Beet Molasses Enhance Salinity Tolerance in Thymus serpyllum—A Study under Greenhouse Condition
Abstract
1. Introduction
2. Results
2.1. Soil pH and EC Analysis
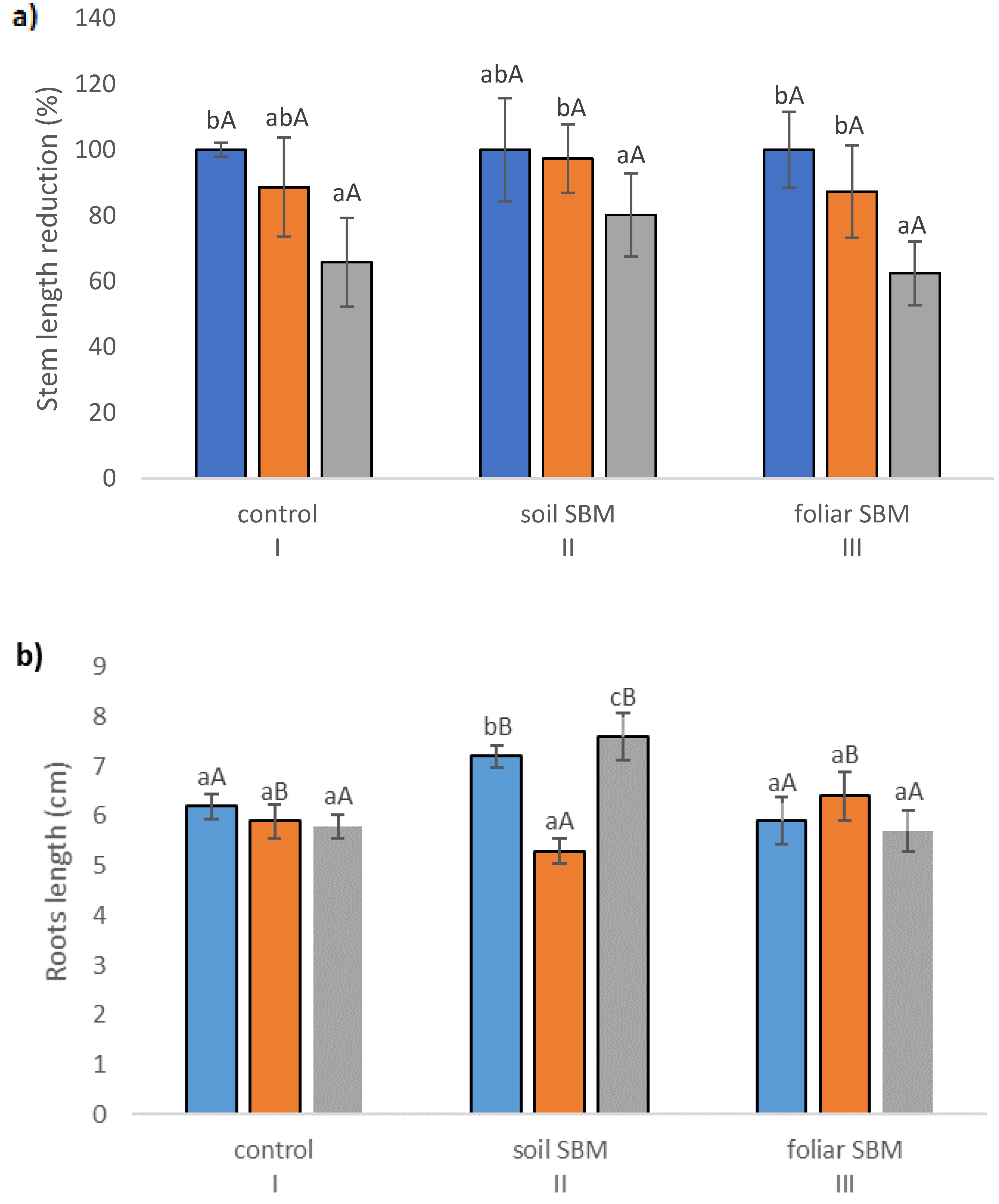
2.2. Growth Parameters
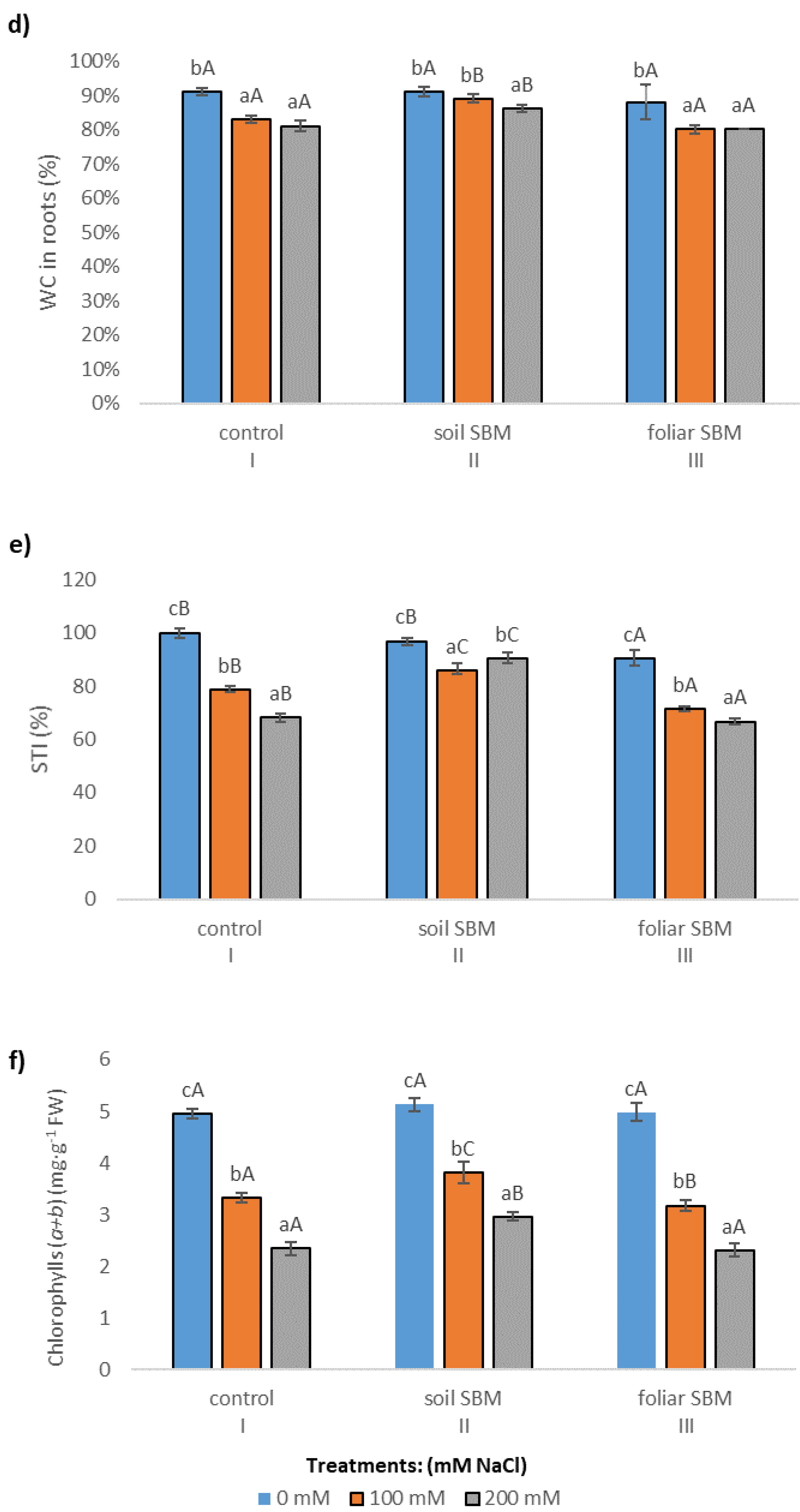
2.3. Biochemical Parameters
2.3.1. The Chlorophyll Content
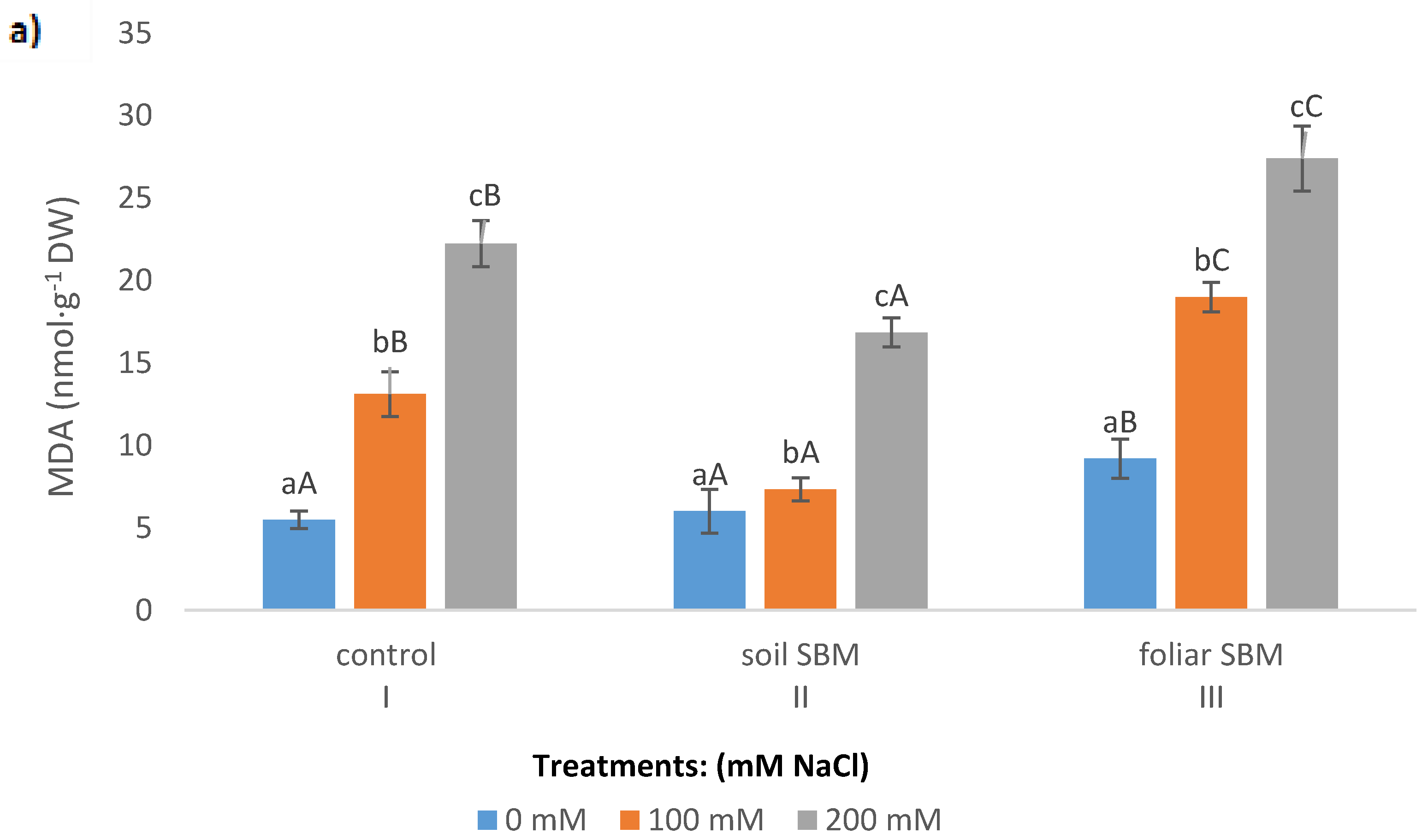
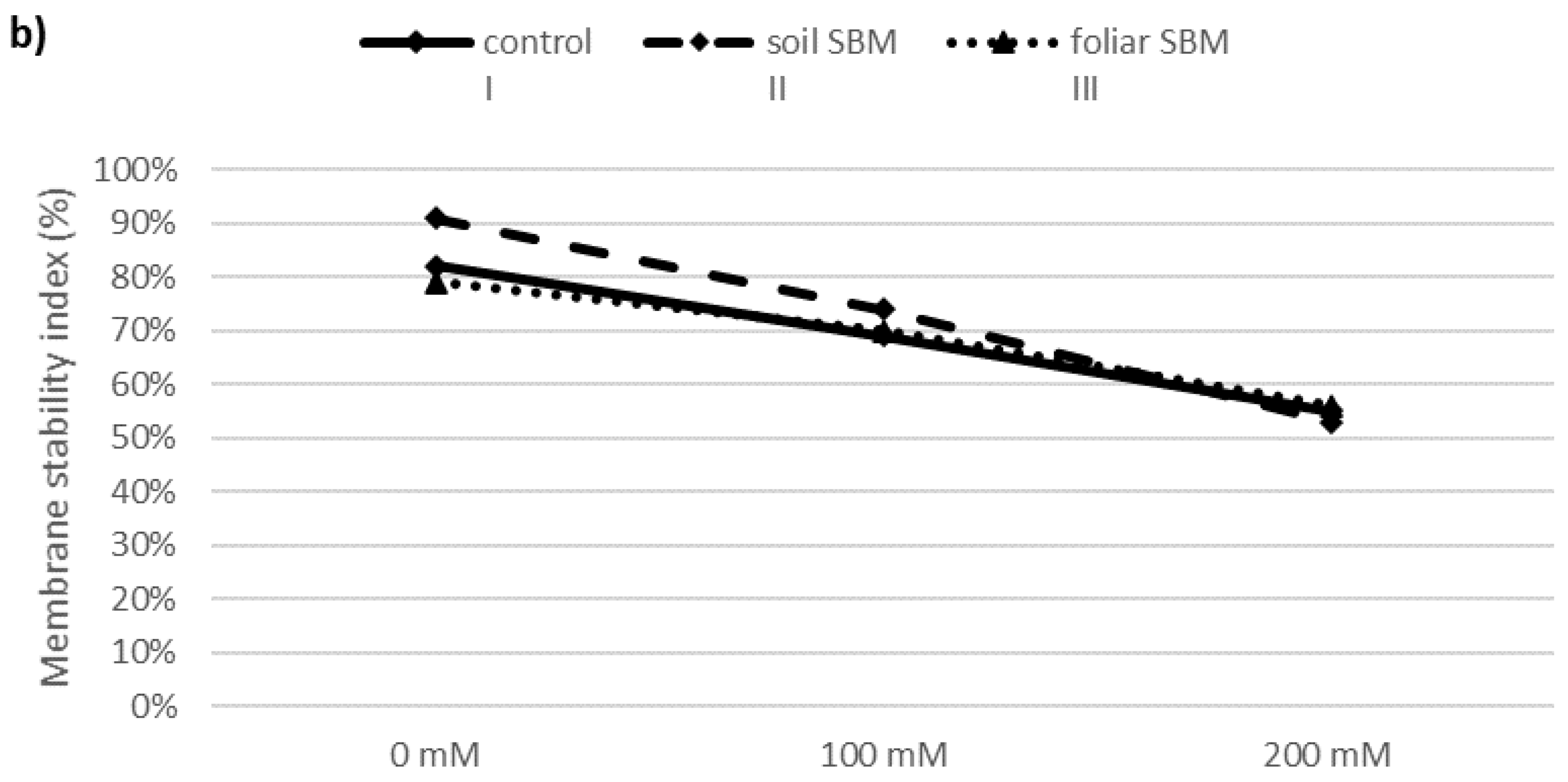
2.3.2. MDA and Membrane Stability
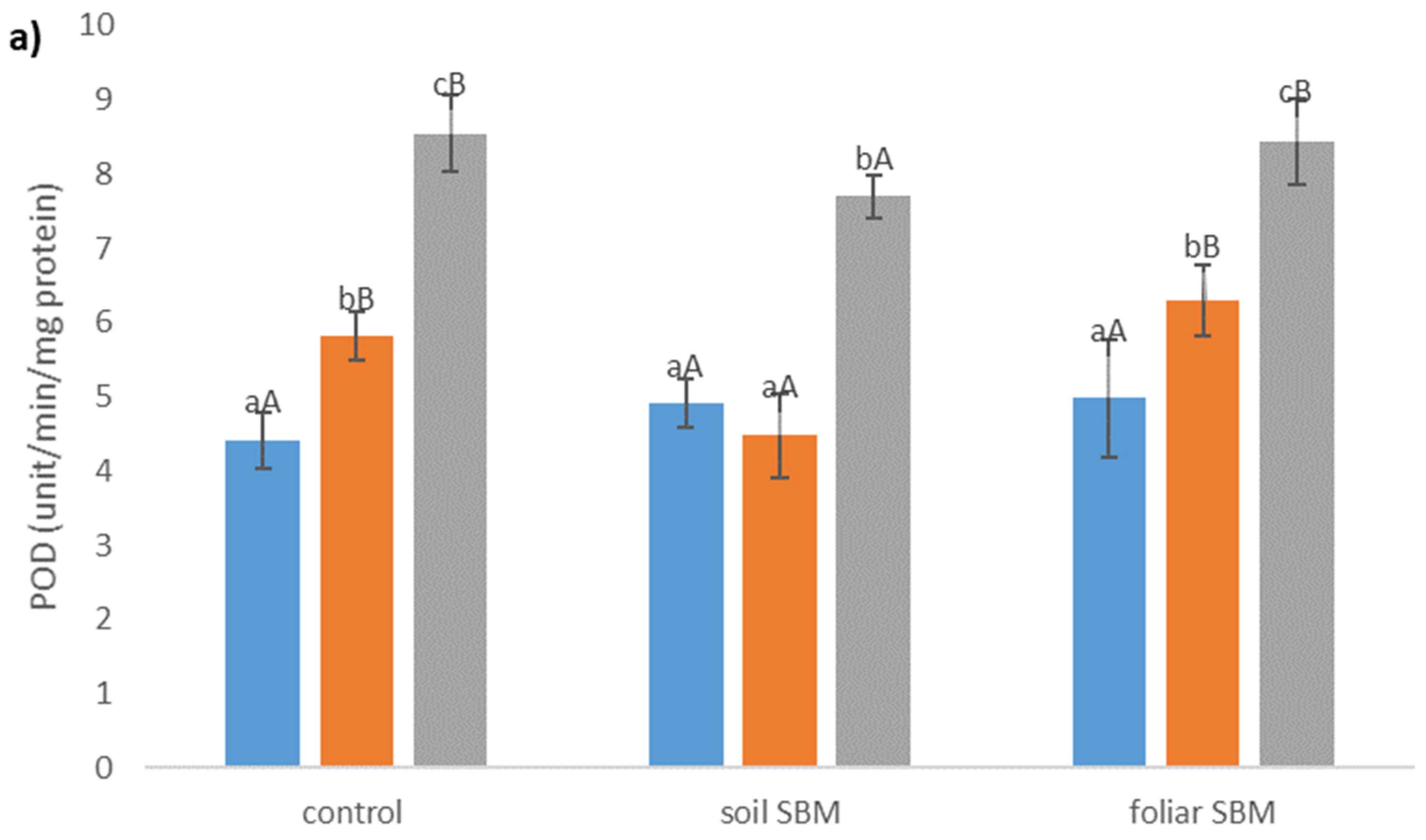
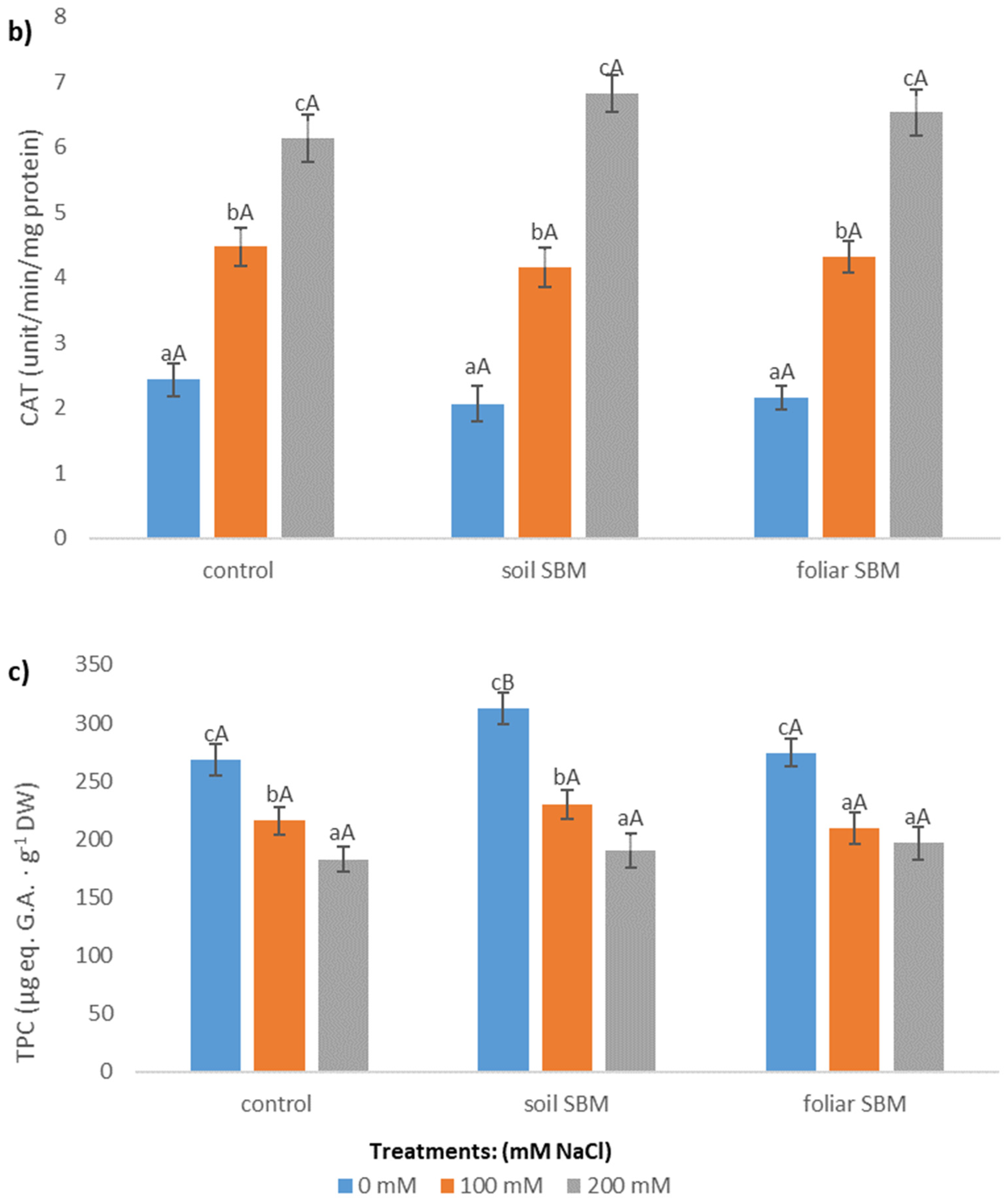
2.3.3. Antioxidants
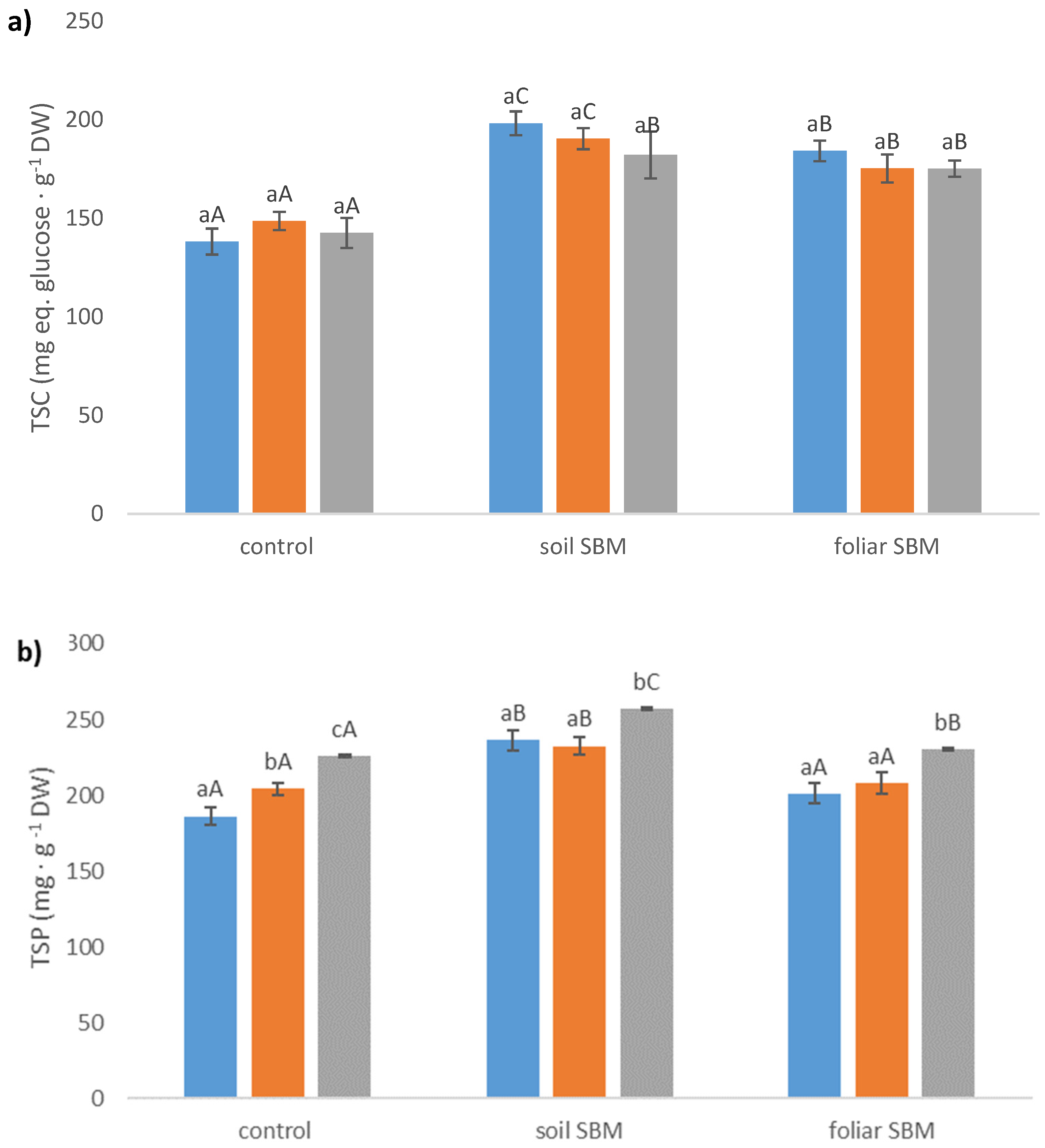
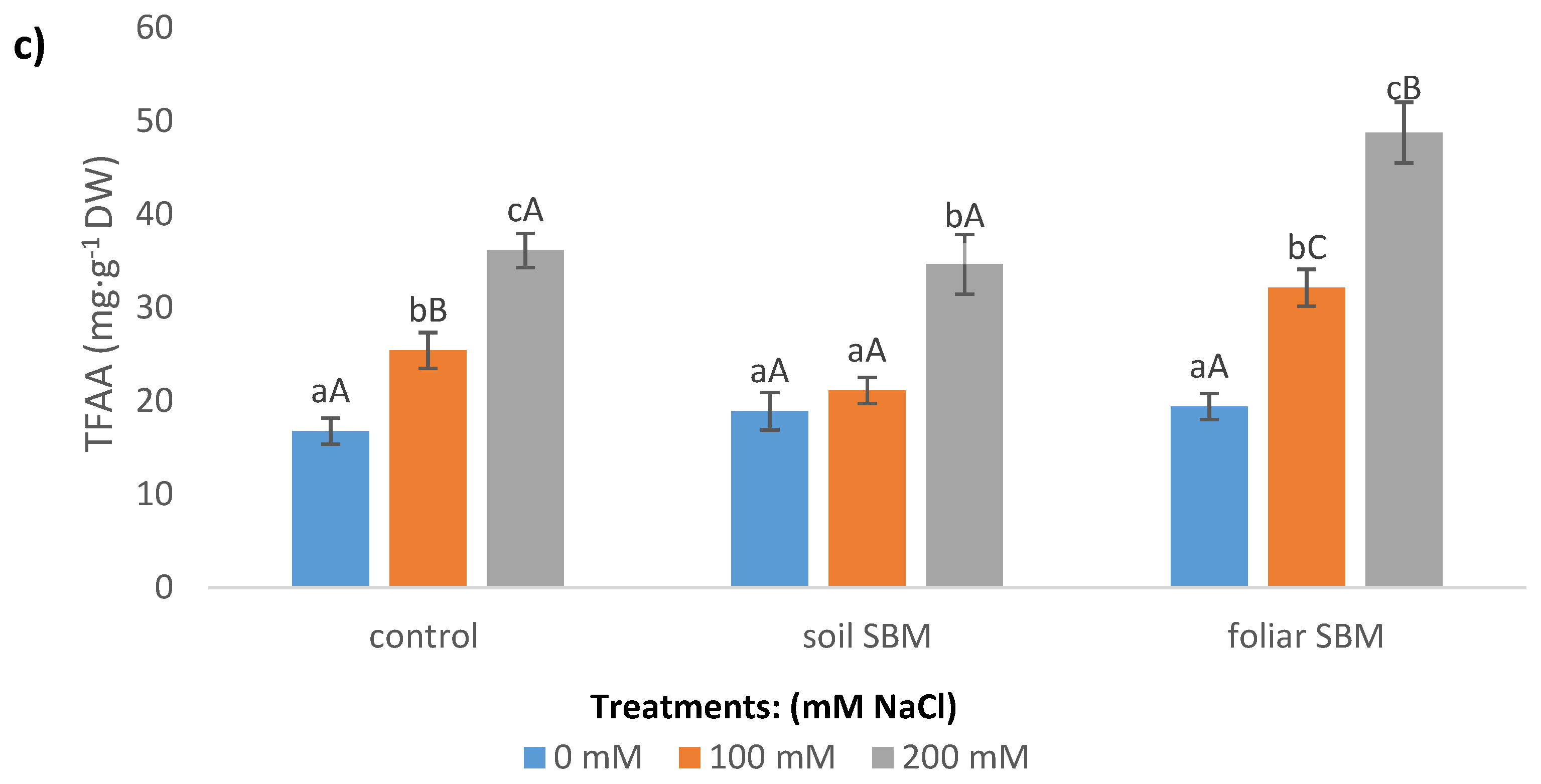
2.3.4. Stress-Related Metabolites
Total Soluble Carbohydrates (TSC)
Total Soluble Protein (TSP)
Total Free Amino Acids (TFAA)
2.3.5. Ions
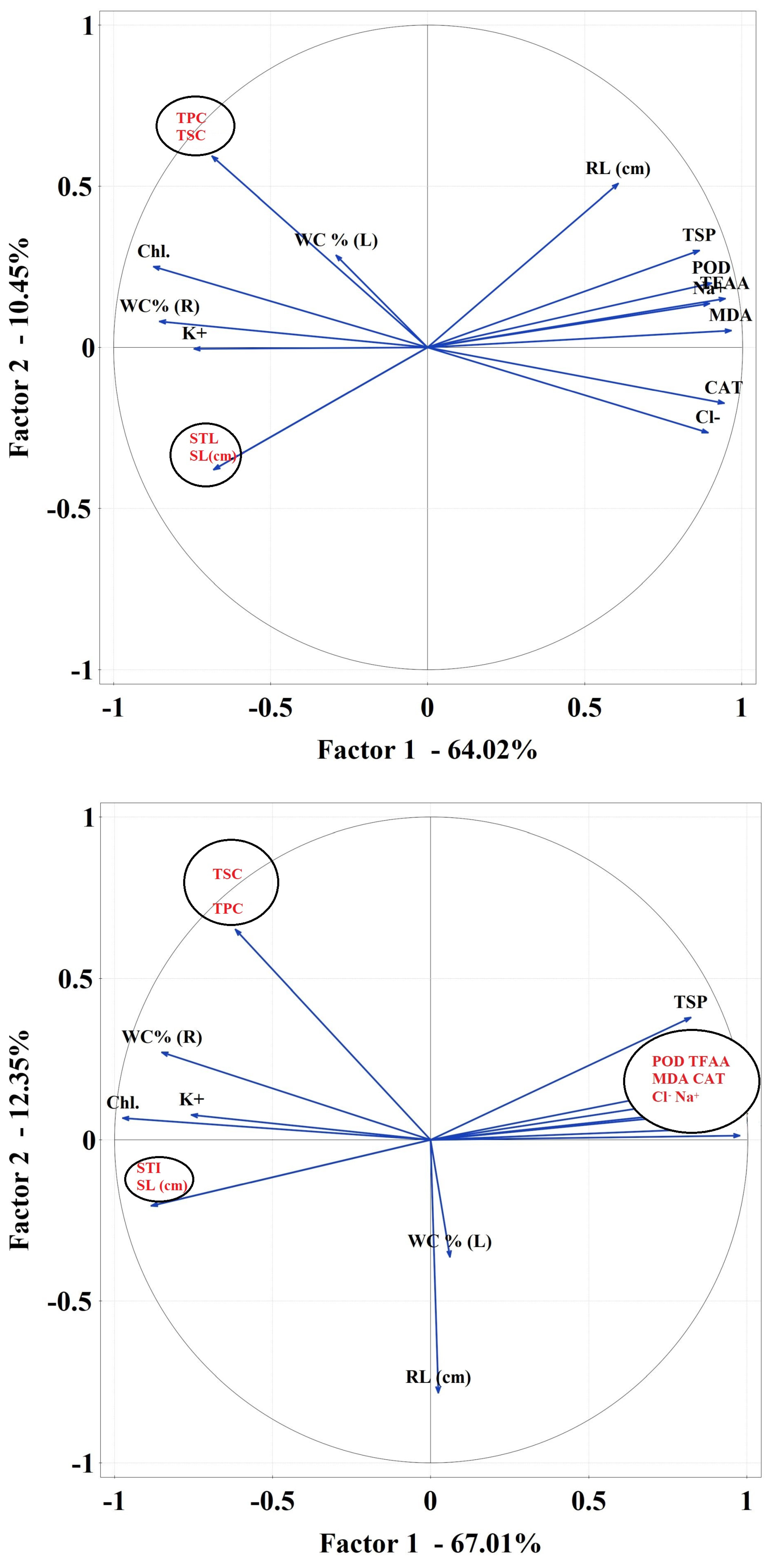
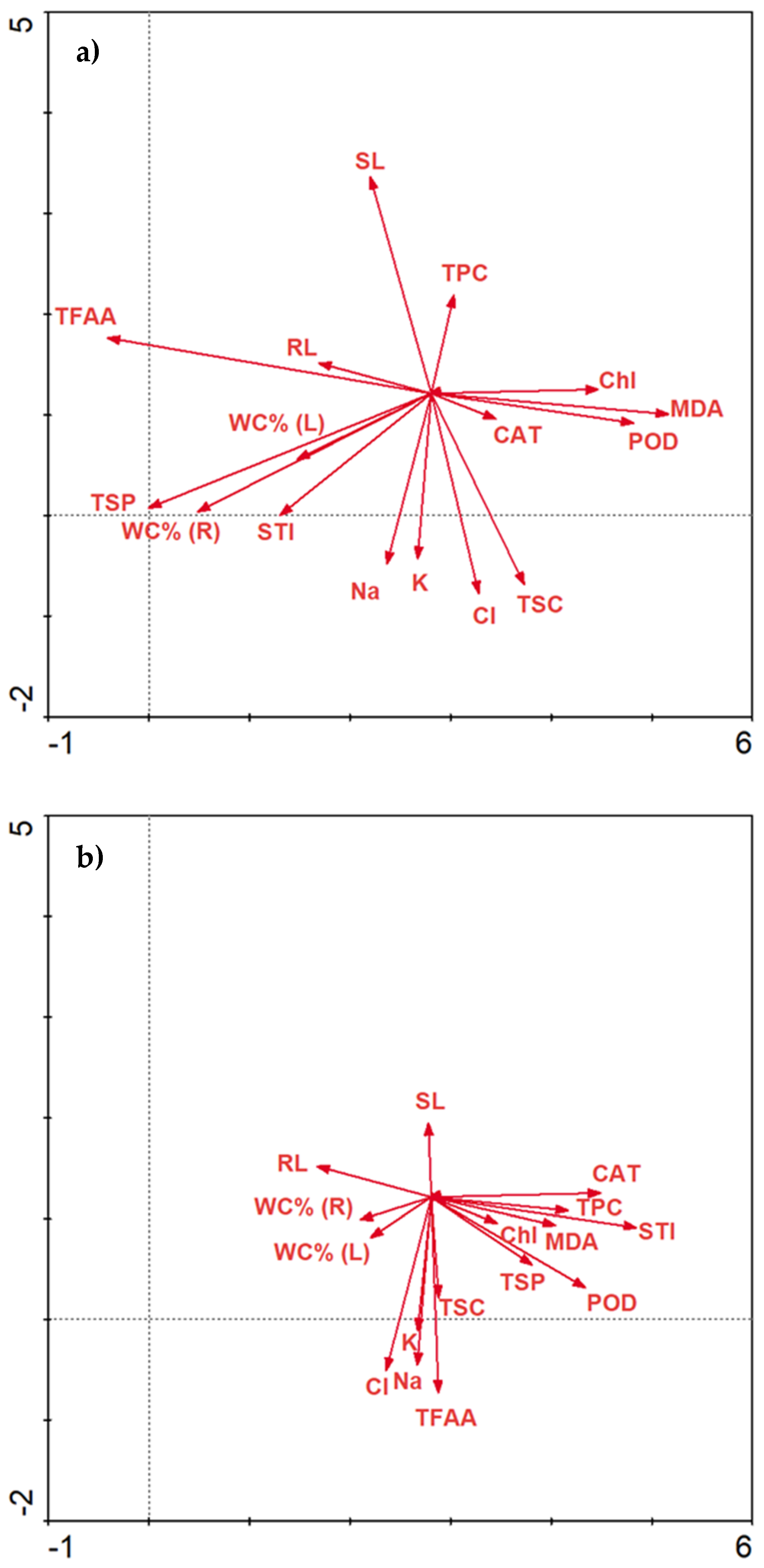
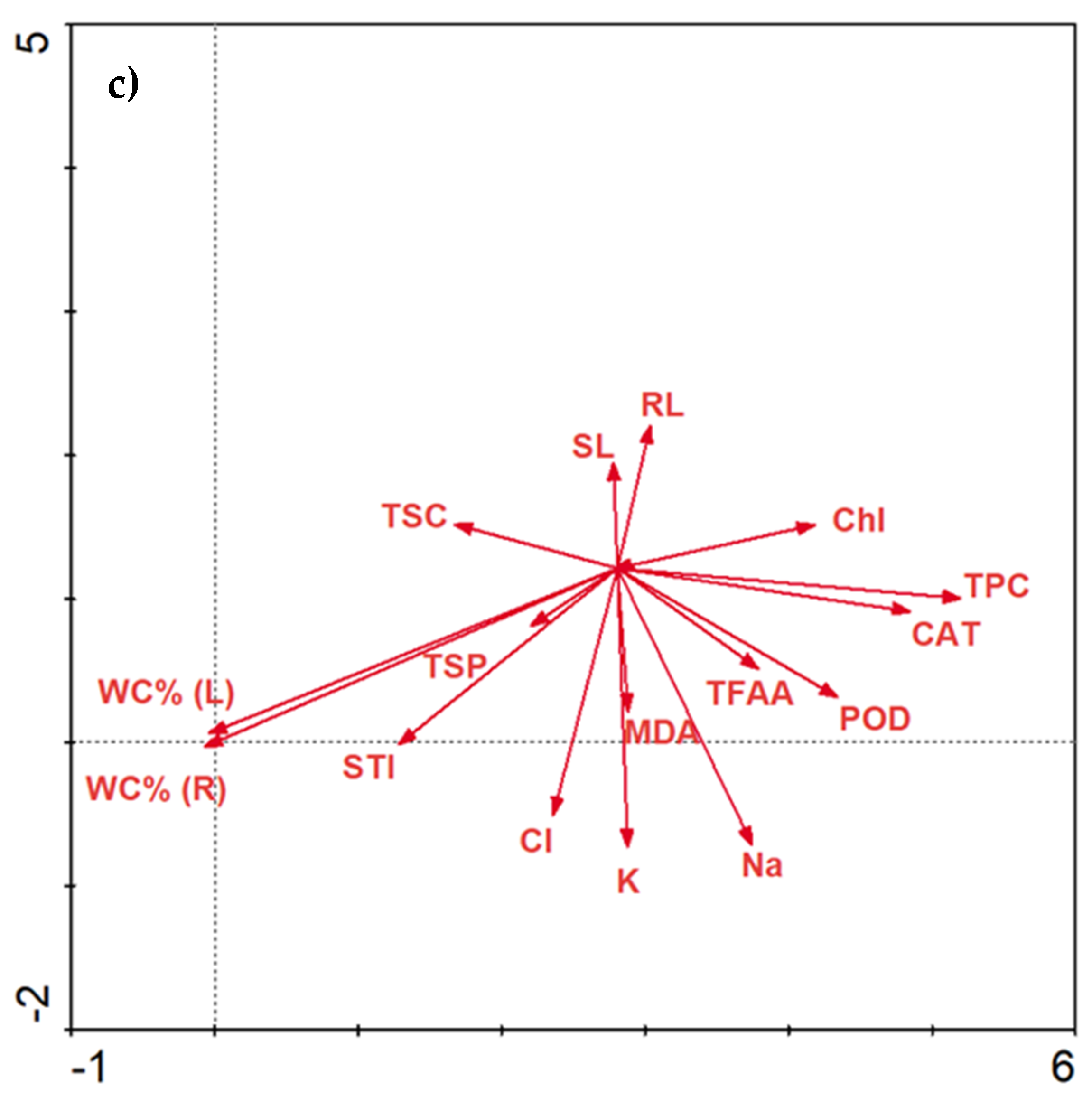
2.3.6. Data Correlations
Correlations Matrix
