Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin
Abstract
:1. Introduction
2. Results and Discussion
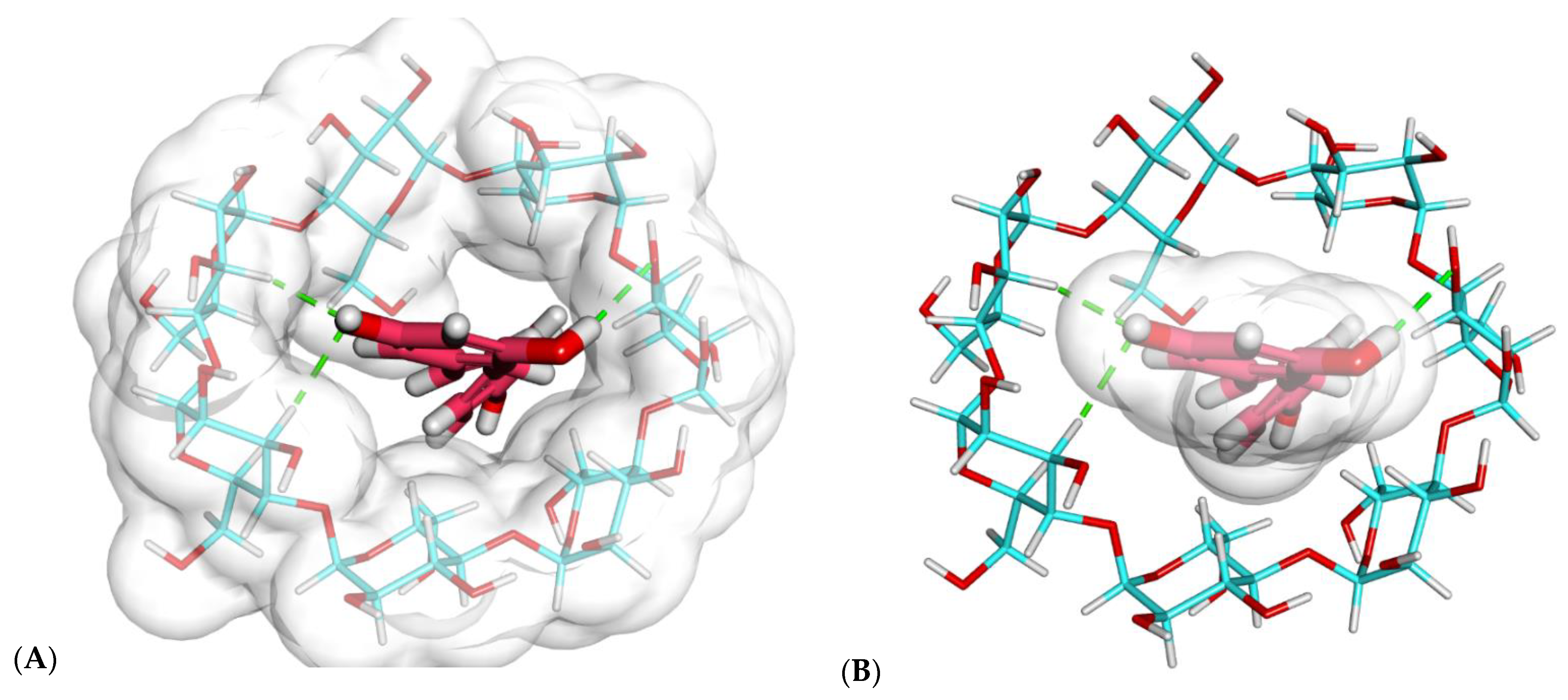
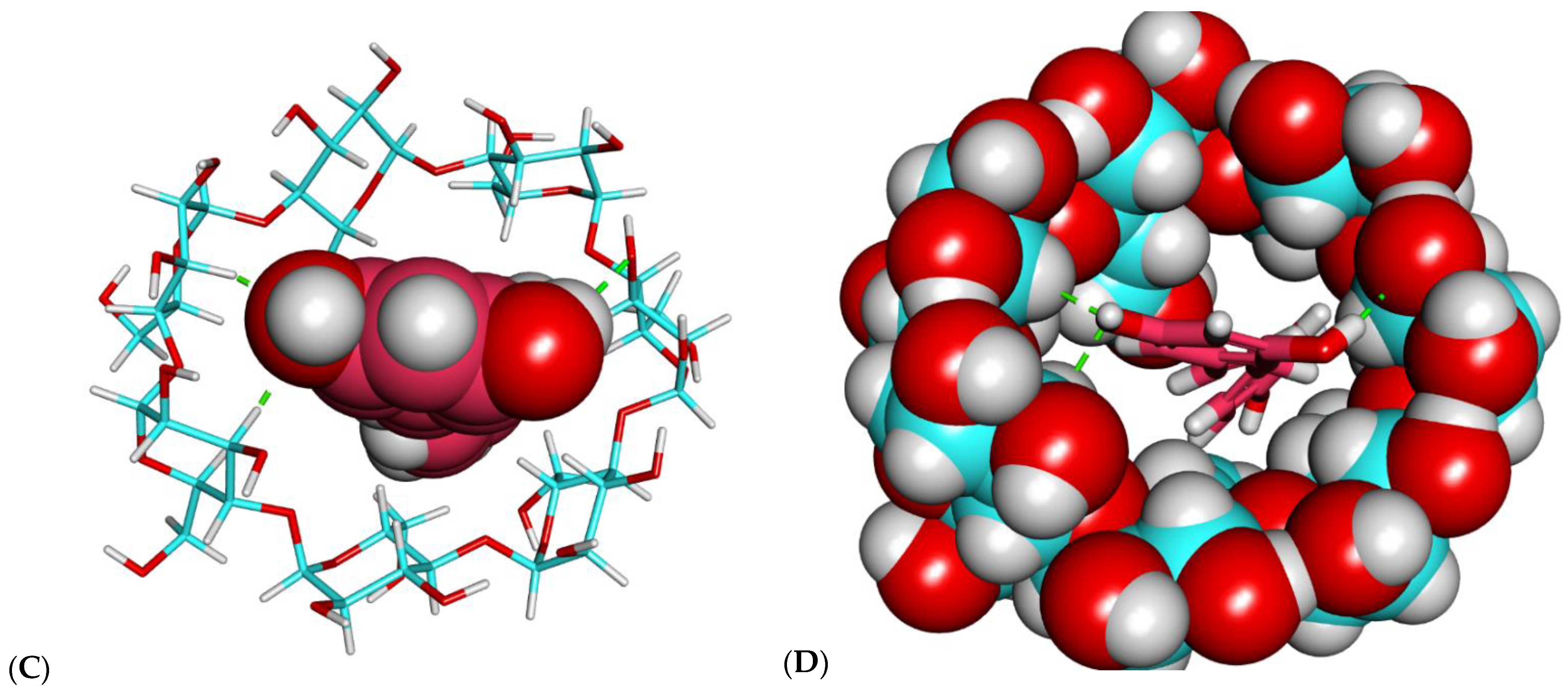
2.1. Molecular Docking Studies
2.1.1. Investigation of Binding Free Energy and Binding Mode
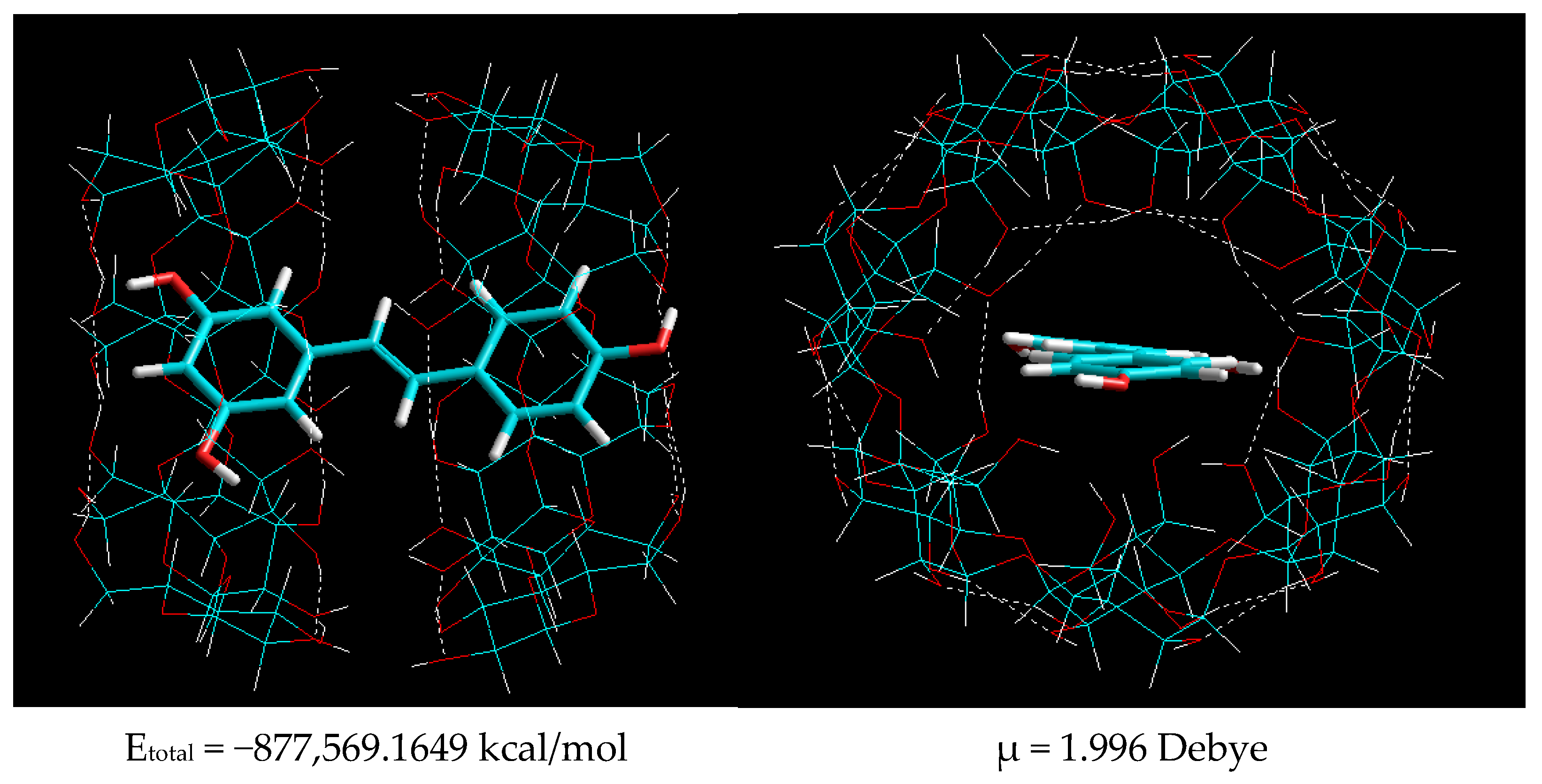
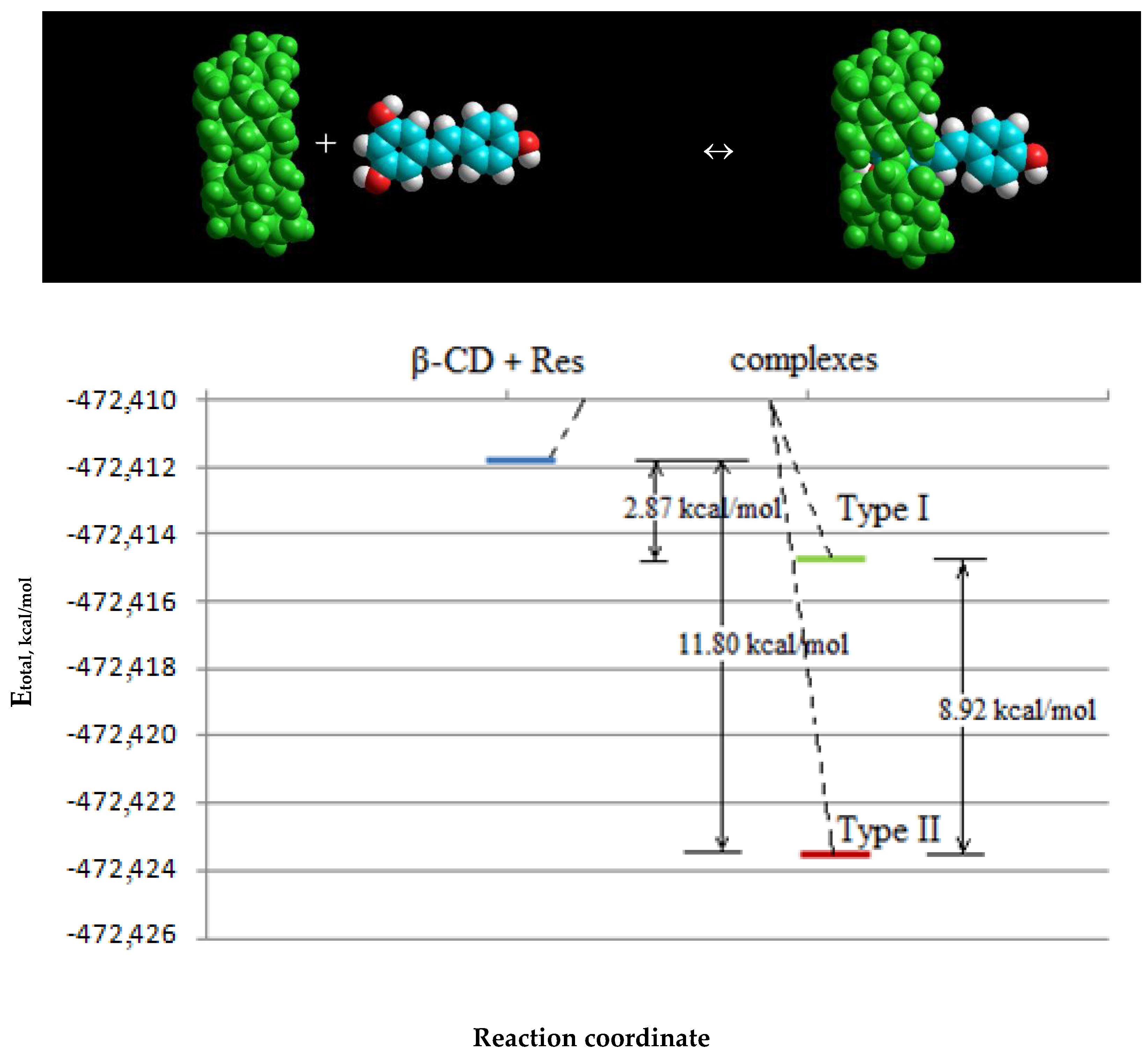
2.1.2. Molecular Modeling of β-CD–Resveratrol Complexes with Different Ratios
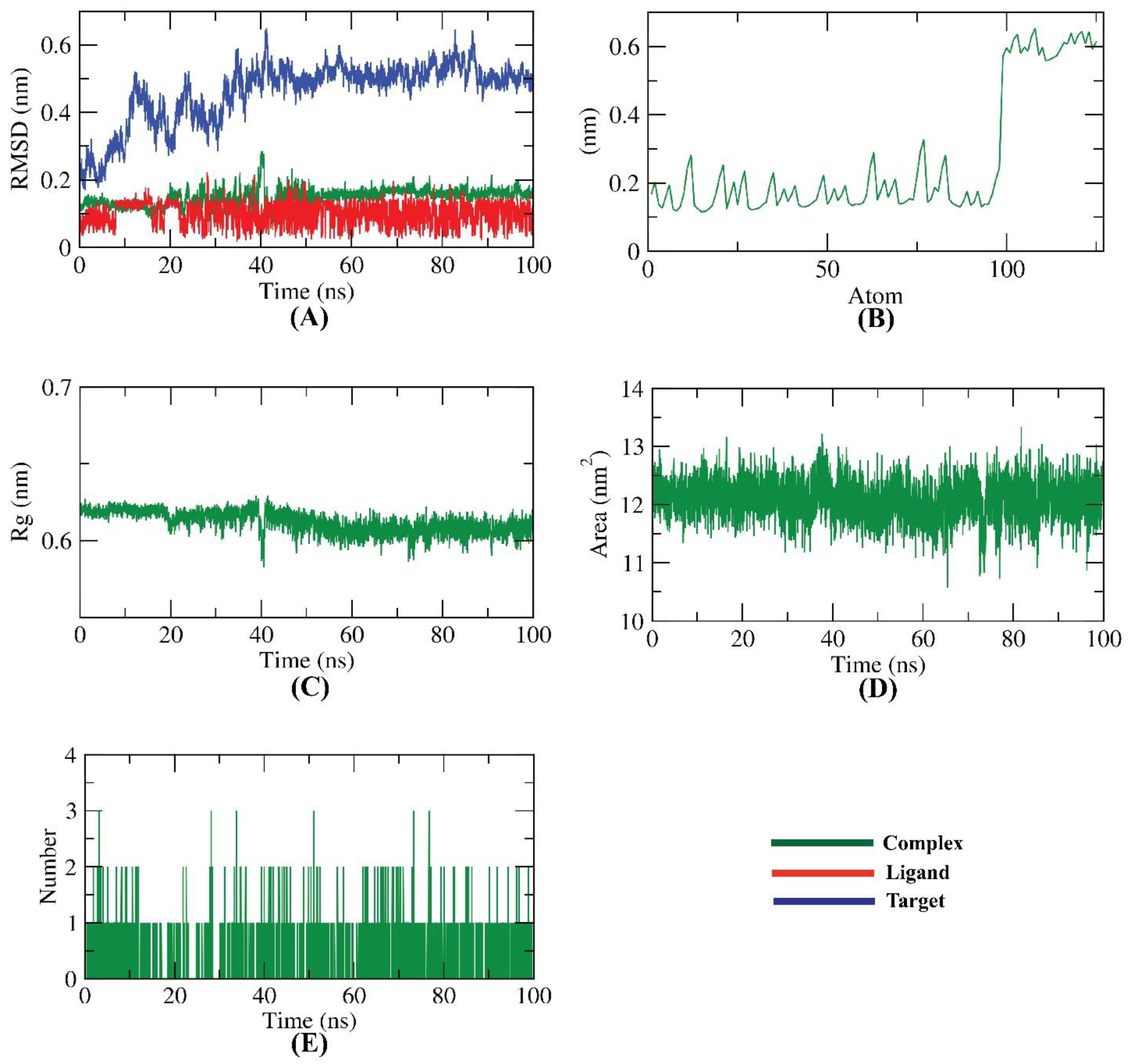
2.2. Molecular Dynamics Simulations
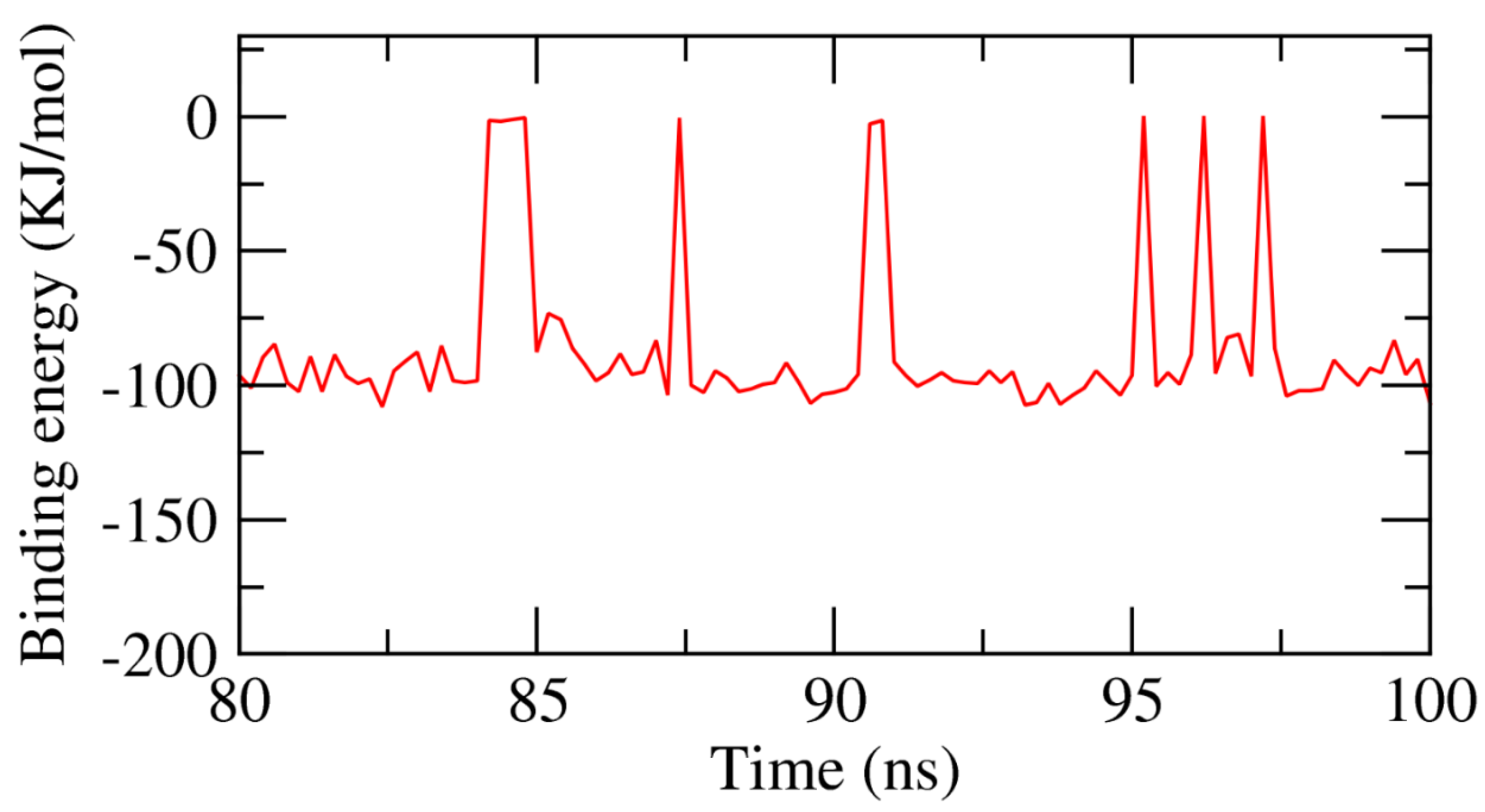
2.3. MMPBSA
2.4. Thermographic Measurements
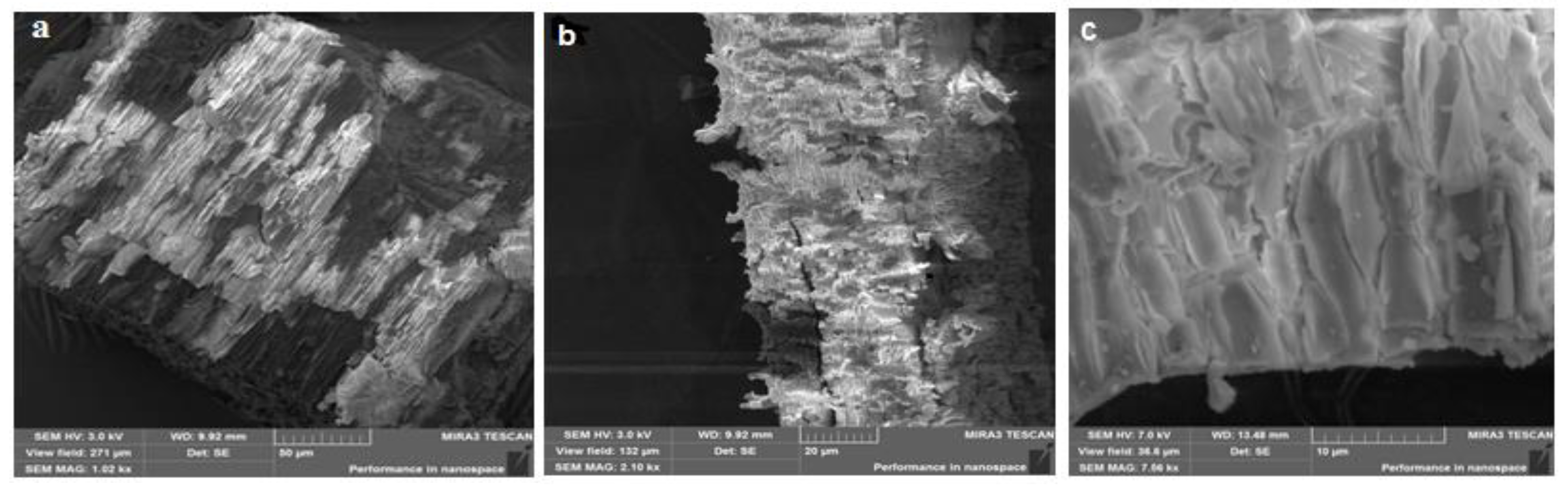
2.5. Morphology of β-CD Particles and the Binary System β-CD–Resveratrol
2.6. FTIR and 1H NMR Measurements
2.7. Antioxidant Properties of Resveratrol and β-CD–Resveratrol (2:1)
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Samuel, V.P.; Gupta, G.; Dahiya, R.; Jain, D.A.; Mishra, A.; Dua, K. Current update on preclinical and clinical studies of resveratrol, a naturally occurring phenolic compound. In Critical Reviews™ in Eukaryotic Gene Expression; Begell: Danbury, CT, USA, 2019; Volume 29. [Google Scholar]
