Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Pisum ssp. Germplasm Origin
2.2. Pathogen Isolate and Multiplication
2.3. Field Experiments and Data Assessments
2.4. Controlled Condition Experiment and Assessments
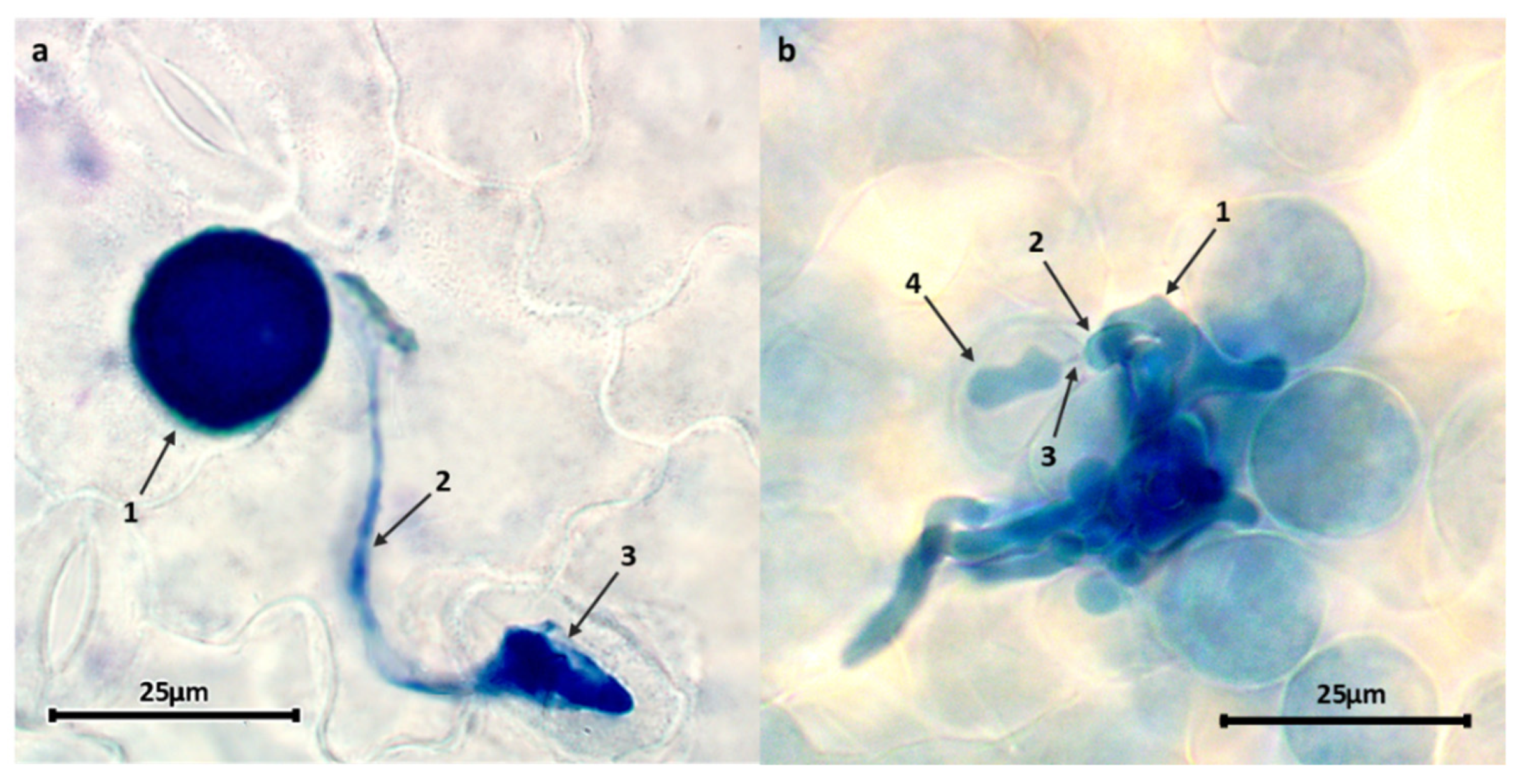
