Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolic and Flavonoids Content
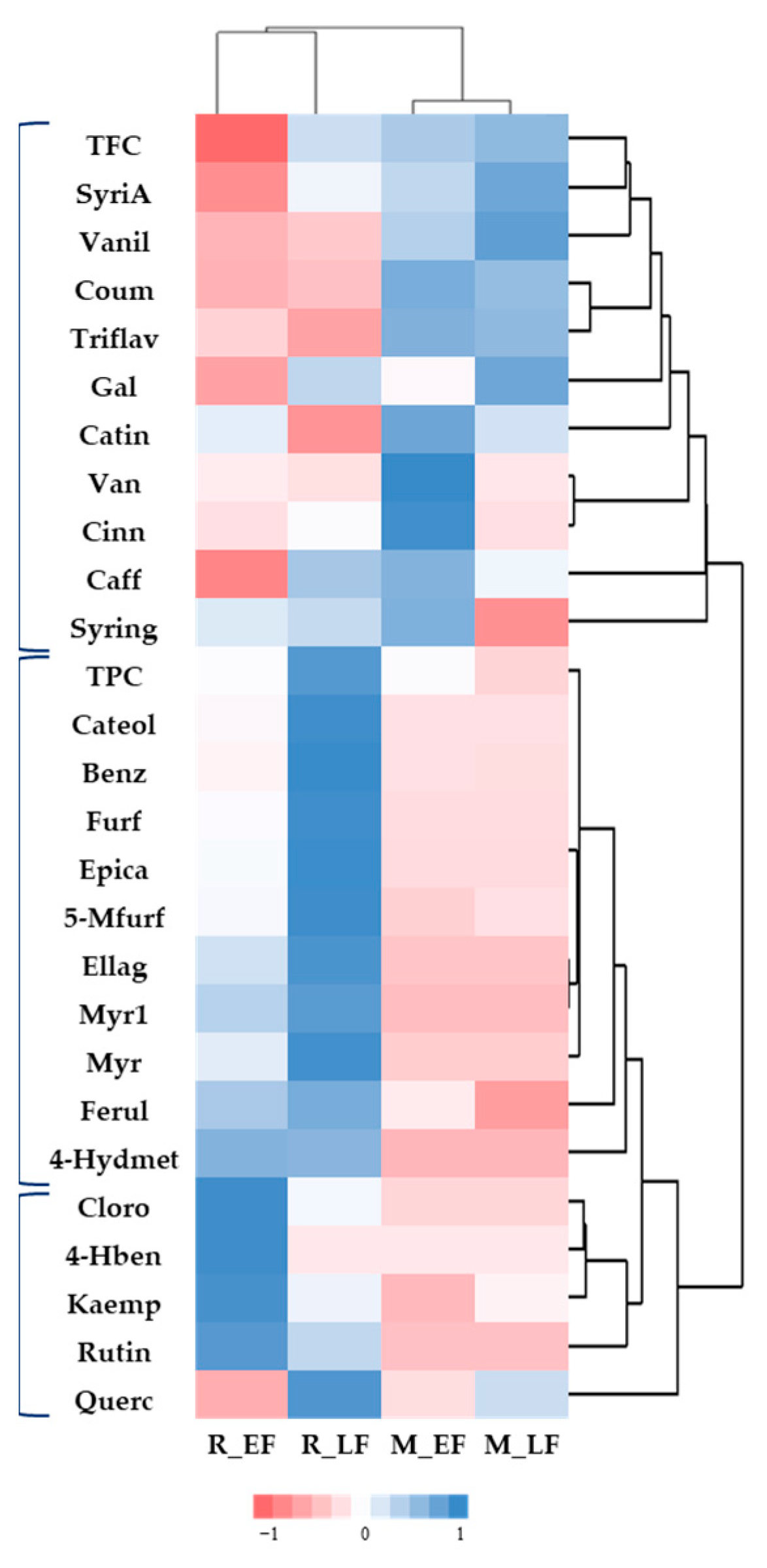
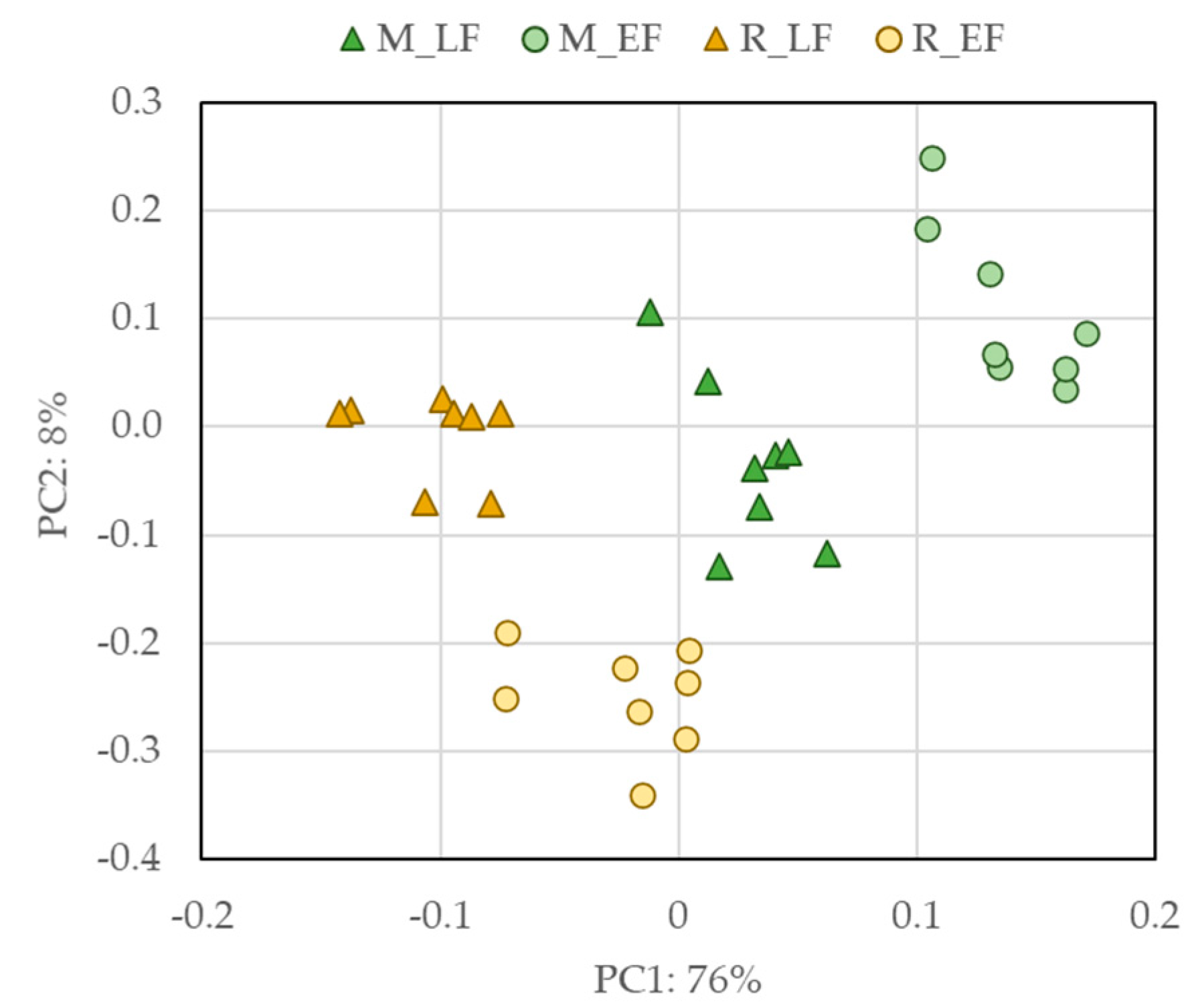
2.2. Targeted and Untargeted Phytochemical Study
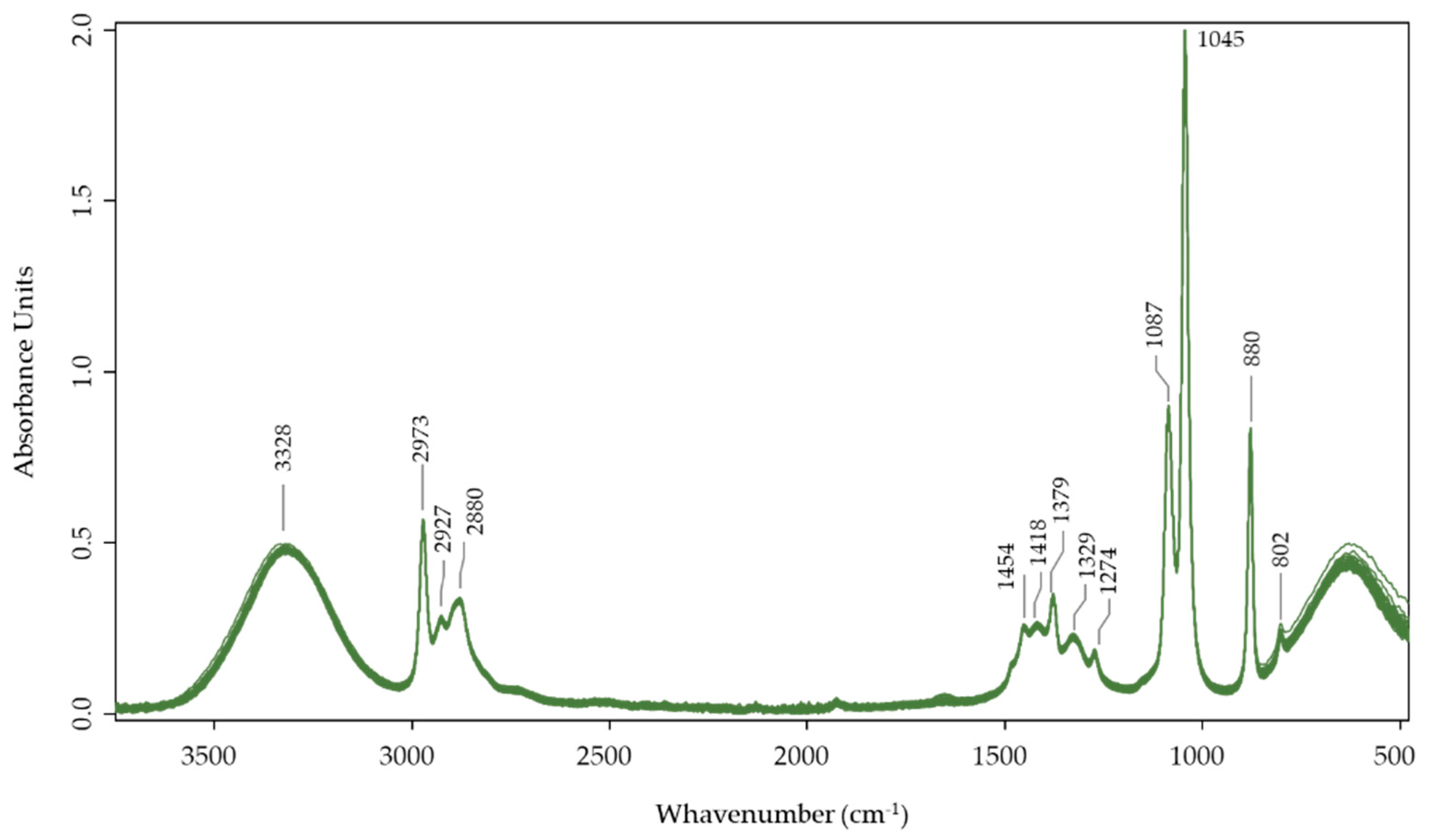
2.3. FTIR-ATR Spectral Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Extraction Conditions
3.4. Determination of Total Phenolic and Flavonoid Contents
3.5. UHPLC/ESI-QTOF-MS Analysis
3.6. HPLC Analysis
3.7. FTIR-ATR Spectral Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seigler, D. Phytochemistry of Acacia—Sensu lato. Biochem. Syst. Ecol. 2003, 31, 845–873. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- El-Hefny, M.; Ashmawy, N.A.; Salem, M.Z.M.; Salem, A.Z.M. Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathog. 2017, 113, 348–356. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.M.; EL-Hefny, M.; Abd El-Kareem, M.S.M.; El-Shanhorey, N.A.; Mohamed, A.A.; Salem, A.Z.M. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathog. 2018, 121, 331–340. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Elansary, H.O.; Nasser, R.A.; El-Settawy, A.A.A.; El Shanhorey, N.; Ashmawy, N.A.; Salem, A.Z.M. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathog. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; Cavaleiro, C. Ambient has become strained. Identification of Acacia dealbata Link volatiles interfering with germination and early growth of native species. J. Chem. Ecol. 2014, 40, 1051–1061. [Google Scholar] [CrossRef]
- Souto, X.C.; Bolaño, J.C.; González, L.; Reigosa, M.J. Allelopathic Effects of Tree Species on Some Soil Microbial Populations and Herbaceous Plants. Biol. Plant. 2001, 44, 269–275. [Google Scholar] [CrossRef]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the valorization of biomass from portuguese invasive Acacia spp. in a biorefinery perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- WFO The World Flora Online. Available online: http://www.worldfloraonline.org/taxon/wfo-0000203882 (accessed on 19 January 2022).
- Roskov, Y.R.; Bisby, F.A.; Zarucchi, J.L.; Schrire, B.D.; White, R.J. International Legume Database and Information Service (ILDIS). Available online: https://ildis.org/cgi-bin/Araneus.pl?version~10.01&LegumeWeb&tno~313&genus~Acacia&species~mearnsii (accessed on 25 January 2022).
- Stevens, P.F. Angiosperm Phylogeny Website, Version 14. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 25 January 2022).
- Missouri Botanical Garden Tropics, Botanical Information System. Available online: http://www.tropicos.org/Name/13037485 (accessed on 25 January 2022).
- POWO Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 19 January 2022).
- Western Australian Herbarium; Species Gallery, Acacia mearnsii De Wild., Pl. Bequaert. 3: 61 (1925). Available online: http://worldwidewattle.com/speciesgallery/species-intro.php?id=17958 (accessed on 25 January 2022).
