Abstract
Apocynum hendersonii is a traditional medicinal plant used primarily as tea. It has a potential health benefit from its rich bioactive substances. This study investigated the reactivity of solvents of different polarities (ethanol, ethyl acetate, n-hexane, methanol, and water) extracts of the A. hendersonii leaf. The phytochemical composition of the extracts was evaluated using a Fourier Transform Infrared spectrophotometer (FT-IR), Gas Chromatography-Mass Spectrometry (GC-MS), UHPLC-MS, and Higher Performance Liquid Chromatography (HPLC). The result revealed the presence of medicinally important bioactive constituents, including phenols, flavonoids, and polysaccharides. Methanol extracts exhibited the highest flavonoid contents (20.11 ± 0.85 mg QE/g DW) and the second-highest in terms of phenolic (9.25 ± 0.03 mg GAE/g DW) and polysaccharide (119.66 ± 2.65 mg GE/g DW). It also had the highest antioxidant capacity with 60.30 ± 0.52% and 4.60 ± 0.02 µmol Fe2+ per g DW based on a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and ferric reducing antioxidant power (FRAP), respectively. Ethanol extract displayed the maximum antibacterial action against Gram-negative and Gram-positive bacteria and the highest inhibition activity against the enzymes tyrosinase and acetylcholinesterase, followed by methanol extract. The principal component analysis revealed a positive correlation between the constituents, bioactivities, and extracts. The overall result showed A. hendersonii as a rich natural source of antimicrobial and antioxidant bioactive compounds and may be used for future applications in pharmaceuticals and food industries.
1. Introduction
Plants are a major and rich source of biomolecules that have multifunctional activities and low drug resistance potential, eliciting few side effects [1]. Bioactive plant materials, such as flavonoids, phenols, polysaccharides, and terpenoids, have tremendous biological applications and have been exploited as medicine, cosmetics, and food additives [2]. Polyphenols and polysaccharides have been reported to have exhibited therapeutic action against oxidative stress scavenging on free radicals [3,4]. Free radicals are generated in living cells as a product of metabolism or due to endogenous and environmental factors [4,5,6]. Oxidative stress leads to diseases such as inflammation, diabetes, and cancer [6,7]. Synthetic antioxidants, such as butylhydroxyanisole and butylhydroxytoluene, are carcinogenic and lead to liver impairment and atherosclerosis [4,8]. Plant-based antioxidants presented a better alternative. Many findings demonstrated a positive correlation between plant polyphenols’ bioactive substances and antioxidant activity, increasing global interest in exploiting plants for medicinal purposes [4]. Traditional uses of plants or those produced from their safety level and availability have been promoted by botanists. Moreover, numerous plants and their products have been reported and scientifically proven to have medicinal significance against many diseases, such as cancer, Alzheimer’s, diabetes, fever, hepatopathy, etc. [9,10]. Due to the increasing demand for bioactive compound-rich plants, the limited number of available wild plants, and prevent endangering of target plant species, several other plants are being investigated for possible medicinal potentials [4,6]. Apocynum hendersonii is one of the best alternatives with significant therapeutic potential.
Apocynum hendersonii (Hook.f.) Woodson, also known as A. pictum (Schrenk) Baillon, belongs to the Apocynaceae family and is used as traditional herbal tea in China and Japan due to its rich quercetin content [11]. It is considered a substitute [12] or complementary to its closely related species—A. venetum, which exhibits broad medicinal functions [13]. The two Apocynum species are often confused together. However, A. hendersonii is distinguished by its comparably narrow leaves and conspicuous white flower, unlike A. venetum, which has a red-colored flower and broader leaves. Both species however have the same geographical distribution [14,15]. Several types of research papers on metabolomics, transcriptomics, genomics, and bioactivities were conducted mainly on A. venetum with only a few on the A. hendersonii. Extract of A. hendersonii flower has been reported to have enhanced adipogenesis of 3T3 L1 cells [12]. Metabolomic analysis of A. hendersonii showed a substantial amount of flavonoids in the plant [16]. Flavonoids are the most diverse and most common forms of phenolic compounds with very effective antioxidants capacity by chelating elements involved in the free radical formation or scavenging reactive oxidative species [13,15,17].
A. hendersonii, in addition to flavonoids or polyphenols, may contain polysaccharides, as A. venetum was recently reported to contain the bioactive substance [18]. Polysaccharides are macromolecules of biological importance with anti-inflammatory and immunomodulatory properties [19]. Studies have also indicated their significance in preventing oxidative damage and as dietary free radical scavenging molecules [20]. Polysaccharides from corn silk have exhibited antifatigue, antihepatoma, antiobesity, and antioxidant activity [21].
Medicinal properties, including antioxidants and antimicrobials of plant and plant products, are associated with their bioactive constituents, mainly phenolic metabolites [22]. Phenolic compounds encompassed phenolic acids, polyphenols (condensed tannins), and flavonoids [23]. Techniques such as the Soxhlet extraction, maceration, subcritical aqueous extraction, supercritical fluid, and ultrasound-assisted extraction methods are available for recovering plants’ polyphenols. The extraction technique employed and the solvents used affect the yield and bioactive components’ property. Solvents of different polarities, such as methanol, ethanol, ethyl acetate, acetone, or aqueous forms, are commonly used [23,24,25]. Selection of suitable solvent is paramount and will enhance extraction efficiency [26,27]. Therefore, this study evaluated the reactivity of extracts obtained from different solvents of A. hendersonii. Total polyphenols comprising phenolics, flavonoids, and tannins, and total polysaccharide contents were determined. FT-IR was used to identify compound functional groups in the extracts, GC-MS and UHPLC to identify various bioactive constituents. HPLC analysis was conducted to quantify some of the active phenolic compounds in the different extracts. Antioxidant activity was determined using FRAP, DPPH, and 2,2- azino- bis (3- ethylbenzo thiazoline-6-sulphonic acid) (ABTS), and antibacterial activity was assayed against Gram-positive and Gram-negative bacteria. Enzyme inhibition activity was also tested against tyrosinase and acetylcholinesterase.
2. Results and Discussions
2.1. Polyphenol Contents
There was comparably higher significant variation (p < 0.05) in the total phenols (TPC), flavonoids (TFC), and tannin contents (TTC) in the various leaf extracts of A. hendersonii, as depicted in Figure 1. Total phenolic content was highest in the water extract (9.86 ± 0.05 mg GAE/g DW), followed by methanol leaf extract, which had phenolic content of 9.25 ± 0.03 mg GAE/g DW and ethanol extracts (6.00 ± 0.04 mg GAE/g DW). The lowest total phenolic content (0.19 ± 0.009 mg GAE/g DW) was obtained in n-hexane leaf extracts, which may not be unconnected to its non-polarity. The higher phenolic content obtained in water extract may have been attributed to higher sugar levels reacting with the Folin–Ciocalteu regent, leading to its overestimation [28], or due to the formation of some complex phenolic compound soluble in methanol or ethanol or from the presence of non-phenolic compounds such as carbohydrate or terpene in the aqueous extract [23]. It might also be due to the higher polarity of the water and comparably higher solubility of the phenols in water from its numerous hydroxyl group [5]. Methanol and ethanol or their aqueous forms are the most common solvents employed in the extraction of plant-based metabolites, and the choice of solvents has a significant role in yield and bioactive properties [23,24,25]. The methanol extract was reported to have had the highest phenolic contents in Cupressus sempervirens [29], Ceropegia spp. [30], and pomegranate peels [31], as obtained in this study.
