Protective Effects of Filtrates and Extracts from Fungal Endophytes on Phytophthora cinnamomi in Lupinus luteus
Abstract
:1. Introduction
2. Results
2.1. In Vitro Experiments
2.1.1. Effect of Filtrates on the Pathogen Mycelial Growth
2.1.2. Minimum Inhibitory Concentration (MIC) of Endophytic Filtrates and Extracts
2.1.3. Effect of Extracts on the Lupinus Seeds Germination
2.2. In Planta Experiments
2.2.1. Effect of the Pre-Emergence Application of Extracts on Lupinus Seedlings in Growth Chamber
2.2.2. Effect of the Post-Emergence Application of Extracts on Lupinus Seedlings in Greenhouse
3. Discussion
4. Materials and Methods
4.1. Fungal and Plant Material
4.2. Culture Conditions and Crude Extract Production from Fungal Endophytes
4.3. Effect of the Fungal Filtrates on the Pathogen Mycelial Growth In Vitro
4.4. Minimum Inhibitory Concentration In Vitro of the Filtrates and Extracts
4.5. In Planta Experiments
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simón, N.; Montes, F.; Díaz-Pinés, E.; Benavides, R.; Roig, S.; Rubio, A. Spatial distribution of the soil organic carbon pool in a Holm oak dehesa in Spain. Plant Soil 2013, 366, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Escribano, A.J.; Escribano, M.; Gaspar, P.; Mesías, F.J. The contribution of organic livestock to sustainable rural development in sensitive areas. IJRSAS 2015, 1, 21–34. [Google Scholar]
- Olea, L.; Miguel-Ayanz, A.S. The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. In Proceedings of the 11st General Meeting of the European Grassland Federation, Badajoz, Spain, 3–6 April 2006; Lloveras, J., González-Rodríguez, A., Vázquez-Yáñez, O., Piñeiro, J., Santamaria, O., Olea, L., Poblaciones, M.J., Eds.; Caja de Badajoz: Badajoz, Spain, 2006; pp. 1–15. [Google Scholar]
- González, F.; Maya, V. Los pastos y su importancia en la comunidad de Extremadura. Métodos de mejora. In Proceedings of the 52nd General Meeting of the Spanish Society for the Study of Pastures, Badajoz, Spain, 8–12 April 2013; Olea, L., Rodrigo, S., Santamaria, O., Eds.; Caja de Badajoz: Badajoz, Spain, 2013; pp. 83–105. [Google Scholar]
- Costa, J.C.; Martín, A.; Fernández, P.; Estirado, M. Dehesas de Andalucía. Caracterización Ambiental. Sevilla, Consejería de Medio Ambiente, Junta de Andalucía. Available online: https://www.juntadeandalucia.es/medioambiente/portal_web/web/servicios/centro_de_documentacion_y_biblioteca/fondo_editorial_digital/documentos_tecnicos/dehesas_andaluzas/dehesas_andaluzas.pdf (accessed on 29 April 2022).
- Serrano, M.S.; Fernández-Rebollo, P.; de Vita, P.; Sánchez, M.E. Susceptibility of common herbaceous crops to Phytophthora cinnamomi and its influence on Quercus root rot in rangelands. Eur. J. Plant Pathol. 2012, 134, 409–414. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Lo Presti, V.; Tudisco, R.; Chiofalo, V.; Grossi, M.; Infascelli, F.; Chiofalo, B. Characterization and effect of year of harvest on the nutritional properties of three varieties of white lupine (Lupinus albus L.). J. Sci. Food. Agric. 2015, 95, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Estadísticas agrarias. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/ (accessed on 29 April 2022).
