High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings
Abstract
1. Introduction
2. Results
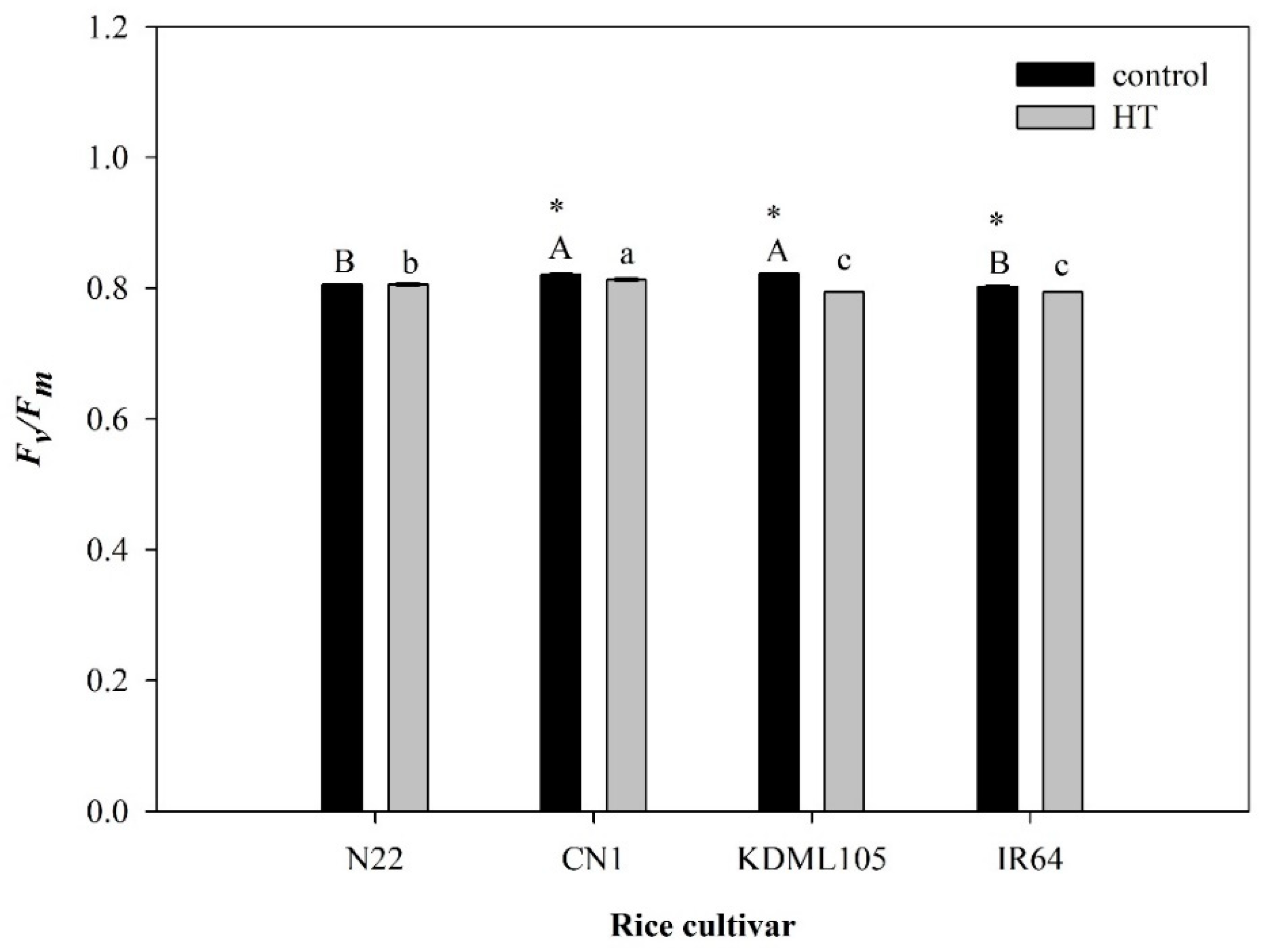
2.1. PSII Efficiency after High Temperature Exposure
2.2. Leaf Membrane Stability after High Temperature Exposure
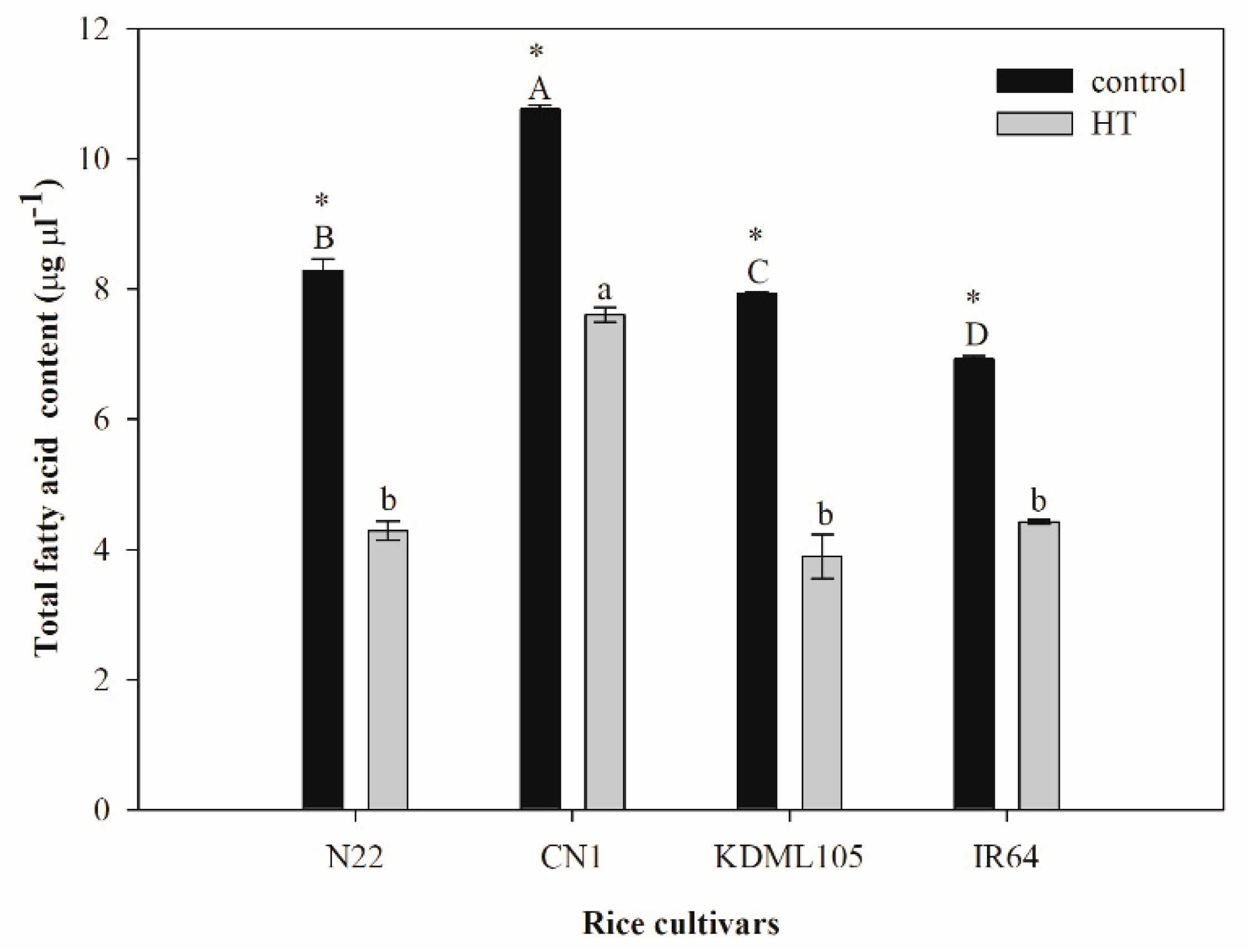
2.3. Alteration of Leaf Total Fatty Acids after High Temperature Exposure
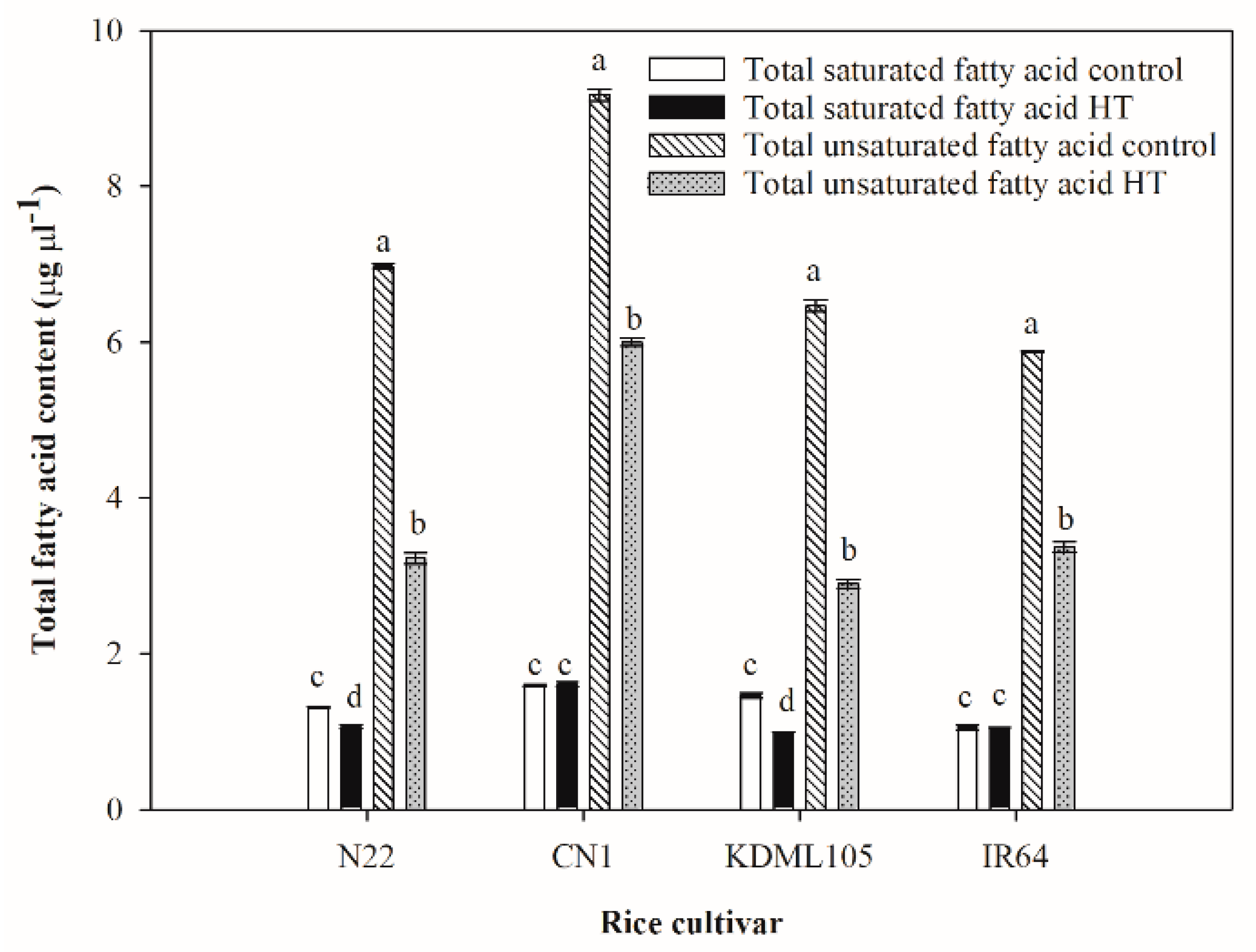
2.4. Alteration of Leaf Saturated Fatty Acid Composition after High Temperature Exposure
2.5. Alteration of Leaf Unsaturated Fatty Acid Composition after High Temperature Exposure
2.6. Alteration of Leaf Ratio of Saturated and Unsaturated Fatty Acids after High Temperature Exposure
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Temperature Condition
4.2. Determination of Photosynthetic Efficiency of PSII
4.3. Determination of Electrolyte Leakage
4.4. Determination of Malondialdehyde Contents
4.5. Determination of Fatty Acid
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, T.; Amombo, E.; Wang, G.; Xie, Y.; Fu, J. The Alleviation of Heat Damage to Photosystem II and Enzymatic Antioxidants by Exogenous Spermidine in Tall Fescue. Front. Plant Sci. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Busheva, M.; Tzonova, I.; Stoitchkova, K.; Andreeva, A. Heat-induced reorganization of the structure of photosystem ii membranes: Role of oxygen evolving complex. J. Photochem. Photobiol. B 2012, 117, 214–221. [Google Scholar] [CrossRef]
- Hu, L.; Bi, A.; Hu, Z.; Amombo, E.; Li, H.; Fu, J. Antioxidant metabolism, photosystem ii, and fatty acid composition of two tall fescue genotypes with different heat tolerance under high temperature stress. Front. Plant Sci. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, S.; Gao, R.; Zhang, Z.; Jiang, C.; Liu, Y. Response of plants photosynthesis to higher temperature. Bull. Bot. Res. Harbin 2002, 22, 205–212. [Google Scholar]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Ausland, M.L.; Smith, K.E.; Price, A.H.; Wilson, Z.A.; Murchie, E.H. Rapid temperature responses of photosystem II efficiency forecast genotypic variation in rice vegetative heat tolerance. Plant J. 2020, 104, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Glatz, A.; Vass, I.; Los, D.A.; Vígh, L. The Synechocystis model of stress: From molecular chaperones to membranes. Plant Physiol. Biochem. 1999, 37, 1–12. [Google Scholar] [CrossRef]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.; Goloubinoff, P. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Huang, B. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrotis stolonifera). Environ. Exp. Bot. 2004, 51, 57–67. [Google Scholar] [CrossRef]