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Soil pH and EC Analysis
4.3. Assessing Plant Growth
4.4. Assessing Plant Biochemical Parameters
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delgado-Adámez, J.; Garrido, M.; Bote, M.E.; Funtes-Pérez, M.C.; Espino, J.; Martín-Vertedor, D.M. Chemical composi-tion and bioactivity of essential oils flom flower and fruit of Thymbra capitata and Thymus species. J. Food Sci. Technol. 2017, 54, 1857–1865. [Google Scholar] [CrossRef]
- Banerjee, P.; Mukherjiee, S.; Bera, K.; Ghosh, K.; Ali, I.; Khawas, S.; Ray, B.; Ray, S. Polysaharides from Thymus vulgaris leaf: Structutal features, antioxidant activity and interactions with bowine serum albumin. Internat. J. Biol. Macromol. 2019, 125, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sim, L.Y.; Rani, N.Z.A.; Husain, K. Lamiaceae: An Insight on Their Anti-Allergic Potential and Its Mechanisms of Action. Front. Pharmacol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Taraweneh, A.H.; Carbal, C.; Gasetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rabaji, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. plants-Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chávez-González, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions—A new paradigm. Trends Food Sci. Technol. 2021, 111, 426–441. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Tohidi, B.; Ahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid content and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z. Salinity: A major agricultural problem-causes, impacts on crop productivity and mamagement strategies. In Plant Abiotic Stress Tolerance, Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Narar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 101–128. [Google Scholar]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Zia, A.; Rezanejad, F.; Safarnejad, A. In