- Wang, B.; Bellot, G.L.; Iskandar, K.; Chong, T.W.; Goh, F.Y.; Tai, J.J.; Schwarz, H.; Wong, S.C.; Pervaiz, S. Resveratrol attenuates TLR-4 mediated inflammation and elicits therapeutic potential in models of sepsis. Sci. Rep. 2020, 10, 18837. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules 2021, 26, 856. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef] [Green Version]
- Ferraz da Costa, D.C.; Fialho, E.; Silva, J.L. Cancer chemoprevention by resveratrol: The p53 tumor suppressor protein as a promising molecular target. Molecules 2017, 22, 1014. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Valentovic, M.A. Evaluation of resveratrol in cancer patients and experimental models. Adv. Cancer Res. 2018, 137, 171–188. [Google Scholar]
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting cancer via resveratrol-loaded nanoparticles administration: Focusing on in vivo evidence. AAPS J. 2019, 21, 57. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The use of micro-and nanocarriers for resveratrol delivery into and across the skin in different skin diseases—A literature review. Pharmaceutics 2021, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: An overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Ma, D.S.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol—potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Hashemi, M.; Amiri, E.; Hassanzadazar, H.; Daneshamooz, S.; Aminzare, M. Evaluation of the synergistic antioxidant effect of resveratrol and Zataria multiflora boiss essential oil in sodium alginate bioactive films. Curr. Pharm. Biotechnol. 2019, 20, 1064–1071. [Google Scholar] [CrossRef]
- Schlich, M.; Lai, F.; Pireddu, R.; Pini, E.; Ailuno, G.; Fadda, A.; Valenti, D.; Sinico, C. Resveratrol proniosomes as a convenient nanoingredient for functional food. Food Chem. 2020, 310, 125950. [Google