Salt Stress Amelioration in Maize Plants through Phosphogypsum Application and Bacterial Inoculation
Abstract
:1. Introduction
2. Results
2.1. Physiological Characteristics in Maize Leaves
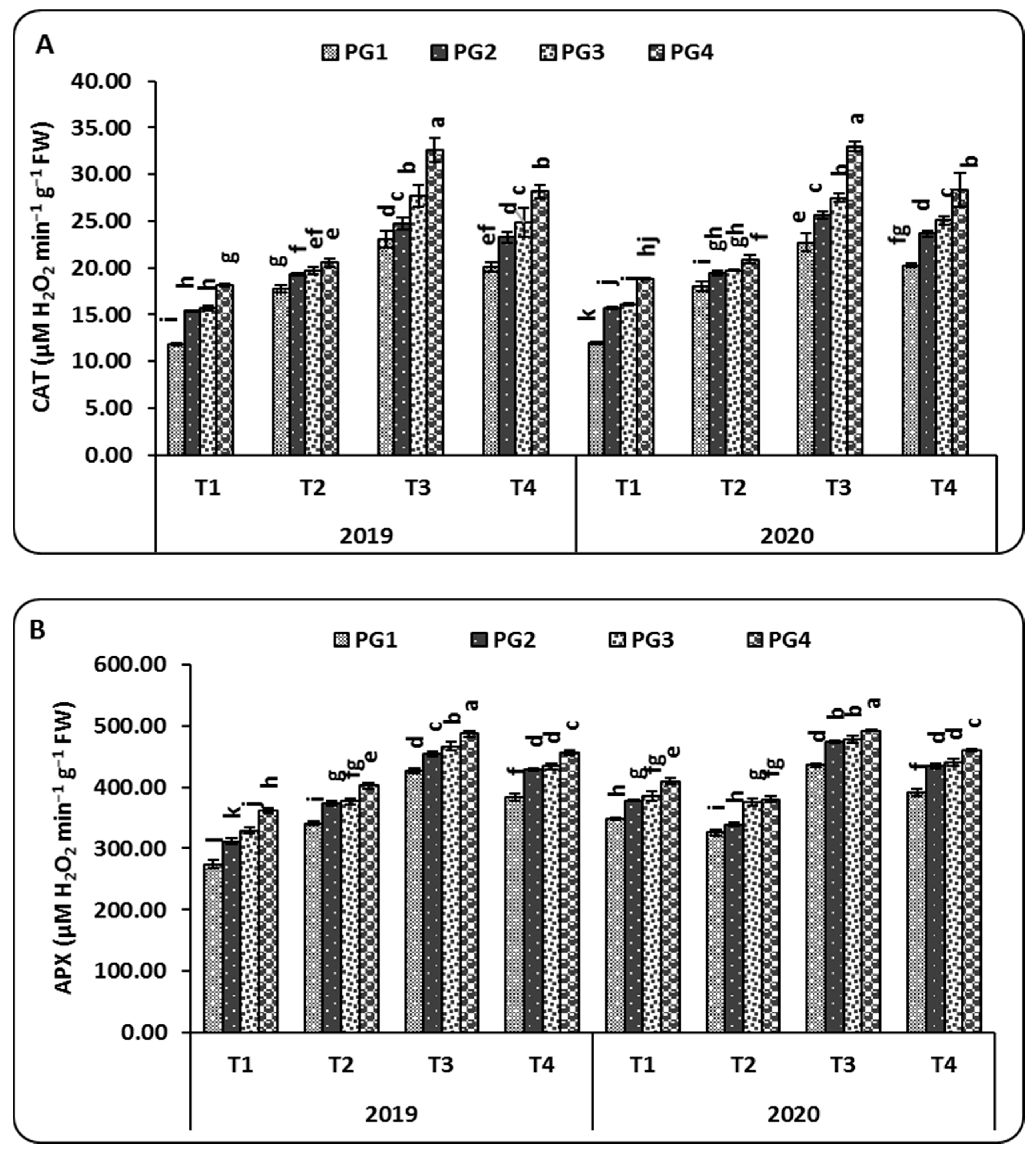
2.2. Antioxidant Enzyme Activities
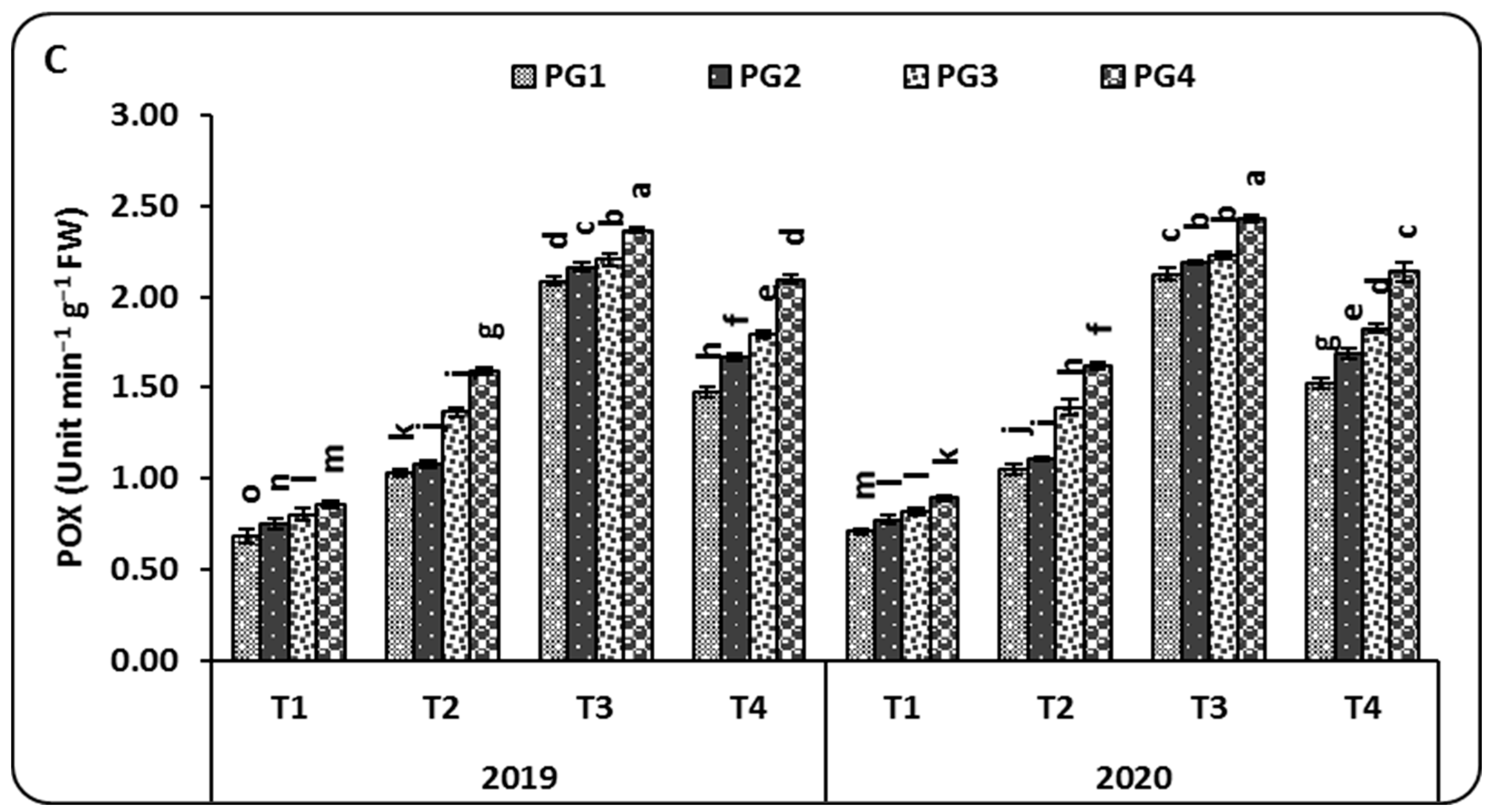
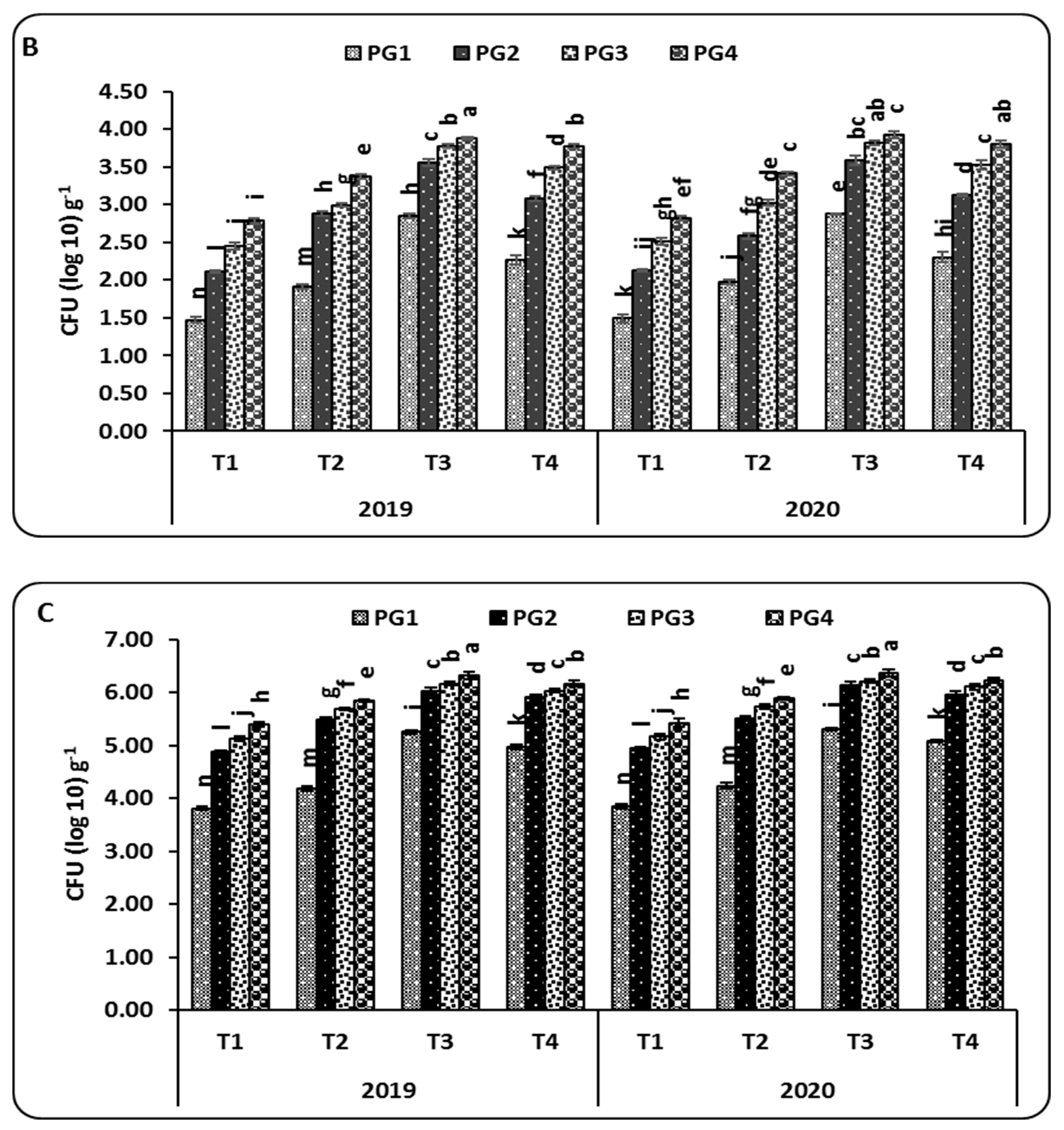
2.3. Microbial Communities
2.4. Soil Enzyme Activities
2.5. Macro-Elements, Na+ and K+/Na+ % in Maize Leaves
2.6. Micro-Elements in Maize Leaves
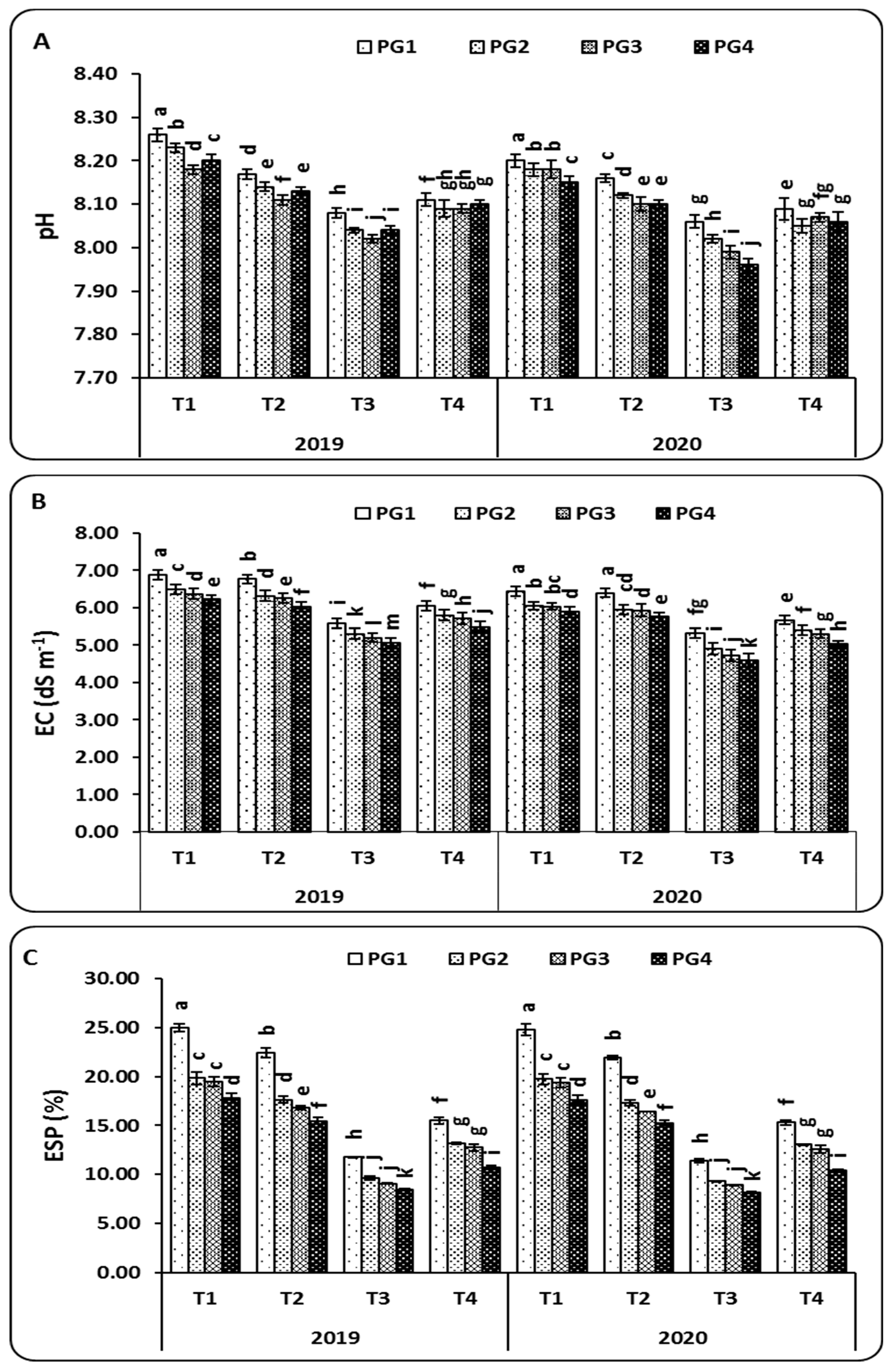
2.7. Soil Physicochemical Characteristics
2.8. Maize Productivity
3. Discussion
3.1. Physiological Characteristics in Maize Leaves
3.2. Antioxidant Enzyme Activities
3.3. Microbial Communities and Soil Enzyme Activities
3.4. The Percentages of Macro-Elements, Na+, and K+/Na+ in Maize Leaves
3.5. Micro-Elements in Maize Leaves
3.6. Soil Physicochemical Characteristics
3.7. Maize Productivity
4. Materials and Methods
4.1. Phosphogypsum (PG)
4.2. Microorganisms and Culture Conditions
4.3. Experimental Setup and Treatments
4.4. Measurements and Analyses
4.4.1. Physiological Characteristics in Maize Leaves
4.4.2. Assay of Antioxidant Enzyme Activities
4.4.3. Microbial Community Estimations
4.4.4. Soil Enzyme Activities
4.4.5. Determinations of Macro and Micro-Nutrients in Maize Leaves
4.4.6. Soil Chemical Characteristics
4.4.7. Maize Productivity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Chaudhary, D.; Narula, N.; Sindhu, S.S.; Behl, R.K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 2013, 19, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Stamford, N.P.; Figueiredo, M.V.B.; Junior, S.D.S.; Santos, A.; Junior, M.A.L. Effect of gypsum and sulfur with Acidithiobacillus on soil salinity alleviation and on cowpea biomass and nutrient status as affected by PK rock biofertilizer. Sci. Hortic. 2015, 192, 287–292. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Nabati, J.; Kafi, M.; Nezami, A.; Moghaddam, P.R.; Ali, M.; Mehrjerdi, M.Z. Effect of salinity on biomass production and activities of some key enzymatic antioxidants in Kochia (Kochia scoparia). Pak. J. Bot. 2011, 43, 539–548. [Google Scholar]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Aghighi Shahverdi, M.; Omidi, H.; Tabatabaei, S.J. Plant growth and steviol glycosides as affected by foliar application of selenium, boron, and iron under NaCl stress in Stevia rebaudiana Bertoni. Ind. Crop. Prod. 2018, 125, 408–415. [Google Scholar] [CrossRef]
- Leal, L.D.S.G.; Pessoa, L.G.M.; de Oliveira, J.P.; Santos, N.A.; Silva, L.F.D.S.; Júnior, G.B.; Freire, M.B.G.D.S.; de Souza, E.S. Do applications of soil conditioner mixtures improve the salt extraction ability of Atriplex nummularia at early growth stage? Int. J. Phytoremediation 2020, 22, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Farid, I.; Hashem, A.N.; El-Aty, A.; Esraa, A.M.; Abbas, M.H.; Ali, M. Integrated approaches towards ameliorating a saline sodic soil and increasing the dry weight of barley plants grown thereon. Environ. Biodivers. Soil Secur. 2020, 4, 31–46. [Google Scholar]
- El-Gaafarey, T.; El-Nahrawy, S.; El-Nahrawy, S.M. Impact of bio-inoculation with Bradyrhizobium spp. on yield and Seed quality of forage cowpea (Vigna unguiculata L.) under soil salinity conditions. Environ. Biodivers. Soil Secur. 2020, 4, 225–238. [Google Scholar] [CrossRef]
- Al-Enazy, A.A.; Al-Barakah, F.; Al-Oud, S.; Usman, A. Effect of phosphogypsum application and bacteria co-inoculation on biochemical properties and nutrient availability to maize plants in a saline soil. Arch. Agron. Soil Sci. 2018, 64, 1394–1406. [Google Scholar] [CrossRef]