vitro selection for NaCl tolerance in Thymus vulgaris. J. Cell Mol. Res. 2010, 2, 86–92. [Google Scholar]
- Ashraf, M.Y.; Mahmood, K.; Akhter, J.; Hussain, F.; Arshad, M. Phytoremediation of Saline Soils for Sustainable Agricultural Productivity. In Plant Adaptation and Phytoremediation; Springer Science and Business Media LLC: Berlin, Germany, 2010; pp. 335–355. [Google Scholar]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Janda, T.G.; Szalai Tari, I.; Paldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Blake, T.J. New Plant Growth Regulators Protect Photosynthesis and Enhance Growth under Drought of Jack Pine Seedlings. J. Plant Growth Regul. 1999, 18, 175–181. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Hegarat, E.; Jiménez-Lao, M.; Lao, M.T. Effects of Exogenous Application of Osmotic Adjustment Substances on Growth, Pigment Concentration, and Physiological Parameters of Dracaena sanderiana Sander under Different Levels of Salinity. Agronomy. 2020, 10, 125. [Google Scholar] [CrossRef]
- Najafian, S.; Khosh-khui, M.; Tavallali, V.; Saharkhiz, M.J. Effect of Salicylic Acid and Salinity in Thyme (Thymus vulgaris L.): Investigation on Changes in Gas Exchange, Water Relations, and Membrane Stabilization and Biomass Accumulation. Aust. J. Basic Appl. Sci. 2009, 3, 2620–2626. [Google Scholar]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef]
- Mustafa, G.; Arshad, M.; Bano, I.; Abbas, M. Biotechnological applications of sugarcane bagasse and sugar beet molasses. Biomass-Convers. Biorefinery 2020, 1–13. [Google Scholar] [CrossRef]
- Olbrich, H. The Molasses; Institut für Zuckerindustrie, Reedition Biotechnologie-Kempe GmbH: Berlin, Germany, 2006; Available online: http://www.biotechnologie-kempe.de/Molasses_OLBRICH.pdf (accessed on 8 May 2021).