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow lupin (Lupinus luteus L.) polysaccharides: Antioxidant, immunomodulatory and prebiotic activities and their structural characterisation. Food Chem. 2018, 267, 319–328. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem. 2006, 98, 291–299. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Analytical nutritional characteristics of seed proteins in six wild Lupinus species from Southern Spain. Food Chem. 2009, 117, 466–469. [Google Scholar] [CrossRef]
- Parra-González, L.B.; Aravena-Abarzúa, G.A.; Navarro-Navarro, C.S.; Udall, J.; Maughan, J.; Peterson, L.M.; Salvo-Garrido, H.E.; Maureira-Butler, I.J. Yellow lupin (Lupinus luteus L.) transcriptome sequencing: Molecular marker development and comparative studies. BMC Genom. 2012, 13, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Frisullo, S.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.O. Phytophthora cinnamomi involved in the decline of Holm Oak (Quercus ilex) stands in Southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Seddaiu, S.; Brandano, A.; Ruiu, P.A.; Sechi, C.; Scanu, B. An Overview of Phytophthora species inhabiting declining Quercus suber stands in Sardinia (Italy). Forests 2020, 11, 971. [Google Scholar] [CrossRef]
- Ruiz-Gómez, F.J.; Miguel-Rojas, C. Antagonistic potential of native Trichoderma spp. against Phytophthora cinnamomi in the control of holm oak decline in dehesas ecosystems. Forests 2021, 12, 945. [Google Scholar] [CrossRef]
- Serrano, M.; Vita, P.; Carbonero, M.; Trapero Casas, A.; Fernández Rebollo, P. Influencia del cultivo de “Lupinus luteus” en la podredumbre radical de las encinas en dehesas. Boletín Sanid. Veg. Plagas 2009, 35, 481–490. [Google Scholar]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N. Fungal endophytes from medicinal plants as a potential source of bioactive secondary metabolites and volatile organic compounds: An overview. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019; pp. 527–537. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Khan, M.A.; Lee, I.J. An endophytic fungus Gliocladium cibotii regulates metabolic and antioxidant system of Glycine max and Helianthus annuus under heat stress. Polish J. Environ. Stud. 2021, 30, 1631–1640. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Halecker, S.; Lledó, S.; Stadler, M. Antagonism between Byssochlamys spectabilis (anamorph Paecilomyces variotii) and plant pathogens: Involvement of the bioactive compounds produced by the endophyte. Ann. Appl. Biol. 2017, 171, 464–476. [Google Scholar] [CrossRef]
- Silva-Valderrama, I.; Toapanta, D.; de los Angeles Miccono, M.; Lolas, M.; Díaz, G.A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2021, 11, 3311. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.B.; Xie, B. Biosynthesis of antibiotic leucinostatins in bio-control fungus Purpureocillium lilacinum and their inhibition on Phytophthora revealed by genome mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.C.; Kharwar, R.N.; Strobel, G.A. Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat. Prod. Commun. 2009, 4, 1934578X0900401114. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, L.; Li, L.; Zheng, C.; Guo, L.; Li, W.; Sun, P.; Qin, L. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 2010, 165, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Leitao, A.L.; Enguita, F.J. Fungal extrolites as a new source for therapeutic compounds and as building blocks for applications in synthetic biology. Microbiol. Res. 2014, 169, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Sasse, F.; Jansen, R.; Murali, T.S. Fungal endophytes and bioprospecting. Fungal Biol. Rev. 2009, 23, 9–19. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A. Fungal endophytes in Mediterranean oak forests: A lesson from Discula quercina. Phytopathology 2008, 98, 380–386. [Google Scholar] [CrossRef] [Green Version]
- D’Errico, G.; Aloj, V.; Flematti, G.R.; Sivasithamparam, K.; Worth, C.M.; Lombardi, N.; Ritieni, A.; Marra, R.; Lorito, M.; Vinale, F. Metabolites of a Drechslera sp. endophyte with potential as biocontrol and bioremediation agent. Nat. Prod. Res. 2020, 35, 4508–4516. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; France Iglesias, A.; Gerding González, M.; Silva Aguayo, G.; Carrasco-Fernández, J.; Castro, J.F.; Ortiz Campos, J. Antifungal activity of Beauveria bassiana endophyte against Botrytis cinerea in two Solanaceae crops. Microorganisms 2019, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Alcock, A.; Elmer, P.; Marsden, R.; Parry, F. Inhibition of Botrytis cinerea by epirodin: A secondary metabolite from New Zealand isolates of Epicoccum nigrum. J. Phytopathol. 2015, 163, 841–852. [Google Scholar] [CrossRef]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2021, 35, 257–265. [Google Scholar] [CrossRef]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating pant defense mechanisms and the expression of crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar] [CrossRef] [PubMed]
- Lledó, S.; Rodrigo, S.; Poblaciones, M.J.; Santamaria, O. Biomass yield, mineral content, and nutritive value of Poa pratensis as affected by non-clavicipitaceous fungal endophytes. Mycol. Prog. 2015, 14, 67. [Google Scholar] [CrossRef]
- de Lima Fávaro, L.C.; Sebastianes, F.L.; Araújo, W.L. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE 2012, 7, e36826. [Google Scholar] [CrossRef]
- Khan, A.L.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Farsi, Z.; Al-Mamari, A.; Waqas, M.; Asaf, S.; Elyassi, A.; Mabood, F.; Shin, J.H.; et al. Endophytic fungi from frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS ONE 2016, 30, e0158207. [Google Scholar] [CrossRef]
- Trzewik, A.; Maciorowski, R.; Klocke, E.; Orlikowska, T. The influence of Piriformospora indica on the resistance of two rhododendron cultivars to Phytophthora cinnamomi and P. plurivora. Biol. Control. 2020, 140, 104121. [Google Scholar] [CrossRef]
- De Lamo, F.J.; Takken, F.L.W. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, K.; Anandaraj, M.; Bhai, R.S. In vitro evaluation of fungal endophytes of black pepper against Phytophthora capsici and Radopholus similis. JOSAC 2016, 25, 113–122. Available online: https://updatepublishing.com/journal/index.php/josac/article/view/5174 (accessed on 29 April 2022).