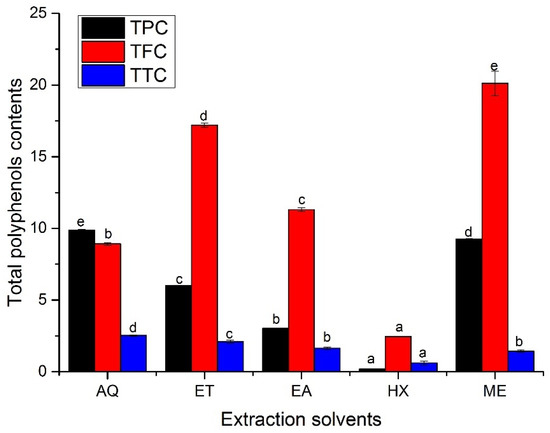
Figure 1.
Total phenols, flavonoids, and tannin contents in the A. hendersonii leaf extracts obtained using various solvents. TPC: total phenol content (mg GAE/g DW); TFC: total flavonoid content (mg QE/g DW); TTC: total tannin content (mg CE/g DW); GAE: gallic acid equivalents; QE: quercetin equivalents; CE: Catechin equivalents. AQ: Water; ET: Ethanol; EA: Ethylacetate, HX: n-Hexane; ME: Methanol. Bars with different letters are statistically different (p < 0.05).
The total flavonoid content in the leaf extracts showed higher significant diversity between the extraction solvents (p < 0.05) and comparably differed from that of total phenolic content, as shown in Figure 1. It ranged from 2.45 ± 0.00 to 20.11 ± 0.85 mg QE/g DW. Methanol leaf extract exhibited the highest total flavonoid content, followed by ethanol extract with 17.20 ± 0.14 mg QE/g DW. n-Hexane leaf extract was the least among the extracts. The low flavonoid content in the n-hexane extract was similar to Limonium delicatulum shoot extract [32], which could have been due to its non-polar characteristics [5]. In another study, the methanol extract was found to have the highest flavonoid content in Meyna spinose leaf extract, which was reflected in its higher antioxidant activity [4]. In contrast, the ethanol extract has had the highest flavonoid content in Limnophila aromatica extract compared to similar extraction solvents [23], which may not be unrelated to the flavonoid constituents in A. hendersonii.
The total tannin contents had different trends compared to the total phenolic and flavonoid contents. The highest (2.52 ± 0.05 mg CE/g DW) and lowest (0.61 ± 0.13 mg CE/g DW) tannin contents were obtained in water and n-hexane extracts, respectively. Ethanol and ethyl acetate extracts with 2.10 ± 0.10 and 1.64 ± 0.08 mg CE/g DW total tannins comparably have higher total tannin contents than methanol extract (1.43 ± 0.08 mg CE/g DW). Nevertheless, ethyl acetate and methanol extracts were statistically similar (p > 0.05). However, methanol extract was reported to have had the highest tannin content in L. delicatulum [32]. Previous studies have shown that polyphenols depend on several factors, such as species, genotypes, and environmental and edaphic factors [33]. In addition, the solvent polarity and degree of phenols polymerization and interaction play a significant role [32].
2.2. Polysaccharide Content
Polysaccharides are monosaccharides polymers joined by glycosidic bonds. They are reported to have antioxidant, antibacterial, antiviral, and antitumor properties, making them essential products in the pharmaceutical, food, and feed as well as cosmetic industries [34,35,36,37]. The polysaccharide content obtained from the various extracts is presented in Figure 2A. There was a significant difference (p < 0.05) among the extraction solvents, in the range of 3.89 ± 0.08 mg GE/g DW-145.74 ± 2.44 mg GE/g DW. Water-based leaf extract had the highest polysaccharide content, followed by methanol leaf extract (119.66 ± 2.65 mg GE/g DW) and ethanol leaf extract (88.33 ± 0.65 mg GE/g DW). The least polysaccharide content was obtained in n-hexane leaf extract. The extraction procedure was reported to affect the structural characteristics and antioxidant capacity of polysaccharides significantly. By extension, chemical composition and types of glycosides bond profoundly impact bioactivity [37]. Ethyl acetate or n-hexane may have distorted the polysaccharides configuration, leading to its low detection.
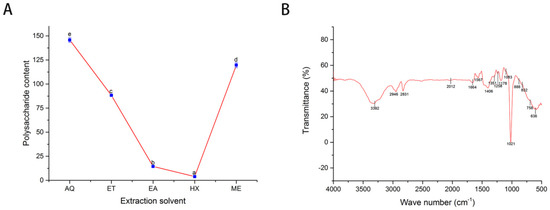
Figure 2.
Polysaccharide contents (mg GE/g DW) of the various solvents (A) and FT-IR spectroscopy analysis of methanol extracts, showing characteristics peaks corresponding to the functional group of the constituents (B). GE: Glucose equivalent; AQ: Water; ET: Ethanol; EA: Ethyl acetate, HX: n-Hexane; ME: Methanol. The different letters in A indicate statistical differences (p < 0.05).
2.3. UV-Visible and Fourier Transform Infrared Spectrophotometers Analyses
UV-Visible spectroscopy, in conjunction with FT-IR, is used to determine plant constituents’ properties [38]. The UV absorption spectra of the extracts (Figure S1) showed three characteristic peaks at 195, 231, and 271 nm, with the absorption at 195 being an expected peak of polysaccharides [39]. The absorption at 271 nm indicates the characteristic peak of flavonoids and their derivatives [38].
Fourier transform spectroscopy is crucial in structural determination of polysaccharides and reliable in obtaining information such as functional group and glycosidic bonds [20]. The result of FT-IR analysis is shown in Figure 2B. The absorption peak at 3392 cm−1 is the characteristics peak of OH groups, and peaks at 2946 and 2831 cm−1 correspond to C-H stretching; putting these together indicated the characteristics peak for polysaccharides [39]. A weak absorption peak at 2012 cm−1 showed the presence of aliphatic C-H stretching [20] and the one at 1664 cm−1 indicated a COO-carboxylate functional group [40]. The strong narrow peaks at 1567 and 1406 cm−1 represent the asymmetrical and symmetrical carboxylate anion stretching group (C=O) [34], and the few absorptions that fall between these two peaks might be due to the stretching vibration of N-H [20]. The peak absorption at 1258 cm−1 corresponded to sulfate vibration stretching of S-O [34], indicating possible sulfation of the A. hendersonii polysaccharide or other constituents. Sulfated polysaccharides have neuroprotective, anti-inflammatory, and immunomodulatory properties and protective properties against diabetic nephropathy [41]. Furthermore, the weak peak at 1351 cm−1 represents amine (C-N), and the strong peak at 1178 cm−1 indicates the presence of flavonoids [26,42].
