Antimicrobial Screening and Fungicidal Properties of Eucalýptus globulus Ultrasonic Extracts
Abstract
:1. Introduction
2. Results
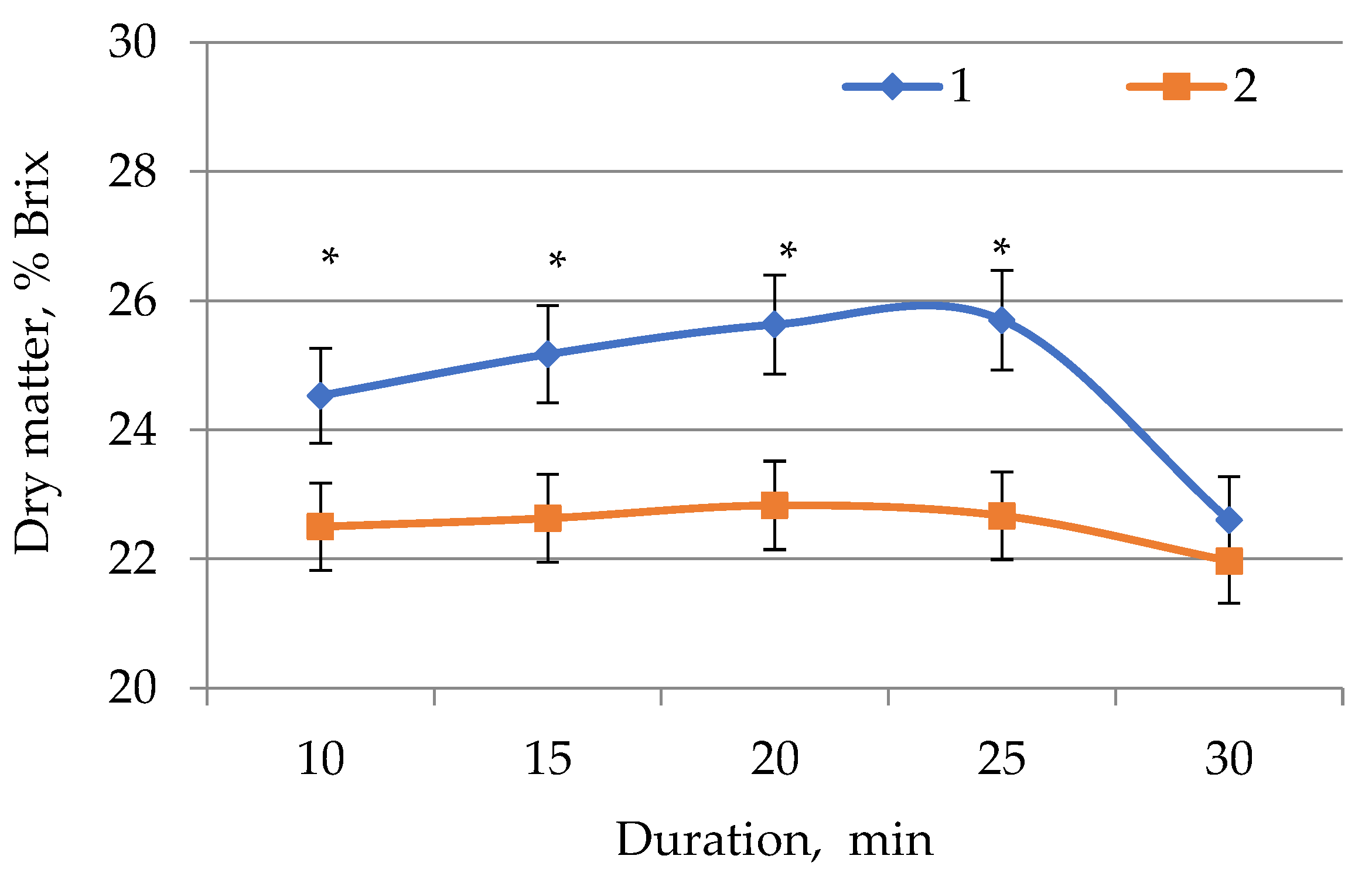
2.1. Determination of Dry Matter in E. globúlus Extracts