- Hafez, E.; Omara, A.E.D.; Ahmed, A. The Coupling Effects of Plant Growth Promoting Rhizobacteria and Salicylic Acid on Physiological Modifications, Yield Traits, and Productivity of Wheat under Water Deficient Conditions. Agronomy 2019, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the Adversely Impacts of Water Deficit and Soil Salinity on Maize Growth and Productivity in Response to the Application of Plant Growth-Promoting Rhizobacteria and Silica Nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.-D.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive Impacts of Beneficial Microbes and Si-Zn Nanocomposite on Growth and Productivity of Soybean Subjected to Water Deficit under Salt-Affected Soil Conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.J.; Ito, O.; Siddique, K.H.M. Advances in drought resistance of rice. Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mchanisms and applications of plantgrowth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- IAEA. Radiation Protection and Management of NORM Residues in the Phosphate Industry; International Atomic Energy Agency: Vienna, Austria, 2013. [Google Scholar]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Mazzilli, B.; Palmiro, V.; Saueia, C.; Nisti, M. Radiochemical characterization of Brazilian phosphogypsum. J. Environ. Radioact. 2000, 49, 113–122. [Google Scholar] [CrossRef]
- Domingo-Olive, F.; Bosch-Serra, A.D.; Yague, M.R.; Poch, R.M.; Boixadera, J. Long term application of dairy cattle manure and pig slurry to winter cereals improves soil quality. Nutr. Cycl. Agroecosyst. 2016, 104, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Mesić, M.; Brezinščak, L.; Zgorelec, Ž.; Perčin, A.; Šestak, I.; Bilandžija, D.; Trdenić, M.; Lisac, H. The application of phosphogypsum in agriculture. Agric. Conspec. Sci. 2016, 81, 7–13. [Google Scholar]
- Al-Enazy, A.R.; Al-Oud, S.S.; Al-Barakah, F.N.; Usman, A.R. Role of microbial inoculation and industrial by-product phosphogypsum in growth and nutrient uptake of maize (Zea mays L.) grown in calcareous soil. J. Sci. Food Agric. 2017, 97, 3665–3674. [Google Scholar] [CrossRef]
- Lee, C.H.; Ha, B.Y.; Lee, Y.B.; Kim, P.J. Effect of alkalized phosphogypsum on soil chemical and biological properties. Commun. Soil Sci. Plant Anal. 2009, 40, 2072–2086. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ghoneim, A.; El Baroudy, A.; Abd El-Kader, N.; Aldhumri, S.A.; Othman, S.; El Khamisy, R. Effects of phosphogypsum and water treatment residual application on key chemical and biological properties of clay soil and maize yield. Soil Use Manag. 2020, 37, 494–503. [Google Scholar] [CrossRef]
- Hafez, E.M.; Kheir, A.M.S.; Badawy, S.A.; Rashwan, E.; Farig, M.; Osman, H.S. Differences in physiological and biochemical attributes of wheat in response to single and combined salicylic acid and biochar subjected to limited water irrigation in saline sodic soil. Plants 2020, 9, 1346. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Lippold, F.; Redestig, H.; Hannah, M.A.; Erban, A.; Krämer, U.; Kopka, J.; Udvardi, M.K. Integrative functional genomics of salt acclimatization in the model legume Lotus japonicus. Plant J. 2008, 53, 973–987. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Lindström, K.; Räsänen, L.A. A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl. Microbiol. Biotechnol. 2016, 100, 2829–2841. [Google Scholar] [CrossRef]
- Alvarez-Campos, O.; Lang, T.A.; Bhadha, J.H.; McCray, J.M.; Glaz, B.; Daroub, S.H. Biochar and mill ash improve yields of sugarcane on a sand soil in Florida. Agric. Ecosyst. Environ. 2018, 253, 122–130. [Google Scholar] [CrossRef]
- Kheir, A.S.; Abouelsoud, H.M.; Hafez, E.M.; Ali, O.A. Integrated effect of nano-Zn, nano-Si, and drainage using crop straw-filled ditches on saline sodic soil properties and rice productivity. Arab. J. Geosci. 2019, 12, 471. [Google Scholar] [CrossRef]