- Marcum, K.B. Cell membrane thermostability and whole plant heat tolerance of Kentucky bluegrass. Crop Sci. 1998, 38, 1214–1218. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Changes in fatty acid composition and saturation in leaves and roots of creeping bentgrass exposed to high soil temperature. J. Am. Soc. Hortic. Sci. 2004, 129, 795–801. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the responses of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Fan, W.; Evans, R.M. 2015. Turning Up the Heat on Membrane Fluidity. Cell 2015, 161, 962–963. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhang, T.J.; Lu, X.X.; Ma, B.L.; Ren, A.; Shi, L.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Membrane fluidity is involved in the regulation of heat stress induced secondary metabolism in Ganoderma lucidum. Environ. Microbiol. 2017, 19, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.K.; Li, Z.J.; Lu, A.Y.; Sun, F.; Chen, S.D.; Rothe, M.; Menze, R.; Sun, F.; Horvitz, H.R. Acyl-CoA dehydrogenase drives heat adaptation by sequestering fatty acids. Cell 2015, 161, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Vigh, L.; Gombos, Z.; Horvath, I.; Joo, F. Saturation of membrane lipids by hydrogenation induces thermal stability in chloroplast inhibiting the heat dependent stimulation of Photosystem I mediated electron transport. Biochim. Biophys. Acta 1989, 979, 361–364. [Google Scholar] [CrossRef]
- Vigh, L.; Los, D.A.; Horvath, I.; Murata, N. The primary signal in the biological perception of temperature: Pd catalysed hydrogenation of the membrane lipids stimulated the expression of the desA gene in Synecchocystis PC6803. Proc. Natl. Acad. Sci. USA 1993, 90, 9090–9094. [Google Scholar] [CrossRef]
- Horvath, I.; Glatz, A.; Varvasovszki, V.; Zsolt, T.; Pali, T.; Balogh, G.; Kovacs, E.; Nadasdi, L.; Benko, S.; Joo, F.; et al. Membrane physical state controls the signalling mechanism of the heat shock response in Syneschocystis PCC 6803: Identification of HSP17 as a fluidity gene. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef]