2.2. Study of the Antimicrobial Activity of E. globúlus Extracts
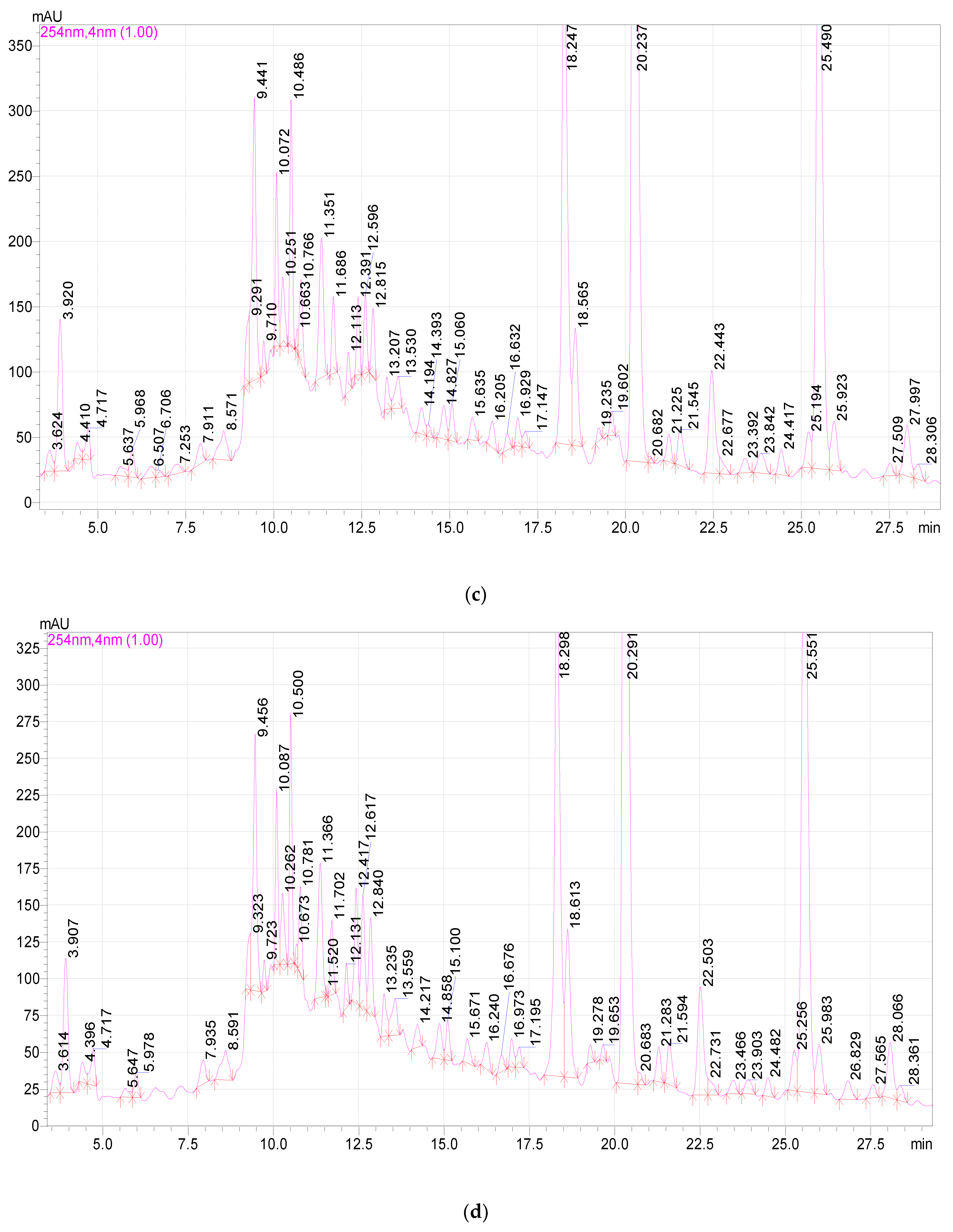
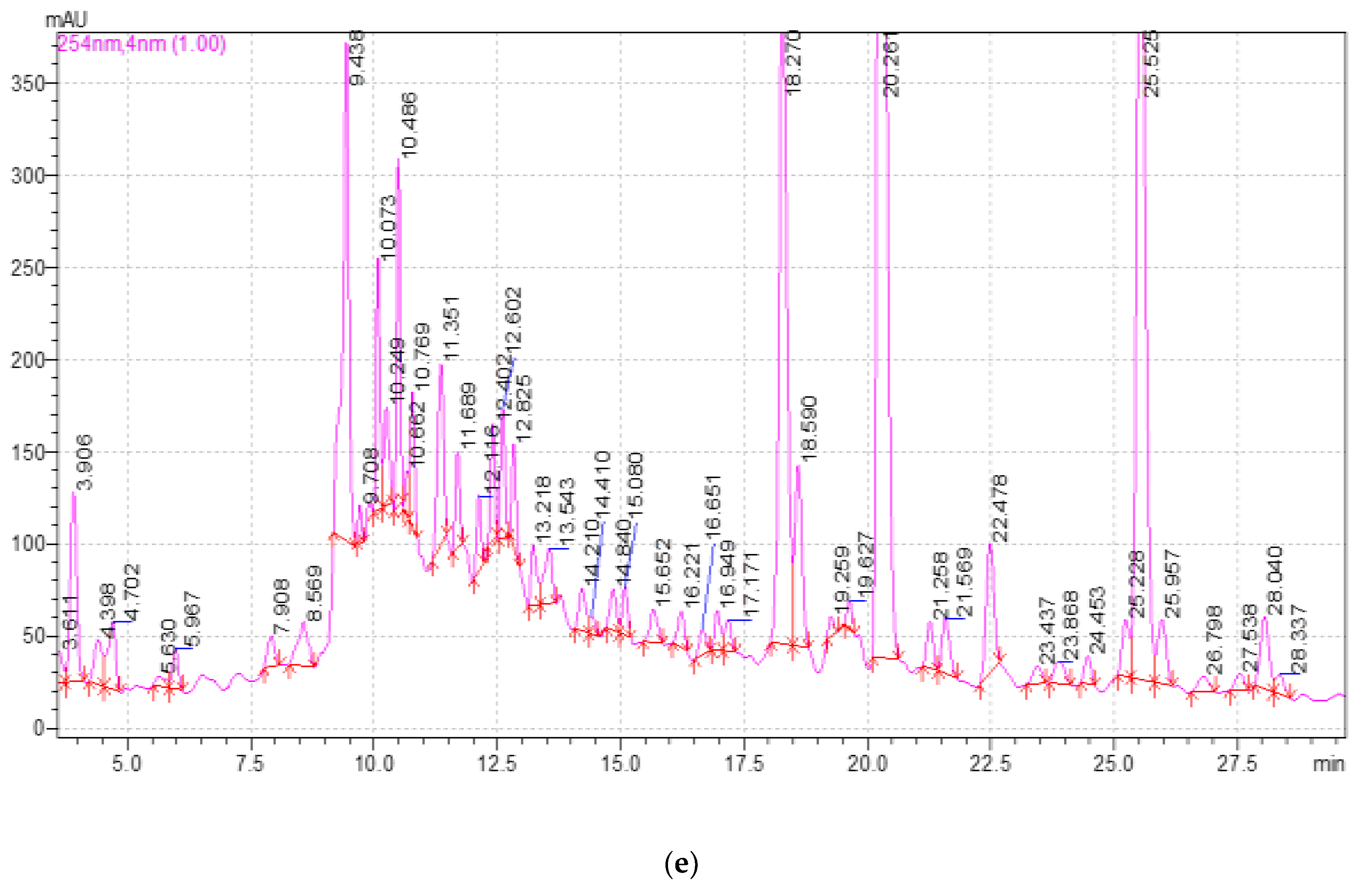
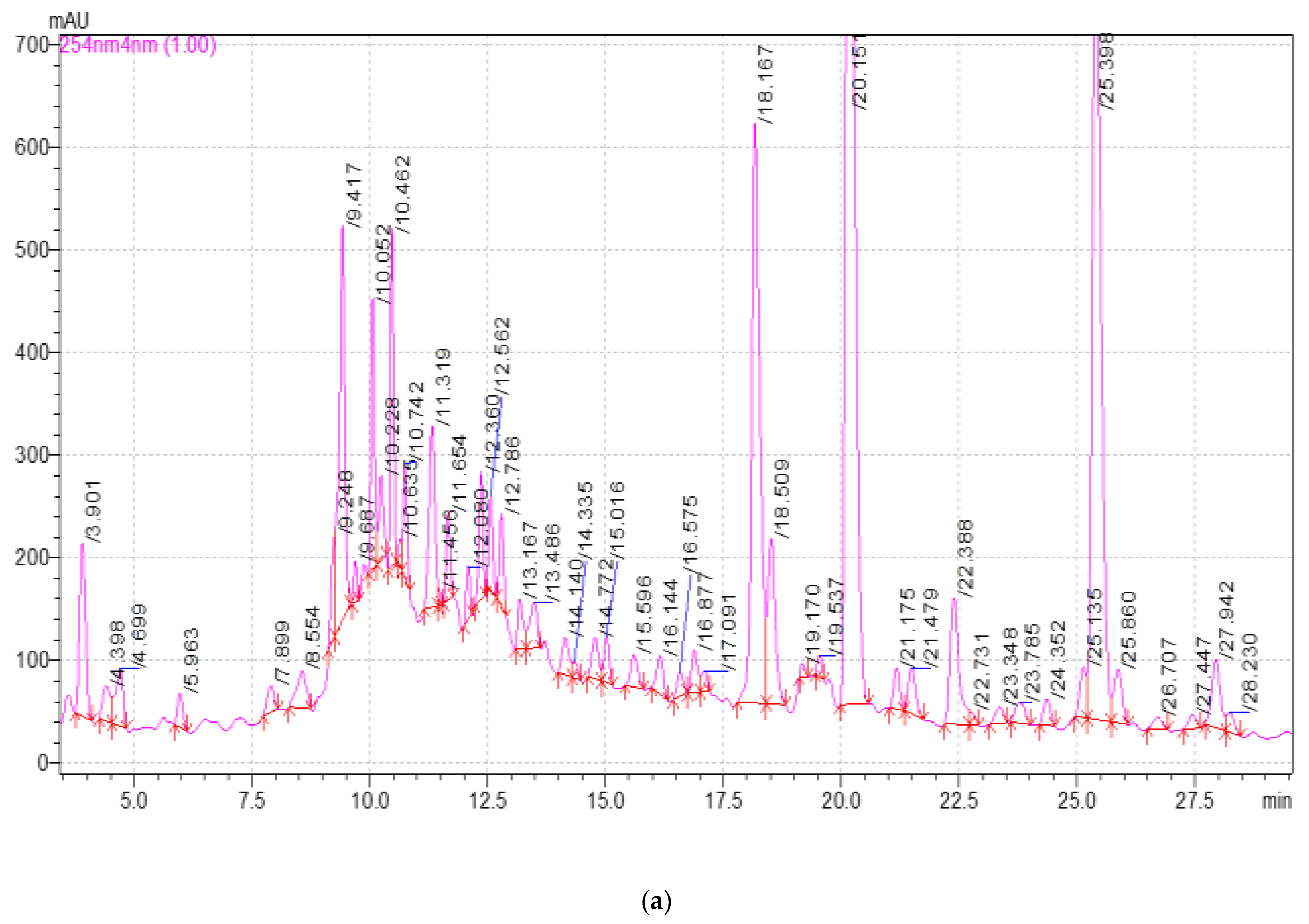
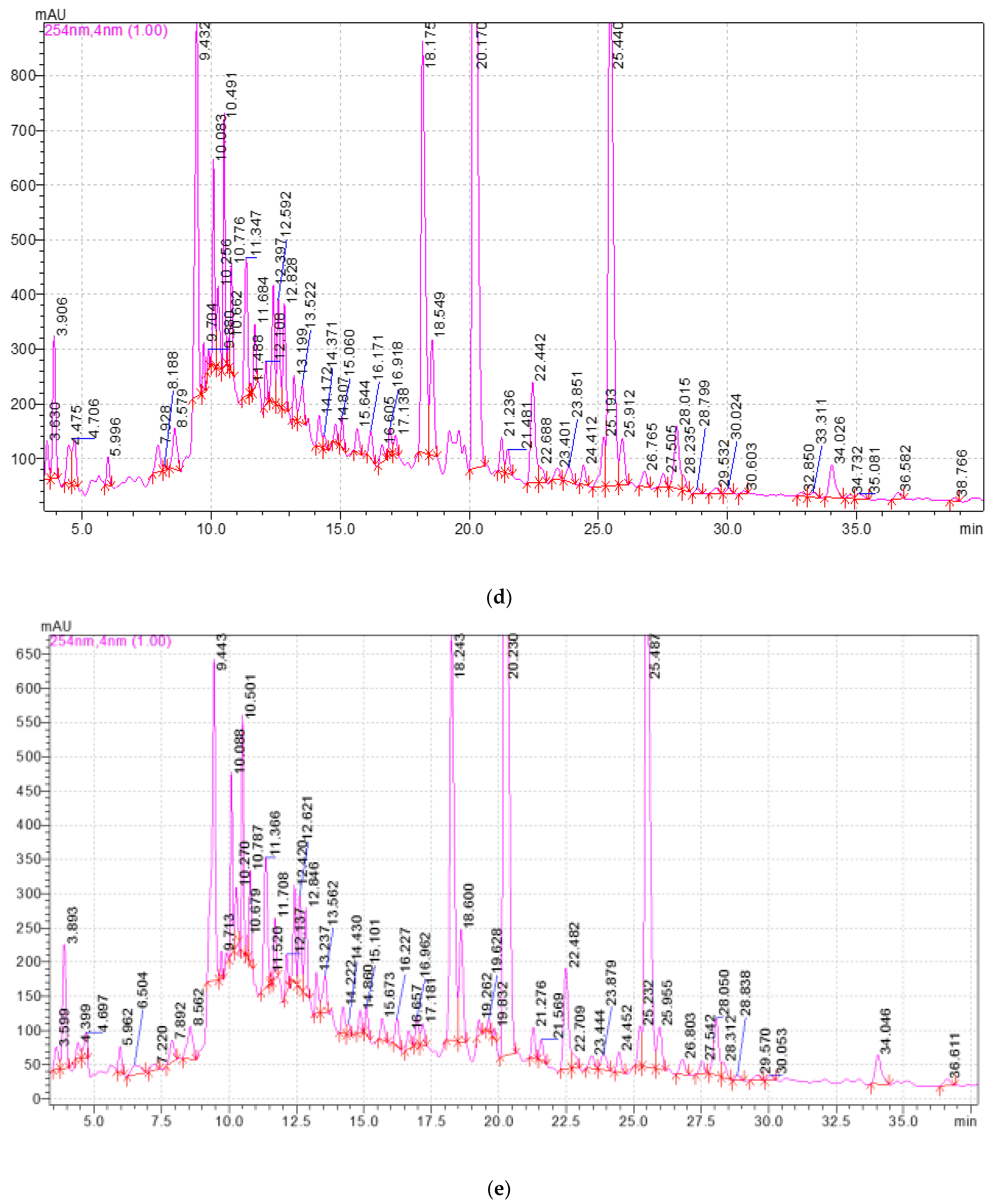
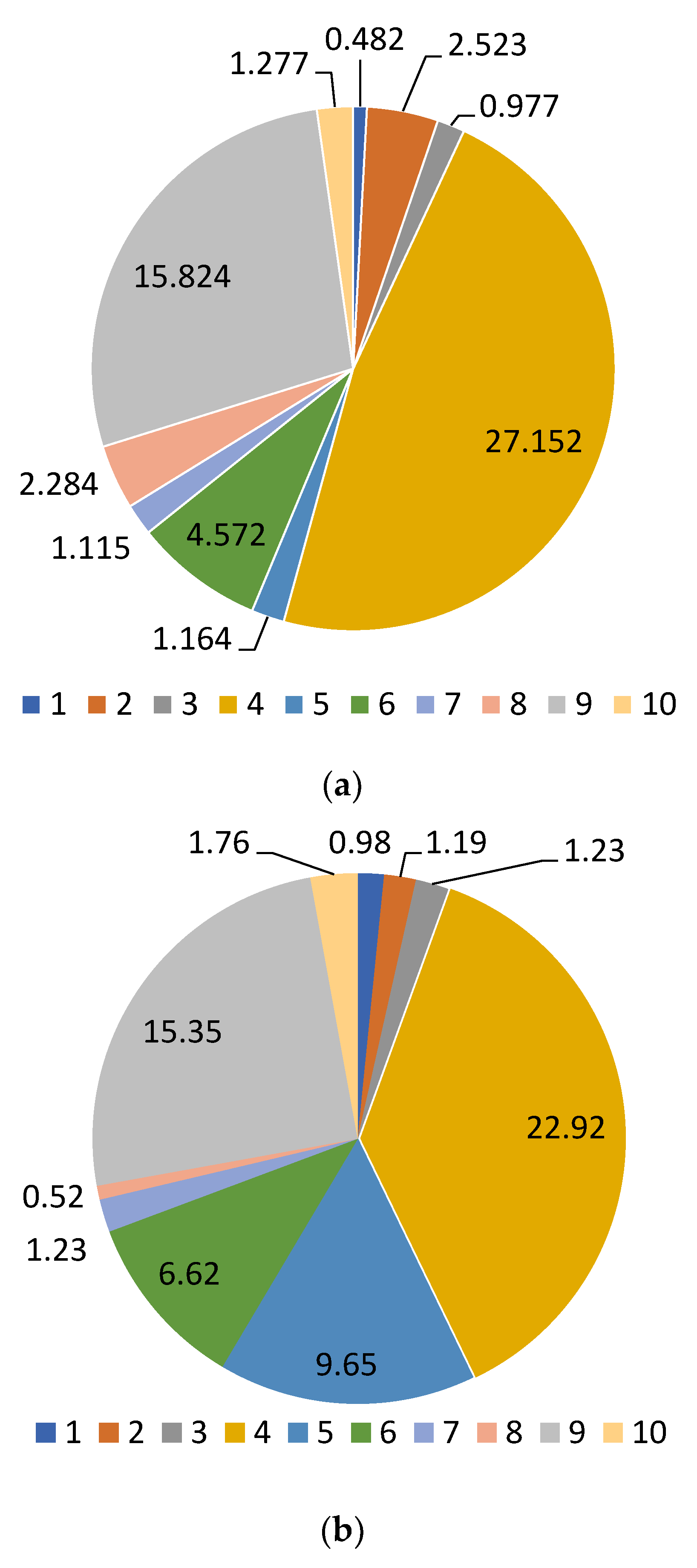
2.3. Study of the Qualitative and Quantitative Composition of BASs in E. globulus Extracts
3. Discussion
4. Materials and Methods
4.1. Object of Research
4.2. Research Methods
4.3. Evaluation of the Quality of Eucalýptus globúlus Leaf Extract
4.3.1. Determination of Dry Residue by Refractometric Method
4.3.2. Method for Determining the Antimicrobial Activity of a Plant Extract
4.3.3. Methodology and Conditions for High-Performance Liquid Chromatography
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regulation of the European Parliament and of the Council of the European Union 1831/2003 of 22.09.2003 on Additives in Animal Feed. Available online: https://fsvps.gov.ru/fsvps-docs/ru/laws/registration/1831-2003.pdf (accessed on 3 May 2022).