- Abd El-Ghany, T.M.; Masrahi, Y.S.; Mohamed, A.; Abboud, A.; Alawlaqi, M.M.; Elhussieny, A. Maize (Zea Mays L.) Growth and Metabolic Dynamics with Plant Growth- Promoting Rhizobacteria under Salt Stress. J. Plant Pathol. Microbiol. 2015, 6, 305. [Google Scholar]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B.M. Enhancing antioxidants defense system of snap bean under NaCl salinity using foliar application of salicylic acid, spermidine and glycine betaine. Am. Eurasian J. Agric. Environ. Sci. 2016, 16, 1200–1210. [Google Scholar]
- Salim, B.B.; Hikal, M.S.; Osman, H.S. Ameliorating the deleterious effects of saline water on the antioxidants defense system and yield of eggplant using foliar application of zinc sulphate. Ann. Agric. Sci. 2019, 64, 244–251. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Agami, R.A.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int. J. Environ. Agric. Res. 2016, 2, 78–92. [Google Scholar]
- Smaoui-Jardak, M.; Kriaa, W.; Maalej, M.; Zouari, M.; Kamoun, L.; Trabelsi, W.; Abdallah, F.B.; Elloumi, N. Effect of the phosphogypsum amendment of saline and agricultural soils on growth, productivity and antioxidant enzyme activities of tomato (Solanum lycopersicum L.). Ecotoxicology 2017, 26, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Machulla, G.; Bruns, M.A.; Scow, K.M. Microbial properties of mine spoil materials in the initial stages of soil development. Soil Sci. Soc. Am. J. Soil Sci. 2005, 69, 1069–1077. [Google Scholar] [CrossRef]
- Nayak, S.; Mishra, C.S.; Guru, B.C.; Rath, M. Effect of phosphogypsum amendment on soil physico-chemical properties, microbial load and enzyme activities. J. Environ. Biol. 2011, 32, 613–617. [Google Scholar] [PubMed]
- Stamford, N.P.; Freitas, A.D.S.; Ferraz, D.S.; Montenegro, A.A.A.; Santos, C.E. Nitrogen fixation and growth of cowpea (Vigna unguiculata) and yam bean (Pachyrhizus erosus) in a sodic soil as affected by gypsum and sulfur inoculated with Thiobacillus and rhizobial inoculation. Trop. Grassl. 2003, 37, 11–19. [Google Scholar]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.K.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Shilev, S.; Azaizeh, H.; Vassilev, N.; Georgiev, D.; Babrikova, I. Interactions in Soil-Microbe-Plant System: Adaptation to Stressed Agriculture. In Microbial Interventions in Agriculture and Environment; Singh, D., Gupta, V., Prabha, R., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Sandmann, G. Consequences of iron deficiency on photosynthetic and respiratory electron transport in blue-green algae. Photosynth. Res. 1985, 6, 261–271. [Google Scholar] [CrossRef]
- Omara, A.; El-Gaafarey, T. Alleviation of Salinity Stress Effects in Forage Cowpea (Vigna unguiculata L.) by Bradyrhizobium sp. Inoculation. Microbiol. Res. J. Int. 2018, 23, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Al-Karaki, G.N.; Al-Omoush, M. Wheat response to phosphogypsum and mycrorrhizal fungi in alkaline soil. J. Plant Nutr. 2002, 25, 873–883. [Google Scholar] [CrossRef]
- Chung, J.-B.; Kang, S.-C.; Park, S. Short term effect of phosphogypsum on soil chemical properties. Korean J. Environ. Agric. 2001, 20, 317–324. [Google Scholar]
- Hassan, T.U.; Bano, A. Role of carrier-based biofertilizer in reclamation of saline soil and wheat growth. Arch. Agron. Soil Sci. 2015, 61, 1719–1731. [Google Scholar] [CrossRef]