The strong and weak absorbance at 1021 and 1083 cm−1, respectively, might be due to the pyranose ring, which has characteristic peaks between 1000–1200 cm−1 [20,40]. The absorption peaks at 888, 832, and 758 cm−1 indicate the polysaccharides’ beta glycosidic, alpha structural configuration, and the asymmetrical ring vibration, respectively [20,39,40,43]. The glycosidic bond linkage, structural branches, composition, and degree of sulfation determined the bioactivity [34].
2.4. GC-MS Analysis
GC-MS is a valuable technique important in detecting, separating, and identifying constituents from a complex mixture [5]. The GC-MS analysis of the methanol leaf extract of A. hendersonii revealed about 20 compounds, as shown in Table 1, which were detected within the retention time of 5 to 55 min (Figure S2). Four sugar components, L-glucose, D-mannose, methylated glucose (3-O-Methyl-d-glucose), and β-Lactose, were detected at 0.87–5.39%. Mannose was the highest among the sugars and third highest among all the identified constituents after only fenpropathrin (8.66%) and palmitic acid (5.86%). Pyranone accounted for 4.76%. Mannose was similarly obtained as the main monosaccharide in the extract of seaweeds Sarcodia ceylonensis and Ulva lactuca [34]. Galactose was, however, reported as the main monosaccharide in Poulownia fortunei [19]. Polysaccharide extract from U. lactuca showed significant antioxidant capacity based on ABTS and hydroxyl radical scavenging assay [34].

Table 1.
GC-MS analysis showing the identified constituents in the methanol extracts A. hendersonii leaf.
2.5. UHPLC-MS Identification of Various Constituents and HPLC-Based Quantification of Some Essential Components in Various Extracts
To further identify more secondary metabolites constituents, including flavonoids, phenols, and sugars and support the GC-MS, UHPLC-MS analysis was carried out with the methanol extract. The compounds identified (Table 2, Figure S3) indicated the presence of medicinally important bioactive components. Many of them formed components of drugs for the treatment of many ailments. Rutin, for example, has antihypertensive, antiplatelet, antibacterial, antiviral, antiprotozoal, antitumor, and anti-inflammatory activity [44]. Epigallocatechin gallate and caffeic acid have exhibited antibacterial and antitubercular potencies [45,46]. Apigenin has antidiabetic activity and results in increased secretion of insulin. It is also neuroprotective, antiamyloidogenic, antidepressive, antitumor, and anti-inflammatory, and prevents insomnia [47]. Kaempferol is cardio-protective and neuroprotective and exhibits therapeutic properties against obesity, asthma, cancer, and diabetes [48,49]. Quercetin exhibits similar prophylactic properties as rutin and apigenin. Recently, in silico and in vitro studies reported that the flavonoid can interfere with various stages of coronavirus entry and replication [50].

Table 2.
UHPLC-MS analysis of A. hendersonii methanol leaf extract.
HPLC analysis was performed to quantity some of the common flavonoid constituents in the various A. hendersonii leaf extracts. There were statistically significant differences (p < 0.05) across the different extracts. The flavonoids kaempferol, rutin, and apigenin were highest in the methanol leaf extract (Table 3). Ethanol leaf extracts had the highest quercetin level of 167.34 ± 2.59 µg/g, followed by methanol extracts with 113.49 ± 0.17 µg/g. n-Hexane leaf extracts had the lowest levels for all the flavonoid constituents quantified, followed by water-based extract. The low level of the flavonoids in water-based extract is in tandem that many flavonoids are not readily soluble in water, but they are in methanol and ethanol, which is directly reflected in the total flavonoids (Figure 1).

Table 3.
HPLC analysis of some common flavonoids constituents in A. hendersonii leaf extracts obtained using various solvents.
2.6. Antioxidant Capacity Assay
The result of the antioxidant activity of the A. hendersonii leaf extracts is presented in Figure 3. The values obtained ranged from 0.14 ± 0.00 to 4.60 ± 0.02 µmol Fe2+ per g DW, 3.19 ± 0.58 to 60.30 ± 0.52%, and 4.49 ± 0.42 to 87.64 ± 0.03% for FRAP, DPPH, and ABTS, respectively. Methanol leaf extract had the highest antioxidant activity, according to FRAP and DPPH. Ethanol leaf extract comes after methanol for DPPH with 54.96 ± 0.87% and third with 3.00 ± 0.01 µmol Fe2+ per g DW after methanol and water (4.50 ± 0.10 µmol Fe2+ per g DW) for FRAP. In both cases, n-hexane performed the least, reflecting its low polyphenols and polysaccharide contents. ABTS radical scavenging rate, however, slightly differed from the other two with results in the order Water > Methanol > Ethanol > Ethyl acetate > n-Hexane. Under acidic conditions, Fe3+-TPTZ is reduced to blue Fe2+-TPTZ, with the color changes reflecting the antioxidant capacity of the reacting substance. For ABTS, the blue color solution fades in the presence of antioxidants. DPPH reacts with antioxidants by pairing off an electron, resulting in the solution’s discoloration. The scavenging activity is reflected by the intensity of the discoloration and is directly dependent on electrons taken up [4]. The result obtained for FRAP is in line with what was obtained in Ceropegia spp., where methanol extracts gave the best radical scavenging activity [30]. It has been reported that extracts obtained from organic solvents have displayed more antioxidant power than water-based extracts obtained from many plant species. In a study on Hibiscus cannabinus leaf extracts, the water-based extract showed the lowest reducing power which was linked to the low solubility of the bioactive constituents in water [26] and this conforms with the result of FRAP and DPPH obtained in this study.
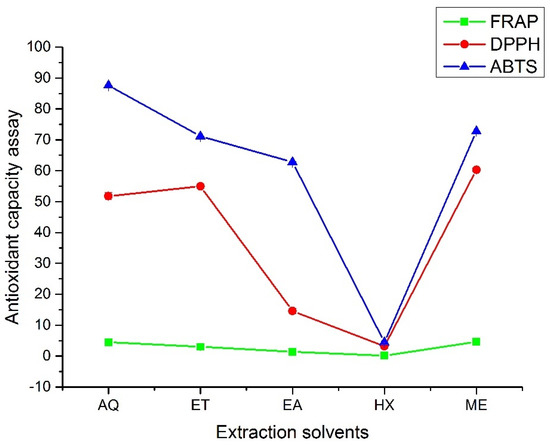
Figure 3.
Antioxidant capacity assays of the A. hendersonii leaf extracts obtained using various solvents. FRAP: Ferric Reducing Antioxidant Potential (µmol Fe2+ per g DW); DPPH: 2,2-Diphenyl-1-picrylhydrazyl (%); ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (%); AQ: Water; ET: Ethanol; EA: Ethylacetate, HX: n-Hexane; ME: Methanol.