- Xiong, J.; Grace, M.H.; Esposito, D.; Komarnytsky, S.; Wang, F.; Lila, M.A. Polyphenols isolated from Acacia mearnsii bark with anti-inflammatory and carbolytic enzyme inhibitory activities. Chin. J. Nat. Med. 2017, 15, 816–824. [Google Scholar] [CrossRef]
- O’Leary, M.C. Review of Acacia retinodes and closely related species, A. uncifolia and A. provincialis (Leguminosae: Mimosoideae: Sect. Phyllodineae). J. Adel. Bot. Gard. 2007, 21, 95–109. [Google Scholar]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC-Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Milena, V.; Tatjana, M.; Gökhan, Z.; Ivana, B.; Aleksandra, C.; Mohammad, M.F.; Marija, R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crops Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Sinan, K.I.; Saftić, L.; Peršurić, Ž.; Pavelić, S.K.; Etienne, O.K.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; Zengin, G. A comparative study of the chemical composition, biological and multivariate analysis of Crotalaria retusa L. stem barks, fruits, and flowers obtained via different extraction protocols. South African J. Bot. 2020, 128, 101–108. [Google Scholar] [CrossRef]
- Pedro, S.I.; Gallardo, E.; Rosado, T.; Simão, A.A.; Gominho, J.A.O. Extraction of phenolic compounds from Acacia flower by Energized Dispersive Guided Extraction. In Proceedings of the ST 2021: 5th International Caparica Christmas Conference on Sample Treatment, Caparica, Portugal, 15–18 November 2021; Capelo-Martinez, J.L., Ed.; PROTEOMASS Scientific Society: Caparica, Portugal, 2021; p. 92. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Phytochemical assessment and antioxidant activities of alcoholic and aqueous extracts of Acacia mearnsii de wild. Int. J. Pharmacol. 2011, 7, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Rababah, T.M.; Ereifej, K.I.; Esoh, R.B.; Al-U’Datt, M.H.; Alrababah, M.A.; Yang, W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 2011, 25, 596–605. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits-an overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, C.A.; Costa, C.K.; Bora, K.; Miguel, M.D.; Miguel, O.G.; Kerber, V.A. Determinação do conteúdo fenólico e avaliação da atividade antioxidante de Acacia podalyriifolia A. Cunn. ex G. Don, Leguminosae-mimosoideae. Rev. Bras. de Farm. 2007, 17, 231–235. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Wang, S.-Y.; Shyur, L.-F.; Kuo, Y.-H.; Chang, S.-T. Phenolic Antioxidants from the Heartwood of Acacia confusa. J. Agric. Food Chem. 2005, 53, 5917–5921. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.Y.; Gonçalves, J.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Rodilla, J.M.; Barroso, M.; Duarte, A.P.; Cristóvão, A.C.; et al. Evaluation of the Cytotoxicity of Ayahuasca Beverages. Molecules 2020, 25, 5594. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Luís, Â.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Domingues, F.; Gallardo, E.; Duarte, A.P. Ayahuasca beverages: Phytochemical analysis and biological properties. Antibiotics 2020, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From ethnobotanical surveys to phytochemical characterization and bioactivities evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ramos, R.; Rosado, T.; Gallardo, E.; Duarte, A.P. Development and validation of a HPLC–DAD method for quantification of phenolic compounds in different sweet cherry cultivars. SN Appl. Sci. 2019, 1, 954. [Google Scholar] [CrossRef] [Green Version]
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gominho, J.; Anjos, O.; Gallardo, E. Insights into the bioactivities and chemical analysis of Ailanthus Altissima (Mill.) swingle. Appl. Sci. 2021, 11, 11331. [Google Scholar] [CrossRef]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef] [Green Version]
- FooDB. Available online: www.foodb.ca (accessed on 2 February 2022).
- CEU Mass Mediator. Available online: http://ceumass.eps.uspceu.es/mediator (accessed on 2 February 2022).