- Grove, A.; Agarwal, M.; Katiyar-Argarwal, S.; Sahi, C.; Argarwal, S. Production of high temperature tolerant transgenic plant through manipulation of membrane lipids. Curr. Sci. 2000, 79, 557–559. [Google Scholar]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: I. high day and high temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef]
- Zoong Lwe, Z.; Sah, S.; Persaud, L.; Li, J.; Gao, W.; Reddy, K.R.; Narayanan, S. Alterations in the leaf lipidome of Brassica carinata under high-temperature stress. BMC Plant Biol. 2021, 21, 404. [Google Scholar]
- Narayanan, S.; Zoong-Lwe, Z.S.; Gandhi, N.; Welti, R.; Fallen, B.; Smith, J.R.; Rustgi, S. Comparative lipidomic analysis reveals heat stress responses of two soybean genotypes differing in temperature sensitivity. Plants 2020, 9, 457. [Google Scholar] [CrossRef]
- Zoong Lwe, Z.S.; Welti, R.; Anco, D.; Naveed, S.; Rustgi, S.; Narayanan, S. Heat stress elicits remodeling in the anther lipidome of peanut. Sci. Rep. 2020, 10, 22163. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, B.R.; Li-Xin, X.U.; And, L.I. Heat stress effects on osmotic potential, membrane fatty acid composition and lipid peroxidation content of two kentucky bluegrass cultivars differing in drought tolerance. Acta Hortic. Sin. 2013, 40, 971–980. [Google Scholar]
- Yoshida, H.; Tanigawa, T.; Kuriyama, I.; Yoshida, N.; Tomiyama, Y.; Mizushina, Y. Variation in Fatty Acid Distribution of Different Acyl Lipids in Rice (Oryza sativa L.) Brans. Nutrients 2011, 3, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.N.; Verma, S.; Goswami, S.P.; Devedee, A.K. Effect of temperature on different growth stages and physiological process of rice crop- as a review. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 162–169. [Google Scholar]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D.; et al. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Cui, L.J.; Li, J.L.; Fan, Y.M.; Xu, S.; Zhang, Z. High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Bot. Stud. 2006, 47, 61–69. [Google Scholar]
- Tang, Y.; Wen, X.; Lu, Q.; Yang, Z.; Cheng, Z.; Lu, C. Heat stress induces an aggregation of the light-harvesting complex of photosystem II in Spinach plants. Plant Physiol. 2007, 143, 629–638. [Google Scholar] [CrossRef]
