Abstract
Many fungi, especially endophytes, have been found to produce multiple benefits in their plant hosts, with many of these benefits associated with the protection of plants against fungal diseases. This fact could be used in the development of new bio-products that could gradually reduce the need for chemical fungicides, which have been associated with multiple health and environmental problems. However, the utilization of the living organism may present several issues, such as an inconsistency in the results obtained and more complicated management and application, as fungal species are highly influenced by environmental conditions, the type of relationship with the plant host and interaction with other microorganisms. These issues could be addressed by using the bioactive compounds produced by the fungus, in cases where they were responsible for positive effects, instead of the living organism. Multiple bioactive compounds produced by fungal species, especially endophytes, with antifungal properties have been previously reported in the literature. However, despite the large amount of these metabolites and their potential, extensive in-field application on a large scale has not yet been implemented. In the present review, the main aspects explaining this limited implementation are analyzed, and the present and future perspectives for its development are discussed.
1. Introduction: Fungal Plant Pathogens and Control Alternatives
Considering world data from the beginning of the 21st century, microbial diseases have been estimated to cause losses of around 16% of crop yields, from which 70–80% is caused by fungal pathogens [1]. These losses are expected to increase under the new scenario created by global change, due to pests and diseases displaying more pathogenic behavior, and lower host fitness developing under the new conditions [2]. Although the traditional use of synthetic fungicides has been shown to be quite effective in fighting against fungal diseases, it presents multiple hazards. The most important among them include human health problems [3,4]; residue accumulation in multiple ecosystems, resulting in serious risks for soil organisms [5,6], aquatic organisms [7,8] and mammals [9]; and the development of fungicide resistance by pathogens [10].
Current societies are becoming increasingly aware of the drawbacks associated with the health and environmental problems derived from those traditional cropping systems. Consequently, there is a growing demand for more environmentally friendly agriculture that can reduce the use of agrochemicals. Nowadays, more than ever in the context of a global pandemic, people want healthier food, produced more respectfully with regard to the environment. These new demands are pushing the research and farming sector to look for new alternatives to fight against fungal diseases. A detailed review examining the recent alternative approaches to the use of agrochemicals to fight against plant phytopathogens was presented by El-Baky and Amara [11]. Among these new alternatives, the use of resistant cultivars may be one of the most effective methods of disease control, both economically and environmentally. However, although phytopathological aspects are a clear goal for plant breeders, there still are a very small number of resistant cultivars for a very limited number of plant pathogens. Likewise, more sustainable agricultural practices can help in avoiding fungal disease outbreaks. This is the case, for instance, for Fusarium graminearum, which is controlled to a great extent in wheat by using suitable crop rotation and/or inversive tillage [12]. Agricultural practices can also be applied for the control of Phytophthora, shown to be possible by maintaining an adequate nutritional status in citrus plants and a correct drainage system to avoid flooding [13]. Sowing cover crops acting as bio-fumigants is also an agricultural technique used against soil fungal pathogens with interesting results. Thus, Rahman et al. [14] found that mustard plants grown in intercropping with tomato, and mowed at early flowering to be incorporated in the first 15 cm of topsoil, reduced the damage from Verticillium dahliae.
Another very interesting alternative used to fight against fungal pathogens is biological control. Numerous organisms, parasites, predators or antagonists of pests and diseases have been reported as plant bio-protectors [15], and many of them are currently being used in the field. However, although this practice is increasingly becoming more accepted, its efficacy is highly dependent on a wide range of abiotic and biotic factors, aspects which do not yet allow for the complete replacement of agrochemicals [16]. Among biocontrol agents, in recent decades, the use of endophytic fungi has become an interesting alternative for organic agriculture. As these fungi occur inside plant tissues, in contrast with epiphytic populations which inhabit the plant surface, they have to establish a closer relationship with their hosts, which may explain the lower richness and diversity in comparison with epiphytes [17]. Although both endophytes and epiphytes have been shown to benefit plant growth and to provide plant protection [18], because epiphytic fungi are more exposed to environmental conditions, their applicability and effectiveness may be more limited. For this reason, the present review is mainly focused on (although not restricted to) fungal endophytes. An example of effective control of plant pathogens by these organisms is given by the endophyte Induratia spp., which has been found to produce an important diminution in the disease severity caused by three common fungal pathogens in bean crops [19]. Similarly, the symptom severity caused by Fusarium moniliforme in Lolium rigidum was reduced by the fungal endophyte Byssochlamys spectabilis (anamorph Paecilomyces variotii) when co-inoculated in plants [20]. These two examples highlight the huge potential of these microorganisms to be used as biocontrol agents, although they have also been shown to produce many other benefits in their host plants, as outlined hereafter.
2. Fungal Endophytes: A Promising Tool in Plant Production and Protection
Fungal endophytes are fungi living asymptomatically within plant tissues [21], both above-ground and in root tissues [22]. These fungi are extremely ubiquitous and diverse and have been found in every examined plant [23,24]. Although the role of many endophytic fungi in their host plants is not completely understood, numerous beneficial effects in their hosts have been reported in the literature. Those beneficial effects can be encompassed in two major groups: (i) those conferring adaptive advantages to the host plant and (ii) those conferring protection to the host plant against biotic or abiotic stresses. Regarding the first major group of effects, endophytes have been found to improve the nutritional status of their host plant [25,26,27,28], as well as to enhance its competitiveness against other plant species [29]. Furthermore, other fungi, such as several species from the genus Trichoderma, have been shown to produce growth promotion effects in their host plants [30]. Other beneficial effects found in the literature deal with increasing photosynthetic efficiency or antioxidant capacity [31,32].
Regarding the second major group of effects, while several endophytic fungi are able to confer a positive response to water stress [33,34] or to high temperatures [35] on their host plants, others have been found to provide resistance to salinity or soil heavy metals [36,37,38,39]. With regard to their protective effects against biotic factors, numerous endophytes have been described to confer resistance toward macroherbivores [40], nematodes [41,42,43], pests [44,45,46] and pathogens [47,48,49,50]. As examples of the antagonism of fungal endophytes towards fungal pathogens, Trichoderma atroviride, Hypoxylon rubiginosum and Metarhizium anisopliae have shown the capacity to protect their host plants against Diplodia pinea, Hymenosciphus fraxineus and Fusarium graminearum, respectively, among others [51,52,53]. A detailed consideration of many other examples of antagonism between fungal endophytes and fungal pathogens can be found in several reviews, such as those of Busby et al. [54], Zivanovic and Rodgers [55] and Pavithra et al. [56].
The mechanisms involved in the protective responses against fungal pathogens differ with each fungal endophyte and with the type of interaction that it establishes with its host. Although a detailed description of these control mechanisms can be found in the work of Gao et al. [57] or Busby et al. [54], they can be summarized in the following four types according to Zabalgogeazcoa [58]: (i) competition for space and nutrients, (ii) direct inhibition through antibiosis, (iii) mycoparasitism or (iv) plant resistance induction by activating the host plant defense system. In relation to the first type (competition for space and nutrients), this mechanism has been widely described in many plant–pathogen systems, such as that practiced by Trichoderma spp. against Botrytis cinerea [59]. The direct inhibition through antibiosis is mainly produced when the endophyte releases different metabolites or volatiles [60]. Regarding mycoparasitism, several genera of endophytes, such as those of Trichoderma spp., have been found to parasitize a wide range of fungal pathogens [61]. Multiple events are produced during mycoparasitism, such as those described by Harman et al. [62] as follows: first, the biocontrol agent has to detect other fungi and grow tropically towards them. After that and before contact, the endophyte may produce an extracellular exochitinase first, and then fungitoxic endochitinases. Once the fungi come into contact, the endophyte attaches to the host and can coil around it, forming appressoria on the host surface. Once in contact, the endophyte may produce several fungitoxic cell wall-degrading enzymes, which may result in the dissolution of the cell walls and the direct entry of the endophyte hyphae into the lumen of the target fungus.