- Masciarelli, O.; Llanes, A.; Luna, V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Salinity Laboratory Staff, US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Döbereiner, J.; Day, J.M. Associative Symbiosis in Tropical Grasses: Characterization of Microorganisms and Dinitrogen Fixing Sites in Sysposium on Nitrogen Fixation; Washington State University Press: Washington, DC, USA, 1976. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 2nd ed.; CRC Press: New York, NY, USA, 1997; p. 1026. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentic Hall Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Mousa, N.A.; Siaguru, P.; Wiryowidagdo, S.; Wagih, M.E. Evaluation and selection of elite clonal genotypes of the sweet crop licorice (Glycyrrhiza glabra) in a new environment. Sugar Technol. 2007, 9, 83–94. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 273–286. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kong, F.X.; Hu, W.; Chao, S.Y.; Sang, W.L.; Wang, L.S. Physiological responses of mexicana to oxidative stress of SO2. Environ. Exp. Bot. 1999, 42, 201–209. [Google Scholar] [CrossRef]
- Cochran, D.S. Estimation of bacteria densities by means of the most probable number. Biometrics 1950, 6, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Allen, O.N. Experiments in Soil Bacteriology; Wisconsin Univeristy Press: Madison, WI, USA, 1959; p. 202. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Snatoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil enzymes in relation to old succession: Amylase, invertase, cellulose, dehydrogenase and urease. Soil Sci. Soc. Am. Proc. 1973, 37, 47–50. [Google Scholar] [CrossRef]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 37–49. [Google Scholar]
- Jones, J.; Wolf, B.J.B.; Mills, H.A. A practical sampling, preparation, analysis, and interpretative guide. In Plant Analysis Handbook; Micro-Macro Publishing: Athens, Greece, 1991. [Google Scholar]
- Peters, J.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; University of Wisconsin Cooperative Extension Publishing: Madison, WI, USA, 2003. [Google Scholar]
- Page, A.I.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velghe, G.; Camerlynck, R. Chemical Analysis of Plants and Soils; Laboratory of Analytical and Agrochemistry, State University: Ghent, Belgium, 1982; pp. 14–24. [Google Scholar]
- Arshad, M.; Lowery, B.; Grossman, B. Physical tests for monitoring soil quality. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Special Publication 49; Soil Science Society of America: Madison, WI, USA, 1997; pp. 123–141. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]

| Year | Phosphogypsum Rates (PG) | Inoculation (I) | Total Chlorophyll (mg g−1 FW) | Carotenoids (μg g−1 FW) | Proline (µmol g−1 FW) |
|---|---|---|---|---|---|
| 2019 | PG1 | T1 | 1.00 ± 0.04 j | 0.297 ± 0.01 l | 9.94 ± 0.15 a |
| T2 | 1.16 ± 0.05 i | 0.377 ± 0.01 j | 9.60 ± 0.20 b | ||
| T3 | 1.29 ± 0.02 h | 0.357 ± 0.01 jk | 8.79 ± 0.20 cd | ||
| T4 | 1.25 ± 0.03 h | 0.330 ± 0.02 kl | 8.97 ± 0.20 c | ||
| PG2 | T1 | 1.74 ± 0.07 g | 0.540 ± 0.07 i | 8.77 ± 0.14 cd | |
| T2 | 1.91 ± 0.02 f | 0.710 ± 0.02 g | 8.30 ± 0.20 e | ||
| T3 | 2.16 ± 0.04 d | 0.730 ± 0.03 fg | 7.49 ± 0.20 ghi | ||
| T4 | 1.90 ± 0.03 f | 0.693 ± 0.04 gh | 7.68 ± 0.20 g | ||
| PG3 | T1 | 1.85 ± 0.03 f | 0.653 ± 0.03 h | 8.59 ± 0.05 d | |
| T2 | 2.06 ± 0.04 e | 0.867 ± 0.04 d | 7.53 ± 0.25 gh | ||
| T3 | 2.47 ± 0.05 b | 0.883 ± 0.03 cd | 6.16 ± 0.08 k | ||
| T4 | 2.07 ± 0.04 e | 0.820 ± 0.02 e | 7.27 ± 0.05 ij | ||
| PG4 | T1 | 2.21 ± 0.03 d | 0.767 ± 0.02 f | 8.13 ± 0.10 ef | |
| T2 | 2.31 ± 0.03 c | 0.927 ± 0.02 b | 8.08 ± 0.05 f | ||
| T3 | 2.73 ± 0.05 a | 0.970 ± 0.01 a | 7.21 ± 0.25 j | ||
| T4 | 2.17 ± 0.03 d | 0.923 ± 0.01 bc | 7.45 ± 0.05 hi | ||
| 2020 | PG1 | T1 | 1.04 ± 0.04 k | 0.32 ± 0.02 k | 9.95 ± 0.03 a |
| T2 | 1.20 ± 0.05 j | 0.40 ± 0.03 i | 9.67 ± 0.11 b | ||
| T3 | 1.33 ± 0.03 i | 0.38 ± 0.01 ij | 8.84 ± 0.08 c | ||
| T4 | 1.29 ± 0.02 i | 0.35 ± 0.01 jk | 8.91 ± 0.10 c | ||
| PG2 | T1 | 1.80 ± 0.07 h | 0.57 ± 0.06 h | 8.84 ± 0.07 c | |
| T2 | 1.92 ± 0.02 g | 0.73 ± 0.02 f | 8.19 ± 0.16 e | ||