- Simamora, A.V.; Hahuly, M.V.; Henuk, J.B. Endophytic fungi as potential biocontrol agents of Phytophthora palmivora in the cocoa plant. Biodiversitas 2021, 22, 2601–2609. [Google Scholar] [CrossRef]
- Kiliç, G.; Tosun, G.; Bozdeveci, A.; Erik, I.; Öztürk, E.; Reis, R.; Sipahi, H.; Cora, M.; Karaoğlu, S.A.; Yaylı, N. Antimicrobial, cytotoxic, antiviral effects, and spectroscopic characterization of metabolites produced by Fusarium oxysporum YP9B. Rec. Nat. Prod. 2021, 15, 547–567. [Google Scholar] [CrossRef]
- Jindal, K.K.; Singh, H.; Meeta, M. Biological control of Phtophthora infestans on potato. Indian J. Plant Pathol. 1988, 6, 59–62. [Google Scholar]
- Ziedan, E.H.E.; Farrag, E.S.H.; Sahab, A.F. First record and preliminary evaluation of Mucor hiemalis as biocontrol agent on inflorescence brown rot incidence of date palm. Arch. Phytopathol. Plant Prot. 2013, 46, 617–626. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Lledó, S.; Rodrigo, S.; Poblaciones, M.J.; Santamaria, O. Biomass yield, nutritive value and accumulation of minerals in Trifolium subterraneum L. as affected by fungal endophytes. Plant Soil 2016, 405, 197–210. [Google Scholar] [CrossRef]
- Santamaria, O.; Rodrigo, S.; Lledó, S.; Poblaciones, M.J. Fungal endophytes associated with Ornithopus compressus growing under semiarid conditions. Plant Ecol. Divers. 2018, 11, 581–595. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Effect of fungal endophytes on plant growth and nutrient uptake in Trifolium subterraneum and Poa pratensis as affected by plant host specificity. Mycol. Prog. 2021, 20, 1217–1231. [Google Scholar] [CrossRef]
- Santamaria, O.; Lledó, S.; Rodrigo, S.; Poblaciones, M.J. Effect of fungal endophytes on biomass yield, nutritive value and accumulation of minerals in Ornithopus compressus. Microb. Ecol. 2017, 74, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Camisón, Á.; Martín, M.Á.; Oliva, J.; Elfstrand, M.; Solla, A. Increased tolerance to Phytophthora cinnamomi in offspring of ink-diseased chestnut (Castanea sativa Miller) trees. Ann. For. Sci. 2019, 76, 119. [Google Scholar] [CrossRef]