Scholar] [CrossRef]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New delivery systems to improve the bioavailability of resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Sapino, S.; Carlotti, M.E.; Caron, G.; Ugazio, E.; Cavalli, R. In silico design, photostability and biological properties of the complex resveratrol/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 171–180. [Google Scholar] [CrossRef]
- Zou, A.; Zhao, X.; Handge, U.A.; Garamus, V.M.; Willumeit-Römer, R.; Yin, P. Folate receptor targeted bufalin/β-cyclodextrin supramolecular inclusion complex for enhanced solubility and anti-tumor efficiency of bufalin. Mater. Sci. Eng. C 2017, 78, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.K.; Brown, M.E. Handbook of Thermal Analysis and Calorimetry; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Pereira, A.N.; Trevisan, O.V. Thermoanalysis and reaction kinetics of heavy oil combustion. J. Braz. Soc. Mech. Sci. Eng. 2014, 36, 393–401. [Google Scholar] [CrossRef]
- Freeman, E.S.; Carroll, B. The application of thermoanalytical techniques to reaction kinetics: The thermogravimetric evaluation of the kinetics of the decomposition of calcium oxalate monohydrate. J. Phys. Chem. 1958, 62, 394–397. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Serra, R.; Nomen, R.; Sempere, J. The non-parametric kinetics a new method for the kinetic study of thermoanalytical data. J. Therm. Anal. Calorim. 1998, 52, 933–943. [Google Scholar] [CrossRef]
- Maazaoui, R.; Abderrahim, R. Applications of cyclodextrins: Formation of inclusion complexes and their characterization. Int. J. Adv. Res. 2015, 3, 1030. [Google Scholar]
- Zhao, D.; Liao, K.; Ma, X.; Yan, X. Study of the supramolecular inclusion of β-cyclodextrin with andrographolide. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 259–264. [Google Scholar] [CrossRef]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Lin, H.-S. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods 2016, 22, 122–131. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Wu, D.; Jiang, K.