Regarding the induction of the host plant defense system, several mechanisms can be found in the literature. For instance, the endophytic fungi Serendipita indica (previously known as Piriformospora indica) enhances resistance in barley to Blumeria graminis and Fusarium culmorum and in rice to Thanatephorus cucumeris (anamorph Rhizoctonia solani). However, in the former case, the induced plants developed structures or strategies that reduced the successful penetration of Blumeria, even causing local cell death [63], while in the latter case, the rice-induced plants increased their antioxidant capacity, thus limiting the severity of the pathogen [64]. Trichoderma harzianum has also been found to induce fungal pathogen resistance [65]. This endophyte enhances the expression of the enzymes involved in lignification in several crops, such as maize, or strengthens the epidermal cell walls in others, such as cucumber, hindering fungal pathogen penetration and colonization. All of these responses seem to be mediated by salicylic acid produced by plants as a signaling molecule. This pathway is characterized by the production of a cascade of pathogenesis-related proteins, including chitinases, glucanases and thaumatins, as well as oxidative enzymes such as peroxidases, polyphenol oxidases and lipoxygenases [62].
Among the aforementioned four types of mechanisms, it is interesting to highlight that in two of them, the fungal endophyte must be inside the plant and needs to establish some kind of interaction with its host to produce the effect. The establishment of such an interaction may require the penetration of the outermost tissues of the host by the endophyte, which may be mediated by the nature of the host and the endophyte and by the environmental conditions. For this, among other aspects, the communication between the endophyte and plant host becomes fundamental [66]. Such communication is mediated by proteins and enzymes secreted by the endophyte, which are recognized by the plant cells. As a response, plants may alter their defenses, allowing for the entrance of the fungus and the establishment of the association [67,68]. Therefore, in those cases, the protective effect is only going to be produced if multiple conditions, environmental and those related to the compatibility between organisms, are produced. In the other two types of mechanisms, the production of metabolites by endophytes is involved in the protective effect, either because they induce defense mechanisms in the host or due to their antimicrobial properties against a wide range of plant pathogens [69,70]. Thus, for a long time, fungal endophytes have been identified as an important source of novel and chemically diverse secondary metabolites [71,72,73].
3. Secondary Metabolites from Fungal Organisms
Fungi, especially fungal endophytes, have been identified as a prolific source of secondary metabolites with very interesting properties in a broad range of applications for different industries, such as pharmacy, the wood and food industry and agronomy [72]. Within the pharmaceutical industry, a clear example of this prolificacy has been shown by endophytic fungi isolated from marine plants, which were found to produce a great number of secondary metabolites of a diverse nature, perhaps derived from the fact that they live under extreme environmental conditions of salinity stress [74]. Thus, as an example, secondary metabolites obtained from Fusarium equiseti isolated from marine seaweed have shown activity against cancer cells [75]. Taritla et al. [76] also reported compounds isolated from the extracts of the marine endophyte Aspergillus sp. that produced cytotoxicity and apoptosis in human cancer lines. Compounds identified from the extracts of the marine endophyte Epicoccum nigrum isolated from seagrass from the Red Sea also showed interesting pharmacological effects, causing the growth inhibition of both Gram-positive and Gram-negative bacteria [77]. However, not only marine endophytes produce interesting secondary metabolites. In their review work, Savidov et al. [78] reported numerous fungi, several endophytes among them, that, regardless of the habitat conditions, produced biologically active terpenoids and steroids, many of them belonging to the class of highly oxygenated isoprenoid lipids (HOILs), which are important compounds involved in numerous life processes. The most important activities of these HOIL compounds reported in the literature include effects ranging from hepatoprotectant to anti-inflammatory, as well as immunosuppressant, chemopreventive or hair growth stimulant, among others [78]. Other compounds produced by fungal endophytes with further beneficial health effects, such as anticancer [79,80], anti-inflammatory, antiarthritic [81] and antiviral [82], have also been reported in the literature.
In relation to the wood and food industries, attention is being focused on various enzymes produced by endophytic fungi, such as phosphatases, esterases, lipases, proteases, cellulases, laccases, xylanases, amylases and phytases, with very interesting properties involved in multiple manufacturing processes [83,84]. In the food industry, there is also great potential for the use of other compounds, such as polyphenols, flavonoids or antioxidants, produced by endophytic fungi [85,86]. For example, several endophytic fungi, such as Chaetomium sp., Curvularia sp., Colletotrichum sp. and Trichoderma sp., isolated from Azadirachta indica, have been found to be an interesting source of antioxidant compounds with high free radical scavenging activity, as well as being a source of tannins and carotenoids [87], which are used in the food industry as food colorants [88] and in pharmacy due to their enhancement of the immune cell function in the body [89].
In agriculture, as active metabolites are often involved in the beneficial effects that endophytes produce on their plant hosts, they can be used to improve the general performance of crops or to protect them against biotic or abiotic stresses, in both cases increasing crop production as a consequence. A clear example of the applicability of bioactive metabolites produced by endophytes is the case of those produced by Penicillium citrinum isolated from Ixeris repens. When these metabolites were applied in Carex kobomugi, they produced better growth, a higher chlorophyll and carotenoid content and higher efficiency in carboxylation and in water use [90]. Another more recent example is the application of the extract produced by the ascomycete Byssochlamys spectabilis, formerly known as Paecilomyces variotii, which improved the utilization efficiency of nitrogen and phosphorus, consequently increasing the yield in rice and maize, under in-field conditions [91,92]. All of these applications evidence an extremely high diversity in the nature of the metabolites produced by fungal endophytes, many of them with antifungal properties. A complete description of the active metabolites produced by fungal endophytes and the nature of their bioactivity can be found in the review carried out by Zheng et al. [93].
4. Secondary Metabolites of Fungi with Antifungal Properties
Within the wide range of secondary metabolites produced by fungi, especially fungal endophytes, many of them have antimicrobial properties, as described in detail by Mousa and Raizada [73]. Among those antimicrobial metabolites, a thorough review carried out by Xu et al. [94] showed their wide chemical diversity, with a total of 132 chemical structures being found with antifungal properties, when analyzed over the past two decades. From the compounds included in the present review, most of them (53%) are included within the polyketide compounds (Figure 1). The predominant chemical family within this category is isocoumarin, followed by furan derivative and acetophenone derivative. Twelve other chemical families are also represented within this group (Figure 1). After polyketides, the categories, ordered according to the number of metabolites with antifungal properties, are the following: terpenoids (13%), nonribosomal peptides (10%), phenols (7%), alkaloids (7%), aliphatic compounds (5%) and other compounds (5%) such as inorganic acids (Figure 1). This information is based on the most relevant substances produced by fungi showing some capacity to control any fungal phytopathogen, which are indicated in Table 1. Table 1 also displays the efficacy of the biological compounds to control the phytopathogen in relation to the corresponding chemical formulation, as well as the conditions in which the studies were performed.
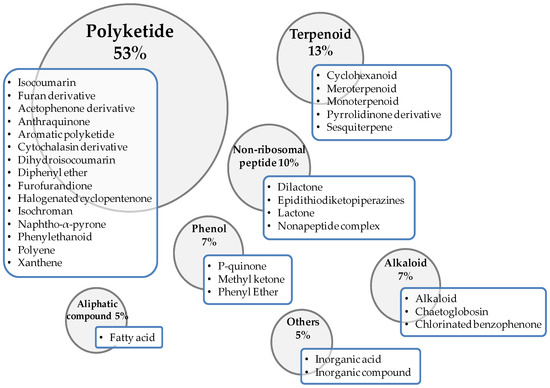
Figure 1.
Graphical abstract of the different categories in which the fungal metabolites with antifungal properties are included, indicating the percentage of compounds in each category.
Historically, one of the first examples found in the literature of a secondary metabolite produced by a fungus with antifungal properties is the case of the griseofulvin compound, which belongs to the class of organic compounds known as benzofurans. This compound, produced by Penicillium sp., was able to inhibit the growth of Botrytis cinerea, causing abnormal hyphal formations and disorientation of growth [95]. Since then, many other substances produced by fungi, especially fungal endophytes, have been described to show antifungal properties. This is the case with ergot alkaloids, produced, for instance, by Claviceps purpurea. These compounds, biosynthetically derived from the amino acid L-tryptophan and dimethylallyl diphosphate, have been found to exhibit, among other properties, strong antifungal activity [96]. Other alkaloids produced by fungal endophytes, such as sceptrin, a cyclobutane-containing substance, have also shown such antifungal activity [97].