| T3 | 2.19 ± 0.04 e | 0.75 ± 0.03 f | 7.51 ± 0.14 g | ||
| T4 | 1.92 ± 0.02 g | 0.72 ± 0.04 f | 7.88 ± 0.04 f | ||
| PG3 | T1 | 1.89 ± 0.04 g | 0.67 ± 0.03 g | 8.66 ± 0.05 d | |
| T2 | 2.11 ± 0.04 f | 0.89 ± 0.06 c | 7.52 ± 0.21 g | ||
| T3 | 2.50 ± 0.05 b | 0.90 ± 0.04 c | 6.17 ± 0.05 j | ||
| T4 | 2.10 ± 0.05 f | 0.85 ± 0.03 d | 7.25 ± 0.08 h | ||
| PG4 | T1 | 2.26 ± 0.04 d | 0.79 ± 0.02 e | 8.10 ± 0.05 e | |
| T2 | 2.34 ± 0.04 c | 0.95 ± 0.02 b | 8.08 ± 0.04 e | ||
| T3 | 2.76 ± 0.06 a | 1.00 ± 0.01 a | 6.98 ± 0.11 i | ||
| T4 | 2.21 ± 0.02 de | 0.95 ± 0.02 b | 7.39 ± 0.05 gh | ||
| F-test | |||||
| Phosphogypsum rates (PG) | *** | *** | *** | ||
| Inoculation (I) | *** | *** | *** | ||
| Interaction (PG × I) | *** | *** | *** | ||
| Year | Phosphogypsum Rates (PG) | Inoculation (I) | DHA (mg TPF g−1 Soil d−1) | Urease (NH4+ − N g−1 Soil Day−1) |
|---|---|---|---|---|
| 2019 | PG1 | T1 | 50.00 ± 4.58 l | 45.33 ± 1.53 j |
| T2 | 74.00 ± 2.65 k | 58.33 ± 3.51 i | ||
| T3 | 80.00 ± 3.00 jk | 89.00 ± 3.61 e | ||
| T4 | 84.00 ± 4.00 j | 71.33 ± 3.79 h | ||
| PG2 | T1 | 104.67 ± 5.51 i | 57.00 ± 2.65 i | |
| T2 | 147.33 ± 5.69 f | 71.00 ± 2.00 h | ||
| T3 | 184.33 ± 4.16 c | 97.00 ± 2.00 d | ||
| T4 | 169.33 ± 5.51 d | 82.67 ± 3.21 f | ||
| PG3 | T1 | 122.33 ± 5.51 h | 68.67 ± 2.52 h | |
| T2 | 158.33 ± 4.04 e | 77.67 ± 3.51 g | ||
| T3 | 201.33 ± 3.51 b | 124.33 ± 3.51 b | ||
| T4 | 176.00 ± 3.61 d | 92.67 ± 3.51 e | ||
| PG4 | T1 | 134.33 ± 4.16 g | 76.33 ± 2.08 g | |
| T2 | 172.00 ± 4.58 d | 89.67 ± 3.06 e | ||
| T3 | 216.00 ± 5.57 a | 138.00 ± 4.58 a | ||
| T4 | 190.00 ± 3.61 c | 106.00 ± 4.36 c | ||
| 2020 | PG1 | T1 | 51.67 ± 3.51 n | 46.67 ± 2.08 l |
| T2 | 77.67 ± 0.58 m | 61.33 ± 1.53 k | ||
| T3 | 81.67 ± 3.21 m | 91.00 ± 3.46 f | ||
| T4 | 87.00 ± 2.65 l | 76.00 ± 3.61 i | ||
| PG2 | T1 | 111.00 ± 2.65 k | 60.33 ± 4.73 k | |
| T2 | 151.33 ± 3.51 h | 72.33 ± 3.21 j | ||
| T3 | 187.00 ± 3.61 d | 99.67 ± 2.08 d | ||
| T4 | 172.33 ± 5.69 f | 82.67 ± 1.15 g | ||
| PG3 | T1 | 129.67 ± 1.53 j | 70.33 ± 3.51 j | |
| T2 | 162.00 ± 3.61 g | 80.00 ± 4.58 gh | ||
| T3 | 209.67 ± 1.15 b | 130.00 ± 3.61 b | ||
| T4 | 179.00 ± 4.00 e | 95.00 ± 4.00 e | ||
| PG4 | T1 | 138.33 ± 4.04 i | 78.67 ± 1.53 hi | |
| T2 | 175.33 ± 3.79 ef | 91.67 ± 3.51 ef | ||
| T3 | 222.67 ± 5.51 a | 143.33 ± 4.51 a | ||
| T4 | 195.00 ± 6.08 c | 112.67 ± 5.69 c | ||
| F-test | Phosphogypsum rates (PG) | *** | *** | |
| Inoculation (I) | *** | *** | ||
| Interaction (PG × I) | *** | *** | ||
| Year | Phosphogypsum Rates (PG) | Inoculation (I) | N | P | K | Na | K/Na |
|---|---|---|---|---|---|---|---|
| 2019 | PG1 | T1 | 1.03 ± 0.03 l | 0.24 ± 0.02 l | 0.98 ± 0.03 k | 2.18 ± 0.04 a | 0.44 ± 0.02 m |
| T2 | 1.07 ± 0.04 l | 0.26 ± 0.02 k | 1.00 ± 0.02 k | 2.08 ± 0.04 b | 0.48 ± 0.02 lm | ||
| T3 | 1.34 ± 0.02 h | 0.37 ± 0.02 i | 1.33 ± 0.03 ef | 1.43 ± 0.02 j | 0.93 ± 0.01 e | ||
| T4 | 1.14 ± 0.03 k | 0.34 ± 0.02 j | 1.25 ± 0.01 g | 1.71 ± 0.03 fg | 0.73 ± 0.02 g | ||
| PG2 | T1 | 1.18 ± 0.03 j | 0.34 ± 0.02 j | 1.05 ± 0.04 j | 1.95 ± 0.02 c | 0.53 ± 0.02 kl | |
| T2 | 1.25 ± 0.03 i | 0.48 ± 0.04 g | 1.16 ± 0.03 hi | 1.88 ± 0.03 d | 0.61 ± 0.02 ij | ||
| T3 | 1.71 ± 0.03 e | 0.65 ± 0.02 c | 1.60 ± 0.04 c | 1.20 ± 0.02 k | 1.33 ± 0.04 c | ||
| T4 | 1.41 ± 0.03 g | 0.52 ± 0.03 f | 1.36 ± 0.03 e | 1.59 ± 0.04 h | 0.85 ± 0.03 f | ||
| PG3 | T1 | 1.33 ± 0.03 h | 0.43 ± 0.02 h | 1.07 ± 0.02 j | 1.83 ± 0.03 e | 0.58 ± 0.01 jk | |
| T2 | 1.35 ± 0.02 h | 0.54 ± 0.02 f | 1.19 ± 0.02 h | 1.68 ± 0.05 g | 0.70 ± 0.01 gh | ||
| T3 | 1.76 ± 0.03 d | 0.68 ± 0.02 b | 1.87 ± 0.02 b | 1.05 ± 0.04 l | 1.78 ± 0.09 b | ||
| T4 | 1.54 ± 0.03 f | 0.58 ± 0.01 e | 1.36 ± 0.03 e | 1.49 ± 0.02 i | 0.91 ± 0.01 ef | ||