-M.; Zhang, D.; Zheng, X.; Wan, C.-P.; Zhu, H.-Y.; Xie, X.-G.; Jin, Y.; Lin, J. Preparation, spectroscopy and molecular modelling studies of the inclusion complex of cordycepin with cyclodextrins. Carbohydr. Res. 2015, 406, 55–64. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, R.; Liu, H.; Hu, Y.; Cheng, B.; Zou, G. Study of the complexation of resveratrol with cyclodextrins by spectroscopy and molecular modeling. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 295–300. [Google Scholar] [CrossRef]








| Complex | Etotal, kcal/mol | ΔЕcomplex, kcal/mol | |
|---|---|---|---|
| Initial Substances | Complex | ||
| Composition 1:1 Type I | −472,411.8326 | −472,423.6333 | 11.80066 |
| Composition 1:1 Type II | −472,411.8326 | −472,414.7083 | 2.8757 |
| Composition 2:1 | −877,529.7433 | −877,569.1649 | 39.42156 |
| Sample | , kJ/mol | |||
|---|---|---|---|---|
| β-CD–resveratrol (2:1) | 135.03 | 2.06 × 103 | 177.65 | 7.12 × 1020 |
| β-CD–resveratrol (4:1) | 131.68 | 1.08 × 103 | 173.25 | 1.65 × 1017 |