The A. hendersonii extract was shown to contain metabolites that cut across the phenols, flavonoids, and polysaccharides (Table 1 and Table 2), most of which possessed strong antioxidant activities. For example, Mannose-6-phosphate protects proteins in the dermis scaffold against oxidation and degradation [51]. Polysaccharides, comprising mannose, glucose and galactose from Siraitia grosvenorii, decreased the reactive oxygen species as well as the percentage of apoptotic and necrotic cells in H2O2 oxide injury PC12 cells [52], and these components were similarly identified in the A. hendersonii extract. Injection of L-fucose in rabbits resulted in increased production of vitamin C and promoted body defense against oxidative stress [53]. Quercetin is currently used in various pharmaceuticals preparation as an antioxidant and in the treatment of age-associated diseases [54]. Its mechanism of action involves the regulation of glutathione (GSH). Its phenyl ring hydroxyl groups bind to amino acid residues of the clinically important enzymes such as butyrylcholinesterase, resulting in a strong inhibitory effect [55]. Pretreatment with quercetin protected hippocampal CA1 pyramidal neurons from ischemic injury in gerbils by promoting the expression of endogenous antioxidants [56]. This further supported the antioxidant properties of the various extracts, as established in this study. Thus, the antioxidant capacity results obtained showed that A. hendersonii has excellent medicinal potential and would be a candidate for the pharmaceutical, food, and cosmetic industries.
2.7. Correlation Analysis of Bioactive Constituents and Antioxidant Capacity
Correlation analysis within the various constituents and with the FRAP, DPPH, and ABTS radical scavenging assays were computed to understand the strength of the relationship. FRAP (r = 0.99), DPPH (r = 0.92), and ABTS (r = 0.87) strongly correlated with total phenolic content as presented in Table 4. Flavonoid content showed a moderate correlation with FRAP (r = 0.677), DPPH (0.79), and ABTS (r = 0.67) compared to the total phenolic as similarly obtained in total tannins. Interestingly, polysaccharide contents correlated strongly with FRAP, DPPH, and ABTS with correlation coefficient values of 0.97, 0.92, and 0.79, respectively. A strong correlation was previously reported between polysaccharide content and total phenolic with antioxidant capacity [3].

Table 4.
Pearson correlation coefficient of the A. hendersonii bioactive constituents and antioxidant capacities.
Similarly, tannin content from leaves extract of Ruta chalepensis showed a higher positive correlation (0.95–0.99) with antioxidant capacity, and total flavonoids from the plant’s flower also correlated with free radical scavenging activity [57]. Flavonoid contents appeared to be the dominant form of polyphenols in the species, as similarly reported in guava [58] and L. aromatica [23]. Extraction solvents have significantly affected polyphenols levels, with methanol being the best solvent for flavonoids and phenolic than ethanol, ethyl acetate, or n-hexane. However, where condensed tannin content is the sole target, ethanol proved the best alternative, followed by methanol or ethyl acetate.
Antioxidant capacity showed a significant and positive correlation with polyphenols and polysaccharide contents, as most of these constituents are proven scavengers of free radicals. Antioxidants enhance the body’s defense system by shielding biomolecules against reactive oxygen species. The reactive oxygen species stressed the system, leading to diseases such as cardiovascular, cancer, liver cirrhosis, etc. Natural antioxidants, such as plant-based antioxidants, are always the best option compared to synthetic ones, which may have some side effects. Many studies have linked plants’ antioxidants and other medicinal properties or their extracts to their polyphenols, principally phenol and flavonoids [4]. The antioxidant property of A. hendersonii leaf extracts could have been due to multiple effects of all the bioactive components investigated, as each has a strong antioxidant capacity [3,23,37]. This confirms the species’ excellent nutraceuticals and pharmaceutical potential. This study might be the first to delve into assessing the polysaccharide content of this species. [4]
2.8. Antimicrobial Activity Assay
The assay result revealed the antagonistic effect of the ethanol, ethyl acetate, and methanol leaf extracts against the two bacterial strains, as given in Table 5. The three different extracts were statistically similar (p > 0.05) against the Escherichia coli, with the zone of inhibition of 10.33 ± 0.32, 9.15 ± 0.51, and 10.15 ± 0.60 mm, respectively. However, a significant difference (p < 0.05) was obtained against the Staphylococcus aureus, with ethanol leaf extract exhibiting the highest effect, having a 10.96 ± 0.15 mm zone of inhibition followed by methanol leaf extract with 8.90 ± 0.06 mm, as depicted in Figure S4. Water- and n-hexane-based extracts had no antibacterial effects against the two bacterial strains, which may not be unconnected to the significantly low flavonoid contents, as seen in Figure 1 and Table 3. The result is indicative of a positive correlation between flavonoid contents of the A. hendersonii extracts and antibacterial activity consistent with the literature [59].

Table 5.
Antibacterial effect of various leaf extracts of A. hendersonii against E. coli and S. aureus.
The higher antibacterial effect of ethanol extract may have been due to the additional effect of the solvent, as its aqueous form is often used as sterilizing agent [60] and as seen in the negative control. Tannin, which has strong bactericidal activity [61], might have strengthened the antibacterial effect of the extract. It may have also been because bioactive components responsible for the antibacterial effect were readily soluble in the ethanol but not in the other solvents [62]. The antibacterial inhibition might have occurred due to extracts interacting with extracellular microbial enzymes, inhibiting substrates required for the bacterial growth and development or inhibiting metabolism via deprivation of oxidative phosphorylation [61]. Though the positive control displayed a higher effect against the bacterial strains with 12.15 ± 0.06 and 11.10 ± 0.13 mm zones of inhibition for E. coli and S. aureus, respectively, the plant extracts are still the best alternative because of the rapid emergence of resistance strains [63]. Ethanol leaf extract of Hibiscus cannibinus was reported to have imparted the highest antibacterial effect against Bacillus cereus, Pseudomonas, Salmonella, and S. epidermis; at the same time, methanol extract displayed the best effect against B. subtilis and S. auerus [26]. The differential antibacterial effects against the two different bacteria might be strain-dependent [57]. The overall result, thus, displayed great antibacterial potentials of the extracts and the plant, by extension.
2.9. Enzyme Inhibition Assay
Tyrosine inhibitors are being targeted as a treatment for Parkinson’s disease. Tyrosine partakes in neuromelanin formation in the human brain leading to dopamine neurotoxicity and neurodegeneration [64]. They also play a significant role in melanin biosynthesis by protecting the skin against ultraviolet light and reactive oxygen species [65]. There is a need for alternative drugs to stop these associated neurodegenerative diseases, with plants being an attractive source [64]. The tyrosinase inhibition assay showed great potential for the A. hendersonii in tyrosinase inhibition. There were, however, significant differences among the extracts, with ethanol extract exhibiting the highest inhibition percentage (62.30 ± 1.11%), followed by methanol (55.97 ± 0.89%) and water (32.10 ± 0.41) extracts. Hexane and ethyl acetate displayed the lowest inhibition percentage (Table 6). The higher variation obtained for the different extracts may be explained by their polyphenols contents, which are consistent with the result. Constituents with strong antioxidant activity tend to have good tyrosinase inhibition [26]. This may be the first report on the tyrosinase inhibition activity of the extracts, and as such, provides a basis and reason to explore many other clinically essential enzymes.

Table 6.
Enzyme inhibition percentage of various A. hendersonii leaf extracts.