- Stadler, M.; Wollweber, H.; Mühlbauer, A.; Henkel, T.; Asakawa, Y.; Hashimoto, T.; Ju, Y.M.; Rogers, J.D.; Wetzstein, H.G.; Tichy, H.V. Secondary metabolite profiles, genetic fingerprints and taxonomy of Daldinia and allies. Mycotaxon 2001, 77, 379–429. [Google Scholar]
- Halecker, S.; Surup, F.; Kuhnert, E.; Mohr, K.I.; Brock, N.L.; Dickschat, J.S.; Junker, C.; Schulz, B.; Stadler, M. Hymenosetin, a 3-decalinoyltetramic acid antibiotic from cultures of the ash dieback pathogen, Hymenoscyphus pseudoalbidus. Phytochemistry 2014, 100, 86–91. [Google Scholar] [CrossRef]
- Santamaría, O.; Pajares, J.A.; Diez, J.J. Physiological and morphological variation of Gremmeniella abietina from Spain. Forest Pathol. 2004, 34, 395–405. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I.; Ciudad, A.G.; Vázquez de Aldana, B.R.; Criado, B.G. Effects of the infection by the fungal endophyte Epichloë festucae in the growth and nutrient content of Festuca rubra. Eur. J. Agron. 2006, 24, 374–384. [Google Scholar] [CrossRef]
- Stracquadanio, C.; Luz, C.; La Spada, F.; Meca, G.; Cacciola, S.O. Inhibition of mycotoxigenic fungi in different vegetable matrices by extracts of Trichoderma species. J. Fungi 2021, 7, 445. [Google Scholar] [CrossRef] [PubMed]
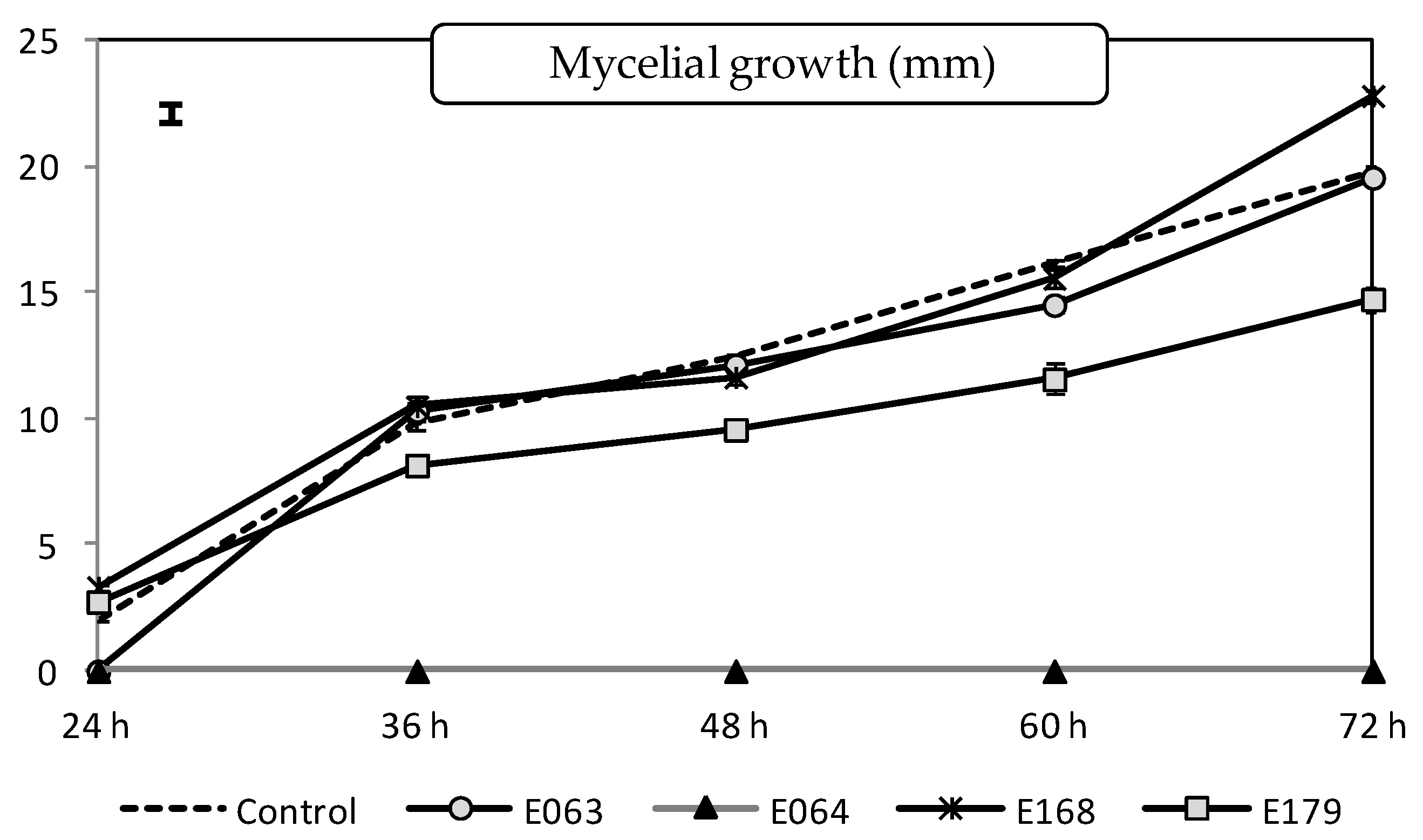

| Treatments | MIC | |
|---|---|---|
| Filtrate Dilution | Extract | |
| B | 0 | 0.00 |
| C | - | 2.34 |
| E063 | 0 | 150.00 |
| E064 | 1/2 | 300.00 |
| E168 | 0 | 300.00 |
| E179 | 1 | 300.00 |
| Treatments | Sl (cm) 1 | Rl (cm) | Leaves # | Roots # | DMs (mg) | DMr (mg) | DMt (mg) |
|---|---|---|---|---|---|---|---|
| B 2 | 7.7 ± 1.0 | 5.5 ± 1.0 c | 3.5 ± 0.6 | 10.8 ± 1.0 b | 72.0 ± 7.4 | 24.8 ± 6.4 | 96.8 ± 13.5 |
| C 3 | 6.3 ± 0.3 | 6.4 ± 0.1 bc | 3.0 ± 0.0 | 9.0 ± 0.5 bc | 53.0 ± 5.2 | 16.0 ± 3.8 | 69.0 ± 9.0 |
| E063 | 7.9 ± 0.7 | 6.9 ± 0.9 bc | 3.8 ± 0.6 | 13.0 ± 0.7 a | 63.5 ± 4.5 | 18.3 ± 2.5 | 81.8 ± 4.9 |
| E064 | 7.5 ± 0.8 | 7.7 ± 0.3 ab | 2.8 ± 0.3 | 10.0 ± 0.8 b | 62.8 ± 10.5 | 16.5 ± 5.3 | 79.3 ± 15.1 |
| E168 | 5.5 ± 0.7 | 7.0 ± 0.6 abc | 3.5 ± 0.6 | 7.8 ± 0.9 c | 56.8 ± 7.9 | 12.5 ± 0.6 | 69.3 ± 7.8 |