From a different chemical family, some other substances with antifungal properties produced by fungi are known as acetylenic metabolites, which are isolated from Penicillium sp., Aspergillus sp. and other filamentous fungi [98,99]. These compounds, which are widespread in nature, although limited to a few groups of plants and fungi [100], are being used as the natural basis of synthetic compounds with fungicide activity [101]. Among the mechanisms of action, acetylenic compounds interfere with fatty acid production and can alter the expression of the genes that are required for fungal growth [102]. Other common chemical compounds, such as ferric chloride, potassium hydroxide and vanillin–sulfuric acid, all of them with antifungal activity, have been isolated from ethyl acetate extracts produced by the endophytic fungi Xylaria allantoidea [103].


Table 1.
Metabolites produced by fungi, especially fungal endophytes, which have shown a certain control of phytopathogens.
Table 1.
Metabolites produced by fungi, especially fungal endophytes, which have shown a certain control of phytopathogens.
| Metabolite(s) | Producer Fungi | Fungal Pathogen(s) | Action 1 | Conditions 2 | Efficacy 3 | Ref. |
|---|---|---|---|---|---|---|
| (12R)-12-Hydroxymonocerin | Exserohilum sp. | Fusarium oxysporum | A | IV | 3% | [104] |
| 3-(5-Oxo-2,5-dihydrofuran-3-yl) propanoic acid | Aspergillus tubingensis | Fusarium graminearum | A | IV | 2-fold | [105] |
| 4-Methylmellein, 4-hydroxymellein, 6-hydroxymellein, tyrosol | Penicillium sp. | Fusarium oxysporum | A | IV | 35% | [106] |
| 4-Prenyloxyclavatol | Nigrospora sphaerica | Colletotrichum gloeosporioides | A | IV | 63% | [107] |
| 5-Methylmellein | Biscogniauxia mediterranea | Phomopsis obscurans Phomopsis viticola | A | IV | 5% | [108] |
| 5-(Undeca-3′,5′,7′-trien-1′-yl) furan-2-ol | Emericella sp. | Verticillium dahliae | A | IV | 49% | [109] |
| 5-(Undeca-3′,5′,7′-trien-1′-yl) furan-2-carbonate | 12% | |||||
| Bicolorin D | Saccharicola bicolor | Sclerotinia sclerotiorum | A | IV | 13% | [110] |
| IP-A | 57% | |||||
| Brefeldin A | Cladosporium sp. | Aspergillus niger | A | IV | 8-fold | [111] |
| Cercosporamide | Cadophora orchidicola | Fusarium oxysporum Pestalotia diospyri Botrytis cinerea Sclerotium rolfsii Penicillium digitatum | A | IV | - | [112] |
| Cuminic acid | Aspergillus spp. | Phytophthora spp. | A | IV | - | [113] |
| Epirodin | Epicoccum nigrum | Brotrytis cinerea | A | IV, IP-A | - | [114] |
| Ergot alkaloids | Claviceps purpurea | - | A | - | - | [96] |
| Exserolide C | Exserohilum sp. | Fusarium oxysporum | A | IV | 3% | [104] |
| Ferric chloride, potassium hydroxide, vanillin–sulfuric acid | Xylaria allantoidea | - | A | IV | - | [103] |
| Geoxantethers A and B | Fungus from Massarinaceae | Microbotryum violaceum | A | IV | 16% | [115] |
| Griseofulvin | Penicillium sp. | Botrytis cinerea | A | IV | - | [95] |
| Guignardianone C | Phyllosticta sp. | Botrytis cinerea | A | IV | 52% | [96] |
| Hexadecanoic acid, 2,3-bis ((trimethylsilyl) oxy) propyl ester | Trichoderma harzanium | Sclerotinia sclerotiorum | A | IV | 25% | [116] |
| Leucinostatins A and B | Purpureocillium lilacinum | Phytophthora sp. | A | IV | - | [117] |
| Macrosporin | Phoma sp. | Fusarium graminearum | A | IV | 10% | [118] |
| Methyl dichloroasterrate | Aspergillus capensis | Botrytis cinerea Monilinia fructicola Sclerotinia sclerotiorum Sclerotinia trifoliorum | A, ISR | IV | 17% | [119] |
| Monocerin | Drechslera sp. | Botrytis cinerea Sclerotinia sclerotiorum | A | IV, IP-A | - | [120] |
| Nigrosporamide A | Nigrospora sphaerica | Colletotrichum gloeosporioides | A | IV | 10.83-fold | [107] |
| Not identified | Metarhizium anisopliae | Fusarium graminearum Fusarium oxysporum | A | IV | - | [121] |
| Not identified | Metarhizium anisopliae | Fusarium graminearum | A | IP-F | - | [53] |
| Palmitic acid, stearic acid, octadecenoic acid | Fusarium oxysporum | - | A, ISR, PGP | IV | - | [82] |
| Penicillither | Aspergillus capensis | Botrytis cinerea Monilinia fructicola Sclerotinia sclerotiorum Sclerotinia trifoliorum | A | IV | 5% | [119] |
| Penochalasin K | Penicillium chrysogenum, | Colletotrichum gloeosporioides | A | IV | 10-fold | [122] |
| Rhizoctonia solani | 2.66-fold | |||||
| Pestalachlorides A, B and C | Pestalotiopsis adusta | Fusarium culmorum Gibberella zeae Verticillium aiboatrum | A | IV | - | [123] |
| Pretrichodermamide A | Trichoderma harzianum Epiccocum nigrum | Ustilago maydis | A | IV | 2-fold | [124] |
| Pseudoanguillosporin A | Pseudoanguillospora sp. | Mycrobotryum violaceum | A | IV | 40% | [125] |
| Pyrenophorol | Lophodermium nitens | Cronartium ribicola | A | IP-G | - | [126] |
| Rosellichalasin | Aspergillus capensis | Botrytis cinerea Monilinia fructicola Sclerotinia sclerotiorum Sclerotinia trifoliorum | A | IV | 32% | [119] |
| Speciosin U | Saccharicola sp. | Cladosporium cladosporioides | A | IV | - | [127] |
| Sporothriolide | Nodulisporium sp. | Rhizoctonia solani Sclerotinia sclerotiorum | A | IV | 60% | [128] |
| IP-G | 79% | |||||
| Trichodermin | Trichoderma brevicompactum | Rhizoctonia solani | A | IV | 1.44-fold | [129] |
| Fusarium solani | 1.21-fold | |||||
| Versicolorin B | Aspergillus versicolor | Colletotrichum musae | A | IV | - | [130] |
| Viriditoxin | Byssochlamys spectabilis | Fusarium spp. | A | IV; IP-G | - | [20] |
1 Mechanism of action involved in the biocontrol: antibiosis (A); induced systemic resistance (ISR); plant growth promotion (PGP). 2 Conditions in which the antifungal bioactivity was obtained: in vitro (IV); in planta (or detached tissues) axenic conditions in laboratory (IP-A); in planta controlled conditions in greenhouse (IP-G); in planta uncontrolled conditions in the field (IP-F). 3 Efficacy: average percentage of efficacy of the biological compound in relation to the positive control (chemical fungicide) used in the corresponding study.
Furthermore, from ethyl acetate extracts (but in this case from Trichoderma harzianum and Epiccocum nigrum), pretrichodermamide A, an epidithiodiketopiperazine, has shown antimicrobial activity towards the plant pathogenic fungus Ustilago maydis [124]. Palmitic, stearic and octadecenoic acids, isolated from the ethyl acetate extracts of the endophyte Fusarium oxysporum [82], have also been identified as fungicides. Against several species of Fusarium, the biocontrol produced by the endophytic fungi Byssochlamys spectabilis when it was artificially inoculated in Lolium rigidum plants was attributed to the production of viriditoxin by the endophyte [20]. This molecule was identified by Wang et al. [131] as a potent bactericide due to the inhibition it causes in the formation of one of the proteins required for cell division, a mechanism that could work similarly against fungi. Leucinostatins produced by Purpureocillium lilacinum have displayed a broad bioactivity against bacteria and fungi. The mechanism which explains the antibiotic effect of these compounds is based on their ability to inhibit ATP synthesis in the mitochondria, as well as different phosphorylation pathways [117]. Metarhizium anisopliae can be considered as a promising biocontrol agent against F. graminearum; it produces fungistatic secondary metabolites, enhances wheat growth and elicits wheat defense responses [53]. Several other plant pathogens, such as Alternaria solani, Rhizoctonia solani, Phytium ultimum and Colletotrichum lagenarium, have shown a susceptibility to some fatty acids produced by fungal endophytes [132,133]. The important phytopathogen Phytophthora can be controlled by cuminic acid, a benzoic acid produced by the Aspergillus genus [113] and other endophytic fungi belonging to different genera [134,135,136]. Finally, Fusarium graminearum and F. oxysporum have been found to be controlled by a crude extract of the fungal endophyte Metarhizium anisopliae [53,121], although the active metabolite responsible for such an effect has not yet been identified. In addition to the already indicated information, the main mechanism of action responsible for the antifungal activity is related to antibiosis in most of the cases (Table 1), although this could be explained by the fact that most of the compounds were only tested in vitro. In the few cases where they were also evaluated in planta, other mechanisms, such as induced systemic resistance (ISR) in monocerin or plant growth promotion (PGP) in the compounds produced by M. anisopliae, might also be involved.