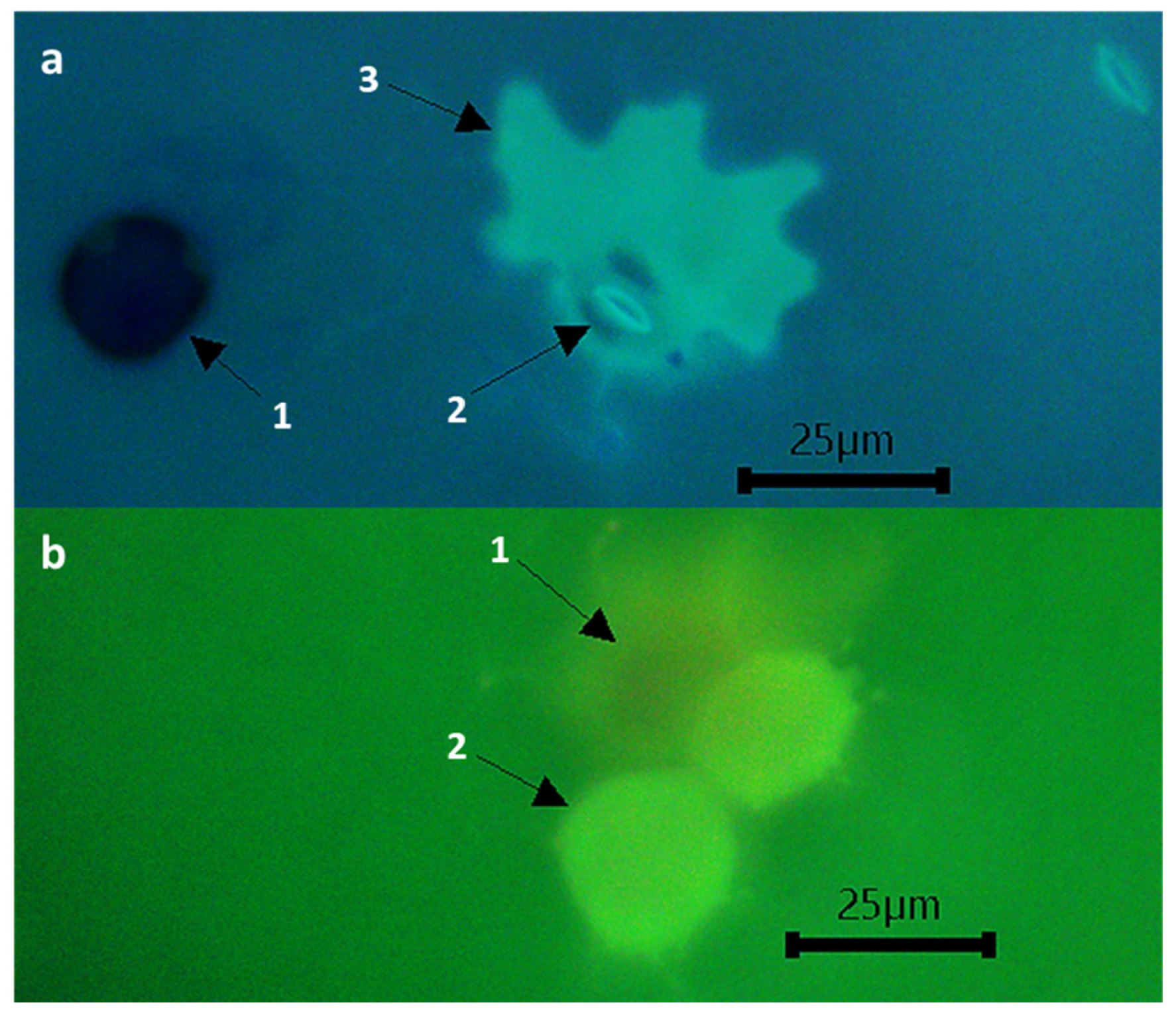
2.5. Histological Assessments
2.6. Data Manipulation and Statistical Analysis
3. Results
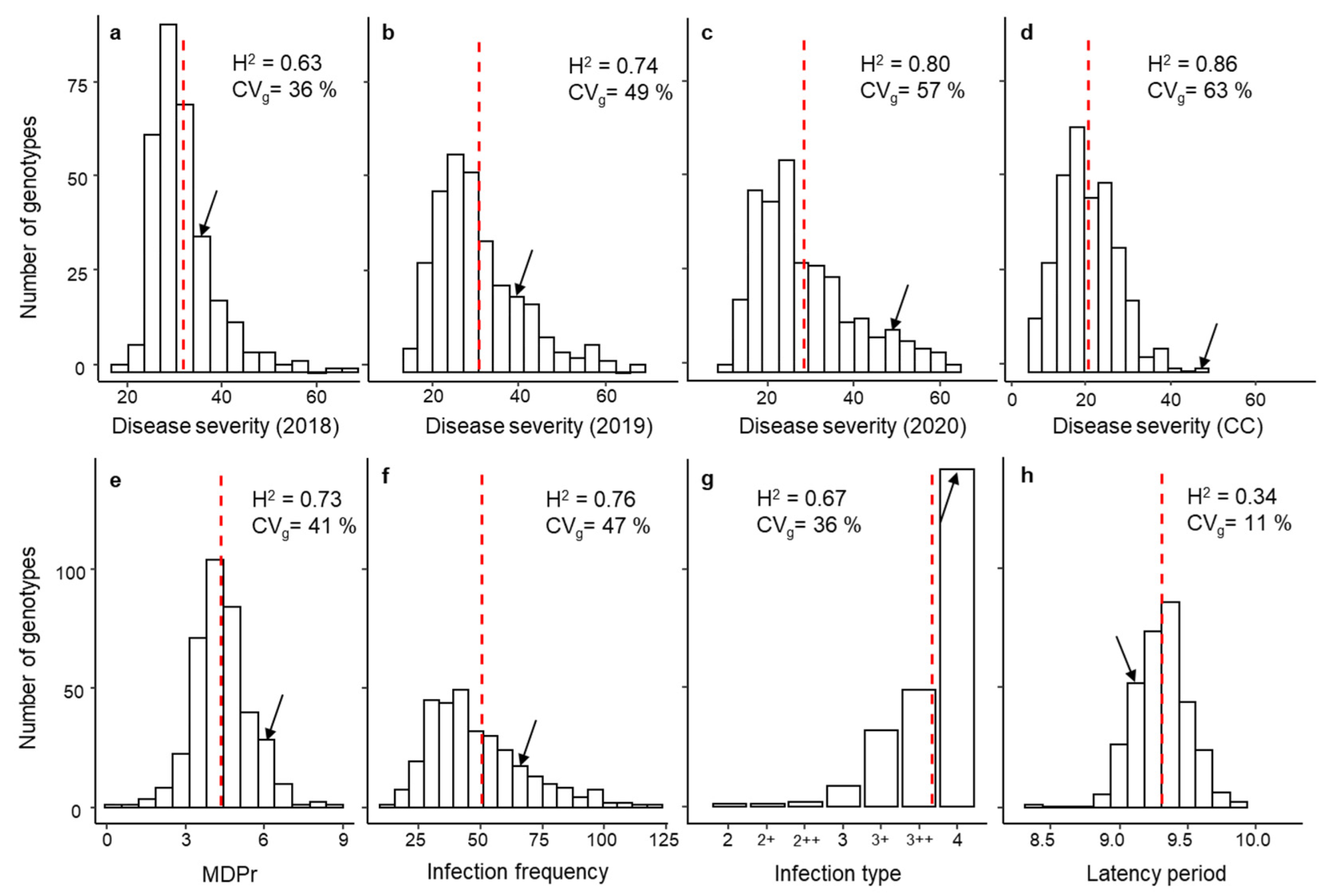
3.1. Phenotypic Response, Variance Components and Broad-Sense Heritability