- Toddenroth, D.; Ganslandt, T.; Castellanos, I.; Prokosch, H.U.; Bürkle, T. Employing heat maps to mine associations in structured routine care data. Artif. Intell. Med. 2014, 60, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Balyklova, K.S.; Gegechkori, V.; Morton, D.W. HPTLC and ATR/FTIR Characterization of Antioxidants in Different Rosemary Extracts. Molecules 2021, 26, 6064. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Grzybek, M.; Strachecka, A.; Jaworska, A.; Ludwiczuk, A. ATR-FTIR-based fingerprinting of some Cucurbitaceae extracts: A preliminary study. Acta Soc. Bot. Pol. 2018, 87, 1–6. [Google Scholar] [CrossRef]
- Wulandari, L.; Retnaningtyas, Y.; Nuri; Lukman, H. Analysis of Flavonoid in Medicinal Plant Extract Using Infrared Spectroscopy and Chemometrics. J. Anal. Methods Chem. 2016, 2016, 4696803. [Google Scholar] [CrossRef] [Green Version]
- Paradkar, M.M.; Sakhamuri, S.; Irudayaraj, J. Comparison of FTIR, FT-Raman, and NIR Spectroscopy in a Maple Syrup Adulteration Study. J. Food Sci. 2002, 67, 2009–2015. [Google Scholar] [CrossRef]
- Izzo, F.C.; Zendri, E.; Bernardi, A.; Balliana, E.; Sgobbi, M. The study of pitch via gas chromatography–mass spectrometry and Fourier-transformed infrared spectroscopy: The case of the Roman amphoras from Monte Poro, Calabria (Italy). J. Archaeol. Sci. 2013, 40, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. Encycl. Anal. Chem. 2000, 10815–10837. [Google Scholar] [CrossRef]
- Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Luís, Â.; Sousa, S.; Duarte, A.P.; Pereira, L.; Domingues, F. Phytochemical characterization, and evaluation of rheological and antioxidant properties of commercially available juices of berries. J. Berry Res. 2018, 8, 11–23. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| FS | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | |
|---|---|---|---|
| A. retinodes | EF | 311.24 ± 23.36 b | 136.47 ± 1.27 a |
| LF | 350.50 ± 13.79 b | 287.91 ± 14.28 b | |
| A. mearnsii | EF | 310.55 ± 12.76 b | 317.97 ± 1.89 c |
| LF | 300.03 ± 2.36 a | 342.73 ± 4.32 d | |
| Species (S) | 49.1 *** | 46.8 *** | |
| Flowering stages (FS) | n.s. | 25.9 *** | |
| SxFS | n.s. | 26.6 *** | |
| Residual | 50.9 | 0.7 | |
| Compound | tR (min) | m/z Experimental |
|---|---|---|
| Polyphenols Analysed in Negative Ionization Mode | ||
| Phenolic acids | ||
| Hydroxybenzoic acids | ||
| Gallic acid | 3.0 | 169.0142 |
| Protocatechuic acid | 6.4 | 153.0193 |
| 4-Hydroxybenzoic acid | 8.3 | 137.0244 |
| Gentisic acid | 8.3 | 153.0193 |
| Salicylic acid | 11.5 | 137.0244 |
| Hydroxy cinnamic acids | ||
| 5-O-Chlorogenic acid | 8.9 | 353.0878 |
| Caffeic acid | 9.3 | 179.0350 |
| p-Coumaric acid | 10.4 | 163.0400 |
| Hydroxycoumarins | ||
| Aesculetin | 9.1 | 177.0193 |
| Flavonoids | ||
| Dihydrochalcones | ||
| Phlorizin | 11.3 | 435.1296 |
| Flavanones | ||
| Naringenin (aglycone) | 12.5 | 271.0612 |
| Flavones | ||
| Chrysin (aglycone) | 15.1 | 253.0506 |
| Flavonols | ||
| (+)-Catechin | 8.5 | 289.0717 |
| Quercetin-3-O-rhaminoside | 9.2 | 447.0933 |