- Suryanarayana, S.; Durga, P. Role of Phytogenic Feed Additives in Swine Production-A Review. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1071–1078. [Google Scholar] [CrossRef]
- Rasskazova, N.T.; Tsoy, Z.V.; Pulinets, A.K. Productivity of rabbits when using the drug «KED+LBA» in their diets. Earth Environ. Sci. 2021, 677, 042001. [Google Scholar] [CrossRef]
- Hossan, M.D.S.; Khan, S.H.; Kazi, M.K.; Anwarul, M.D.; Haque, B. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef]
- Raj, M.G.; Basharat, S.; Sudipto, H.; Chasity, P. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, U.; Kuter, E.; Raza, I.; Köksal, B.H.; Cengiz, Ö.; Yıldız, M.; Kızanlık, P.K.; Kaya, M.; Tatlı, O.; Sevim, Ö. Dietary Supplementation of Different Levels of Phytogenic Feed Additive in Broiler Diets: The Dynamics of Growth Performance, Caecal Microbiota, and Intestinal Morphometry. Rev. Bras. Cienc. Avic. 2018, 20, 737–745. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Peters-Kennedy, J. Chapter 6—Integumentary System. In Jubb Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 509–736. [Google Scholar] [CrossRef]
- Reis, J.H.; Gebert, R.R.; Barreta, M.; Baldissera, M.D.; dos Santos, I.D.; Wagner, R.; Campigotto, G.; Jaguezeski, A.M.; Gris, A.; de Lima, J.L.F.; et al. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathog. 2018, 6, 10. [Google Scholar] [CrossRef]
- Flees, J.J.; Ganguly, B.; Dridi, S. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism–related signaling pathways. Poult. Sci. 2021, 100, 100963. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, S.M. From Medicinal Plant Raw Material to Herbal Remedies. In Aromatic and Medicinal Plants-Back to Nature; IntechOpen: London, UK, 2017; pp. 269–288. Available online: https://www.intechopen.com/chapters/53301 (accessed on 27 May 2022).
- Xie, X.; Zhu, D.; Zhang, W.; Huaic, W.; Wanga, K.; Huanga, X.; Zhoud, L.; Fan, H. Microwave-assisted aqueous two-phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of four flavonoids in Crotalaria sessiliflora L. Ind. Crops Prod. 2017, 95, 63–642. [Google Scholar] [CrossRef]
- Savun-Hekimoğlu, B.A. Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 42. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, H.; Li, Y.; Ohl, C.D.; Yu, H.; Li, D. Effects of surface tension on the dynamics of a single micro bubble near a rigid wall in an ultrasonic field. Ultrason. Sonochem. 2021, 78, 105735. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-theart review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar] [CrossRef]
- Kirkpatrick, J.; Gibson, N. Towards an explanation of the altitudinal distributions of three species of Eucalyptus in central Tasmania. Aust. J. Ecol. 2009, 24, 123–131. [Google Scholar] [CrossRef]
- Maza, P.K.; Bonfim-Melo, A.; Padovan, A.C.B.; Mortara, R.A.; Orikaza, C.M.; Ramos, L.M.D.; Moura, T.R.; Soriani, F.M.; Almeida, R.S.; Suzuki, E.; et al. Candida albicans: The Ability to Invade Epithelial Cells and Survive under Oxidative Stress Is Unlinked to Hyphal Length. Front. Microbiol. 2017, 8, 1235. [Google Scholar] [CrossRef]
- Pan, M.; Pan, M.; Lei, Q.; Zhang, H. Prediction and confirmation of active ingredients in Eucalyptus globulus Labill leaves. Ind. Crops Prod. 2020, 154, 112631. [Google Scholar] [CrossRef]