| Sample | |||
|---|---|---|---|
| β-CD–resveratrol (2:1) | 5.4 | 177.43 | 6.85 × 1016 |
| β-CD–resveratrol (4:1) | 5.0 | 173.24 | 3.15 × 1015 |
| Proton | δ0, ppm (β-CD) | δ, ppm (β-CD: Resveratrol) | Δδ (δ − δ0), ppm | |||
|---|---|---|---|---|---|---|
| δ (1Н) | δ (13С) | δ (1Н) | δ (13С) | δ (1Н) | δ (13С) | |
| H-1 | 4.780 | 102.416 | 4.770 | 102.493 | −0.010 | 0.076 |
| H-2 | 3.312 | 72.853 | 3.268 | 72.974 | −0.044 | 0.121 |
| H-3 | 3.452 | 73.514 | 3.358 | 73.608 | −0.094 | 0.094 |
| H-4 | 3.283 | 82.021 | 3.267 | 82.125 | −0.016 | 0.104 |
| H-5 | 3.330 | 72.493 | 3.315 | 72.595 | −0.015 | 0.102 |
| H-6 | 3.58 | 60.408 | 3.57 | 60.492 | −0.017 | 0.084 |
| Samples | IC50 (μg/mL) |
|---|---|
| Resveratrol | 14.3 ± 0.4 |
| β-CD–resveratrol (2:1) | 12.1 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskineyeva, A.; Fazylov, S.; Bakirova, R.; Sarsenbekova, A.; Pustolaikina, I.; Seilkhanov, O.; Alsfouk, A.A.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M. Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin. Plants 2022, 11, 1678. https://doi.org/10.3390/plants11131678
Iskineyeva A, Fazylov S, Bakirova R, Sarsenbekova A, Pustolaikina I, Seilkhanov O, Alsfouk AA, Elkaeed EB, Eissa IH, Metwaly AM. Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin. Plants. 2022; 11(13):1678. https://doi.org/10.3390/plants11131678
Chicago/Turabian StyleIskineyeva, Ainara, Serik Fazylov, Ryszhan Bakirova, Akmaral Sarsenbekova, Irina Pustolaikina, Olzhas Seilkhanov, Aisha A. Alsfouk, Eslam B. Elkaeed, Ibrahim H. Eissa, and Ahmed M. Metwaly. 2022. "Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin" Plants 11, no. 13: 1678. https://doi.org/10.3390/plants11131678
APA StyleIskineyeva, A., Fazylov, S., Bakirova, R., Sarsenbekova, A., Pustolaikina, I., Seilkhanov, O., Alsfouk, A. A., Elkaeed, E. B., Eissa, I. H., & Metwaly, A. M. (2022). Combined In Silico and Experimental Investigations of Resveratrol Encapsulation by Beta-Cyclodextrin. Plants, 11(13), 1678. https://doi.org/10.3390/plants11131678










