Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest
Abstract
:1. Introduction
2. Results and Discussion
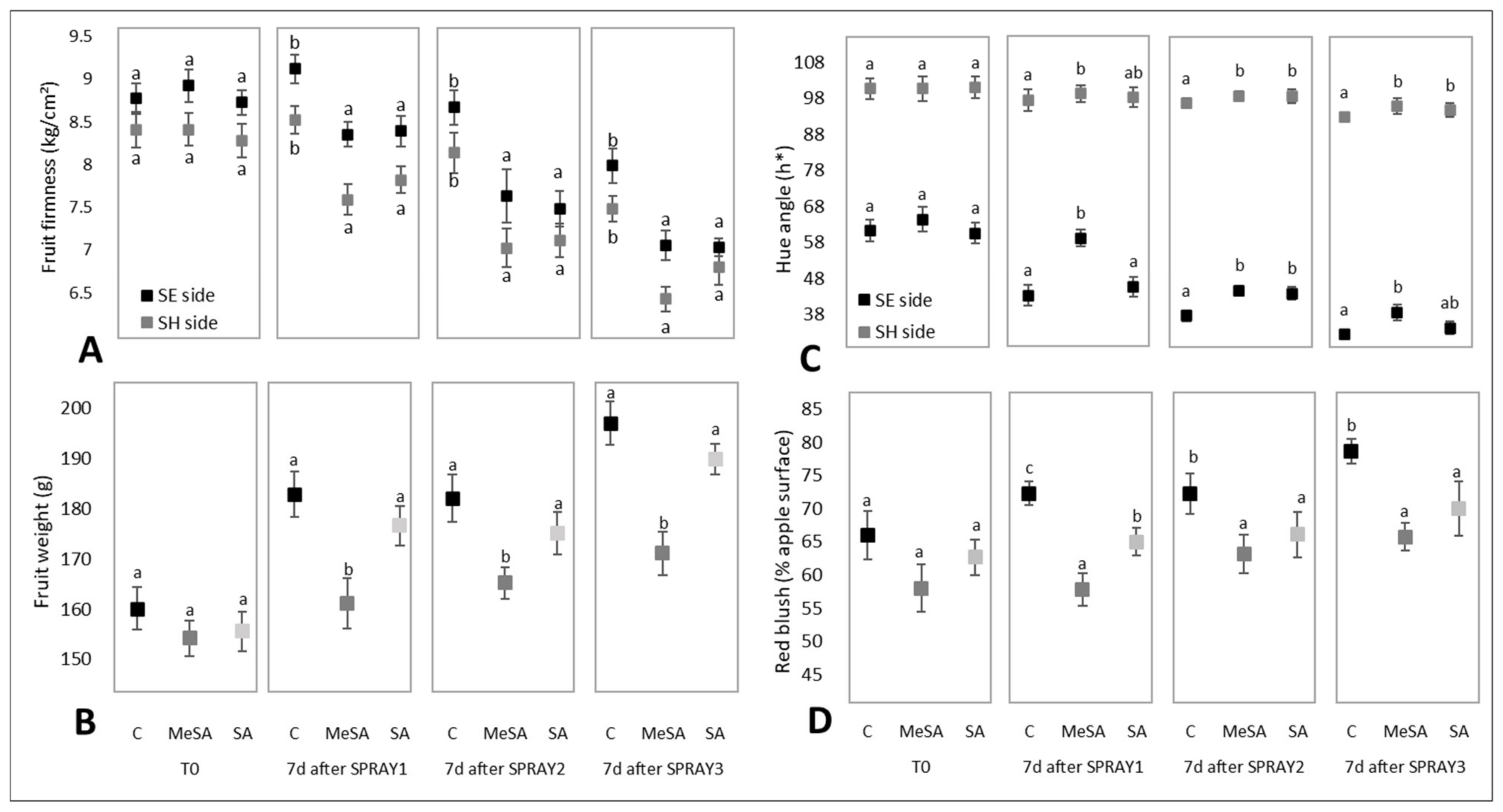