| (-)-Epicatechin | 9.5 | 289.0717 |
| Quercetin-3-O-galactoside | 11.0 | 463.0882 |
| Quercetin-3-O-glucoside | 11.0 | 463.0882 |
| Quercetin-3-O-rutinoside | 11.0 | 609.1461 |
| Kaempferol 3-O-glucoside | 11.5 | 447.0933 |
| Kaempferol 3-O-rutinoside | 11.5 | 593.1512 |
| Quercetin (aglycone) | 12.0 | 301.0354 |
| Luteolin (aglycone) | 12.7 | 285.0404 |
| Kaempferol (aglycone) | 13.3 | 285.0404 |
| Other polyphenols | ||
| Catechol | 6.8 | 109.0295 |
| Polyphenols analysed in positive ionization mode | ||
| Anthocyanines | ||
| Delphinidin 3-O-rutinoside * | 9.1 | 610.1539 |
| Cyanidin-3-O-glucoside | 9.4 | 449.1084 |
| Delphinidin (aglycone) | 11.1 | 304.0578 |
| Peonidin 3-O-glucoside | 18.4 | 463.1240 |
| Compound | tR (min) | λmax (nm) | A. retinodes | A. mearnsii | ||
|---|---|---|---|---|---|---|
| EF | LF | EF | LF | |||
| Simple phenolics | ||||||
| Catechol | 5.7 | 280 | 4.53 ± 0.23 c | 6.20 ± 0.21 d | 2.50 ± 0.08 a | 3.64 ± 0.10 b |
| Hydroxybenzoic acids | ||||||
| Gallic acid | 3.1 | 280 | 1.45 ± 0.15 a | 4.38 ± 1.27 a | 5.88 ± 3.35 a | 6.07 ± 1.06 a |
| 4-Hydroxybenzoic acid | 9.8 | 255 | 4.86 ± 0.63 a | 7.51 ± 0.38 b | <LOQ ▪ | <LOQ ▪ |
| Hydroxybenzoic aldehydes | ||||||
| 4-Hydroxybenzaldehyde | 12.6 | 280 | 4.80 ± 0.07 a | 7.50 ± 0.42 b | <LOQ ▪▪ | <LOQ ▪▪ |
| Vanillin | 16.6 | 280 | 7.55 ± 0.52 a | 31.8 ± 4.24 a | 194.93 ± 10.52 b | 310.71 ± 65.68 c |
| Syringaldehyde | 20.9 | 322 | 1.51 ± 0.55 ab | 1.76 ± 0.70 ab | 2.45 ± 0.83 b | 0.36 ± 0.25 a |
| Hydroxycinnamic acids | ||||||
| Chlorogenic acid | 10.0 | 280 | 65.84 ± 3.15 b | 12.16 ± 2.35 a | <LOQ ▪▪ | <LOQ ▪▪ |
| Caffeic acid | 12.9 | 322 | 7.59 ± 1.16 b | 5.84 ± 0.89 ab | 6.92 ± 2.46 ab | 3.40 ± 0.34 a |
| p-Coumaric acid | 19.8 | 291 | 0.74 ± 0.02 a | 1.45 ± 0.21 a | 11.94 ± 2.30 b | 10.35 ± 1.05 b |
| trans-Cinnamic acid | 33.5 | 280 | 0.68 ± 0.06 a | 1.66 ± 0.09 a | 9.65 ± 3.30 b | 16.17 ± 3.91 b |
| Hydroxycinnamic aldehydes | ||||||
| Coniferaldehyde | 29.2 | 322 | 0.44 ± 0.01 a | 0.43 ± 0.05 a | <LOQ ▪▪ | <LOQ ▪▪ |
| Furans | ||||||
| Furfural | 5.7 | 2.63 | 0.48 ± 0.03 a | 3.68 ± 0.99 b | <LOQ ▪▪▪ | <LOQ ▪▪▪ |
| 5-Methylfurfural | 11.6 | 255 | 42.99 ± 6.29 b | 219.71 ± 20.14 c | 1.71 ± 1.14 a | 15.09 ± 2.01 ab |
| Flavonoids | ||||||
| Flavanols | ||||||
| (+)-Catechin | 9.0 | 280 | <LOQ ▪▪▪ | <LOQ ▪▪▪ | 0.26 ± 0.12 a | 0.15 ± 0.01 a |
| (-)-Epicatechin | 10.6 | 280 | 9.43 ± 0.82 c | 14.38 ± 0.70 d | 3.05 ± 0.08 a | 7.07 ± 0.43 b |
| Flavonols | ||||||
| Rutin | 31.9 | 255 | 0.70 ± 0.04 a | 4.31 ± 0.72 b | <LOQ ▪▪ | <LOQ ▪▪ |
| Myricitrin | 33.4 | 263 | 39.49 ± 3.54 a | 70.39 ± 3.54 b | <LOQ ▪▪ | <LOQ ▪▪ |
| Myricetin | 34.5 | 360 | 2.63 ± 0.10 a | 8.71 ± 0.09 b | <LOQ ▪▪ | <LOQ ▪▪ |
| Quercetin | 41.0 | 360 | 1.11 ± 0.02 a | 5.54 ± 0.11 c | 0.94 ± 0.08 a | 2.78 ± 0.19 b |
| Kaempferol | 45.3 | 360 | 7.62 ± 1.23 b | 2.32 ± 1.32 a | <LOQ ▪▪ | <LOQ ▪▪ |
| Flavones | ||||||
| 4′,5,7-Trihydroxyflavanone | 43.5 | 280 | 2.35 ± 0.20 a | 1.53 ± 0.42 a | 5.92 ± 0.98 b | 5.59 ± 0.53 b |
| Compound | Specie (S) | Flowering Stages (FS) | S × FS | Residual |
|---|---|---|---|---|