- Paethaisong, W.; Lontom, W.; Dondsansuk, A. Impact of short-term exposure to elevated temperatures on physiology of Thai rice (cv. Riceberry). IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012083. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Garcés-Varón, G.; Restrepo-Díaz, H. Biochemical and physiological characterization of three rice cultivars under different daytime temperature conditions. Chil. J. Agric. Res. 2014, 74, 373–379. [Google Scholar] [CrossRef][Green Version]
- Dongsansuk, A.; Theerakulpisut, P.; Pongdontri, P. Short-term heat exposure effect on PSII efficiency and growth of rice (Oryza sativa L.). Pertanika J. Trop. Agric. Sci. 2017, 40, 621–628. [Google Scholar]
- Pansarakham, P.; Pongdontri, P.; Theerakulpisut, P.; Dongsansuk, A. Effect of short-term heat exposure on physiological traits of rice indica at grain-filling stage. Acta Physiol. Plant. 2018, 40, 173. [Google Scholar] [CrossRef]
- Borriboon, W.; Lontom, W.; Pongdontri, P.; Theerakulpisut, P.; Dongsansuk, A. Effects of Short- and Long-Term Temperature on Seed Germination. Oxidative Stress and Membrane Stability of Three Rice Cultivars (Dular, KDML105 and Riceberry). Pertanika J. Trop. Agric. Sci. 2018, 41, 151–162. [Google Scholar]
- Dongsansuk, A.; Paethaisong, W.; Theerakulpisut, P. Membrane stability and antioxidant enzyme activity of rice seedlings in response to short-term high temperature treatments. Chil. J. Agric. Res. 2021, 81, 607–617. [Google Scholar] [CrossRef]
- Pearcy, R.W. Effect of growth temperature on the fatty acid composition of the leaf lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1978, 61, 484–486. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Mathur, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). Biochim. Biophys. Acta 2011, 1807, 22–29. [Google Scholar] [CrossRef]
- Dongsansuk, A.; Neuner, G. Temperature optimum, stress temperature range and thermal limits of quantum yeid of PSII in tropical versus temperate plants. 2013. Trends Photochem. Photobiol. 2013, 15, 77–87. [Google Scholar]
- Lang, N.T.; Ha, P.T.T.; Tru, P.C.; Toam, T.B.; Buu, B.C.; Cho, Y.C. Breeding for heat tolerance rice based on marker-assisted backcrossing in Vietnam. Plant Breed. Biotech. 