5. Current and Future Perspectives for a Large-Scale Application
Despite the vast number of studies, mostly in vitro, but also in planta, showing the antifungal properties of many fungal species, especially endophytes, which have partially been summarized in the present review, very few isolates are currently authorized by the European Union (EU) to be used against fungal phytopathogens (Table 2). Of the current few fungus-based products authorized by the EU as fungicides, none are metabolites. Therefore, the antifungal properties of many fungal species, especially endophytes, present great potential for use as biological fungicides [137], although so far they remain a research curiosity rather than a trait of commercial significance, at least with regard to agricultural purposes, as indicated by Card et al. [138] in their review on the antagonism toward plant pathogens by Epichloë endophytes.

Table 2.
Fungal species authorized by the European Union to be applied against fungal pathogens.
The reasons explaining the very limited large-scale usage of fungal species as biocontrol agents against fungal pathogens of plants are many and can be analyzed from several points of view: efficiency on a large scale, food security, ecological impact, farmer acceptance, scaling-up of production and commercialization. Regarding efficiency on a large scale, there are numerous cases in which the promising results obtained in vitro (or even in planta under greenhouse conditions) are not translated under in-field conditions [139], where there are numerous unpredictable factors. However, the real problem lies in the fact that we do not actually know the importance of this lack of transferability, as very few studies include such in-field conditions in their research.
In-field experiments are much more complicated to carry out, as there are many uncontrolled variables that can ruin the assay, there is much more uncertainty regarding the achievement of good results and those results are usually obtained after a longer experimental time. All of these aspects, in addition to the fact that results obtained under controlled environments (either in vitro or in planta in the greenhouse) are already publishable in many scientific journals, discourage researchers from evaluating the actual applicability on a large scale of the endophytes studied. In the few cases where the biocontrol potential of fungal species was evaluated in the field, the causes for the lack of transferability, or at least for the inconsistency in the results, are related to the fact that fungi, and the effects they produce in plants, are highly influenced by environmental conditions (especially in epiphytes, but also in endophytes), the type of relationship with the plant host (which is also influenced by the environment) and the interactions with other microorganisms involved [140].
In the case of endophytic fungi (the most important ones in plant protection), the environmental conditions may be important not only to establish the association between endophyte and plant host, but also to produce the defensive response by the endophyte once the association has been established [141,142]. The variability of endophyte and host genotypes and the variability of the environmental conditions may result in a significant inconsistency in the responses observed. In some cases, even the same endophyte–host association has been found to be beneficial for the host under specific environmental conditions, while detrimental under others [143,144]. This fact may reduce the effectiveness of the association, and consequently limit its eventual application on a large scale in the field, despite the potential for endophytes to be biocontrol agents against phytopathogens. However, these above-mentioned problems are mostly derived directly from the use of the living organism. An interesting way to address such inconveniences may be the utilization of the bioactive compounds produced by the fungus (in the cases where they were responsible for a positive effect) as their efficacy may not be as dependent on environmental conditions, and the establishment of the endophyte–plant host association may not need to be produced. This fact can be observed in the efficacy of the metabolite sporothriolid (in Table 1), which was relatively similar under both in vitro and greenhouse conditions. Nevertheless, as in the case of the living organism, metabolites have also been little studied to date under in-field conditions (Table 1). Therefore, further efforts should be made to evaluate the transferability of the positive results found under laboratory conditions to the field.
With respect to food security and ecological impact, it may be important to evaluate if the application of the fungal species produces harmful effects in humans, livestock, wildlife, flora, mycorrhizal fungi, invertebrates, beneficial microorganisms or ground water. This is a vital issue as many substances that are produced by fungi have been found to present toxic effects that could affect non-target organisms, such as insects [46], plants [145] or mammals [146]. Therefore, although a fungus may exhibit strong antifungal activity, its utilization as a biocontrol agent should be avoided or limited if toxicity for other organisms is detected. Furthermore, toxicity should be evaluated under different conditions and hosts because, when a living organism is used, several other substances could be produced by the fungus in addition to those causing the antifungal activity, as the production of multiple metabolite types is quite common in many fungal species, especially endophytes [110,147]. In the case of directly using the metabolite instead of the living organism, this multiple production may not be produced, thus facilitating the evaluation process. In any case, strict regulations in different countries (such as those in the EU) for the utilization of active substances as fungicides, which undoubtedly serve to protect our health, have also somewhat limited further development of this type of product.
Another reason for the scarce utilization of this type of product in the field is the still low acceptance by farmers, who are still quite skeptical about the efficiency of its application in comparison with chemical pesticides. This founded perception is based on the fact that most of the fungi-based products are able to reduce the incidence of disease, but they do not produce a complete suppression of the pathogen. In very few cases, the efficiency of the biological product is superior to the chemical fungicide. Only when using the metabolite instead of the living organism, such as in some synthetic derivatives from griseofulvin, a better efficiency has been found in comparison with chemical fungicides, such as hymexazol, thiophanate-methyl, ketoconazole or bifonazole, for the control of several phytopathogens [148,149]. Nevertheless, the cases in which the biological compound has been found to be more efficient than the corresponding chemical substance are mainly limited to in vitro experiments (Table 1). Further experiments in the field should be conducted to evaluate if biological control can be maintained under such uncontrolled conditions. In any case, most of the cases present an efficacy percentage below 50% (Table 1). Therefore, while these new fungi-based products do not present at least the same efficacy as that of the chemical options, farmers may be unwilling to use these types of substances, unless consumers are able to pay a higher price for foods produced under such conditions. Likewise, the management and application processes of a living organism are usually more complicated than those of a chemical compound, due to the storage requirements, the application medium preparation and the application procedures, which may be onerous for farmers. This fact may aggravate the already low motivation of farmers to use these types of products. Once again, the use of metabolites instead of living organisms may facilitate such utilization, as the procedure for managing and applying the product may be similar to that of the chemical substances. Furthermore, as explained earlier, the metabolite effect may not be as dependent on external conditions, so its efficiency may be higher than that of the living organism.
Finally, commercializing companies are also very important actors in the large-scale implementation of this type of product, as they are responsible for the development, production and commercialization of the product. Therefore, the scarce utilization of fungi-derived products so far may be partially because many conventional fungicide-manufacturing companies could be unwilling to adapt to the different procedures that this form of manufacturing requires. A major investment is required for such an adaptation in a sector perhaps not mature enough yet, as the farmers’ unwillingness to use these products may indicate. Nevertheless, several studies, such as that of Ganeshan et al. [150], have tried to help the industry translate the research from a laboratory scale to a large-scale commercial production manufacturing process by including the main steps for the optimization processes in bioreactors. However, more important than the production process may be the initial development of the active substance to be further produced and commercialized. This development could take a very long time, requiring strong investment and, most importantly, very sophisticated know-how, along with a technological and fully researched background. All of these important aspects discourage many companies from undertaking the long and winding road to the development of a new product. A suitable option for companies interested in the production of these products may be the establishment of consortia with universities or scientific centers that can manage and take the lead with regard to the most technological tasks and the research. In several countries, such as Spain, the most recent governmental policies encouraged these kinds of associations [151] in order to favor small or medium-sized companies that might be unable to accomplish such a development on their own.