| Appear in booth Acacia species | ||||
| Gallic acid | n.s. | n.s. | n.s. | -- |
| Vanillin | 89.1 *** | 7.2 * | n.s. | 3.7 |
| Syringaldehyde | n.s. | n.s. | n.s. | -- |
| Caffeic acid | n.s. | n.s. | n.s. | -- |
| p-Coumaric acid | 96.9 *** | n.s. | n.s. | 0.6 |
| trans-Cinnamic acid | 91.1 ** | n.s. | n.s. | 8.9 |
| 5-Methyfurfural | 40.2 *** | 24.0 *** | 35.3 *** | 0.6 |
| Quercetin | 14.0 *** | 64.2 *** | 21.6 *** | 0.2 |
| 4′,5,7-Trihydroxyflavanone | 95.2 *** | n.s. | n.s. | 4.8 |
| Catechol | 71.9 *** | 27.0 *** | 1.0 *** | 0.1 |
| (-)-Epicatechin | 69.8 *** | 29.6 *** | 0.3 *** | 0.2 |
| Appear only in A. retinodes | ||||
| Furfural | 90.9 * | 9.1 | ||
| 4-Hydroxybenzoic acid | 99.9 *** | 0.1 | ||
| Chlorogenic acid | 99.5 ** | 0.5 | ||
| 4-Hydroxybenzaldehyde | 97.5 * | 2.5 | ||
| Myricitrin | 97.0 * | 3.0 | ||
| Rutin | 100 *** | 0.0 | ||
| Kaempferol | n.s. | -- | ||
| Myricetin | 100 *** | 0.0 | ||
| Appear only in A. mearnsii | ||||
| (+)-Catechin | n.s. | -- | ||
| Coniferaldehyde | n.s. | -- |
| Compound | A. retinodes | A. mearnsii | ||
|---|---|---|---|---|
| EF | LF | EF | LF | |
| Analysed in Negative Ionization Mode | ||||
| Aesculetin | 2.48 × 10−2 ± 3.36 × 10−4 | 0.014 ± 0.2 × 10−2 | 0.097 ± 0.2 × 10−3 | 0.014 ± 0.1 × 10−3 |
| Chrysin (aglycone) | 7.65 × 10−5 ± 8.06 × 10−6 | 1.15 × 10−4 ± 1.02 × 10−5 | 1.04 × 10−4 ± 1.61 × 10−6 | 1.28 × 10−4 ± 8.90 × 10−7 |
| Delphinidin 3-O-rutinoside | 1.25 ± 0.10 | 2.46 ± 0.05 | 0.42 ± 0.002 | 1.87 ± 0.03 |
| Gentisic acid | 0.31 ± 0.3 × 10−2 | 0.32 ± 0.1 × 10−2 | 0.50 ± 0.02 | 0.50 ± 0.02 |
| kaempferol (aglycone) | 11.18 ± 0.51 | 32.93 ± 0.34 | 2.40 ± 0.01 | 1.94 ± 0.09 |
| kaempferol 3-O-glucoside | 0.80 ± 0.02 | 1.47 ± 0.02 | 9.63 ± 0.48 | 9.43 ± 0.18 |
| Luteolin (aglycone) | 0.95 ± 0.02 | 1.19 ± 0.001 | 0.55 ± 0.02 | 0.52 ± 0.01 |
| Naringenin (aglycone) | 0.72 ± 0.02 | 0.63 ± 0.04 | 1.14 ± 0.02 | 1.16 ± 0.03 |
| Nicotiflorin | 0.03 ± 0.1 × 10−2 | 0.04 ± 0.3 × 10−2 | 0.01 ± 0.1 × 10−3 | 0.52 × 10−2 ± 0.7 × 10−2 |
| Phlorizin | 49.70 ± 4.65 | 56.09 ± 1.51 | 37.43 ± 16.28 | 40.98 ± 21.49 |
| Protocatechuic acid | 0.02 ± 0.1 × 10−2 | 0.45 ± 0.02 | 0.06 ± 0.21 × 10−3 | 0.04 ± 2.2 × 10−3 |
| salicyclic acid | 0.05 ± 0.2 × 10−2 | 0.08 ± 0.2 × 10−2 | 0.45 x 10−2± 0.1 × 10−3 | 0.04 ± 0.2 × 10−2 |
| Quercetin (aglycone) | 2.08 ± 0.32 | 11.59 ± 0.64 | 0.70 ± 0.003 | 1.55 ± 0.01 |
| Quercetin-3-O-glucoside + Quercetin-3-O-galactoside (sum of isomers) | 0.75 ± 0.02 | 4.21 ± 0.01 | 1.96 ± 0.01 | 3.59 ± 0.04 |
| Quercetin-3-O-rhaminoside | 0.79 ± 0.001 | 0.83 ± 0.11 | 17.60 ± 0.72 | 21.82 ± 0.62 |
| Analysed in positive ionization mode | ||||
| Chrysanthemin | nd | nd | 12.56 ± 1.36 | 14.42 ± 0.29 |
| Peonidin 3-O-glucoside | 0.37 ± 0.01 | 0.83 ± 0.002 | 0.49 ± 0.14 | 0.90 ± 0.09 |
| Delphinidin | 2.96 ± 0.02 | 11.46 ± 0.17 | 4.68 ± 0.01 | 9.44 ± 0.44 |
| A. mearnsii Extracts | |||||||
|---|---|---|---|---|---|---|---|
| Monoisotopic Mass | Area | tR (min) | [M−H]− | [M+H]+ | Tentative Annotation | Annotation Level | |
| (m/z) | (m/z) | ||||||
| Hydroxycinnamic acid glycosides | |||||||