3.2. Trait Correlations
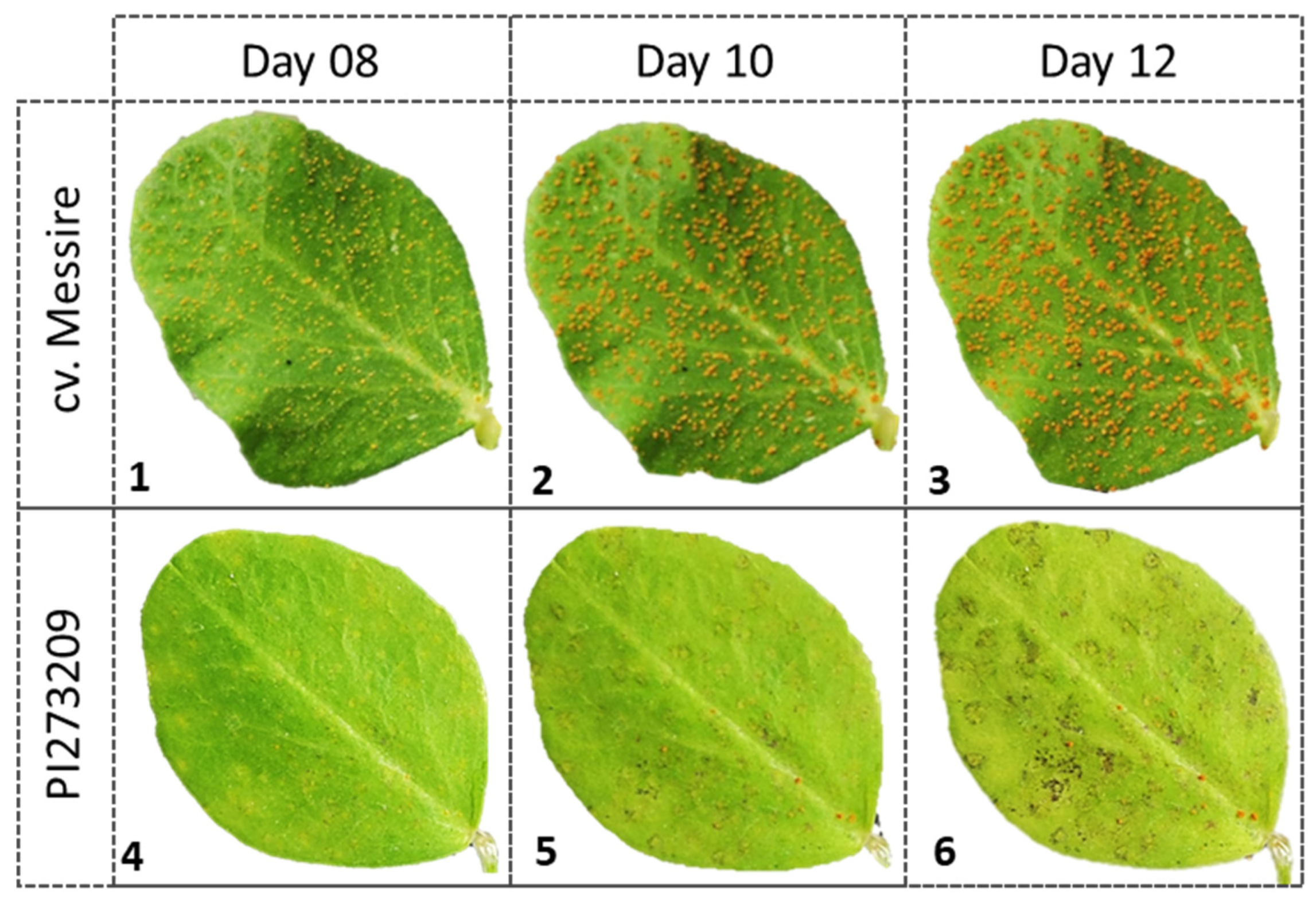
3.3. Selected Rust Resistant Accessions
3.4. Pea Resistance Mechanisms against U. pisi
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat (accessed on 4 February 2022).