6. Concluding Remarks
Despite the huge potential for using fungal species, especially endophytes or their active metabolites, in the control of fungal diseases once a commercial bio-pesticide is developed, the interest in their application is relatively recent, coinciding with increasing societal concern about environmental issues and their relationship with human health. For this reason, the literature explaining the techniques for industrializing the production of endophytes or their metabolites is still scarce, especially for a large-scale application of metabolites. One of the few examples can be found in the work conducted by Wang et al. [152], where a procedure for industrializing the production of metabolites from Paecilomyces variotii was established. In general, the development of a commercial bio-fungicide based on the metabolites produced by fungal species could involve the following stages: (i) isolation and identification of novel fungal species, especially endophytes, from sources that could favor further positive effects; (ii) screening and selecting fungi that could potentially develop the target effect (in our case antifungal activity); (iii) evaluation of artificial inoculation of the selected fungal species in target plant hosts; (iv) production of extracts under various conditions; (v) isolation and identification of the metabolites presented in the extracts; (vi) evaluation of the bioactivity of each isolated metabolite; (vii) optimization of the fermentation conditions to maximize the production of the active metabolite; (viii) development of the commercial formulations by evaluating the best ingredients; (ix) evaluation of the efficiency of the different formulations in the field; and (x) establishment of a marketing strategy and commercialization.
Most of the research studies have been focused on one or several of the points from (i) to (vi). However, the little effort made so far by researchers and companies to develop the points from (vii) to (x) may explain the minimal relevance that this type of product currently has. One of the few works, in this case involving point (vii) (optimization of the fermentation conditions), is the aforementioned study carried out by Ganeshan et al. [150], where a review of the concepts, challenges and perspectives regarding the scale-up production of plant endophytes in bioreactors is given. Therefore, these points should be strengthened in order to successfully implement these bio-products in agriculture. In our opinion, the cooperation between research institutes and companies could be of key importance for the achievement of this goal, which undoubtedly could also be favored by public policies.
Author Contributions
Conceptualization, O.S.; writing—original draft preparation, S.R.; writing—review and editing, O.S., S.R. and C.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
Carlos García-Latorre has been financed by a pre-doctoral grant (PD18037) from the Regional Government of Extremadura (Spain) and by the European Social Fund (ESF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, D.; Robson, G.D.; Trinci, A.P.J. 21st Century Guidebook to Fungi, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 1–610. [Google Scholar]
- Loo, J.A. Ecological impacts of non-native invertebrates and fungi on terrestrial ecosystems. Biol. Invasions 2009, 11, 81–96. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.L.; Simon, J.M.; McCoy, E.S.; Salazar, G.; Fragola, G.; Zylka, M.J. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat. Commun. 2016, 7, 11173. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.C.; Semple, K.T.; Bending, G.D. Isolation and characterisation of azoxystrobin degrading bacteria from soil. Chemosphere 2014, 95, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Leitão, S.; Cerejeira, M.J.; Van den Brink, P.J.; Sousa, J.P. Effects of azoxystrobin, chlorothalonil, and ethoprophos on the reproduction of three terrestrial invertebrates using a natural Mediterranean soil. Appl. Soil Ecol. 2014, 76, 124–131. [Google Scholar] [CrossRef]
- Warming, T.P.; Mulderij, G.; Christoffersen, K.S. Clonal variation in physiological responses of Daphnia magna to the strobilurin fungicide azoxystrobin. Environ. Toxicol. Chem. 2009, 28, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.T.; Lopes, I.; Pardal, M.A. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: A review. Environ. Int. 2013, 53, 18–28. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual; British Crop Protection Council: Farnham, UK, 2000. [Google Scholar]
- Leroch, M.; Kretschmer, M.; Hahn, M. Fungicide resistance phenotypes of Botrytis cinerea isolates from commercial vineyards in South West Germany. J. Phytopathol. 2011, 159, 63–65. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A.A.A.F. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Feichtenberger, E. Citrus phytophthora diseases: Management challenges and successes. J. Citrus Pathol. 2015, 2, 27203. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Biocontrol agent, biofumigation, and grafting with resistant rootstock suppress soil-borne disease and improve yield of tomato in West Virginia. Crop Prot. 2021, 145, 105630. [Google Scholar] [CrossRef]
- Romeralo, C.; Santamaría, O.; Pando, V.; Diez, J.J. Fungal endophytes reduce necrosis length produced by Gremmeniella abietina in Pinus halepensis seedlings. Biol. Control 2015, 80, 30–39. [Google Scholar] [CrossRef]
- Rebek, E.J.; Frank, S.D.; Royer, T.A.; Bográn, C.E. Alternatives to chemical control of insect pests. In Insecticides—Basic and Other Applications; Solonesky, S., Larramendy, M.L., Eds.; InTech: London, UK, 2012; pp. 171–196. [Google Scholar] [CrossRef][Green Version]
- Dong, C.; Wang, L.; Li, Q.; Shang, Q. Epiphytic and endophytic fungal communities of tomato plants. Hortic. Plant J. 2021, 7, 38–48. [Google Scholar] [CrossRef]
- Muller, T.; Ruppel, S. Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol. Ecol. 2014, 87, 2–17. [Google Scholar] [CrossRef]
- Mota, S.F.; Padua, P.F.; Ferreira, A.N.; Gomes, L.D.W.; Dias, M.A.; Souza, E.A.; Pereira, O.L.; Cardoso, P.G. Biological control of common bean diseases using endophytic Induratia spp. Biol. Control 2021, 159, 104629. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaría, O.; Halecker, S.; Lledó, S.; Stadler, M. Antagonism between Byssochlamys spectabilis (anamorph Paecilomyces variotii) and plant pathogens: Involvement of the bioactive compounds produced by the endophyte. Ann. Appl. Biol. 2017, 171, 464–476. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Jin, Q.; Peng, H.; Zhu, T.; Ye, H. Effect of a fungus, Hypoxylon spp., on endophytes in the roots of Asparagus. FEMS Microbiol. Lett. 2019, 366, fnz207. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Maynard, Z.; Gilbert, G.S.; Coley, P.D.; Kursar, T.A. Are tropical fungal endophytes hyperdiverse? Ecol. Lett. 2000, 3, 267–274. [Google Scholar] [CrossRef]
- Saikkonen, K.; Ahlholm, J.; Helander, M.; Lehtimäki, S.; Niemeläinen, O. Endophytic fungi in wild and cultivated grasses in Finland. Ecography 2000, 23, 360–366. [Google Scholar] [CrossRef]
- Lledó, S.; Rodrigo, S.; Poblaciones, M.J.; Santamaria, O. Biomass yield, nutritive value and accumulation of minerals in Trifolium subterraneum L. as affected by fungal endophytes. Plant Soil 2016, 405, 197–210. [Google Scholar] [CrossRef]
- Whithe, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagycycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- García-Latorre, C.; Rodrigo, S.; Santamaría, O. Endophytes as plant nutrient uptake-promoter in plants. In Endophytes: Mineral Nutrient Management, Volume 3. Sustainable Development and Biodiversity; Maheshwari, D.K., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2021; Volume 26, pp. 247–265. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Effect of fungal endophytes on plant growth and nutrient uptake in Trifolium subterraneum and Poa pratensis as affected by plant host specificity. Mycol. Progress 2021, 20, 1217–1231. [Google Scholar] [CrossRef]