- Sibirtsev, V.S.; Nechiporenko, U.Y. Tonkie Khimicheskie Tekhnologii Method of electrochemical biotesting for comparative analysis of probiotic and antibiotic properties of various plant extracts. Fine Chem. Technol. 2020, 15, 34–43. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Asyakina, L.K.; Babich, O.O.; Dyshlyuk, L.; Sukhikh, S.; Popov, A.D.; Kostyushina, N.V. Physicochemical properties and biological activity of extracts of dried biomass of callus and suspension cells and in vitro root cultures. Food Processing Tech. Technol. 2020, 50, 480–492. [Google Scholar] [CrossRef]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updat. 2020, 51, 100695. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern Trends in the In Vitro Production and Use of Callus, Suspension Cells and Root Cultures of Medicinal Plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic Composition, Antioxidant Capacity and Antibacterial Activity of White Wormwood (Artemisia herba-alba). Plants 2021, 10, 164. [Google Scholar] [CrossRef]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Ngoc, P.C.; Leclercq, L.; Rossi, J.-C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.-S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules 2019, 24, 4420. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Li, L.Y.; He, G.H. Optimization of Microwave-Assisted Extraction Conditions for Five Major Bioactive Compounds from Flos Sophorae Immaturus (Cultivars of Sophora japonica L.) Using Response Surface Methodology. Molecules 2016, 21, 296. [Google Scholar] [CrossRef] [Green Version]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Nemytin, Y.V. Antimicrobial and Antifungal Drugs; Remedium: Moscow, Russia, 2002; pp. 57–59. [Google Scholar]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Campillo, M.; Gabaldon, J.A.; Castillo, J. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomed. Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Dezsi, Ș.; Bădărău, A.S.; Bischin, C.; Vodnar, D.C.; Silaghi-Dumitrescu, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L. Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.). Molecules 2015, 20, 4720–4734. [Google Scholar] [CrossRef] [Green Version]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal Activity of Essential Oil of Eucalyptus camaldulensis Dehnh. against Selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.F.; Wu, J.Y.; Hsu, Y.W. Protective Effects of Rosmarinic Acid against Selenite-Induced Cataract and Oxidative Damage in Rats. Int. J. Med. Sci. 2019, 16, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative Approaches for Recovery of Phytoconstituents from Medicinal/Aromatic Plants and Biotechnological Production. Molecules 2020, 25, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dey, A.; Rose, M.K.; Dahiya, S.S. Impact of Dietary Phytogenic Composite Feed Additives on Immune Response, Antioxidant Status, Methane Production, Growth Performance and Nutrient Utilization of Buffalo (Bubalus bubalis) Calves. Antioxidants 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]







| Extraction Method | Extraction Time, min | Microorganism Strain | |||
|---|---|---|---|---|---|
| Bacillus subtilis | Pseudomonasaeruginosa | Candida albicans | |||
| Without ultrasonic treatment | 10 | 6.0 | 11.0 | 10.5 | |
| 15 | 6.0 | 13.0 | 8.5 | ||
| 20 | 9.0 | 14.0 | 7.5 | ||
| 25 | 7.0 | 10.0 | 8.5 | ||
| 30 | 7.0 | 7.0 | 8.5 | ||
| With ultrasonic treatment | 10 | 10.0 | 6.0 | 12.5 | |
| 15 | 11.0 | 5.0 | 9.5 | ||
| 20 | 10.0 | 13.0 | 12.5 | ||
| 25 | 9.0 | 11.0 | 15.5 | ||
| 30 | 10.0 | 11.0 | 11.0 | ||
| Ampicillin | - | 32.5 | 34.0 | 31.0 | |
| Fluconazole | - | - | - | 31.0 | |