2015, 3, 274–281. [Google Scholar] [CrossRef]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Roa, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated Physiological, Biochemical, and Molecular Analysis Identifies Important Traits and Mechanisms Associated with Differential Response of Rice Genotypes to Elevated Temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem ii: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.C.; Hahne, G.; Liu, J.R.; Stewart, C.N., Jr. Plant lipid biology and biotechnology. Plant Cell Rep. 2015, 34, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Janila, P.; Pandey, M.K.; Shasidhar, Y.; Variath, M.T.; Sriswathi, M.; Khera, P.; Manohar, S.S.; Nagesh, P.; Vishwakarma, M.K.; Mishra, G.P.; et al. Molecular breeding for introgression of fatty acid desaturase mutant alleles (ahFAD2A and ahFAD2B) enhances oil quality in high and low oil containing peanut genotypes. Plant Sci. 2016, 242, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Endo, K.; Wada, H. Specific distribution of phosphatidylglycerol to photosystem complexes in the thylakoid membrane. Front. Plant Sci. 2017, 8, 1991. [Google Scholar] [CrossRef]
- Hernández, M.L.; Cejudo, F.J. Chloroplast lipids metabolism and function. a redox perspective. Front. Plant Sci. 2021, 12, 712022. [Google Scholar] [CrossRef]
- Van Eerden, F.J.; de Jong, D.H.; de Vries, A.H.; Wassenaar, T.A.; Marrink, S.J. Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. Biochim. Biophys. Acta 2014, 1848, 1319–1330. [Google Scholar] [CrossRef]
- Mongrand, S.; Bessoule, J.-J.; Cabantous, F.; Cassagne, C. The C16:3\C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry 1998, 49, 1049–1064. [Google Scholar] [CrossRef]
- Chapman, D.J.; De-Felice, J.; Barber, J. Growth Temperature Effects on Thylakoid Membrane Lipid and Protein Content of Pea Chloroplasts. Plant Physiol. 1983, 72, 225–228. [Google Scholar] [CrossRef]
- Popov, V.N.; Antipina, O.V.; Pchelkin, V.P.; Tsydendambaev, V.D. Changes in Fatty Acid Composition of lipids in chloroplast membranes of tobacco plants during cold hardening. Russ. J. Plant Physiol. 2017, 64, 156–161. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell: Oxford, UK, 2006; pp. 145–181. [Google Scholar]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Coast, O.; Murdoch, A.J.; Ellis, R.H.; Hay, F.R.; Jagadish, K.S.V. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress. Plant Cell Environ. 2016, 39, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview; Springer: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar]