AChE inhibitors are also used to treat neurodegenerative diseases such as Alzheimer’s, prevalent in older adults. The available synthetic drugs used to treat such ailments are not without side effects and limited bioavailability [66]. In addition, such synthetic drugs do not stop the diseases but slow their progression via symptomatic treatment, which calls for better alternatives, with the plant being an attractive source [64]. The result obtained for AChE inhibition of the various A. hendersonii extracts followed similar trends with the tyrosinase inhibition activity, with ethanol extract displaying the highest effect (30.65 ± 0.98%) followed by methanol extract (28.60 ± 0.04%). Nevertheless, the two extracts were statistically similar (p < 0.05). The two results indicated that A. hendersonii is a potential candidate for inhibition of clinically important enzymes, and the variation in the extracts relates to the component bioactive substance.
2.10. Multivariate Analyses
Multivariate analyses were carried out to understand further the overall variations obtained among the solvents’ reactivity. The univariate analysis revealed a tremendous variability in the bioactive constituents and bioactivities. Principle component analysis (PCA) and hierarchical cluster analysis (HCA) were computed to explain the correlation between the various components. PCA was obtained using the Kaiser Criterion, and the Eigenvalue is given in Figure 4A. TPC, TFC, TTC, the flavonoids apigenin, kaempferol, quercetin, rutin, and the FRAP, DPPH, and ABTS accounted for the variations in PC1 (63.44%). PC2 (16.39%) accounted for the variations in the PSC (Figure 4B). The considerable variability among the extraction solvents can be seen from the scatter plot, with methanol, ethanol, and ethyl acetate seeming very close. The result of HCA is presented in Figure 4C, which shows all the extracts clustering into five unique clusters in tandem with PCA proving that the extraction solvents have tremendously affected the bioactive constituents and activities. The result is similar to the findings on Sphaeranthus indicus [10]. Therefore, in selecting extraction solvents for plant bioactive components, the target constituents and their activities should be considered to preserve quality and maintain chemical structure [10].
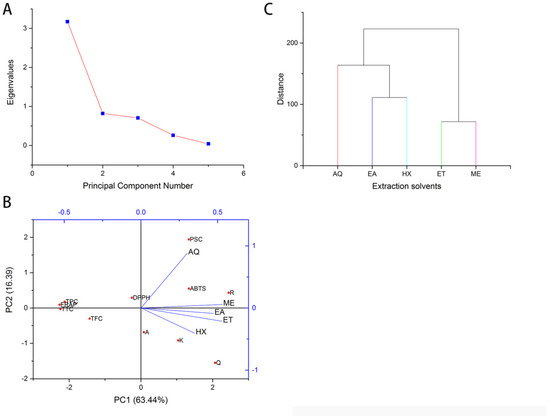
Figure 4.
Principal component analysis and hierarchical cluster analysis of the various extracts, constituents, and bioactivities. (A) Eigenvalues of the variances. (B). Contribution of bioactive constituents to the principal components 1 and 2. (C). Dendogram of cluster analysis. FRAP: Ferric Reducing Antioxidant Potential; DPPH: 2,2-Diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (%); AQ: Water; ET: Ethanol; EA: Ethyl acetate, HX: n-Hexane; ME: Methanol; A: apigenin; R: rutin; Q: quercetin; K: kaempferol.
3. Materials and Methods
3.1. Chemicals
Hexane and methanol were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China and ethyl acetate and ethanol from Hunan Huihong Reagent Co., Ltd. (Hunan, China). Folin-Ciocalteu reagent and vanillin were purchased from Coolaber Science and Technology, Beijing, China. DPPH, ABTS, and T-AOC (FRAP), apigenin, (+)-catechin, gallic acid, glucose, kaempferol, quercetin, luteolin, naringenin, and rutin, were all purchased from Solarbio Life Sciences, Beijing, China. HPLC grade acetic acid and acetonitrile were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China).
3.2. Plant Material and Extraction Procedure
A. hendersonii leaf was obtained from the Xinjiang Western Centre of the Chinese Academy of Agricultural Sciences, Changji, Xinjiang province. The leaf was freeze-dried and pulverized using a mill grinder and sieved to a fine powder.
According to a previous report [3], five different solvents comprising ethanol, ethyl acetate, n-hexane, methanol, and water were used for the extraction process. The sample-to-solvent ratio was 1:10. The mixture was refluxed for 2 h at 85 °C, and the supernatant was collected and centrifuged for 10 min at 10,000 rpm. The extract was then evaporated to dryness at reduced pressure or lyophilized and stored at −20 °C for subsequent analysis.
3.3. Determination of Polyphenols Contents
Total phenolic content was determined using the Folin–Ciocalteau method [67] as reported by Medini et al. [32], with slight modifications. In total, 1.5 mL 20% Folin–Ciocalteu reagent was added to 0.2 mL extracts (10 mg/mL), mixed, and allowed to stay for 5 min at room temperature. Next, 4 mL 7% sodium carbonate (Na2CO3) and distilled water to a final volume of 10 mL were added, and the mixture was kept for 90 min in the dark at room temperature. Absorbance was then recorded at 760 nm using a spectrophotometer. The gallic acid standard curve (0.05–0.3 mg/mL, R2 = 0.99) was used to express the total phenolic as milligram gallic acid equivalent per gram sample dry weight (mg GAE/g DW).
Total flavonoid content was determined using Aluminum Chloride (AlCl3) calorimetric method as reported by Rguez et al. [29] with some modifications. In total, 0.2 mL extracts (10 mg/mL) were diluted with 4 mL distilled water followed by the addition of 0.3 mL 5% NaNO2 and kept for 5 min at room temperature. Then, 0.3 mL 10% AlCl3 was added, and the mixture was kept for another 6 min. Later, 2 mL 4% sodium hydroxide was added, and the final volume was filled up to 10 mL. Absorbance was measured at 510 nm using a spectrophotometer. The quercetin standard curve (0.025–0.25 mg/mL, R2 = 0.99) was used to express the total flavonoids as milligram quercetin equivalent per gram sample dry weight (mg QE/g DW).
Total condensed tannins (proanthocyanidins) were determined according to the procedure reported by Rguez et al. [29] with few modifications. Next, 0.1 mL extracts (10 mg/mL) were diluted with 1 mL distilled water, and 6 mL 4% vanillin-methanol (w/v) added, followed by 3 mL concentrated sulphuric acid. Absorbance was recorded at 500 nm using a spectrophotometer, and the (+)-catechin standard curve (0.05–0.3 mg/mL, R2 = 0.99) was used to compute the total condensed tannins as milligram catechin equivalent per gram dry weight (mg CE/g DW).
3.4. Determination of Polysaccharide Contents
The polysaccharide content was determined using phenol-sulfuric acid calorimetry according to the literature [68]. A total of 1 mL 5% (w/v) phenol was added to 2 mL of the diluted extracts, followed by 5 mL concentrated sulphuric acid, which was mixed and boiled for 15 min. It was allowed to cool down to room temperature, and absorbance was taken at 490 nm using a spectrophotometer. A d-glucose standard curve (0.05–0.3 mg/mL, R2 = 0.99) was used to express the polysaccharide content as milligram glucose equivalent per gram sample dry weight (mg GE/g DW).