| PG4 | T1 | 1.67 ± 0.03 e | 0.49 ± 0.02 g | 1.13 ± 0.03 i | 1.73 ± 0.04 f | 0.65 ± 0.03 hi | |
| T2 | 1.92 ± 0.03 c | 0.62 ± 0.02 d | 1.30 ± 0.03 f | 1.75 ± 0.02 f | 0.74 ± 0.02 g | ||
| T3 | 2.11 ± 0.04 a | 0.76 ± 0.03 a | 1.97 ± 0.02 a | 0.85 ± 0.03 m | 2.31 ± 0.06 a | ||
| T4 | 2.04 ± 0.05 b | 0.67 ± 0.02 bc | 1.45 ± 0.04 d | 1.39 ± 0.03 j | 1.04 ± 0.04 d | ||
| 2020 | PG1 | T1 | 1.05 ± 0.02 k | 0.28 ± 0.03 l | 1.01 ± 0.03 k | 2.16 ± 0.02 a | 0.45 ± 0.01 l |
| T2 | 1.07 ± 0.02 k | 0.30 ± 0.02 l | 1.04 ± 0.03 l | 2.01 ± 0.07 b | 0.50 ± 0.02 kl | ||
| T3 | 1.40 ± 0.02 h | 0.40 ± 0.02 j | 1.36 ± 0.01 l | 1.39 ± 0.02 i | 0.97 ± 0.00 e | ||
| T4 | 1.20 ± 0.02 j | 0.36 ± 0.03 k | 1.32 ± 0.02 efg | 1.62 ± 0.02 g | 0.81 ± 0.03 g | ||
| PG2 | T1 | 1.22 ± 0.02 j | 0.39 ± 0.01 jk | 1.06 ± 0.03 g | 1.91 ± 0.03 c | 0.55 ± 0.03 k | |
| T2 | 1.30 ± 0.03 i | 0.49 ± 0.01 h | 1.19 ± 0.05 kl | 1.81 ± 0.02 d | 0.65 ± 0.01 ij | ||
| T3 | 1.75 ± 0.02 d | 0.69 ± 0.02 c | 1.64 ± 0.03 hi | 1.18 ± 0.02 k | 1.38 ± 0.08 c | ||
| T4 | 1.49 ± 0.06 g | 0.57 ± 0.02 f | 1.40 ± 0.08 e | 1.53 ± 0.02 h | 0.91 ± 0.02 f | ||
| PG3 | T1 | 1.31 ± 0.05 i | 0.46 ± 0.02 i | 1.10 ± 0.01 jk | 1.79 ± 0.03 d | 0.61 ± 0.01 j | |
| T2 | 1.39 ± 0.01 h | 0.56 ± 0.03 f | 1.23 ± 0.04 h | 1.65 ± 0.06 fg | 0.74 ± 0.02 h | ||
| T3 | 1.81 ± 0.03 c | 0.72 ± 0.02 b | 1.91 ± 0.03 b | 1.00 ± 0.02 l | 1.91 ± 0.04 b | ||
| T4 | 1.63 ± 0.02 f | 0.62 ± 0.02 e | 1.39 ± 0.02 ef | 1.43 ± 0.01 i | 0.97 ± 0.01 e | ||
| PG4 | T1 | 1.69 ± 0.03 e | 0.53 ± 0.02 g | 1.16 ± 0.02 ij | 1.68 ± 0.02 ef | 0.69 ± 0.00 i | |
| T2 | 2.03 ± 0.05 b | 0.66 ± 0.01 d | 1.34 ± 0.03 fg | 1.70 ± 0.02 e | 0.78 ± 0.01 gh | ||
| T3 | 2.19 ± 0.03 a | 0.80 ± 0.03 a | 1.99 ± 0.02 a | 0.80 ± 0.01 m | 2.48 ± 0.02 a | ||
| T4 | 2.15 ± 0.04 a | 0.69 ± 0.03 c | 1.48 ± 0.04 d | 1.35 ± 0.03 j | 1.09 ± 0.02 d | ||
| F-test | Phosphogypsum rates (PG) | *** | *** | *** | *** | *** | |
| Inoculation (I) | *** | *** | *** | *** | *** | ||
| Interaction (PG × I) | *** | *** | *** | *** | *** | ||
| Year | Phosphogypsum Rates (PG) | Inoculation (I) | Zn | Mn | Fe | Cu |
|---|---|---|---|---|---|---|
| 2019 | PG1 | T1 | 23.73 ± 0.32 m | 23.55 ± 0.38 n | 55.04 ± 0.22 j | 7.17 ± 0.06 h |
| T2 | 25.36 ± 0.45 l | 24.43 ± 0.27 m | 61.75 ± 0.11 i | 7.44 ± 0.04 g | ||
| T3 | 35.67 ± 0.32 h | 26.42 ± 0.32 k | 75.31 ± 0.19 efg | 7.74 ± 0.05 e | ||
| T4 | 28.82 ± 0.20 k | 25.19 ± 0.17 l | 66.29 ± 0.10 h | 7.60 ± 0.09 f | ||
| PG2 | T1 | 29.84 ± 0.44 j | 27.45 ± 0.33 j | 67.69 ± 0.17 h | 7.59 ± 0.04 f | |
| T2 | 32.79 ± 0.41 i | 29.95 ± 0.11 i | 73.04 ± 0.15 fg | 7.72 ± 0.03 e | ||
| T3 | 47.56 ± 0.42 c | 32.24 ± 0.29 g | 86.13 ± 0.12 c | 8.18 ± 0.05 c | ||
| T4 | 39.88 ± 0.40 f | 31.38 ± 0.14 h | 75.77 ± 6.80 ef | 7.91 ± 0.04 d | ||
| PG3 | T1 | 37.69 ± 0.45 g | 31.34 ± 0.14 h | 72.40 ± 0.08 g | 7.77 ± 0.07 e | |
| T2 | 41.37 ± 0.22 e | 33.79 ± 0.07 f | 77.68 ± 0.19 e | 7.96 ± 0.07 d | ||
| T3 | 51.55 ± 0.50 b | 37.56 ± 0.28 c | 91.13 ± 0.14 b | 8.26 ± 0.03 b | ||
| T4 | 46.99 ± 0.11 cd | 35.21 ± 0.06 e | 84.09 ± 0.08 cd | 8.12 ± 0.04 c | ||
| PG4 | T1 | 40.13 ± 0.33 f | 33.71 ± 0.05 f | 81.09 ± 0.10 d | 7.92 ± 0.03 d | |
| T2 | 46.69 ± 0.47 d | 36.49 ± 0.06 d | 85.36 ± 0.34 c | 8.16 ± 0.05 c | ||
| T3 | 59.38 ± 0.15 a | 40.48 ± 0.04 a | 94.24 ± 0.46 a | 8.48 ± 0.04 a | ||
| T4 | 51.33 ± 0.33 b | 38.65 ± 0.42 b | 90.52 ± 0.17 b | 8.28 ± 0.05 b | ||
| 2020 | PG1 | T1 | 24.21 ± 0.02 l | 23.74 ± 0.37 n | 55.45 ± 0.38 h | 7.25 ± 0.07 i |
| T2 | 25.80 ± 0.13 k | 24.65 ± 0.25 m | 61.90 ± 0.09 gh | 7.80 ± 0.12 g | ||
| T3 | 36.32 ± 0.37 g | 26.82 ± 0.25 k | 75.67 ± 0.26 de | 7.90 ± 0.03 f | ||
| T4 | 29.33 ± 0.60 j | 25.41 ± 0.32 l | 66.55 ± 0.29 fg | 7.76 ± 0.08 gh | ||
| PG2 | T1 | 30.59 ± 0.45 i | 27.87 ± 0.05 j | 67.99 ± 0.10 efg | 7.70 ± 0.04 h | |