| Component | Release Time, min | Extract Samples | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 3,4-dihydroxybenzoic acid | 5.980 | 19.16 ± 0.2 a | 28.30 ± 0.2 b | 25.00 ± 0.2 b | 27.55 ± 0.2 b | 21.28 ± 0.2 a |
| Catechin | 9.689 | 157.86 ± 1.2 a | 212.64 ± 1.2 b | 209.52 ± 1.2 b | 282.54 ± 1.2 c | 183.19 ± 1.2 d |
| Chlorogenic acid | 10.464 | 256.46 ± 1.2 a | 261.88 ± 1.2 a | 294.16 ± 1.2 ab | 342.14 ± 1.2 b | 274.38 ± 1.2 a |
| Caffeic acid | 10.727 | 17.96 ± 0.2 a | 17.64 ± 0.2 a | 20.17 ± 0.2 a | 26.54 ± 0.2 b | 22.90 ± 0.2 ab |
| Hyperoside | 19.537 | 9.51 ± 0.1 a | 8.90 ± 0.1 a | 16.67 ± 1.2 b | 33.38 ± 0.2 c | 11.26 ± 0.2 a |
| Rutin | 19.537 | 16.16 ± 0.2 a | 15.03 ± 0.2 a | 60.13 ± 0.2 b | 69.79 ± 0.1 b | 14.07 ± 0.2 a |
| Quercetin-3D-Glycoside | 20.151 | 1246.82 ± 12.3 a | 1281.48 ± 12.3 a | 1477.78 ± 12.3 b | 1703.30 ± 12.3 c | 1350.34 ± 12.3 ab |
| Astragalin | 25.398 | 1283.64 ± 12.3 a | 1381.04 ± 12.3 a | 1521.44 ± 12.3 b | 1737.82 ± 12.3 c | 1358.68 ± 12.3 a |
| Apigenin | 26.710 | 7.42 ± 0.1 a | 14.26 ± 0.2 b | 16.36 ± 0.2 bc | 20.28 ± 0.2 c | 13.04 ± 0.2 b |
| Rosmarinic acid | 28.191 | 26.22 ± 1.2 a | 27.4 ± 0.2 a | 30.56 ± 0.2 ab | 36.39 ± 0.2 b | 27.83 ± 0.2 a |
| Component | Release Time, min | Extract Samples | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 3,4-dihydroxybenzoic acid | 5.980 | 13.98 ± 0.2 a | 13.25 ± 0.2 a | 13.26 ± 0.2 a | 10.44 ± 0.2 a | 13.39 ± 0.2 a |
| Catechin | 9.689 | 87.34 ± 0.2 a | 79.77 ± 0.2 b | 105.09 ± 1.2 c | 95.15 ± 0.2 a | 92.75 ± 0.2 a |
| Chlorogenic acid | 10.464 | 136.12 ± 1.2 a | 145.93 ± 1.2 ab | 151.70 ± 1.2 b | 139.67 ± 1.2 a | 151.09 ± 1.2 b |
| Caffeic acid | 10.727 | 9.00 ± 0.1 a | 8.56 ± 0.1 a | 10.34 ± 0.2 a | 10.22 ± 0.2 a | 11.32 ± 0.2 a |
| Hyperoside | 19.537 | 4.80 ± 0.1 a | 5.26 ± 0.1 a | 5.78 ± 0.1 a | 6.94 ± 0.1 a | 5.94 ± 0.1 a |
| Rutin | 19.537 | 9.19 ± 0.1 a | 7.22 ± 0.1 a | 16.09 ± 0.2 b | 8.60 ± 0.1 a | 12.16 ± 0.2 b |
| Quercetin-3D-Glycoside | 20.151 | 672.92 ± 1.2 a | 708.34 ± 1.2 a | 845.28 ± 1.2 b | 761.78 ± 1.2 b | 806.94 ± 1.2 b |
| Astragalin | 25.398 | 686.00 ± 1.2 a | 692.06 ± 1.2 a | 750.88 ± 1.2 b | 732.50 ± 1.2 ab | 723.80 ± 1.2 ab |
| Apigenin | 26.710 | 5.09 ± 0.1 a | 5.48 ± 0.1 a | 3.56 ± 0.1 b | 8.01 ± 0.1 c | 5.05 ± 0.1 a |
| Rosmarinic acid | 28.191 | 14.44 ± 0.2 a | 15.18 ± 0.2 a | 16.29 ± 0.2 a | 15.25 ± 0.2 a | 15.20 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, S.; Ivanova, S.; Babich, O.; Larina, V.; Krol, O.; Prosekov, A.; Popov, A.; Kriger, O. Antimicrobial Screening and Fungicidal Properties of Eucalýptus globulus Ultrasonic Extracts. Plants 2022, 11, 1441. https://doi.org/10.3390/plants11111441
Sukhikh S, Ivanova S, Babich O, Larina V, Krol O, Prosekov A, Popov A, Kriger O. Antimicrobial Screening and Fungicidal Properties of Eucalýptus globulus Ultrasonic Extracts. Plants. 2022; 11(11):1441. https://doi.org/10.3390/plants11111441
Chicago/Turabian StyleSukhikh, Stanislav, Svetlana Ivanova, Olga Babich, Viktoria Larina, Olesia Krol, Alexander Prosekov, Alexander Popov, and Olga Kriger. 2022. "Antimicrobial Screening and Fungicidal Properties of Eucalýptus globulus Ultrasonic Extracts" Plants 11, no. 11: 1441. https://doi.org/10.3390/plants11111441
APA StyleSukhikh, S., Ivanova, S., Babich, O., Larina, V., Krol, O., Prosekov, A., Popov, A., & Kriger, O. (2022). Antimicrobial Screening and Fungicidal Properties of Eucalýptus globulus Ultrasonic Extracts. Plants, 11(11), 1441. https://doi.org/10.3390/plants11111441