3.5. UV-Visible and Fourier Transform Infrared Spectrophotometers Analyses
The extract was diluted to a 1 mg/mL final concentration and used for UV spectra. The UV-visible spectrum was obtained from 190–400 nm using a UV-3600 Plus UV-VIS-NIR Spectrophotometer (Shimadzu, Japan).
The structural-functional group of compounds in the extract was obtained using IRSpirit Fourier Transform Infrared spectrophotometer (Shimadzu, Japan). A drop of the extract was placed on the diamond crystal to form a thin film. The spectral scanning was taken at the frequency range of 4000 to 400 cm−1 at 4 cm−1 at room temperature.
3.6. Gas Chromatography-Mass Spectrometry Analysis
Bioactive constituents in the methanol extract of the A. hendersonii leaf were obtained using 7890A Agilent Technology Gas chromatography-mass spectrometry (GC-MS). The instrument was equipped with an HP-5ms capillary column (30 m × 0.25 mm × 0.25 μm) with helium (purity > 99.99%) as carrier gas. The flow rate was set at 1.2 mL/min, and the sample injection volume was 1 μL with 1:5 diversion ratios. The injection and detection temperatures were set at 250 °C and 280 °C, respectively. The initial temperature was maintained at 60 °C for 2 min, before it was increased to 280 °C at 5 °C /min and kept for 9 min. The ionization mode was 70 eV with a scanning range of 40–400 amu, a scanning rate of 3.99, and a 3 min solvent delay. The fragmentation pattern was compared to the NIST database, and percentages were computed from the chromatogram peak area.
3.7. UHPLC-MS Analysis
UHPLC-MS analysis was performed for the methanol extract using ACQUITY UHPLC coupled with Triple TOF 5600 systems in both positive and negative modes, as previously reported [69], and the details are given in the Supplementary. The tentative constituents were identified by METLIN metabolite and Human Metabolome databases.
3.8. HPLC-Based Quantitative Identification of Flavonoids Constituents
Higher performance liquid chromatography (HPLC) analysis was used to quantify some common flavonoid constituents in the different extracts. The extracts were filtered using a 0.45 µm filter into small vials for HPLC analysis. The analysis was performed on SHIMADZU LC-20A, column Shim-pack GIST C18 5 µm (4.6 I.D. × 250 mm) with the following conditions: flow rate 0.8 mL/min and 10 µL injection volume at 25 °C. The mobile phases were 0.04% acetic acid in water (mobile phase A) and 0.04% acetic acid in acetonitrile (mobile phase B). The column was initially developed isocratically with 5% solvent B, followed by a linear gradient from 5 to 95% for 20 min. It was then isocratically washed with 95% B for 2 min, followed by 95 to 5% linear gradient for 0.1 min. The column was then developed isocratically with 5% B for 5 min [70]. Chromatogram was obtained at 330 nm, and apigenin, kaempferol, naringenin, luteolin, quercetin, and rutin were used as flavonoid standards for calibration.
3.9. Antioxidant Capacity
3.9.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
DPPH assay was conducted following the report of Liang et al. [15] with slight modification. A total of 0.5 mL of samples of various concentrations were mixed with 2 mL of 1 mM DPPH in an ethanol working solution and allowed to stay for 30 min at room temperature. Absorbance was obtained at 515 nm using a spectrophotometer, and radical scavenging capacity was expressed in the form of an inhibition percentage as follows:
where Ab = the absorbance of the DPPH working solution without the extract and Ae = absorbance of the reaction mixture.
Inhibition (%) = (Ab − Ae)/Ab) × 100
3.9.2. 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-sulphonic Acid) (ABTS) Radical Scavenging Capacity Assay
A radical scavenging capacity assay was conducted following the method reported by Shang et al. [37] with slight modifications. Different sample extract (0.1 mL) was mixed with 1 mL ABTS working solution, kept in the dark, and allowed to stay for 6 min. Absorbance was obtained at 405 nm. Vitamin C was used as a positive control, and all tests were repeated three times. Radical scavenging capacity was computed using the above equation (Equation (1)), with the absorbance of ABTS working solution as blank.
3.9.3. Ferric Reducing Antioxidant Power (FRAP) Total Antioxidant Capacity Assay
In acidic conditions, the Fe3+-TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) are reduced to Fe2+-TPTZ, resulting in a change of the colorless former mixture to blue, which reflects the antioxidant capacity of the sample used to bring about the color changes. A FRAP assay was conducted following the report of Yi et al. [68] with some modifications. The working solution was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM Iron (III) chloride in a 7:1:1 ratio. Next, 900 µL of the working solution was mixed with 30 µL extracts followed by 90 µL distilled water to obtain a final volume of 1 mL. The mixture was thoroughly mixed and allowed to stay for 10 min at room temperature. Absorbance was measured at 593 nm against a blank that contained the working solution without the extract. FeSO4 standard curve (0.05–0.00156 µmol/mL, R2 = 0.99) was used and the results expressed as µmol Fe2+/g DW. The entire test was replicated three times.
3.10. Antimicrobial Activity Assay
The antimicrobial assay was carried out using the disc diffusion method according to previous reports [3] on Luria Bertani agar plates. Two bacterial strains, Escherichia coli, ATCC8739—a Gram-negative strain—and Staphylococcus aureus, ATCC6538—a Gram-positive strain—were used. The bacterial inoculums suspension of each (100 µL, 1 × 107 CFU) was uniformly swabbed on the plates and 10 µL of each of the extracts (10 mg/mL), later loaded on an individual sterile disc (6 mm), allowed to rest for 30 min at room temperature, followed by incubation at 37 °C for 18 h. Ampicillin (100 µM) was used as positive control and respective solvents used in the extraction process were used as a negative control. The circular zone formed around the disc was considered a zone of inhibition due to the extract and was measured using a Vernier caliper. All tests were replicated three times.
3.11. Enzyme Inhibition Assay
Enzyme inhibition assay was carried out for all the extracts against acetylcholinesterase (AChE) and tyrosinase. AChE inhibition activity was determined as reported by Orhan et al. [66], and the result was expressed as a percentage using the equation:
where ‘Ac’ is the absorbance of the control without the sample and ‘As’ is the absorbance of the mixture containing the sample.
For tyrosinase inhibition activity, L-tyrosine (100 µL, 5 mM), sodium phosphate buffer (20 µL, 0.1 M, pH 6.8), and the sample (40 µL) were loaded onto a 96-well microplate. This was followed by adding tyrosinase (200 units/mL) and incubation at 37 °C for 20 min [71]. Absorbance was obtained at 450 nm using a microplate reader. A blank containing all the components except for the enzyme was also recorded and subtracted from the samples [72]. The tyrosinase inhibition was expressed as a percentage using the above equation.
3.12. Statistical Analysis
All the assays were repeated three times, and the experimental data obtained were expressed as mean ± standard error of the mean (n = 3). The means of the total polyphenols, polysaccharide contents, and the results of antioxidant capacity assay were subjected to one-way analysis of variance using GenStat 17th edition and means separated using the Student–Newman–Keul method. Correlation analysis was performed using Statistix analytical software version 8.3. Principal component and hierarchical cluster analyses were carried out using Origin Pro for further understanding the bioactive constituents’ variability and their activities.