- Bajji, M.; Lutts, S.; Kinet, J.M. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, S.G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Metcalfe, L.D.; Schmitz, A.A.; Pelka, J.R. Rapid preparation of fatty acid esters from lipids gas chromatographic analysis. Anal. Chem. 1996, 38, 514–515. [Google Scholar] [CrossRef]


| Saturated Fatty Acids (µg µL−1) | N22 | CN1 | KDML105 | IR64 | ||||
|---|---|---|---|---|---|---|---|---|
| Control | HT | Control | HT | Control | HT | Control | HT | |
| Capric acid (C10:0) | 0.000f | 0.000f | 0.006f | 0.008d * | 0.006f | 0.010c * | 0.000e | 0.000g |
| Lauric acid (C12:0) | 0.076c * | 0.047d | 0.093c * | 0.087c | 0.080e * | 0.050c | 0.050d | 0.052e |
| Myristic acid (C14:0) | 0.038d * | 0.023e | 0.028e | 0.030d * | 0.021f | 0.031c | 0.015e | 0.018f |
| Pentadecanoic acid (C15:0) | 0.017e * | 0.012ef | 0.025e | 0.029d * | 0.019f | 0.021c | 0.015e | 0.015f |
| Palmitic acid (C16:0) | 0.768a * | 0.498a | 1.048a * | 0.951a | 0.762a * | 0.550a | 0.655a * | 0.548a |
| Stearic acid (C18:0) | 0.161b * | 0.119b | 0.185b * | 0.150b | 0.156bc | 0.138b | 0.119b * | 0.089d |
| Arachidic acid (C20:0) | 0.088c * | 0.128b | 0.057d | 0.104c * | 0.131cd * | 0.057c | 0.060cd | 0.109c * |
| Behenic acid (C22:0) | 0.080c * | 0.133b | 0.055d | 0.110c * | 0.109de * | 0.035c | 0.066cd | 0.116b * |
| Lignoceric acid (24:0) | 0.085c | 0.104c | 0.097c | 0.138b * | 0.178b * | 0.103b | 0.071c | 0.108c * |
| Total | 1.313C * | 1.064b | 1.594A | 1.606a | 1.462B * | 0.995b | 1.050D | 1.055b |
| Unsaturated Fatty Acids (µg µL−1) | N22 | CN1 | KDML105 | IR64 | ||||
|---|---|---|---|---|---|---|---|---|
| Control | HT | Control | HT | Control | HT | Control | HT | |
| Linoleic acid (C18:2) | 0.709b * | 0.298b | 1.012b * | 0.625b | 0.668b | 0.567b | 0.622b * | 0.381b |
| γ-Linolenic acid (C18:3n6) | 0.027c * | 0.010b | 0.033d * | 0.022c | 0.023d * | 0.012c | 0.018d * | 0.012c |
| Linolenic acid (C18:3) | 6.137a * | 2.855a | 7.981a * | 5.174a | 5.613a * | 2.199a | 5.099a | 2.910a |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.003c * | 0.000c | 0.005d * | 0.000c | 0.005d * | 0.000c | 0.003d * | 0.000c |
| cis-5,8,11,14,17-Eicosapentaenoic acid (C20:5) | 0.008c * | 0.008c | 0.002d | 0.013c * | 0.012d * | 0.008c | 0.006d | 0.010c |
| Palmitoleic acid (C16:1) | 0.004c | 0.006c * | 0.000d | 0.085c * | 0.000d | 0.046c * | 0.005d | 0.006c * |