- Vázquez-de-Aldana, B.R.; Zabalgogeazcoa, I.; García-Ciudad, A.; García-Criado, B. An Epichloë endophyte affects the competitive ability of Festuca rubra against other grassland species. Plant Soil 2013, 362, 201–213. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Cortes-Penagos, C.; Lopez-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Spiering, M.J.; Greer, D.H.; Schmid, J. Effects of the fungal endophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of in planta endophyte concentration. Ann. Bot. 2006, 98, 379–387. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Bauerle, T.L. A new currency for mutualism? Fungal endophytes alter antioxidant activity in hosts responding to drought. Fungal Divers. 2012, 54, 39–49. [Google Scholar] [CrossRef]
- Ghabooli, M.; Khatabi, B.; Ahmadi, F.S.; Sepehri, M.; Mirzaei, M.; Amirkhani, A.; Jorrín-Novo, J.V.; Salekdeh, G.H. Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J. Proteomics 2013, 94, 289–301. [Google Scholar] [CrossRef]
- Zhou, X.R.; Dai, L.; Xu, G.F.; Shen, H. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 2021, 11, 7637. [Google Scholar] [CrossRef]
- Ismail, I.; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Khan, M.A.; Lee, I.J. An endophytic fungus Gliocladium cibotii regulates metabolic and antioxidant system of glycine max and helianthus annuus under heat stress. Pol. J. Environ. Stud. 2021, 30, 1631–1640. [Google Scholar] [CrossRef]
- Zamani, N.; Sabzalian, M.R.; Khoshgoftarmanesh, A.; Afyuni, M. Neotyphodium endophyte changes phytoextraction of zinc in Festuca arundinacea and Lolium perenne. Int. J. Phytoremediat. 2015, 17, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Moghaddam, M.S.H.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Ważny, R.; Rozpądek, P.; Domka, A.; Jędrzejczyk, R.J.; Nosek, M.; Hubalewska-Mazgaj, M.; Lichtscheidl, I.; Kidd, P.; Turnau, K. The effect of endophytic fungi on growth and nickel accumulation in Noccaea hyperaccumulators. Sci. Total Environ. 2021, 768, 144666. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Verma, V.C.; Wheeler, T.A.; Woodward, J.E.; Starr, J.L.; Sword, G.A. Tapping into the cotton fungal phytobiome for novel nematode biological control tools. Phytobiomes J. 2020, 4, 19–26. [Google Scholar] [CrossRef]
- Kumar, K.K.; Dara, S.K. Fungal and bacterial endophytes as microbial control agents for plant-parasitic nematodes. Int. J. Environ. Res. Public. Health 2021, 18, 4269. [Google Scholar] [CrossRef]
- Hartley, S.; Gange, A. Impacts of plant symbiotic fungi on insect herbivores: Mutualism in a multitrophic context. Annu. Rev. Entomol. 2009, 54, 323–342. [Google Scholar] [CrossRef]
- García Parisi, P.A.; Grimoldi, A.A.; Omacini, M. Endophytic fungi of grasses protect other plants from aphid herbivory. Fungal Ecol. 2014, 9, 61–64. [Google Scholar] [CrossRef]
- Ramakuwela, T.; Hatting, J.; Bock, C.; Vega, F.E.; Wells, L.; Mbata, G.N.; Shapiro-Ilan, D. Establishment of Beauveria bassiana as a fungal endophyte in pecan (Carya illinoinensis) seedlings and its virulence against pecan insect pests. Biol. Control 2020, 140, 104102. [Google Scholar] [CrossRef]
- Santamaría, O.; González, M.A.; Pajares, J.A.; Diez, J.J. Effect of fungicides, endophytes and fungal filtrates on in vitro growth of Spanish isolates of Gremmeniella abietina. For. Pathol. 2007, 37, 251–262. [Google Scholar] [CrossRef]
- Farhat, H.; Urooj, F.; Tariq, A.; Sultana, V.; Ansari, M.; Ahmad, V.U.; Ehteshamul-Haque, S. Evaluation of antimicrobial potential of endophytic fungi associated with healthy plants and characterization of compounds produced by endophytic Cephalosporium and Fusarium solani. Biocatal. Agric. Biotechnol. 2019, 18, 101043. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Silva-Valderrama, I.; Toapanta, D.; de los Angeles Miccono, M.; Lolas, M.; Díaz, G.A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2021, 11, 3311. [Google Scholar] [CrossRef]
- Santamaria, O.; Smith, D.R.; Stanosz, G.R. Interaction between Diplodia pinea or Diplodia scrobiculata and fungal endophytes isolated from pine shoots. Can. J. For. Res. 2012, 42, 1819–1826. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Hao, Q.; Albaghdady, D.M.D.; Xiao, Y.; Xiao, X.; Mo, C.; Tian, T.; Wang, G. Endophytic Metarhizium anisopliae is a potential biocontrol agent against wheat Fusarium head blight caused by Fusarium graminearum. J. Plant Pathol. 2021, 103, 875–885. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef]
- Zivanovic, A.; Rodgers, L. The role of fungal endophytes in plant pathogen resistance. Bios 2018, 89, 192–197. [Google Scholar] [CrossRef]
- Pavithra, G.; Sumant, B.; Meenakshi, R.; Seweta, S. Role of endophytic microbes against plant pathogens: A review. Asian J. Plant Sci. 2020, 19, 54–62. [Google Scholar] [CrossRef]
- Gao, F.-K.; Dai, C.-C.; Liu, X.-Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I. Fungal endophytes and their interaction with plant pathogens. Span. J. Agric. Res. 2008, 6, 138–146. [Google Scholar] [CrossRef]
- Elad, Y. Mechanisms involved in the biological control of Botrytis cinerea incited diseases. Eur. J. Plant Pathol. 1996, 102, 719–732. [Google Scholar] [CrossRef]
- Bailey, B.; Bae, H.; Strem, M.; Crozier, J.; Thomas, S.; Samuels, G.; Vinyard, B.; Holmes, K. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol. Control. 2008, 46, 24–35. [Google Scholar] [CrossRef]
- Zeilinger, S.; Galhaup, C.; Payer, K.; Woo, S.L.; Mach, R.L.; Fekete, C.; Lorito, M.; Kubicek, C.P. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet. Biol. 1999, 26, 131–140. [Google Scholar] [CrossRef]
- Harman, G.; Howell, C.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Molitor, A.; Zajic, D.; Voll, L.M.; Pons-Kühnemann, J.; Samans, B.; Kogel, K.-H.; Waller, F. Barley leaf transcriptome and metabolite analysis reveals new aspects of compatibility and Piriformospora indicamediated systemic induced resistance to powdery mildew. Mol. Plant Microbe Interact. 2011, 24, 1427–1439. [Google Scholar] [CrossRef]
- Nassimi, Z.; Taheri, P. Endophytic fungus Piriformospora indica induced systemic resistance against rice sheath blight via affecting hydrogen peroxide and antioxidants. Biocontrol Sci. Technol. 2017, 3157, 256–267. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, Q.; Chen, C.; Zhang, C. Differential communications between fungi and host plants revealed by secretome analysis of phylogenetically related endophytic and pathogenic fungi. PLoS ONE 2016, 11, e0163368. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Tellier, A. Plant-parasite coevolution: Bridging the gap between genetics and ecology. Annu. Rev. Phytopathol. 2011, 49, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef]
- Clarke, B.B.; White, J.F.; Hurley, H.; Torres, M.S.; Sun, S.; Huff, D.R. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Dis. 2006, 90, 994–998. [Google Scholar] [CrossRef]
- Istifadah, N.; McGee, P.A. Endophytic Chaetomium globosum reduces development of tan spot in wheat caused by Pyrenophora tritici-repentis. Australas. Plant Pathol. 2006, 35, 411–418. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: An interdisciplinary perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef]
- Sahoo, S.; Subban, K.; Chelliah, J. Diversity of marine macro-algicolous endophytic fungi and cytototix potential of Biscogniauxia petrensis metabolites against cancer cell lines. Front. Microbiol. 2021, 12, 650177. [Google Scholar] [CrossRef]
- Adhila Haji Basheerudeen, M.; Mushtaq, S.A.; Soundhararajan, R.; Kumar Nachimuthu, S.; Srinivasan, H. Marine endophytic fungi mediated Silver nanoparticles and their application in plant growth promotion in Vigna radiata L. Int. J. Nano Dimens. 2021, 12, 1–10. [Google Scholar]
- Taritla, S.; Jumari, M.; Kamat, S.; Bhat, S.G.; Jayabaskaran, C. Optimization of physicochemical parameters for production of cytotoxic secondary metabolites and apoptosis induction activities in the culture extract of a marine algal-derived endophytic fungus Aspergillus sp. Front. Pharmacol. 2021, 12, 542891. [Google Scholar] [CrossRef]