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.; Asavajaru, P.; Lam, A. Pea: A Sustainable Vegetable Protein Crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 145–164. [Google Scholar]
- Clemente, A.; Olias, R. Beneficial Effects of Legumes in Gut Health. Curr. Opin. Food Sci. 2017, 14, 32–36. [Google Scholar] [CrossRef]
- Borges Martínez, J.E.; Concha, D.d.R.M.; Gallardo Velázquez, T.G.; Martínez, C.J.; Ruiz Ruiz, J.C. Anti-Inflammatory Properties of Phenolic Extracts from Phaseolus vulgaris and Pisum sativum during Germination. Food Biosci. 2021, 42, 101067. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. CRC Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, R.; Singh, B.D.; Chand, R.; Srivastava, C.P. Validation of SSR Markers Associated with Rust (Uromyces fabae) Resistance in Pea (Pisum sativum L.). Physiol. Mol. Biol. Plants 2015, 21, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Barilli, E.; Sillero, J.C.; Fernández-Aparicio, M.; Rubiales, D. Identification of Resistance to Uromyces pisi (Pers.) Wint. in Pisum spp. Germplasm. Field Crops Res. 2009, 114, 198–203. [Google Scholar] [CrossRef]
- Emeran, A.A.; Sillero, J.C.; Niks, R.E.; Rubiales, D. Infection Structures of Host-Specialized Isolates of Uromyces viciae-fabae and of Other Species of Uromyces Infecting Leguminous Crops. Plant Dis. 2005, 89, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pfunder, M.; Roy, B.A. Pollinator-Mediated Interactions between a Pathogenic Fungus, Uromyces pisi (Pucciniaceae), and Its Host Plant, Euphorbia Cyparissias (Euphorbiaceae). Am. J. Bot. 2000, 87, 48–55. [Google Scholar] [CrossRef]
- EPPO Standards Pea. EPPO Bulletin. Available online: https://www.eppo.int/RESOURCES/eppo_standards/pp2_gpp. (accessed on 14 May 2022).
- Emeran, A.A.; Sillero, J.C.; Fernández-Aparicio, M.; Rubiales, D. Chemical Control of Faba Bean Rust (Uromyces viciae-fabae). Crop. Prot. 2011, 30, 907–912. [Google Scholar] [CrossRef]
- Barilli, E.; Cimmino, A.; Masi, M.; Evidente, M.; Rubiales, D.; Evidente, A. Inhibition of Early Development Stages of Rust Fungi by the Two Fungal Metabolites Cyclopaldic Acid and Epi-Epoformin. Pest. Manag. Sci. 2017, 73, 1161–1168. [Google Scholar] [CrossRef]
- Barilli, E.; Rubiales, D.; Castillejo, M.Á. Comparative Proteomic Analysis of BTH and BABA-Induced Resistance in Pea (Pisum sativum) toward Infection with Pea Rust (Uromyces pisi). J. Proteom. 2012, 75, 5189–5205. [Google Scholar] [CrossRef]
- Barilli, E.; Prats, E.; Rubiales, D. Benzothiadiazole and BABA Improve Resistance to Uromyces pisi (Pers.) Wint. in Pisum sativum L. with an Enhancement of Enzymatic Activities and Total Phenolic Content. Eur. J. Plant Pathol. 2010, 128, 483–493. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A.; Singh, A.K. Influence of Planting Time, Planting Geometry, Intercropping and Row Direction on Rust (Uromyces viciae-fabae) Pers. de Bary of Field Pea (Pisum sativum L.). Legum. Res. 2014, 37, 542–546. [Google Scholar] [CrossRef]
- Shtaya, M.J.Y.; Emeran, A.A.; Fernández-Aparicio, M.; Qaoud, H.A.; Abdallah, J.; Rubiales, D. Effects of Crop Mixtures on Rust Development on Faba Bean Grown in Mediterranean Climates. Crop. Prot. 2021, 146, 105686. [Google Scholar] [CrossRef]
- Barilli, E.; Sillero, J.C.; Prats, E.; Rubiales, D. Resistance to Rusts (Uromyces pisi and U. viciae-fabae) in Pea. Czech. J. Genet. Plant Breed. 2014, 50, 135–143. [Google Scholar] [CrossRef]
- Barilli, E.; Sillero, J.C.; Moral, A.; Rubiales, D. Characterization of Resistance Response of Pea (Pisum spp.) against Rust (Uromyces pisi). Plant Breed. 2009, 128, 665–670. [Google Scholar] [CrossRef]
- Barilli, E.; Satovic, Z.; Rubiales, D.; Torres, A.M. Mapping of Quantitative Trait Loci Controlling Partial Resistance against Rust Incited by Uromyces pisi (Pers.) Wint. in a Pisum fulvum L. Intraspecific Cross. Euphytica 2010, 175, 151–159. [Google Scholar] [CrossRef]
- Barilli, E.; Cobos, M.J.; Carrillo, E.; Kilian, A.; Carling, J.; Rubiales, D. A High-Density Integrated DArTseq SNP-Based Genetic Map of Pisum fulvum and Identification of QTLs Controlling Rust Resistance. Front. Plant Sci. 2018, 9, 167. [Google Scholar] [CrossRef]
- Sillero, J.C.; Moreno, M.T.; Rubiales, D. Characterization of New Sources of Resistance to Uromyces viciae-fabae in a Germplasm Collection of Vicia faba. Plant Pathol. 2000, 49, 389–395. [Google Scholar] [CrossRef]
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of Physiologic Races of Puccinia Graminis Var. Tritici; USDA Agricultural Research Service: Washington, DC, USA, 1962; p. 54.