| E179 | 7.5 ± 0.5 | 8.6 ± 0.4 a | 3.0 ± 0.3 | 9.5 ± 0.3 bc | 66.0 ± 5.1 | 19.8 ± 0.3 | 85.8 ± 4.9 |
| df | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| F | 2.24 | 3.63 | 0.97 | 8.02 | 1.21 | 1.53 | 1.48 |
| p-value | 0.0943 | 0.0191 | 0.4613 | 0.0004 | 0.3451 | 0.2316 | 0.2448 |
| Treatments | AUDPC 1 | Rl (cm) | Roots # | DMs (mg) | DMr (mg) | DMt (mg) |
|---|---|---|---|---|---|---|
| B 2 | 0.0 ± 0.0 d | 19.6 ± 2.7 c | 14.2 ± 0.4 ab | 456.3 ± 51.8 c | 271.9 ± 26.0 bc | 728.3 ± 69.8 b |
| C 3 | 168.0 ± 0.0 a | 14.7 ± 1.4 d | 6.2 ± 1.0 d | 415.4 ± 42.6 c | 242.9 ± 17.6 c | 658.3 ± 44.2 b |
| E063 | 103.2 ± 5.4 b | 20.2 ± 0.6 bc | 14.6 ± 0.6 a | 427.0 ± 33.7 c | 266.7 ± 21.2 c | 693.7 ± 54.1 b |
| E064 | 105.6 ± 13.0 b | 25.9 ± 0.9 a | 12.8 ± 0.4 bc | 455.6 ± 34.5 c | 259.8 ± 41.1 c | 715.4 ± 73.6 b |
| E168 | 45.6 ± 10.7 c | 25.2 ± 1.9 a | 11.6 ± 0.6 c | 637.3 ± 37.9 b | 353.9 ± 51.0 ab | 991.3 ± 81.8 a |
| E179 | 81.6 ± 14.3 b | 24.2 ± 0.8 ab | 13.6 ± 0.4 ab | 746.4 ± 28.2 a | 365.0 ± 20.0 a | 1111.4 ± 30.5 a |
| df | 5 | 5 | 5 | 5 | 5 | 5 |
| F | 47.53 | 9.44 | 32.29 | 15.35 | 3.34 | 11.56 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0197 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Latorre, C.; Rodrigo, S.; Santamaria, O. Protective Effects of Filtrates and Extracts from Fungal Endophytes on Phytophthora cinnamomi in Lupinus luteus. Plants 2022, 11, 1455. https://doi.org/10.3390/plants11111455
García-Latorre C, Rodrigo S, Santamaria O. Protective Effects of Filtrates and Extracts from Fungal Endophytes on Phytophthora cinnamomi in Lupinus luteus. Plants. 2022; 11(11):1455. https://doi.org/10.3390/plants11111455
Chicago/Turabian StyleGarcía-Latorre, Carlos, Sara Rodrigo, and Oscar Santamaria. 2022. "Protective Effects of Filtrates and Extracts from Fungal Endophytes on Phytophthora cinnamomi in Lupinus luteus" Plants 11, no. 11: 1455. https://doi.org/10.3390/plants11111455
APA StyleGarcía-Latorre, C., Rodrigo, S., & Santamaria, O. (2022). Protective Effects of Filtrates and Extracts from Fungal Endophytes on Phytophthora cinnamomi in Lupinus luteus. Plants, 11(11), 1455. https://doi.org/10.3390/plants11111455