| T2 | 33.18 ± 0.19 h | 30.69 ± 0.45 i | 61.16 ± 20.67 gh | 7.88 ± 0.04 f | ||
| T3 | 48.56 ± 0.49 c | 32.40 ± 0.33 g | 86.43 ± 0.31 abc | 8.26 ± 0.04 d | ||
| T4 | 40.84 ± 0.18 e | 31.54 ± 0.13 h | 79.13 ± 1.75 cd | 8.05 ± 0.07 e | ||
| PG3 | T1 | 38.77 ± 0.38 f | 31.65 ± 0.21 h | 72.70 ± 0.28 def | 7.87 ± 0.07 f | |
| T2 | 42.24 ± 0.55 d | 34.03 ± 0.08 f | 77.97 ± 0.07 cd | 8.06 ± 0.06 e | ||
| T3 | 52.12 ± 0.23 b | 37.76 ± 0.17 c | 91.95 ± 0.08 ab | 8.34 ± 0.04 c | ||
| T4 | 47.77 ± 0.67 c | 35.62 ± 0.39 e | 84.55 ± 0.29 bc | 8.24 ± 0.05 d | ||
| PG4 | T1 | 40.58 ± 0.43 e | 33.92 ± 0.07 f | 81.36 ± 0.27 cd | 8.06 ± 0.06 e | |
| T2 | 47.90 ± 1.65 c | 36.82 ± 0.23 d | 85.65 ± 0.33 abc | 8.25 ± 0.05 d | ||
| T3 | 60.14 ± 0.18 a | 41.39 ± 0.34 a | 94.40 ± 0.36 a | 8.58 ± 0.02 a | ||
| T4 | 52.00 ± 0.13 b | 38.79 ± 0.44 b | 90.91 ± 0.12 ab | 8.43 ± 0.05 b | ||
| F-test | Phosphogypsum rates (PG) | *** | *** | *** | *** | |
| Inoculation (I) | *** | *** | *** | *** | ||
| Interaction (PG × I) | *** | *** | *** | *** | ||
| Year | Phosphogypsum Rates (PG) | Inoculation (I) | Ear Length (cm) | Ear Diameter (cm) | Grains/Ear | 100-Grain Weight (g) | Grain Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|
| 2019 | PG1 | T1 | 15.97 ± 0.45 j | 3.53 ± 0.12 j | 387.33 ± 4.73 k | 31.70 ± 0.10 l | 4746.66 ± 45.09 n |
| T2 | 17.17 ± 0.21 i | 3.70 ± 0.10 i | 405.00 ± 3.61 j | 32.16 ± 0.21 k | 5117.66 ± 14.01 k | ||
| T3 | 20.20 ± 0.26 d | 3.90 ± 0.10 h | 425.00 ± 3.61 h | 36.20 ± 0.70 c | 5729.66 ± 28.50 e | ||
| T4 | 19.17 ± 0.15 e | 4.13 ± 0.06 fg | 428.66 ± 1.53 g | 33.50 ± 0.40 fg | 5443.00 ± 42.14 h | ||
| PG2 | T1 | 17.33 ± 0.42 hi | 4.00 ± 0.10 gh | 422.00 ± 3.00 i | 32.36 ± 0.15 jk | 5006.33 ± 16.26 l | |
| T2 | 18.87 ± 0.15 e | 4.16 ± 0.06 f | 429.33 ± 1.53 g | 32.70 ± 0.10 ij | 5359.00 ± 51.12 i | ||
| T3 | 22.63 ± 0.15 b | 4.56 ± 0.06 abc | 449.66 ± 1.15 b | 37.66 ± 0.21 b | 5936.66 ± 20.82 c | ||
| T4 | 20.03 ± 0.21 d | 4.43 ± 0.06 cde | 437.66 ± 1.53 de | 33.86 ± 0.15 f | 5619.66 ± 17.56 f | ||
| PG3 | T1 | 17.67 ± 0.21 gh | 4.10 ± 0.10 fg | 426.66 ± 3.21 gh | 32.60 ± 0.10 ij | 4910.00 ± 30.00 m | |
| T2 | 17.90 ± 0.10 fg | 4.36 ± 0.06 de | 435.33 ± 2.08 ef | 33.13 ± 0.21 gh | 5431.66 ± 33.08 h | ||
| T3 | 22.87 ± 0.06 b | 4.63 ± 0.12 ab | 452.00 ± 1.00 b | 37.93 ± 0.25 b | 6028.66 ± 23.07 b | ||
| T4 | 20.20 ± 0.26 d | 4.56 ± 0.06 abc | 439.00 ± 2.00 d | 34.53 ± 0.58 e | 5752.33 ± 45.35 e | ||
| PG4 | T1 | 18.23 ± 0.21 f | 4.33 ± 0.06 e | 433.33 ± 3.51 f | 32.96 ± 0.12 hi | 5236.33 ± 25.11 j | |
| T2 | 19.17 ± 0.21 e | 4.50 ± 0.10 bcd | 436.66 ± 2.31 de | 33.50 ± 0.26 fg | 5534.66 ± 26.76 g | ||
| T3 | 24.20 ± 0.26 a | 4.70 ± 0.10 a | 458.00 ± 1.00 a | 38.46 ± 0.15 a | 6235.33 ± 20.43 a | ||
| T4 | 22.10 ± 0.10 c | 4.50 ± 0.10 bcd | 445.00 ± 1.00 c | 35.70 ± 0.10 d | 5846.33 ± 21.22 d | ||
| 2020 | PG1 | T1 | 16.13 ± 0.40 j | 3.60 ± 0.10 g | 389.00 ± 3.61 j | 31.83 ± 0.21 h | 4805.67 ± 45.09 n |
| T2 | 17.27 ± 0.21 i | 4.03 ± 0.61 f | 414.00 ± 12.49 i | 32.33 ± 0.06 gh | 5186.67 ± 14.01 k | ||
| T3 | 20.10 ± 0.26 d | 4.00 ± 0.10 f | 427.00 ± 4.36 gh | 35.00 ± 2.00 bc | 5802.00 ± 31.05 e | ||
| T4 | 19.30 ± 0.10 e | 4.20 ± 0.10 def | 431.00 ± 1.73 fg | 33.57 ± 0.35 ef | 5515.00 ± 40.73 h | ||
| PG2 | T1 | 17.37 ± 0.38 i | 3.97 ± 0.06 f | 422.33 ± 4.51 h | 32.37 ± 0.06 gh | 5065.33 ± 16.26 l | |
| T2 | 18.90 ± 0.20 f | 4.23 ± 0.06 c–f | 430.67 ± 2.08 fg | 32.73 ± 0.06 fgh | 5428.00 ± 51.12 i | ||
| T3 | 22.63 ± 0.12 bc | 4.53 ± 0.15 abc | 453.33 ± 2.89 b | 37.80 ± 0.20 a | 6010.67 ± 20.82 c | ||
| T4 | 20.23 ± 0.21 d | 4.47 ± 0.06 a–d | 439.67 ± 2.08 de | 33.97 ± 0.15 de | 5690.67 ± 17.56 f | ||
| PG3 | T1 | 17.77 ± 0.12 h | 4.10 ± 0.10 ef | 427.33 ± 3.06 gh | 32.63 ± 0.15 gh | 4969.00 ± 30.00 m | |