- Sillero, J.C.; Rubiales, D. Histological Characterization of Resistance to Uromyces viciae-fabae in Faba bean. Phytopathology 2002, 92, 294–299. [Google Scholar] [CrossRef]
- DeLacy, I.H.; Basford, K.E.; Cooper, M.; Bull, J.; McLaren, C.G. Analysis of Multi-Environment Trials-an Historical Perspective. In Plant Adaptation and Crop Improvement, 1st ed.; Cooper, M., Hammer, G.L., Eds.; Cab International: Wallingford, UK, 1993; pp. 104–119. [Google Scholar]
- Toker, C. Estimates of broad-sense heritability for seed yield and yield criteria in faba bean (Vicia faba L.). Hereditas 2004, 140, 222–225. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Toward an Effective Multivariate Selection in Biological Experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.F. A discriminant function for plant selection. Ann. Eugen. 1936, 7, 240–250. [Google Scholar] [CrossRef]
- Rocha, J.R.D.A.S.D.C.; Machado, J.C.; Carneiro, P.C.S. Multitrait Index Based on Factor Analysis and Ideotype-Design: Proposal and Application on Elephant Grass Breeding for Bioenergy. Glob. Chang. Biol. Bioenerg. 2018, 10, 52–60. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A Superiority Measure of Cultivar Performance for Cultivar × Location Data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Olivoto, T.; Lúcio, A.D.C. Metan: An R Package for Multi-Environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Emeran, A.A.; Roman, B.; Sillero, J.C.; Satovic, Z.; Rubiales, D. Genetic Variation among and within Uromyces Species Infecting Legumes. J. Phytopathol. 2008, 156, 419–424. [Google Scholar] [CrossRef]
- Anil-Kumar, T.B.; Rangaswamy, K.T.; Ravi, K. Assessment of Tall Field Pea Genotypes for Slow Rusting Resistance. Legum. Sci. 1994, 17, 79–82. [Google Scholar]
- Singh, D.; Tripathi, H.; Singh, A. Influence of Environmental Factors on Development of Field Pea Rust Caused by Uromyces viciae-fabae. J. Plant Dis. Sci. 2012, 7, 13–17. [Google Scholar]
- Das, A.; Parihar, A.K.; Saxena, D.; Singh, D.; Singha, K.D.; Kushwaha, K.P.S.; Chand, R.; Bal, R.S.; Chandra, S.; Gupta, S. Deciphering Genotype-by- Environment Interaction for Targeting Test Environments and Rust Resistant Genotypes in Field Pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 825. [Google Scholar] [CrossRef]
- More, P.E.; Deokar, C.D.; Bhalerao, V.K. Effect of Meteorological Factors on Rust Severity of Pea at Rahuri, Maharashtra. J. Agrometeorol. 2019, 21, 110–111. [Google Scholar] [CrossRef]
- Negussie, T.; Pretorius, Z.A.; Bender, C.M. Effect of Some Environmental Factors on In Vitro Germination of Urediniospores and Infection of Lentils by Rust. J. Phytopathol. 2005, 153, 43–47. [Google Scholar] [CrossRef]
- More, P.E.; Deokar, C.D.; Ilhe, B.M. Effect of Leaf Wetness and Soil Temperatures on Pea Rust Development Caused by Uromyces viciae-fabae (Pers.) de Bary. J. Agrometeorol. 2020, 22, 207–211. [Google Scholar] [CrossRef]
- Fondevilla, S.; Chattopadhyay, C.; Khare, N.; Rubiales, D. Erysiphe Trifolii Is Able to Overcome Er1 and Er3, but Not Er2, Resistance Genes in Pea. Eur. J. Plant Pathol. 2013, 136, 557–563. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Rubiales, D. Identification and Characterization of Partial Resistance to Rust in a Germplasm Collection of Lathyrus sativus L. Plant Breed. 2009, 128, 495–500. [Google Scholar] [CrossRef]
- Rubio, J.; Rubiales, D. Resistance to Rusts and Broomrape in One-Flowered Vetch (Vicia articulata). Euphytica 2021, 217, 9. [Google Scholar] [CrossRef]
- Précigout, P.A.; Claessen, D.; Makowski, D.; Robert, C. Does the Latent Period of Leaf Fungal Pathogens Reflect Their Trophic Type? A Meta-Analysis of Biotrophs, Hemibiotrophs, and Necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef]