| 326.2282 | 3.38 × 106 | 9.1 | 325.0935 | 327.1358 | p-coumaroyl hexose | MS/MS | |
| 356.2498 | 2.28 × 106 | 9.3 | 355.104 | 357.1155 | feruloyl hexose | MS/MS | |
| Flavanones O-glycosides | |||||||
| 434.3828 | 3.26 × 107 | 10.2/10.9/11.5/11.9 | 433.115 | 435.0927 | naringenin O-hexose isomers | MS/MS | |
| Flavonol O-glycosides | |||||||
| 448.2944 | 3.11 × 106 | 11.6 | 447.0943 | 449.1090 | quercetin O-hexoside | MS1 | |
| A. retinodes extracts | |||||||
| Monoisotopic mass | Area | tR (min) | [M−H]− | [M+H]+ | Tentative annotation | Annotation level | |
| (m/z) | (m/z) | ||||||
| Hydroxycinnamic acid glycosides | |||||||
| 326.2282 | 3.38 × 106 | 9.1 | 325.0935 | 327.1358 | p-coumaroyl hexose | MS/MS | |
| 356.2498 | 2.28 × 106 | 9.3 | 355.104 | 357.1155 | feruloyl hexose | MS/MS | |
| Flavanones O-glycosides | |||||||
| 434.3828 | 4.94 × 106 | 11.1/11.3/11.5 | 433.115 | 435.0927 | naringenin O-hexose isomers | MS/MS | |
| Flavonol O-glycosides | |||||||
| 788.2021 | 9.94 × 105 | 9.7 | 787.1949 | 789.2089 | quercetin O-triglucoside | MS1 | |
| 642.1445 | 1.27 × 106 | 9.8 | 641.1372 | 643.1517 | myricetin O-dihexoside isomer | MS1 | |
| 626.1483 | 3.92 × 106 | 10.1/10.3 | 625.1423 | 627.1571 | quercetin O-dihexoside isomer | MS1 | |
| 480.0904 | 2.29 × 106 | 10.5 | 479.0839 | 481.0982 | myricetin O-hexoside isomer | MS1 | |
| 450.0798 | 2.50 × 106 | 10.8 | 449.0703 | 451.0879 | myricetin O-hexoside isomer | MS1 | |
| 464.0962 | 2.05 × 106 | 10.9/11.1 | 463.0861 | 465.1034 | quercetin O-hexoside isomer | MS1 | |
| 448.2944 | 3.11 × 106 | 11.6 | 447.0943 | 449.1090 | quercetin O-hexoside isomer | MS1 | |
| 318.0378 | 6.74 × 106 | 12.4 | 317.0305 | 319.0454 | myricetin (aglycone) | MS/MS | |
| 302.0431 | 1.47 × 106 | 12.5 | 301.0358 | 303.0502 | quercetin (aglycone) | MS/MS | |
| Jasmonic acids | |||||||
| 388.1742 | 1.39 × 106 | 9.8 | 387.17 | 389.18 | 11-hydroxyjasmonic acid glucoside | MS1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, S.I.; Rosado, T.; Barroca, C.; Neiva, D.; Alonso-Herranz, V.; Gradillas, A.; García, A.; Gominho, J.; Gallardo, E.; Anjos, O. Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts. Plants 2022, 11, 1442. https://doi.org/10.3390/plants11111442
Pedro SI, Rosado T, Barroca C, Neiva D, Alonso-Herranz V, Gradillas A, García A, Gominho J, Gallardo E, Anjos O. Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts. Plants. 2022; 11(11):1442. https://doi.org/10.3390/plants11111442
Chicago/Turabian StylePedro, Soraia I., Tiago Rosado, Celina Barroca, Duarte Neiva, Vanesa Alonso-Herranz, Ana Gradillas, Antonia García, Jorge Gominho, Eugenia Gallardo, and Ofélia Anjos. 2022. "Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts" Plants 11, no. 11: 1442. https://doi.org/10.3390/plants11111442
APA StylePedro, S. I., Rosado, T., Barroca, C., Neiva, D., Alonso-Herranz, V., Gradillas, A., García, A., Gominho, J., Gallardo, E., & Anjos, O. (2022). Characterisation of the Phenolic Profile of Acacia retinodes and Acacia mearnsii Flowers’ Extracts. Plants, 11(11), 1442. https://doi.org/10.3390/plants11111442