- Quader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and antibiofilm activities of the fungal metaboiltes isolated form the marine endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Savidov, N.; Gloriozov, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Gond, S.K. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Mazumder, K.; Ruma, Y.N.; Akter, R.; Aktar, A.; Hossain, M.M.; Shahiina, Z.; Mazumdar, S.; Kerr, P.G. Identification of bioactive metabolites and evaluation of in vitro anti-inflammatory and in vivo antinociceptive and antiarthritic activities of endophyte fungi isolated from Elaeocarpus floribundus blume. J. Ethnopharmacol. 2021, 273, 113975. [Google Scholar] [CrossRef]
- Kiliç, G.; Tosun, G.; Bozdeveci, A.; Erik, I.; Öztürk, E.; Reis, R.; Sipahi, H.; Cora, M.; Karaoğlu, S.A.; Yaylı, N. Antimicrobial, cytotoxic, antiviral effects, and spectroscopic characterization of metabolites produced by Fusarium oxysporum YP9B. Rec. Nat. Prod. 2021, 15, 547–567. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S. Afr. J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Vukelic, I.D.; Prokic, L.T.; Racic, G.M.; Pešic, M.B.; Bojovic, M.M.; Sierka, E.M.; Kalaji, H.M.; Pankovic, D.M. Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt. J. Basic Appl. Sci. 2018, 5, 303–312. [Google Scholar] [CrossRef]
- Palupi, K.D.; Ilyas, M.; Agusta, A. Endophytic fungi inhabiting Physalis angulata L. plant: Diversity, antioxidant, and antibacterial activities of their ethyl acetate extracts. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 823–829. [Google Scholar] [CrossRef]
- Kumaresan, S.; Karthi, V.; Senthilkumar, V.; Balakumar, B.S.; Stephen, A. Biochemical constituents and antioxidant potential of endophytic fungi isolated from the leaves of Azadirachta indica A. Juss (neem) from Chennai, India. J. Acad. Ind. Res. 2015, 3, 355–361. [Google Scholar]
- Bauerfeind, J.C. Carotenoids as Colorants and Vitamin A Precursors; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Marie, C.F.; Regine, M.Q.; Stephane, W.; Emilie, T.; Joseph, R.; Brigitte, W.R.; Edmond, R.; Marie, P.V. Immune status is more affected by age than by carotenoid depletionrepletion in healthy human subjects. Br. J. Nutr. 2012, 108, 2054–2065. [Google Scholar] [CrossRef]
- Hwang, J.S.; You, Y.H.; Bae, J.J.; Khan, S.A.; Kim, J.G.; Choo, Y.S. Effects of endophytic fungal secondary metabolites on growth and physiological response of Carex kobomugi. J. Coast. Res. 2011, 27, 544–548. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.L.; Qu, Z.M.; Zhou, H.Y.; Qi, Y.J.; Liu, Z.G.; Zhang, M. Maize yield and root morphological characteristics affected by controlled release diammonium phosphate and Paecilomyces variotii extracts. Field Crops Res. 2020, 255, 107862. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Chen, B.; Zhang, M.; Liu, Z.; Wang, Q.; Ma, J. Paecilomyces variotii extracts and controlled-release urea synergistically increased nitrogen use efficiency and rice yield. ACS Omega 2020, 22, 13303–13311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological activities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.-Q.; Zhao, D.-L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef]
- Huber, F.M.; Gottlieb, D. The mechanism of action of griseofulvin. Can. J. Microbiol. 1968, 14, 111–118. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Naturally occurring bioactive Cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef]
- Sergeiko, A.; Poroikov, V.V.; Hanuš, L.O.; Dembitsky, V.M. Cyclobutane-containing alkaloids: Origin, synthesis, and biological activities. Open Med. Chem. J. 2008, 2, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef] [PubMed]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef]
- Seigler, D.S. Acetylenic compounds. In Plant Secondary Metabolism; Seigler, D.S., Ed.; Springer: Boston, MA, USA, 1998; pp. 42–50. [Google Scholar] [CrossRef]
- Azerang, P.; Sardari, S. Antifungal activity of enynediesters and acetylenic compounds obtained by synthesis and in silico prediction pattern. J. Mycol. Med. 2012, 22, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Tripathi, S.K.; Feng, Q.; Lorenz, M.C.; Wright, M.A.; Jacob, M.R.; Mask, M.M.; Baerson, S.R.; Li, X.C.; Clark, A.M.; et al. A potent plant-derived antifungal acetylenic acid mediates its activity by interfering with fatty acid homeostasis. Antimicrob. Agents Chemother. 2012, 56, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.D.; Gomes, R.R.; Gonçalves, R.; Fornari, G.; Maia, B.H.L.N.S.; Schmidt-Dannert, C.; Gaascht, F.; Glienke, G.; Schneider, G.X.; Colombo, I.R.; et al. Molecular identification and antimicrobial activity of foliar endophytic fungi on the Brazilian pepper tree (Schinus terebinthifolius) reveal new species of Diaporthe. Curr. Microbiol. 2021, 78, 3218–3229. [Google Scholar] [CrossRef]
- Li, R.; Chen, R.; Niu, S.; Guo, L.; Yin, J.; Che, Y. Exserolides A–F, new isocoumarin derivatives from the plant endophytic fungus Exserohilum sp. Fitoterapia 2014, 96, 88–94. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, N.N.; Kang, Y.F.; Ma, Y.M. A new furan derivative from an endophytic Aspergillus tubingensis of Decaisnea insignis (Griff.) Hook.f. & Thomson. Nat. Prod. Res. 2019, 33, 2777–2783. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Goda, A.M. Antifungal and cytotoxic constituents from the endophytic fungus Penicillium sp. Bull. Fac. Pharmacy Cairo Univ. 2017, 55, 85–89. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Zhu, S.; He, Y.; Ding, W.; Li, C. Two new compounds from Nigrospora sphaerica ZMT05, a fungus derivated from Oxya chinensis Thunber. Nat. Prod. Res. 2017, 32, 2375–2381. [Google Scholar] [CrossRef]
- Silva-Hughes, A.F.; Wedge, D.E.; Cantrell, C.L.; Carvalho, C.R.; Pan, Z.; Moraes, R.M.; Madoxx, V.L.; Rosa, L.H. Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol. Res. 2015, 175, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pang, X.J.; Xu, L.L.; Zhao, T.; Long, X.Y.; Zhang, Q.Y.; Qin, H.L.; Yang, D.F.; Yang, X.L. Two new alkylated furan derivatives with antifungal and antibacterial activities from the plant endophytic fungus Emericella sp. XL029. Nat. Prod. Res. 2018, 32, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, D.L.; Liu, G.H.; Fu, X.; Gu, Y.G.; Ding, L.S.; Zhou, Y. Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy. J. Agric. Food Chem. 2020, 68, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.W.; Jiao, R.H.; Cheng, A.B.; Tan, S.H.; Song, Y.C. Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin A obtained from Cladosporium sp. World J. Microbiol. Biotechnol. 2007, 23, 79–83. [Google Scholar] [CrossRef]
- Wang, L.; Shen, J.; Xu, L.; Gao, J.; Zhang, C.; Wang, Y.; Chen, F. A metabolite of endophytic fungus Cadophora orchidicola from Kalimeris indica serves as a potential fungicide and TLR4 agonist. J. Appl. Microbiol. 2019, 126, 1383–1390. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, Y.; Hou, Y.; Yu, H.; Zhu, L.; Sun, Y.; Zhou, M.; Chen, Y.; Dong, M. Two new benzoic acid derivatives from endophytic fungus Aspergillus versicolor. Nat. Prod. Res. 2020, Online ahead of print. [CrossRef]
- Alcock, A.; Elmer, P.; Marsden, R.; Parry, F. Inhibition of Botrytis cinerea by Epirodin: A secondary metabolite from New Zealand isolates of Epicoccum nigrum. J. Phytopathol. 2015, 163, 841–852. [Google Scholar] [CrossRef]
- Richardson, S.N.; Nsiama, T.K.; Walker, A.K.; McMullin, D.R.; Miller, J.D. Antimicrobial dihydrobenzofurans and xanthenes from a foliar endophyte of Pinus strobus. Phytochemistry 2015, 117, 436–443. [Google Scholar] [CrossRef]