- Dehghani, H.; Moghaddam, M. Genetic Analysis of the Latent Period of Stripe Rust in Wheat Seedlings. J. Phytopathol. 2004, 152, 325–330. [Google Scholar] [CrossRef]
- Suffert, F.; Thompson, R.N. Some Reasons Why the Latent Period Should Not Always Be Considered Constant over the Course of a Plant Disease Epidemic. Plant Pathol. 2018, 67, 1831–1840. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef]
- Pariaud, B.; Goyeau, H.; Halkett, F.; Robert, C.; Lannou, C. Variation in Aggressiveness Is Detected among Puccinia triticina Isolates of the Same Pathotype and Clonal Lineage in the Adult Plant Stage. Eur. J. Plant Pathol. 2012, 134, 733–743. [Google Scholar] [CrossRef]
- Sillero, J.C.; Moreno-Alías, I.; Rubiales, D. Identification and Characterization of Resistance to Rust (Uromyces ciceris-arietini (Grognot) Jacz. & Boyd) in a Germplasm Collection of Cicer spp. Euphytica 2012, 188, 229–238. [Google Scholar]
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Njau, P.; Wanyera, R.; Herrera-Foessel, S.A.; Ward, R.W. Will Stem Rust Destroy the World’s Wheat Crop? Adv. Agron. 2008, 98, 271–309. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Rosewarne, G.M.; Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Lan, C.X.; He, Z.H. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 2013, 126, 2427–2449. [Google Scholar] [CrossRef]
- Barilli, E.; Sillero, J.C.; Serrano, A.; Rubiales, D. Differential response of pea (Pisum sativum) to rusts incited by Uromyces viciae-fabae and U. pisi. Crop. Prot. 2009, 28, 980–986. [Google Scholar] [CrossRef]
- Talhinhas, P.; Carvalho, R.; Figueira, R.; Ramos, A.P. An annotated checklist of rust fungi (Pucciniales) occurring in Portugal. Sydowia 2019, 71, 65–84. [Google Scholar]
- Kosterin, O.E.; Bogdanova, V.S.; Galieva, E.R. Reciprocal compatibility within the genus Pisum L. as studied in F1 hybrids: 2. Crosses involving P. fulvum Sibth. et Smith. Genet. Resour. Crop. Evol. 2019, 66, 383–399. [Google Scholar] [CrossRef]
- Coyne, C.J.; Kumar, S.; von Wettberg, E.J.B.; Marques, E.; Berger, J.D.; Redden, R.J.; Ellis, T.H.N.; Brus, J.; Zablatzká, L.; Smýkal, P. Potential and Limits of Exploitation of Crop Wild Relatives for Pea, Lentil, and Chickpea Improvement. Legum. Sci. 2020, 2, e36. [Google Scholar] [CrossRef]
- Clemente, A.; Arques, M.C.; Dalmais, M.; Le Signor, C.; Chinoy, C.; Olias, R.; Rayner, T.; Isaac, P.G.; Lawson, D.M.; Bendahmane, A.; et al. Eliminating Anti-Nutritional Plant Food Proteins: The Case of Seed Protease Inhibitors in Pea. PLoS ONE 2015, 10, e0134634. [Google Scholar]
- Martins, D.C.; Rubiales, D.; Vaz Patto, M.C. Association Mapping of Lathyrus sativus Disease Response to Uromyces pisi Reveals Novel Loci Underlying Partial Resistance. Front. Plant Sci. 2022, 13, 842545. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Rojas, J.J.; Crossa, J. The Statistical Theory of Linear Selection Indices from Phenotypic to Genomic Selection. Crop. Sci. 2022, 62, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Prunier, J.G.; Colyn, M.; Legendre, X.; Nimon, K.F.; Flamand, M.C. Multicollinearity in Spatial Genetics: Separating the Wheat from the Chaff Using Commonality Analyses. Mol. Ecol. 2015, 24, 263–283. [Google Scholar] [CrossRef]
- Niks, R.E.; Rubiales, D. Potentially Durable Resistance Mechanisms in Plants to Specialised Fungal Pathogens. Euphytica 2002, 124, 201–216. [Google Scholar] [CrossRef]
- Kushwaha, C.; Chand, R.; Singh, A.K.; Rai, R.; Srivastava, C.P.; Singh, B.D.; Mohapatra, C. Lignification and Early Abortive Fungal Colonies as Indicators of Partial Resistance to Rust in Pea. Trop. Plant Pathol. 2016, 41, 91–97. [Google Scholar] [CrossRef]
- Rubiales, D.; Moral, A. Prehaustorial Resistance against Alfalfa Rust (Uromyces striatus) in Medicago truncatula. Eur. J. Plant Pathol. 2004, 110, 239–243. [Google Scholar] [CrossRef]