| T2 | 18.00 ± 0.17 gh | 4.40 ± 0.10 b–e | 437.67 ± 1.15 de | 33.23 ± 0.29 efg | 5500.67 ± 33.08 h | ||
| T3 | 23.00 ± 0.10 b | 4.73 ± 0.12 a | 450.67 ± 5.51 b | 38.30 ± 0.20 a | 6102.67 ± 23.07 b | ||
| T4 | 20.47 ± 0.21 d | 4.57 ± 0.06 ab | 442.00 ± 2.00 cd | 34.50 ± 0.44 cd | 5823.33 ± 45.35 e | ||
| PG4 | T1 | 18.30 ± 0.20 g | 4.23 ± 0.15 c–f | 434.33 ± 3.06 ef | 33.10 ± 0.26 efg | 5295.33 ± 25.11 j | |
| T2 | 19.13 ± 0.42 ef | 4.53 ± 0.06 abc | 438.67 ± 3.21 de | 33.63 ± 0.21 def | 5603.67 ± 26.76 g | ||
| T3 | 24.27 ± 0.31 a | 4.73 ± 0.06 a | 460.00 ± 1.00 a | 38.70 ± 0.26 a | 6309.33 ± 20.43 a | ||
| T4 | 22.50 ± 0.20 c | 4.67 ± 0.06 ab | 447.33 ± 2.08 bc | 35.87 ± 0.12 b | 5917.33 ± 21.22 d | ||
| F-test | Phosphogypsum rates (PG) | *** | *** | *** | *** | *** | |
| Inoculation (I) | *** | *** | *** | *** | *** | ||
| Interaction (PG × I) | *** | *** | *** | *** | *** | ||
| Season | Character | |||
|---|---|---|---|---|
| pH (1:2.5) | EC (dS m−) | O.M (%) | ESP (%) | |
| 2019 | 8.22 ± 0.02 | 7.33 ± 0.04 | 1.56 ± 0.01 | 21.27 ± 0.17 |
| 2020 | 8.18 ± 0.04 | 7.41 ± 0.03 | 1.65 ± 0.04 | 20. 72 ± 0.32 |
| Season | Particle Size Distribution (%) | |||
| Sand | Silt | Clay | Texture grade | |
| 2019 | 28.22 ± 1.66 | 24.11 ± 1.83 | 47.67 ± 2.01 | Clayey |
| 2020 | 28.76 ± 1.78 | 24.60 ± 1.92 | 46.64 ± 2.11 | Clayey |
| Season | Soluble Cations (meq L−1) | |||
| Ca2+ | Mg2+ | Na+ | K+ | |
| 2019 | 7.32 ± 0.66 | 5.12 ± 1.22 | 24.22 ± 2.09 | 0.38 ± 0.09 |
| 2020 | 8.11 ± 0.51 | 5.38 ± 1.38 | 23.67 ± 2.29 | 0.34 ± 0.05 |
| Season | Soluble Anions (meq L−1) | |||
| CO32− | HCO3− | Cl− | SO42− | |
| 2019 | - | 4.01 ± 0.55 | 20.00 ± 1.29 | 13.03 ± 2.11 |
| 2020 | - | 3.98 ± 0.37 | 21.13 ± 1.86 | 12.39 ± 2.02 |
| Season | Available Macronutrients (mg kg−1) | |||
| N | P | K | ||
| 2019 | 8.81 ± 0.71 | 7.12 ± 1.12 | 322 ± 17.23 | |
| 2020 | 9.29 ± 0.45 | 7.96 ±1.32 | 341 ± 14.11 | |
| Season | Total Counts of Microbes (CFU × 105 g−1 dry soil) | |||
| Bacillus * | Azospirillum | |||
| 2019 | 44 ± 2.12 | 23 ± 1.25 | ||
| 2020 | 58 ± 2.65 | 35 ± 1.44 | ||
| Season | 2019 | ||||
|---|---|---|---|---|---|
| Month | Temperature (°C) | Wind Speed (km day−1) | RH (%) | Rainfall (mm month−1) | |
| Max | Min | ||||
| May | 33.7 | 16.2 | 122.3 | 70.1 | 0.0 |
| June | 35.4 | 18.4 | 117.5 | 66.3 | 0.0 |
| July | 36.5 | 22.5 | 101.2 | 65.7 | 0.0 |
| August | 36.7 | 19.2 | 94.2 | 63.2 | 0.0 |
| September | 32.3 | 18.2 | 84.7 | 67.8 | 0.0 |
| Season | 2020 | ||||
| Month | Temperature (°C) | Wind Speed (km day−1) | RH (%) | Rainfall (mm month−1) | |
| Max | Min | ||||
| May | 33.1 | 17.2 | 125.3 | 68.5 | 0.0 |
| June | 36.2 | 16.4 | 115.2 | 65.7 | 0.0 |
| July | 35.4 | 22.3 | 105.8 | 64.2 | 0.0 |
| August | 38.6 | 23.1 | 93.2 | 62.0 | 0.0 |
| September | 37.1 | 22.5 | 85.2 | 45.9 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, T.; Elbagory, M.; Omara, A.E.-D. Salt Stress Amelioration in Maize Plants through Phosphogypsum Application and Bacterial Inoculation. Plants 2021, 10, 2024. https://doi.org/10.3390/plants10102024
Khalifa T, Elbagory M, Omara AE-D. Salt Stress Amelioration in Maize Plants through Phosphogypsum Application and Bacterial Inoculation. Plants. 2021; 10(10):2024. https://doi.org/10.3390/plants10102024
Chicago/Turabian StyleKhalifa, Tamer, Mohssen Elbagory, and Alaa El-Dein Omara. 2021. "Salt Stress Amelioration in Maize Plants through Phosphogypsum Application and Bacterial Inoculation" Plants 10, no. 10: 2024. https://doi.org/10.3390/plants10102024
APA StyleKhalifa, T., Elbagory, M., & Omara, A. E.-D. (2021). Salt Stress Amelioration in Maize Plants through Phosphogypsum Application and Bacterial Inoculation. Plants, 10(10), 2024. https://doi.org/10.3390/plants10102024