- Valli, V.; Gómez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar Cane and Sugar Beet Molasses, Antioxidant-rich Alternatives to Refined Sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Samavat, S.; Samavat, S. Effects of fulvic acid and sugarcane molasses on yeild and qualities of tomato. Int. Res. J. Appl. Basic Sci. 2014, 8, 266–268. [Google Scholar]
- Wynne, A.T.; Meyer, J.H. An economic assessment of using molasses and condensed molasses soluble as a fertilizer in the South African sugar industry. Proc. S. Afr. Sugar Technol. Assoc. 2002, 76, 71–78. [Google Scholar]
- Suganya, K.; Rajannan, G. Effect of onetime post-sown and pre-sown application of distillery spentwash on the growth and yield of maize crop. Bot. Res. Int. 2009, 2, 288–294. [Google Scholar]
- Li, S.; Zhao, X.; Ye, X.; Zhang, L.; Shi, L.; Xu, F.; Ding, G. The Effects of Condensed Molasses Soluble on the Growth and Development of Rapeseed through Seed Germination, Hydroponics and Field Trials. Agriculture 2020, 10, 260. [Google Scholar] [CrossRef]
- Satyawali, Y.; Balakrishnan, M. Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: A review. J. Environ. Manag. 2008, 86, 481–497. [Google Scholar] [CrossRef]
- Srivastava, P.C.; Singh, R.K.; Srivastava, P.; Shrivastava, M. Utilization of molasses based distillery effluent for fertigation of sugarcane. Biodegradation 2012, 23, 897–905. [Google Scholar] [CrossRef]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 1–18. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Ezz El-Din, A.A.; Aziz, E.E.; Hendawy, S.F.; Omer, E.A. Response of Thymus vulgaris L. to salt and Alar (B9) in newly reclaimed soil. J. Appl. Sci. 2009, 5, 2165–2170. [Google Scholar]
- Castiglione, S.; Oliva, G.; Vigliotta, G.; Novello, G.; Gamalero, E.; Lingua, G.; Cicatelli, A.; Guarino, F. Effects of Compost Amendment on Glycophyte and Halophyte Crops Grown on Saline Soils: Isolation and Characterization of Rhizobacteria with Plant Growth Promoting Features and High Salt Resistance. Appl. Sci. 2021, 11, 2125. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Aziz, E.E.; Rezk, A.I.; Omer, E.A.; Nofal, O.A.; Salama, Z.A.; Fouad, H.; Fouad, R. Chemical composition of Mentha pulegium L. (Pennyroyal) plant as influenced by foliar application of different sources of zinc. Egypt. Pharm. J. 2019, 18, 53. [Google Scholar] [CrossRef]
- Lakshmi Madhuri, M.L.; Kumari, S.S.; Swami, D.V.; Gridhar, K.; Salomi Suneetha, D.R.; Uma Krishna, K. Influence of nutrition and elicitation on vegetative, herbage and oil yield in Ocimum (Ocimium sanctum L.). Pharma Innov. J. 2021, 10, 478–486. [Google Scholar]
- Hanafy, M.S.; Ashour, H.A.; Sedek, F.M. Effect of some Bio-stimulants and Micronutrients on Growth, Yield and Essential Oil Production of Majorana hortensis plants. Int. J. Environ. 2018, 7, 37–52. [Google Scholar]
- Fahmy, A.A.; Nosir, W.S. Influence of chitosan and micronutrients (fe + zn) concentrations on growth, yield components and volatile oil of lavender plant. Sci. J. Flowers Ornam. Plants 2021, 8, 87–100. [Google Scholar] [CrossRef]
- Aljabri, M.; Alharbi, S.; Al-Qthanin, R.N.; Ismaeil, F.M.; Chen, J.; Abou-Elwafa, S.F. Recycling of beet sugar byproducts and wastes enhances sugar beet productivity and salt redistribution in saline soils. Environ. Sci. Pollut. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Singh, R.; Yavdav, M.; Kumar, V.; Singh, M.; Upadhyay, S.K. Effect of Molasses on the Growth of Okra Abelmoschus esculentus (L.) Moench (Dicotylenoae: Malvaceae). Bio-Sci. Res. Bull. 2021, 37, 4–11. [Google Scholar] [CrossRef]
- Walker, G.E. Effects of organic amendments, fertilizers and feanamiphos on parasitic and free-living nematodes, tomato growth and yield. Nematol. Medit. 2007, 35, 131–136. [Google Scholar]