- Rubiales, D.; Castillejo, M.A.; Madrid, E.; Barilli, E.; Rispail, N. Legume Breeding for Rust Resistance: Lessons to Learn from the Model Medicago truncatula. Euphytica 2011, 180, 89–98. [Google Scholar] [CrossRef][Green Version]
- Negussie, T.G.; Bender, C.M.; Van Wyk, P.W.J.; Pretorius, Z.A. Hypersensitivity of Rust Resistance in Lentil. S. Afr. J. Plant Soil 2012, 29, 25–29. [Google Scholar] [CrossRef]
- Rubiales, D.; Rojas-Molina, M.M.; Sillero, J.C. Identification of Pre- and Posthaustorial Resistance to Rust (Uromyces viciae-fabae) in Lentil (Lens culinaris) Germplasm. Plant Breed. 2013, 132, 676–680. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Khazaei, H.; Ghaouti, L.; Maalouf, F.; Vandenberg, A.; Link, W.; O’Sullivan, D.M. Conventional and Molecular Breeding Tools for Accelerating Genetic Gain in Faba bean (Vicia faba L.). Front. Plant Sci. 2021, 12, 744259. [Google Scholar] [CrossRef]
- Stavely, J.R. New Pathogenic Variability in Uromyces appendiculatus in North America. Plant Dis. 1989, 73, 428. [Google Scholar] [CrossRef]
- Rubiales, D.; Khazaei, H. Advances in Disease and Pest Resistance in Faba bean. Theor. Appl. Genet. 2022. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.M.; Sillero, J.C.; Rubiales, D.; Moreno, M.T.; Torres, A.M. Identification of RAPD Markers Linked to the Uvf-1 Gene Conferring Hypersensitive Resistance against Rust (Uromyces viciae-fabae) in Vicia faba L. Theor. Appl. Genet. 2003, 107, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, U.; Sudheesh, S.; Kaur, S.; Sadeque, A.; Bariana, H.; Bansal, U.; Adhikari, K. Mapping of Two New Rust Resistance Genes Uvf-2 and Uvf-3 in Faba bean. Agronomy 2021, 11, 1370. [Google Scholar] [CrossRef]
- Yu, N.; Kim, M.; King, Z.R.; Harris, D.K.; Buck, J.W.; Li, Z.; Diers, B.W. Fine Mapping of the Asian Soybean Rust Resistance Gene Rpp2 from Soybean PI 230970. Theor. Appl. Genet. 2015, 128, 387–396. [Google Scholar] [CrossRef]
| Environments | Season | Site (Decimal Degrees Coordinates) | Soil Type | Soil pH | Organic Matter (g/110 g) | Available Phosphorus (mg/kg) | C:N Ratio | Average Tmax (°C) | Average Tmin (°C) | Average RH (%) * | Rain (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Co-18 | 2017–2018 | 37.862875, −4.791796 | Vertisol | - | - | - | - | 18.37 | 6.17 | 75.2 | 472 |
| Co-19 | 2018–2019 | 37.864470, −4.789733 | Vertisol | 7.8 | 0.7 | 9.9 | 7.25 | 21.04 | 5.78 | 63.4 | 127 |
| Co-20 | 2019–2020 | 37.866372, −4.787661 | Vertisol | - | - | - | - | 21.00 | 8.45 | 73.9 | 382 |
| Trait | Mean ± SE | Skewness | Min | Max | Accuracy | LRT | CVi | |
|---|---|---|---|---|---|---|---|---|
| Controlled Conditions | MDPr | 5.7 ± 0.01 | 0.28 | 2.66 | 8.75 | 0.87 | 280 | - |
| IF | 50.3 ± 1.19 | 2.33 | 1.00 | 387.00 | 0.87 | 264 | - | |
| IT | 3.8 ± 0.01 | −2.05 | 2.00 | 4.00 | 0.82 | 180 | - | |
| LP50 | 9.3 ± 0.02 | −0.02 | 6.71 | 12.56 | 0.58 | 11 | - | |
| DSCC | 20.1 ± 0.29 | 0.53 | 2.00 | 60.00 | 0.93 | 549 | - | |
| Field Season | DS2018 | 27.5 ± 0.49 | 1.73 | 2.00 | 60.00 | 0.79 | 111 | 27 |
| DS2019 | 26.2 ± 0.56 | 1.06 | 1.00 | 65.00 | 0.86 | 205 | 11 | |
| DS2020 | 28.8 ± 0.59 | 0.95 | 1.50 | 65.00 | 0.89 | 299 | 17 |
| Field Conditions | Controlled Conditions | ||||||
|---|---|---|---|---|---|---|---|
| DS2018 | DS2019 | DS2020 | MDPr | IF | LP50 | DSCC | |
| Field conditions | |||||||
| DS2019 | 0.46 *** | ||||||
| DS2020 | 0.46 *** | 0.55 *** | |||||
| Controlled conditions | |||||||
| MDPr | 0.21 *** | 0.44 *** | 0.39 *** | ||||
| IF | 0.21 *** | 0.46 *** | 0.40 *** | 0.96 *** | |||
| LP50 | −0.04 | 0.01 | −0.04 | −0.21 ** | −0.05 | ||
| DS | 0.14 * | 0.28 *** | 0.30 *** | 0.58 *** | 0.57 *** | −0.20 ** | |