4. Conclusions
This study showed all the different extracts obtained with the various solvents to have a substantive amount of polyphenols and significant antioxidant properties, except for n-hexane, which has a minimal amount of polyphenols and displayed the lowest antioxidant capacity. Polysaccharide content in n-hexane and ethyl acetate extracts was comparably smaller than that of other solvents, reflecting its non-polar nature and indicating that polar solvents might be the best option for recovering polysaccharides in A. hendersonii. Methanol gave a better result with its extracts based having the highest flavonoids and comparably higher phenolic and showed the best antioxidant capacity. A strong positive correlation was obtained between the antioxidant capacity assays and polyphenols and polysaccharide contents of the extracts indicating the total impacts of the constituents in conferring the medicinal significance of the A. hendersonii. Furthermore, HPLC analysis of some of the flavonoid constituents revealed apigenin, kaempferol, quercetin, and rutin in substantive amounts. Polysaccharides mannose, glucose, and lactose were identified by GC-MS and UHPLC-MS and supported with FT-IR analysis. The extracts obtained using ethanol, methanol, and ethyl acetate showed antibacterial capability against E. coli and S. aureus, while water-and n-hexane-based extracts displayed no antibacterial property. Promising enzyme inhibition activity against tyrosinase and acetylcholinesterase, more prominent in the ethanol and methanol extracts, was established. This study might be the first to report the enzyme inhibition activity of the A. hendersonii extracts. This study, thus, ascertains the medicinal potentiality of A. hendersonii. In addition to polyphenols, the polysaccharides are also significant components in the species and may be exploited for future applications in pharmaceuticals and food industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11151964/s1, Figure S1: UV-visible spectrophotometer analysis; Figure S2: GC-MS absorption spectrum; Figure S3: UHPLC-MS spectra of the identified constituents (neg. ion mode); Figure S4: Antibacterial effects of ethanol extract of A. hendersonii against Staphylococcus aureus (A) and Escherichia coli (B).
Author Contributions
Conceptualization, A.Z. and A.S.A.; Data curation, X.H., G.G., X.F. and C.Y.; Formal analysis, A.S.A., X.H., P.C. and J.C.; Investigation, A.S.A., X.H.; Methodology, A.S.A. and Z.M.B.; Writing an original draft, A.S.A. and X.H.; Writing review and editing, X.H., Z.M.B., G.G. and A.Z.; Supervision, A.Z. and G.G.; Validation, K.C. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Science (CAAS-ASTIP-IBFC04) and the Science and Technology Project of Changsha City (kq2107017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piątczak, E.; Owczarek, A.; Lisiecki, P.; Gonciarz, W.; Kozłowska, W.; Szemraj, M.; Chmiela, M.; Kiss, A.K.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Identification and quantification of phenolic compounds in Salvia cadmica boiss. and their biological potential. Ind. Crops Prod. 2021, 160, 113113. [Google Scholar] [CrossRef]
- Li, Q.; Jia, E.; Yan, Y.; Ma, R.; Dong, J.; Ma, P. Using the strategy of inducing and genetically transforming plant suspension cells to produce high value-added bioactive substances. J. Agric. Food Chem. 2022, 70, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Birhanie, Z.M.; Xiao, A.; Yang, D.; Huang, S.; Zhang, C.; Zhao, L.; Liu, L.; Li, J.; Chen, A.; Tang, H.; et al. Polysaccharides, Total phenolic, and flavonoid content from different kenaf (Hibiscus cannabinus L.) genotypes and their antioxidants and antibacterial properties. Plants 2021, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa roxb., an Indian medicinal plant. Chin. J. Nat. Med. 2013, 11, 149–157. [Google Scholar] [CrossRef]
- Adnan, M.d.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.-H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) leaves and seed as a potential source of the bioactive compounds: Effects of various extraction solvents on biological properties. Life 2020, 10, 223. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella-Foenum Graecum) seed oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Tavakoly, R.; Maracy, M.R.; Karimifar, M.; Entezari, M.H. Does fenugreek (Trigonella Foenum-Graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur. J. Integr. Med. 2018, 18, 13–17. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica Subsp. smyrnaea for the management of oxidative stress, alzheimer’s disease, hyperglycemia, and melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Nadeem, M.F.; Shoaib Khan, H.M.; Sarfraz, M.; Saleem, H.; Khurshid, U.; Locatelli, M.; Ashraf, M.; Akhtar, N.; Zainal Abidin, S.A.; et al. Phytopharmacological evaluation of different solvent extract/fractions from Sphaeranthus indicus L. flowers: From traditional therapies to bioactive compounds. Front. Pharmacol. 2021, 12, 708618. [Google Scholar] [CrossRef]
- Ma, M.; Hong, C.; An, S.; Li, B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J. Agric. Food Chem. 2003, 51, 2390–2393. [Google Scholar] [CrossRef]
- Morikawa, T.; Imura, K.; Miyake, S.; Ninomiya, K.; Matsuda, H.; Yamashita, C.; Muraoka, O.; Hayakawa, T.; Yoshikawa, M. Promoting the effect of chemical constituents from the flowers of Poacynum hendersonii on adipogenesis in 3T3-L1 cells. J. Nat. Med. 2012, 66, 39–48. [Google Scholar] [CrossRef]
- Abubakar, A.S.; Gao, G.; Zhu, A. Apocynum venetum, a bast fiber plant with medicinal significances and potentials for drought tolerance and phytoremediation studies—A review. J. Nat. Fibers 2021, 1–13. [Google Scholar] [CrossRef]
- Chan, C.-O.; Lau, C.-C.; Ng, Y.-F.; Xu, L.-J.; Chen, S.-B.; Chan, S.-W.; Mok, D. Discrimination between leave of Apocynum venetum and its adulterant, A. pictum based on antioxidant assay and chemical profiles combined with multivariate statistical analysis. Antioxidants 2015, 4, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Yue, W.; Li, Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef]
- Gao, G.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Liu, N.; Yu, C.; Zhu, A. Genomic survey, transcriptome, and metabolome analysis of Apocynum venetum and Apocynum hendersonii to reveal major flavonoid biosynthesis pathways. Metabolites 2019, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Kumar, S.; Pandey, A.K. Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci. World J. 2013, 2013, 292934. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zou, P.; Jing, C.; Xu, Z.; Zhou, S.; Li, Y.; Zhang, C.; Yuan, Y. Chemical characterization and bioactivities of polysaccharides from Apocynum venetum Leaves extracted by different solvents. J. Food Meas. Charact. 2020, 14, 244–253. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, X.; Zhu, L.; Xu, Y.; Cui, W.; He, X.; Wei, K.; Zhu, R. A polysaccharide found in Paulownia fortunei Flowers can enhance cellular and humoral immunity in chickens. Int. J. Biol. Macromol. 2019, 130, 213–219. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Wang, Z. Extraction optimization of polysaccharides from corn silk and their antioxidant activities in vitro and in vivo. Front. Pharmacol. 2021, 12, 738150. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa Mill. by-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Wijekoon, M.M.J.O.; Bhat, R.; Karim, A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal. 2011, 24, 615–619. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Jess Ong, W.T.; Nyam, K.L. Effect of various solvents on the pulsed ultrasonic assisted extraction of phenolic compounds from Hibiscus cannabinus L. Leaves. Ind. Crops Prod. 2019, 140, 111708. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Ramdane, F.; Essid, R.; Mkadmini, K.; Hammami, M.; Fares, N.; Mahammed, M.H.; El Ouassis, D.; Tabbene, O.; Limam, F.; Ould Hadj, M.D. Phytochemical composition and biological activities of Asteriscus graveolens (Forssk) extracts. Process Biochem. 2017, 56, 186–192. [Google Scholar] [CrossRef]