- Khalid, A.K.; Zaghloul, S.M.; Yassen, A.A. Responses of thyme (Thymus vulgaris L.) to magnesium application. Med. Ina. Ar. Plant Sci. Biotechnol. 2009, 3, 52–57. [Google Scholar]
- Formann, S.; Hahn, A.; Janke, L.; Stinner, W.; Stäuber, H.; Logroňo, W.; Nikolausz, M. Beyond Sugar and Etanol Production: Value Generation Opportunities Through Sugacane Residues. Front. En. Res. 2020, 8, 579577. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct evidence using a controlled greenhouse study for threshold effects of soil organic matter on crop growth. Ecol. Appl. 2020, 30, e02073. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Yamatsu, T. Molasses melanoidin-like products enhance phytoextraction of lead through three Brassica species. Int. J. Phytorem. 2018, 20, 552–559. [Google Scholar] [CrossRef]
- Perrino, E.V.; Brunetti, G.; Farrag, K. Plant Communities in Multi-Metal Contaminated Soils: A Case Study in the National Park of Alta Murgia (Apulia Region-Southern Italy). Int. J. Phytoremediation 2014, 16, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, E.; Di Gioia, F.; Hong, J.C.; Pisani, C.; Kokalis-Burelle, N. Organic Amendments for Pathogen and Nematode Control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Guglielminetti, L.; Angelini, L.G. The effect of soil moisture depletion on Stevia (Stevia rebaudiana Bertoni) grown in greenhouse conditions: Growth, steviol glycosides content, soluble sugars and total antioxidant capacity. Sci. Hortic. 2015, 183, 93–99. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.; Al-Qurainy, F.; Foolad, M. Drought Tolerance. Adv. Agron. 2011, 111, 249–296. [Google Scholar] [CrossRef]
- Hall, J.D.; Barr, R.; AL-Abbas, A.H.; Crane, F.L. The ultrastructure of choroplasts in mineral deficient maize leaves. Plant Physiol. 1972, 50, 404–440. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Wang, Y.F.; Zhu, A.J.; Peng, F.L.; Wang, L.S. Exogenous sucrose can enhance tolerance of Arabidopsis thaliana seedlings to salt stress. Biol. Plant. 2014, 58, 611–617. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yanga, M.T.; Lia, X.; Zhoua, Z.Q.; Wanga, X.J.; Baia, J.G. Exogenous sucrose increases chilling tolerance in cucumber seedlings by modulating antioxidant enzyme activity and regulating proline and soluble sugar contents. Sci. Hort. 2014, 179, 67–77. [Google Scholar] [CrossRef]
- Altuntas, C.; Sezgin, A.; Demiralay, M.; Terzi, R.; Saglam, A.; Kadioglu, A. Application of sucrose modulates the expressions of genes involved in proline and polyamine metabolism in maize seedlings exposed to drought. Biol. Plant. 2019, 63, 247–252. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, M.; Bi, L.; Li, Y.; Wang, W.; Ye, Y. Effect of irrigating vinaase waste liquor on the activity of three kinds of enzymes and agronomic characters at seedling stage in sugarcane. Southwest China J. Agric. Sci. 2006, 19, 482–485. [Google Scholar]
- Changes in Free Amino Acids and Stress Protein Synthesis in Two Genotypes of Green Gram under Salt Stress. J. Plant Sci. 2005, 1, 56–66. [CrossRef][Green Version]
- Zhang, Z.; Mao, C.; Shi, Z.; Kou, X. The Amino Acid Metabolic and Carbohydrate Metabolic Pathway Play Important Roles during Salt-Stress Response in Tomato. Front. Plant Sci. 2017, 8, 1231. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein Contribution to Plant Salinity Response and Tolerance Acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef]