- Chowdhary, K.; Sharma, S. Plant growth promotion and biocontrol potential of fungal endophytes in the inflorescence of Aloe vera L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 1045–1055. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.B.; Xie, B. Biosynthesis of antibiotic leucinostatins in bio-control fungus Purpureocillium lilacinum and their inhibition on Phytophthora revealed by genome mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Li, F.; Zhou, D.; Xu, L.; Li, C. Identification and antifungal activity of metabolites from the mangrove fungus Phoma sp. L28. Chem. Nat. Compd. 2017, 53, 237–240. [Google Scholar] [CrossRef]
- Qin, J.; Lyu, A.; Zhang, Q.H.; Yang, L.; Zhang, J.; Wu, M.D.; Li, G.Q. Strain identification and metabolites isolation of Aspergillus capensis CanS-34A from Brassica napus. Mol. Biol. Rep. 2019, 46, 3451–3460. [Google Scholar] [CrossRef]
- d’Errico, G.; Aloj, V.; Flematti, G.R.; Sivasithamparam, K.; Worth, C.M.; Lombardi, N.; Ritieni, A.; Marra, R.; Lorito, M.; Vinale, F. Metabolites of a Drechslera sp. endophyte with potential as biocontrol and bioremediation agent. Nat. Prod. Res. 2021, 35, 4508–4516. [Google Scholar] [CrossRef]
- Ravindran, K.; Chitra, S.; Wilson, A.; Sivaramakrishnan, S. Evaluation of antifungal activity of Metarhizium anisopliae against plant phytopathogenic fungi. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 251–255. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, D.; Liang, F.; Wu, Z.; Shi, Z.; Li, C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semisynthesis. Fitoterapia 2017, 123, 23–28. [Google Scholar] [CrossRef]
- Li, E.; Jiang, L.; Guo, L.; Zhang, H.; Che, Y. Pestalachlorides A–C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 2008, 17, 7894–7899. [Google Scholar] [CrossRef] [PubMed]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrüugge, M.; Müller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2019, 35, 257–265. [Google Scholar] [CrossRef]
- Kock, I.; Draeger, S.; Schulz, B.; Elsässer, B.; Kurtán, T.; Kenéz, Á.; Antus, S.; Pescitelli, G.; Salvadori, P.; Speakman, J.B.; et al. Pseudoanguillosporin A and B: Two new isochromans isolated from the endophytic fungus Pseudoanguillospora sp. Eur. J. Org. Chem. 2009, 9, 1427–1434. [Google Scholar] [CrossRef]
- McMullin, D.R.; Nguyen, H.D.T.; Daly, G.J.; Menard, B.S.; Miller, J.D. Detection of foliar endophytes and their metabolites in Picea and Pinus seedling needles. Fungal Ecol. 2018, 31, 1–8. [Google Scholar] [CrossRef]
- Chapla, V.M.; Honório, A.E.; Gubiani, J.R.; Vilela, A.F.L.; Young, M.C.M.; Cardoso, C.L.; Pavan, F.R.; Cicarelli, R.M.; Michel Pinheiro Ferreira, P.; Bolzani, V.; et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020, 39, 116–123. [Google Scholar] [CrossRef]
- Cao, L.L.; Zhang, Y.Y.; Liu, Y.J.; Yang, T.T.; Zhang, J.L.; Zhang, Z.G.; Shen, L.; Liu, J.Y.; Ye, Y.H. Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp. A21 in Ginkgo biloba. Pestic. Biochem. Physiol. 2016, 129, 7–13. [Google Scholar] [CrossRef]
- Shentu, X.; Zhan, X.; Ma, Z.; Yu, X.; Zhang, C. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz. J. Microbiol. 2014, 45, 248–254. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Liu, Z. Antifungal activity of an endophytic fungus Aspergillus versicolor DYSJ3 from Aphanamixis grandifolia Blume against Colletotrichum musae. Mycobiology 2021, 49, 498–506. [Google Scholar] [CrossRef]
- Wang, J.; Galgoci, A.; Kodali, S.; Herath, K.B.; Jayasuriya, H.; Dorso, K.; Vicente, F.; González, A.; Cully, D.; Bramhill, D.; et al. Discovery of a small molecule that inhibits cell division by blocking FtsZ, a novel therapeutic target of antibiotics. J. Biol. Chem. 2003, 278, 44424–44428. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Rojas Torres, M. Biocontrol de moniliophthora roreri y phytophthora palmivora Patógeno de Cacao (theobroma cacao l.) Con Hongos Endífitos Procedentes del Alto Amazonas. Master’s Thesis, Universidad Nacional de San Martin, Tarapoto, Perú, 2013. [Google Scholar]
- Myrchiang, P.; Dkhar, M.S.; Devi, H.R. Studies on endophytic fungi associated with medicinally important aromatic plant Artemisia nilagirica (C.B. Clarke) Pamp. and their antagonistic activity against Phytophthora infestans. J. Adv. Lab. Res. Biol. 2014, 5, 112–119. Available online: https://e-journal.sospublication.co.in/index.php/jalrb/article/view/202 (accessed on 1 December 2021).
- Simamora, A.V.; Hahuly, M.V.; Henuk, J.B. Endophytic fungi as potential biocontrol agents of Phytophthora palmivora in the cocoa plant. Biodiversitas 2021, 22, 2601–2609. [Google Scholar] [CrossRef]
- Soković, M.D.; Glamoclija, J.M.; Ciric, A.D. Natural products from plants and fungi as fungicides. In Fungicides-Showcases of Integrated Plant Disease Management from Around the World; Nita, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Card, S.D.; Bastías, D.A.; Caradus, J.R. Antagonism to Plant Pathogens by Epichloë Fungal Endophytes—A Review. Plants 2021, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Wäli, P.R.; Helander, M.; Nissinen, O.; Saikkonen, K. Susceptibility of endophyte-infected grasses to winter pathogens (snow molds). Botany 2006, 84, 1043–1051. [Google Scholar] [CrossRef]
- Xu, X.; Jeger, M. More ecological research needed for effective biocontrol of plant pathogens. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; de Cal, A., Melgarejo, P., Magan, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 15–30. [Google Scholar] [CrossRef]
- Ahlholm, J.U.; Helander, M.; Lehtimäki, S.; Wäli, P.; Saikkonen, K. Vertically transmitted fungal endophytes: Different responses of host-parasite systems to environmental conditions. Oikos 2002, 99, 173–183. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A. Fungal endophytes in Mediterranean oak forests: A lesson from Discula quercina. Phytopathology 2008, 98, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Delaye, L.; García-Guzmán, G.; Heil, M. Endophytes versus biotrophic and necrotrophic pathogens—are fungal lifestyles evolutionarily stable traits? Fungal Divers. 2013, 60, 125–135. [Google Scholar] [CrossRef]
- Guo, Q.-Y.; Cheng, L.; Zhu, H.-X.; Li, W.; Wei, Y.-H.; Chen, H.-Y.; Guo, L.-Z.; Weng, H.; Wang, J. Herbicidal activity of Aureobasidium pullulans PA-2 on weeds and optimization of its solid-state fermentation conditions. J. Integr. Agric. 2020, 19, 173–182. [Google Scholar] [CrossRef]
- Caradus, J.R.; Card, S.D.; Finch, S.C.; Hume, D.E.; Johnson, L.J.; Mace, W.J.; Popay, A.J. Ergot alkaloids in New Zealand pastures and their impact. N. Z. J. Agric. Res. 2020, 63, 1–41. [Google Scholar] [CrossRef]
- González-Menéndez, V.; Crespo, G.; Toro, C.; Martín, J.; de Pedro, N.; Tormo, J.R.; Genilloud, O. Extending the metabolite diversity of the endophyte Dimorphosporicola tragani. Metabolites 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.B.; Gao, Y.Q.; Nie, X.D.; Tuong, T.M.; Li, D.; Gao, J.M. Antifungal activity of griseofulvin derivatives against phytopathogenic fungi in vitro and in vivo and three-dimensional quantitative structure-activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Kartsev, V.; Geronikaki, A.; Petrou, A.; Lichitsky, B.; Kostic, M.; Smiljkovic, M.; Soković, M.; Sirakanyanet, S. Griseofulvin derivatives: Synthesis, molecular docking and biological evaluation. Curr. Top. Med. Chem. 2019, 19, 1145–1161. [Google Scholar] [CrossRef]
- Ganeshan, S.; Kim, S.H.; Vujanovic, V. Scaling-up production of plant endophytes in bioreactors: Concepts, challenges and perspectives. Bioresour. Bioprocess. 2021, 8, 63. [Google Scholar] [CrossRef]
- Ministry of Science and Innovation of Spain. Gubernamental Strategy for Research, Technology and Innovation 2021–2023. Secretaría General Técnica del Ministerio de Ciencia e Innovación, Gobierno de España. 2021. Available online: https://www.ciencia.gob.es/gesdamdoc-servlet/?uuid=e1f1deb1-7321-4dd9-b8ca-f97ece358d1c&workspace=dam&formato=pdf (accessed on 1 December 2021).
- Wang, Q.; Peng, C.; Shi, L.; Liu, Z.; Zhou, D.; Meng, H.; Zhao, H.; Li, F.; Zhang, M. A technical system for the large-scale application of metabolites from Paecilomyces variotii SJ1 in agriculture. Front. Bioeng. Biotechnol. 2021, 9, 671879. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).