| Oleic acid (C18:1) | 0.072c * | 0.030c | 0.132c * | 0.069c | 0.133c * | 0.058c | 0.109c * | 0.046c |
| Erucic acid (C22:1) | 0.014c | 0.016c | 0.009d | 0.013c * | 0.017d * | 0.008c | 0.014d * | 0.004c |
| Total | 6.974B * | 3.224bc | 9.174A * | 6.002a | 6.471C * | 2.897c | 5.875D * | 3.370b |
| Rice Cultivars | Total Sat | Total Unsat | Sat:Unsat | % Increase in Sat:Unsat after HT | |||
|---|---|---|---|---|---|---|---|
| Control | HT | Control | HT | Control | HT | ||
| N22 | 1.312 | 1.064 | 6.974 | 3.224 | 0.188b | 0.330a * | +75%a |
| CN1 | 1.595 | 1.606 | 9.174 | 6.002 | 0.174b | 0.268b * | +54%b |
| KDML105 | 1.462 | 0.995 | 6.471 | 2.897 | 0.226a | 0.346a * | +53%b |
| IR64 | 1.050 | 1.055 | 5.875 | 3.370 | 0.179b | 0.313ab * | +75%a |
| No. | Time (h) | Temperature (°C) | Relative Humidity (%) |
|---|---|---|---|
| 1 | 00:00–03:00 | 27 | 66 |
| 2 | 03:00–07:00 | 26 | 70 |
| 3 | 07:00–09:00 | 29 | 61 |
| 4 | 09:00–11:00 | 36 | 49 |
| 5 | 11:00–15:00 | 42 | 42 |
| 6 | 15:00–17:00 | 40 | 40 |
| 7 | 17:00–18:00 | 37 | 37 |
| 8 | 18:00–21:00 | 33 | 33 |
| 9 | 21:00–00:00 | 27 | 32 |
| No. | Time (h) | * Light Intensity (µmol m−2 s−1) |
|---|---|---|
| 1 | 06:00–07:00 | 70 |
| 2 | 07:00–08:00 | 115 |
| 3 | 08:00–09:00 | 200 |
| 4 | 09:00–10:00 | 265 |
| 5 | 10:00–11:00 | 340 |
| 6 | 11:00–13:00 | 390 |
| 7 | 13:00–14:00 | 340 |
| 8 | 14:00–15:00 | 265 |
| 9 | 15:00–16:00 | 200 |
| 10 | 16:00–17:00 | 115 |
| 11 | 17:00–18:00 | 70 |
| 12 | 18:00–06:00 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasertthai, P.; Paethaisong, W.; Theerakulpisut, P.; Dongsansuk, A. High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings. Plants 2022, 11, 1454. https://doi.org/10.3390/plants11111454
Prasertthai P, Paethaisong W, Theerakulpisut P, Dongsansuk A. High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings. Plants. 2022; 11(11):1454. https://doi.org/10.3390/plants11111454
Chicago/Turabian StylePrasertthai, Paphitchaya, Warunya Paethaisong, Piyada Theerakulpisut, and Anoma Dongsansuk. 2022. "High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings" Plants 11, no. 11: 1454. https://doi.org/10.3390/plants11111454
APA StylePrasertthai, P., Paethaisong, W., Theerakulpisut, P., & Dongsansuk, A. (2022). High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings. Plants, 11(11), 1454. https://doi.org/10.3390/plants11111454