- Arefian, M.; Vessal, S.; Malekzadeh-Shafaroudi, S.; Siddique, K.; Bagheri, A. Comparative proteomics and gene expression analyses revealed responsive proteins and mechanisms for salt tolerance in chickpea genotypes. BMC Plant Biol. 2019, 19, 1–26. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Mäkelä, P. Agro-industrial uses of glycinebetaine. Sugar Tech. 2004, 6, 207–212. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Boine, B. Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures. Agronomy 2020, 10, 210. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Janicka-Russak, M.; Kabała, K. The Role of Plasma Membrane H+-ATPase in Salinity Stress of Plants. In Progress in Botany; Lüttge, U., Beyschlag, W., Eds.; Progress in Botany (Genetics—Physiology—Systematics—Ecology); Springer: Cham, Switzerland, 2015; pp. 77–92. [Google Scholar] [CrossRef]
- Page, M.J.; Di Cera, E. Role of Na+ and K+ In Enzyme Function. Physiol. Rev. 2006, 86, 1049–1092. [Google Scholar] [CrossRef] [PubMed]
- Šarić, L.; Filipčev, B.; Šimurina, O.; Plavšić, D.V.; Šarić, B.; Lazarević, J.; Milovanovic, I. Sugar beet molasses: Properties and applications in osmotic dehydration of fruits and vegetables. Food Feed Res. 2016, 43, 135–144. [Google Scholar] [CrossRef]
- Mozafar, A.; Oertli, J.J. Uptake and Transport of Thiamin (Vitamin B 1) by Barley and Soybean. J. Plant Physiol. 1992, 139, 436–442. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Kanaya, M.; Ogata, S. Cell Membrane Stability and Leaf Surface Wax Content as Affected by Increasing Water Deficits in Maize. J. Exp. Bot. 1991, 42, 167–171. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 584–593. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lück, H. Peroxidase. In Methoden Derenzymatischen Analyse; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1962; pp. 895–897. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Colorimet-ric Method for Determination of Sugars and Related Substances. Anal. Chem. ACS 1956, 26, 350. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Colowick, S.P.; Kaplan, N.O. Determination of amide residues by chemical methods. In Methods of Enzymology; Hirsh, C.H.W., Ed.; Academic Press: New York, NY, USA, 1967. [Google Scholar]

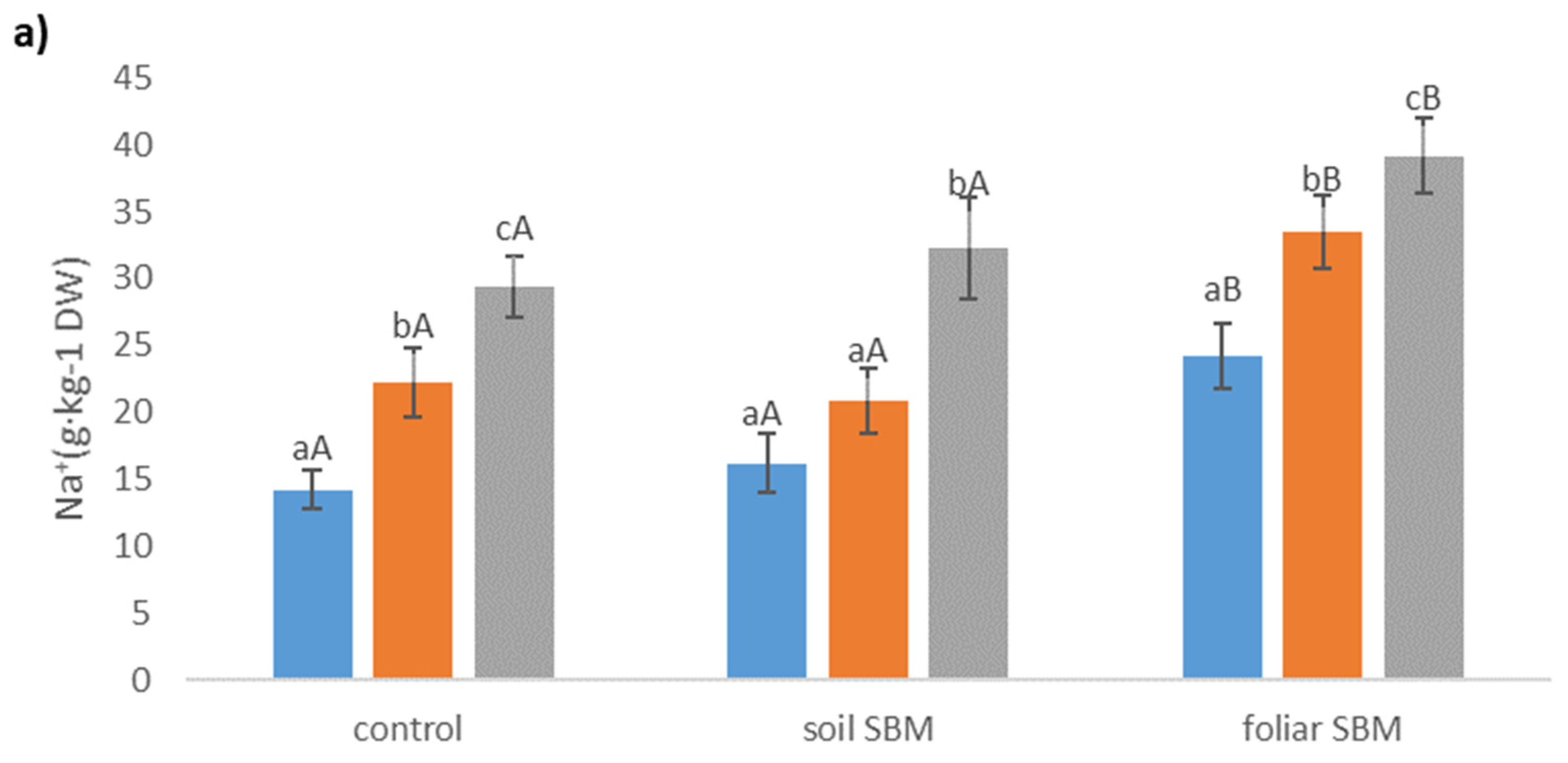
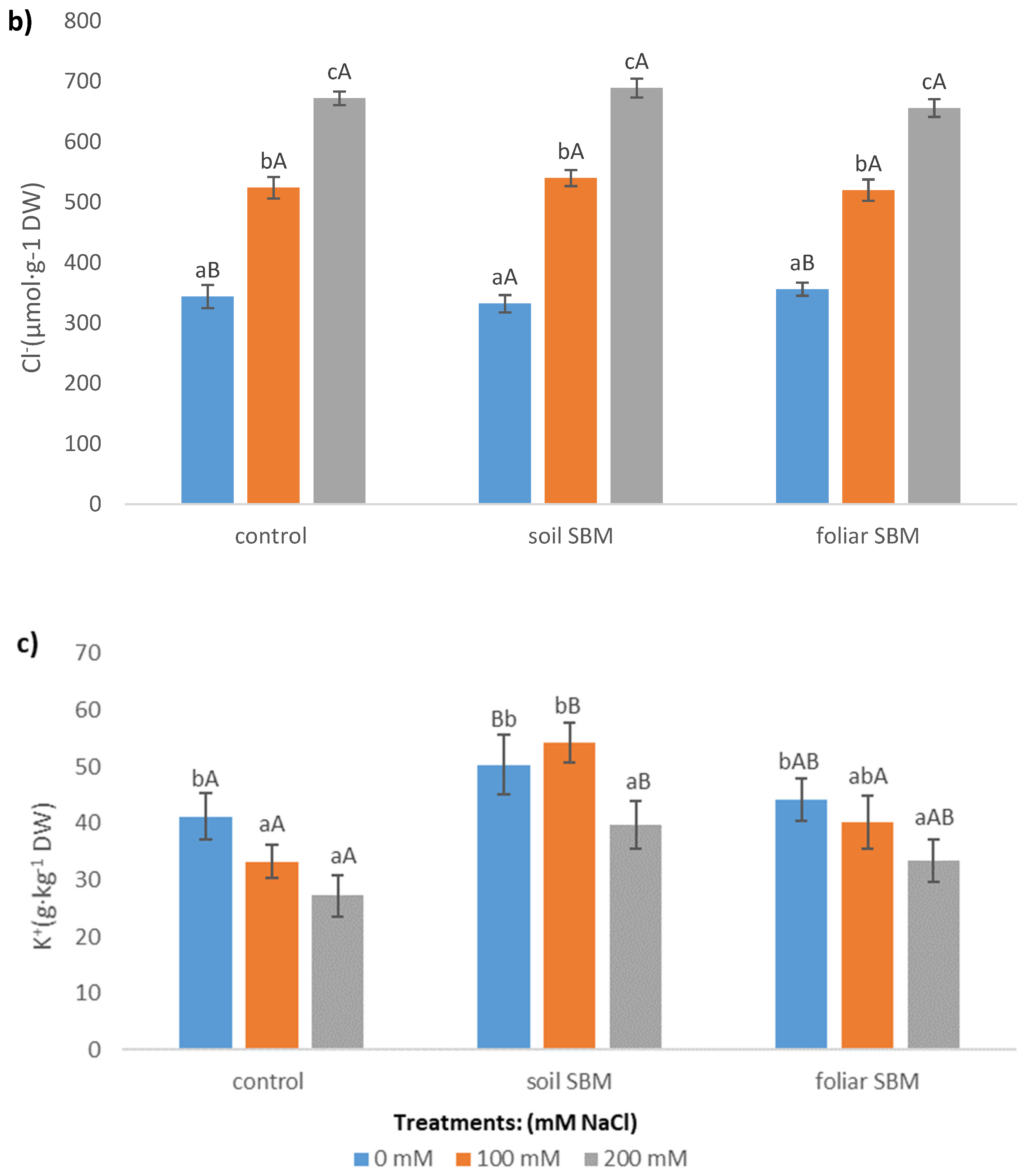
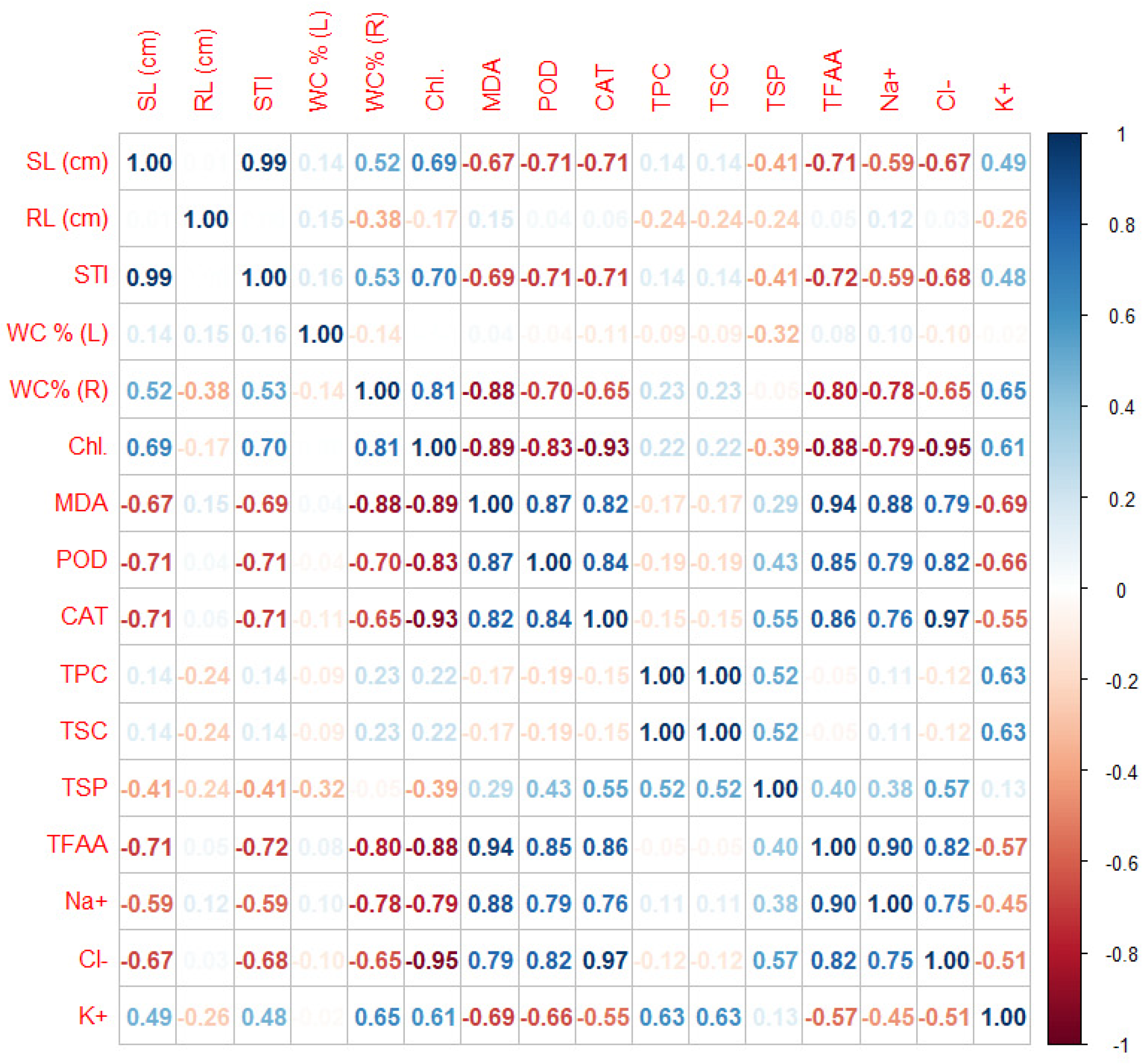
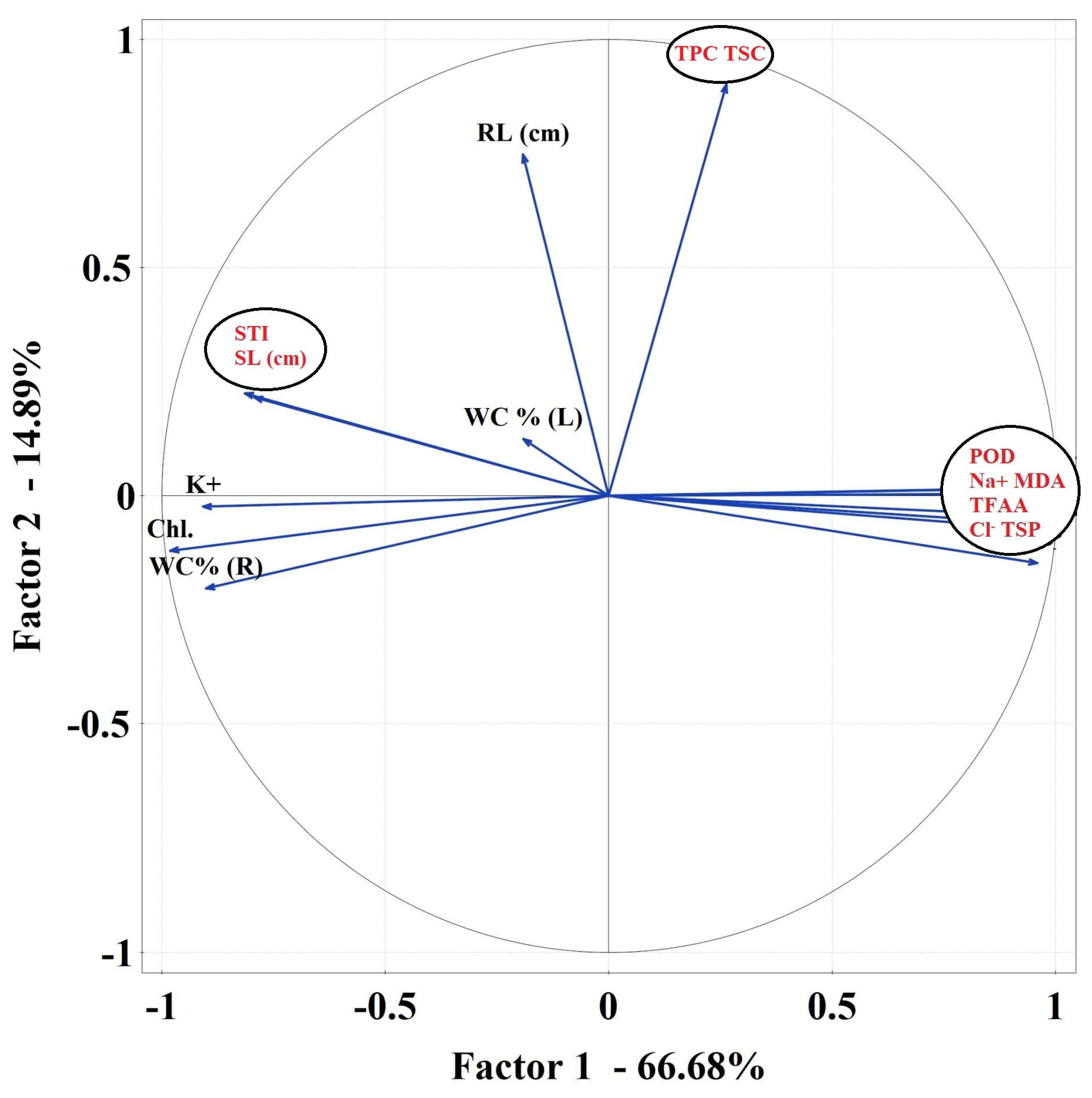
| Treatments | pH | EC (dS m−1) |
|---|---|---|
| I non SBM | ||
| 0 mM NaCl | 5.54 aA | 0.98 aA |
| 100 mM NaCl | 6.08 bA | 3.22 bA |
| 200 mM NaCl | 6.44 bA | 4.64 cA |
| II soil SBM | ||
| 0 mM NaCl | 5.38 aA | 1.10 aA |
| 100 mM NaCl | 5.82 aA | 3.34 aA |
| 200 mM NaCl | 6.34 aA | 4.81±aA |
| III foliar SBM | ||
| 0 mM NaCl | 5.46 aA | 0.95 aA |
| 100 mM NaCl | 5.90 aA | 3.14 aA |
| 200 mM NaCl | 6.40 aA | 4.72 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koźmińska, A.; Hanus-Fajerska, E.; Halecki, W.; Ciarkowska, K. Beet Molasses Enhance Salinity Tolerance in Thymus serpyllum—A Study under Greenhouse Condition. Plants 2021, 10, 1819. https://doi.org/10.3390/plants10091819
Koźmińska A, Hanus-Fajerska E, Halecki W, Ciarkowska K. Beet Molasses Enhance Salinity Tolerance in Thymus serpyllum—A Study under Greenhouse Condition. Plants. 2021; 10(9):1819. https://doi.org/10.3390/plants10091819
Chicago/Turabian StyleKoźmińska, Aleksandra, Ewa Hanus-Fajerska, Wiktor Halecki, and Krystyna Ciarkowska. 2021. "Beet Molasses Enhance Salinity Tolerance in Thymus serpyllum—A Study under Greenhouse Condition" Plants 10, no. 9: 1819. https://doi.org/10.3390/plants10091819
APA StyleKoźmińska, A., Hanus-Fajerska, E., Halecki, W., & Ciarkowska, K. (2021). Beet Molasses Enhance Salinity Tolerance in Thymus serpyllum—A Study under Greenhouse Condition. Plants, 10(9), 1819. https://doi.org/10.3390/plants10091819