- Rguez, S.; Essid, R.; Adele, P.; Msaada, K.; Hammami, M.; Mkadmini, K.; Fares, N.; Tabbene, O.; Elkahoui, S.; Portelli, D.; et al. Towards the use of Cupressus sempervirens L. organic extracts as a source of antioxidant, antibacterial and antileishmanial biomolecules. Ind. Crops Prod. 2019, 131, 194–202. [Google Scholar] [CrossRef]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P.R.; Dixit, G.B. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from western ghats of India. S. Afr. J. Bot. 2013, 88, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of Pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi Pirbalouti, A.; Siahpoosh, A.; Setayesh, M.; Craker, L. Antioxidant activity, total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iran. J. Med. Food 2014, 17, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Luiz, C.; da Rocha Neto, A.C.; Franco, P.O.; Di Piero, R.M. Emulsions of essential oils and aloe polysaccharides: Antimicrobial activity and resistance inducer potential against Xanthomonas fragariae. Trop. Plant Pathol. 2017, 42, 370–381. [Google Scholar] [CrossRef]
- Nazeam, J.A.; Gad, H.A.; Esmat, A.; El-Hefnawy, H.M.; Singab, A.-N.B. Aloe arborescens polysaccharides: In vitro immunomodulation and potential cytotoxic activity. J. Med. Food 2017, 20, 491–501. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Singh, R.; Mendhulkar, V.D. FTIR studies and spectrophotometric analysis of natural antioxidants, polyphenols and flavonoids in Abutilon indicum (Linn) sweet leaf extract. J. Chem. Pharm. Res. 2015, 7, 205–211. [Google Scholar]
- Peng, L.; Huang, Y.; Tan, W.; Wei, Z.; Zhang, L. Physi-Chemical Property Research of Polysaccharides from Pomegranate flowers. Agric. Sci. 2021, 12, 59–67. [Google Scholar] [CrossRef]
- Han, Q.; Yu, Q.-Y.; Shi, J.; Xiong, C.-Y.; Ling, Z.-J.; He, P.-M. Structural characterization and antioxidant activities of 2 water-soluble polysaccharide fractions purified from tea (Camellia sinensis) flower. J. Food Sci. 2011, 76, C462–C471. [Google Scholar] [CrossRef]
- Geng, L.; Zhang, Q.; Wang, J.; Jin, W.; Zhao, T.; Hu, W. Glucofucogalactan, a heterogeneous low-sulfated polysaccharide from Saccharina japonica and its bioactivity. Int. J. Biol. Macromol. 2018, 113, 90–97. [Google Scholar] [CrossRef]
- Oladunmoye, M.; Ayantola, K.; Agboola, A.; Olowe, B.; Adefemi, O. Antibacterial and ftir spectral analysis of methanolic extract of Gliricidia sepium Leaves. J. Adv. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Pawar, H.A.; Lalitha, K.G. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. 2014, 65, 167–175. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–479. ISBN 978-0-12-813820-5. [Google Scholar]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial activity of Pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.; Ghosh, S.; Ray, R.; Hazra, B. Polyphenolic secondary metabolites synergize the activity of commercial antibiotics against clinical isolates of β-lactamase-producing Klebsiella pneumoniae. Phytother. Res. 2016, 30, 272–282. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review: Chemo-preventive and therapeutic effect of kaempferol: A comprehensive. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Commun. 2020, 15, 1934578X2097629. [Google Scholar] [CrossRef]
- Meunier, M.; Chapuis, E.; Lapierre, L.; Auriol, P.; Paulus, C.; Elbaum, B.; Don Simoni, E.; Sandré, J.; Auriol, D.; Scandolera, A.; et al. Mannose-6-phosphate complex and improvement in biomechanical properties of the skin. J. Cosmet. Dermatol. 2021, 20, 1598–1610. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Pan, L.-C.; Zhang, L.-J.; Yin, Y.; Zhu, Z.-Y.; Sun, H.-Q.; Liu, C.-Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Al-Safi, K.A.; Ahmed, B.A. Evaluation the antioxidant effect of α-L-fucose injection into Rabbit periodontium. J. Baghdad Coll. Dent. 2013, 25, 119–124. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Park, J.; Ahn, J.; Cho, J.; Kim, I.; Lee, J.; Won, M.-H.; Lee, C.-H.; Hwang, I.; Kim, J.-D.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Mahboubi, A.; Asgarpanah, J.; Sadaghiyani, P.N.; Faizi, M. Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. Pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases. BMC Complement. Altern. Med. 2015, 15, 366. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.S.; Pudake, R.N. Sterilization procedure and callus regeneration in black turmeric (Curcuma caesia). Agric. Sci. Dig.-Res. J. 2019, 6, 4714. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Salem, W. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef] [Green Version]
- Salisu, I.; Abubakar, A.; Abdullahi, M. A novel biosynthesis, characterization and antimicrobial activity of silver nanoparticles using leaves extract of Aloe vera plant. Int. J. Sci. Res. IJSR 2014, 3, 311–314. [Google Scholar]
- Yılmaz, B.S.; Altun, M.L.; Orhan, I.E.; Ergene, B.; Citoglu, G.S. Enzyme inhibitory and antioxidant activities of Viburnum tinus L. relevant to its neuroprotective potential. Food Chem. 2013, 141, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Voştinaru, O.; Păltinean, R.; Mihali, C.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Mocan, A.; Crişan, O.; Nicula, C.; et al. Chemical composition, diuretic, and antityrosinase activity of traditionally used romanian cerasorum stipites. Front. Pharmacol. 2021, 12, 647947. [Google Scholar] [CrossRef]
- Orhan, I.E.; Kucukboyaci, N.; Calis, I.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis freitag, vural & adigüzel and molecular modeling studies on N-acetyltryptophan. Phytochem. Lett. 2017, 20, 373–378. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Yi, Y.; Hua, H.; Sun, X.; Guan, Y.; Chen, C. Rapid determination of polysaccharides and antioxidant activity of Poria cocos using near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 240, 118623. [Google Scholar] [CrossRef]
- Feng, X.; Gao, G.; Yu, C.; Zhu, A.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Chen, P. Transcriptome and metabolome analysis reveals anthocyanin biosynthesis pathway associated with ramie (Boehmeria nivea (L.) Gaud. leaf color formation. BMC Genom. 2021, 22, 684. [Google Scholar] [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S.; et al. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 2017, 8, 1975. [Google Scholar] [CrossRef]
- Liang, C.; Lim, J.-H.; Kim, S.-H.; Kim, D.-S. Dioscin: A synergistic tyrosinase inhibitor from the roots of Smilax china. Food Chem. 2012, 134, 1146–1148. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).