | *** | *** | *** | *** | *** | *** | 0.27 ± 0.03 | ** | 0.16 ± 0.03 | ** | 0.23 ± 0.03 | ** | 0.07 ± 0.03 | ** | |
| Epidermal flavonoids content | *** | *** | *** | *** | *** | *** | *** | 1.23 ± 0.12 | ** | 1.10 ± 0.15 | ** | 1.22 ± 0.11 | ** | 0.95 ± 0.14 | ** | |
| Nitrogen balance index | *** | *** | *** | *** | *** | *** | *** | 20.14 ± 3.79 | ** | 31.37 ± 6.06 | ** | 20.12 ± 4.00 | ** | 42.75 ± 9.13 | ** | |
| Maximum quantum efficiency of PSII (Fv/Fm) | *** | *** | *** | * | *** | *** | *** | 0.73 ± 0.03 | ** | 0.67 ± 0.05 | ** | 0.76 ± 0.03 | ** | 0.68 ± 0.04 | ** | |
| Roots | Angles of brace roots (°) | *** | ns | *** | *** | *** | *** | *** | 35.80 ± 5.92 | ** | 42.52 ± 7.16 | ** | 33.53 ± 4.96 | ** | 46.37 ± 7.53 | ** |
| Angles of crown roots (°) | *** | ** | *** | *** | *** | *** | *** | 41.91 ± 6.64 | ** | 44.61 ± 8.29 | ** | 41.36 ± 7.15 | ** | 47.41 ± 6.59 | ** | |
| Number of brace roots | ns | *** | *** | ns | *** | *** | *** | 10.84 ± 2.30 | ** | 10.76 ± 1.76 | ** | 11.83 ± 2.20 | ** | 12.06 ± 2.24 | ** | |
| Number of crown roots | *** | *** | *** | * | *** | *** | *** | 16.24 ± 3.62 | ** | 20.73 ± 3.56 | ** | 16.82 ± 5.01 | ** | 22.59 ± 4.57 | ** | |
| Density of brace roots | ns | *** | *** | ns | *** | *** | *** | 5.48 ± 0.89 | ** | 5.31 ± 1.08 | ** | 5.54 ± 0.90 | ** | 5.81 ± 1.24 | ** | |
| Density of crown roots | *** | ns | *** | *** | *** | *** | *** | 5.39 ± 0.73 | ** | 4.72 ± 0.96 | ** | 4.90 ± 0.90 | ** | 4.99 ± 1.31 | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, J.T.; Amaral Junior, A.T.d.; Kamphorst, S.H.; Lima, V.J.d.; Santos Junior, D.R.d.; Alves, U.O.; Azeredo, V.C.; Pereira, J.L.; Bispo, R.B.; Schmidt, K.F.M.; et al. All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes. Plants 2022, 11, 2275. https://doi.org/10.3390/plants11172275
Leite JT, Amaral Junior ATd, Kamphorst SH, Lima VJd, Santos Junior DRd, Alves UO, Azeredo VC, Pereira JL, Bispo RB, Schmidt KFM, et al. All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes. Plants. 2022; 11(17):2275. https://doi.org/10.3390/plants11172275
Chicago/Turabian StyleLeite, Jhean Torres, Antônio Teixeira do Amaral Junior, Samuel Henrique Kamphorst, Valter Jário de Lima, Divino Rosa dos Santos Junior, Uéliton Oliveira Alves, Valdinei Cruz Azeredo, Jacymara Lopes Pereira, Rosimeire Barboza Bispo, Katia Fabiane Medeiros Schmidt, and et al. 2022. "All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes" Plants 11, no. 17: 2275. https://doi.org/10.3390/plants11172275
APA StyleLeite, J. T., Amaral Junior, A. T. d., Kamphorst, S. H., Lima, V. J. d., Santos Junior, D. R. d., Alves, U. O., Azeredo, V. C., Pereira, J. L., Bispo, R. B., Schmidt, K. F. M., Viana, F. N., Viana, A. P., Vieira, H. D., Ramos, H. C. C., Ribeiro, R. M., & Campostrini, E. (2022). All Are in a Drought, but Some Stand Out: Multivariate Analysis in the Selection of Agronomic Efficient Popcorn Genotypes. Plants, 11(17), 2275. https://doi.org/10.3390/plants11172275