| IT | 0.18 * | 0.29 *** | 0.25 *** | 0.45 *** | 0.42 *** | −0.20 ** | 0.33 *** |
| Rust Level | Bank Code | Species | Origin | DSfield (%) | DSCC (%) | Ranking Indices (1–320) | |||
|---|---|---|---|---|---|---|---|---|---|
| FAI-BLUP | MGIDI | SH | LIN | ||||||
| Resistant | CGN10206 | P. sativum subsp. elatius | Unknown | 3.0 | 5.2 | 1 | 1 | 1 | 4 |
| CGN10205 | P. sativum subsp. elatius | Turkey | 6.0 | 5.5 | 2 | 2 | 2 | 1 | |
| PI273209 | P. sativum subsp. elatius | Russia | 3.3 | 3.0 | 3 | 3 | 3 | 2 | |
| IFPI3260 | P. fulvum | Syria | 4.3 | 6.7 | 4 | 4 | 6 | 3 | |
| BGE004710 | P. sativum subsp. sativum | Portugal | 7.7 | 6.5 | 7 | 8 | 7 | 10 | |
| JI199 | P. sativum subsp. elatius | Israel | 5.0 | 6.5 | 5 | 5 | 4 | 5 | |
| JI198 | P. sativum subsp. elatius | Israel | 5.8 | 11.6 | 6 | 6 | 8 | 6 | |
| PI347321 | P. sativum | India | 11.0 | 4.5 | 9 | 9 | 10 | 11 | |
| JI224 | P. fulvum | Israel | 4.3 | 10.0 | 10 | 10 | 12 | 9 | |
| Intermediate | PI347372 | P. sativum | India | 16.3 | 16.1 | 155 | 155 | 162 | 150 |
| PI143483 | P. sativum | Azerbaijan | 17.0 | 22.1 | 160 | 160 | 167 | 156 | |
| PI324705 | P. sativum | France | 15.7 | 21.0 | 175 | 175 | 184 | 169 | |
| Susceptible | PI162910 | P. sativum | Paraguay | 34.5 | 27.6 | 318 | 318 | 318 | 320 |
| PI204667 | P. sativum subsp. sativum | Netherland | 30.0 | 45.8 | 319 | 319 | 319 | 318 | |
| JI1210 | P. sativum subsp. sativum | France | 40.7 | 28.6 | 320 | 320 | 320 | 315 | |
| Bank Code | DSCC (%) | IT | Infection Unit Area (µm²) | Infection Unit Perimeter (µm) | No. Hyphal Tips/Infection Unit | Early Abortion (%) | No. Haustoria/Established Colony | Established Colonies Associated with Host Cell Necrosis (%) |
|---|---|---|---|---|---|---|---|---|
| PI347321 | 4.5 d | 3++ | 490.8 c | 184.0 d | 3.3 c | 8.3 c | 1.9 ab | 0 d |
| JI224 | 10.0 bc | 3++ | 792.5 b | 302.3 b | 5.8 ab | 12.5 b | 1.7 a | 2.5 c |
| BGE004710 | 6.5 c | 3 | 592.6 bc | 211.6 cd | 3.6 c | 10.6 c | 1.6 a | 4.3 bc |
| PI273209 | 3.0 d | 2 | 784.3 b | 277.5 b | 5.4 b | 10.0 c | 1.9 ab | 20.0 a |
| JI198 | 11.6 bc | 3 | 941.2 ab | 374.3 a | 6.8 a | 23.7 a | 1.8 ab | 0 d |
| JI199 | 6.5 c | 3 | 765.0 b | 270.3 bc | 5.0 b | 23.3 a | 1.6 a | 6.7 b |
| CGN10205 | 5.5 cd | 3 | 545.4 c | 195.7 d | 3.8 c | 13.3 b | 1.6 a | 0 d |
| CGN10206 | 5.2 cd | 3 | 606.4 bc | 240.2 c | 4.8 bc | 13.3 b | 1.5 a | 0 d |
| IFPI 3260 | 6.7 c | 4 | 497.5 c | 190.8 d | 3.7 c | 23.3 a | 1.7 a | 0 d |
| PI143483 | 22.1 ab | 4 | 1170.7 a | 360.5 ab | 6.1 ab | 0 d | 2.3 b | 0 d |
| PI347372 | 16.1 b | 4 | 1074.9 a | 356.5 ab | 7.7 a | 12.9 b | 2.3 b | 0 d |
| PI324705 | 21.0 ab | 4 | 662.6 b | 222.6 c | 3.8 c | 0 d | 2.3 b | 0 d |
| PI162910 | 27.6 ab | 4 | 1202.0 a | 395.7 a | 7.9 a | 0 d | 3.4 c | 0 d |
| PI204667 | 45.8 a | 4 | 1024.8 a | 334.0 b | 6.2 ab | 0 d | 3.5 c | 0 d |
| JI1210 | 28.6 ab | 4 | 1006.1 a | 292.0 b | 5.9 ab | 0 d | 3.2 c | 0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuna-Caballero, S.; Rispail, N.; Barilli, E.; Rubiales, D. Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp. Plants 2022, 11, 2268. https://doi.org/10.3390/plants11172268
Osuna-Caballero S, Rispail N, Barilli E, Rubiales D. Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp. Plants. 2022; 11(17):2268. https://doi.org/10.3390/plants11172268
Chicago/Turabian StyleOsuna-Caballero, Salvador, Nicolas Rispail, Eleonora Barilli, and Diego Rubiales. 2022. "Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp." Plants 11, no. 17: 2268. https://doi.org/10.3390/plants11172268
APA StyleOsuna-Caballero, S., Rispail, N., Barilli, E., & Rubiales, D. (2022). Identification and Characterization of Novel Sources of Resistance to Rust Caused by Uromyces pisi in Pisum spp. Plants, 11(17), 2268. https://doi.org/10.3390/plants11172268