2.1. Effects of MeSA and SA on External Quality Characteristics of the Apple Fruit
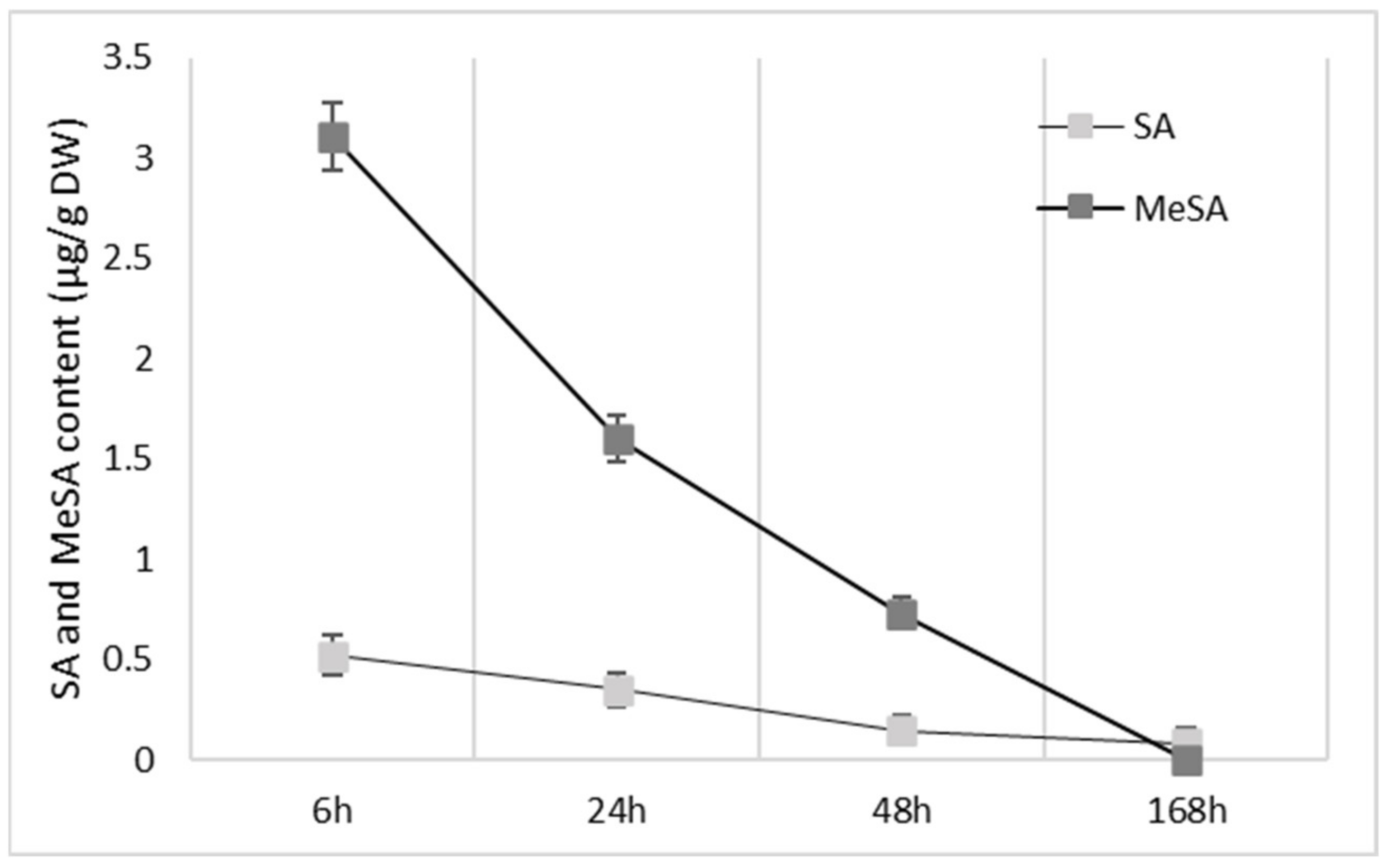
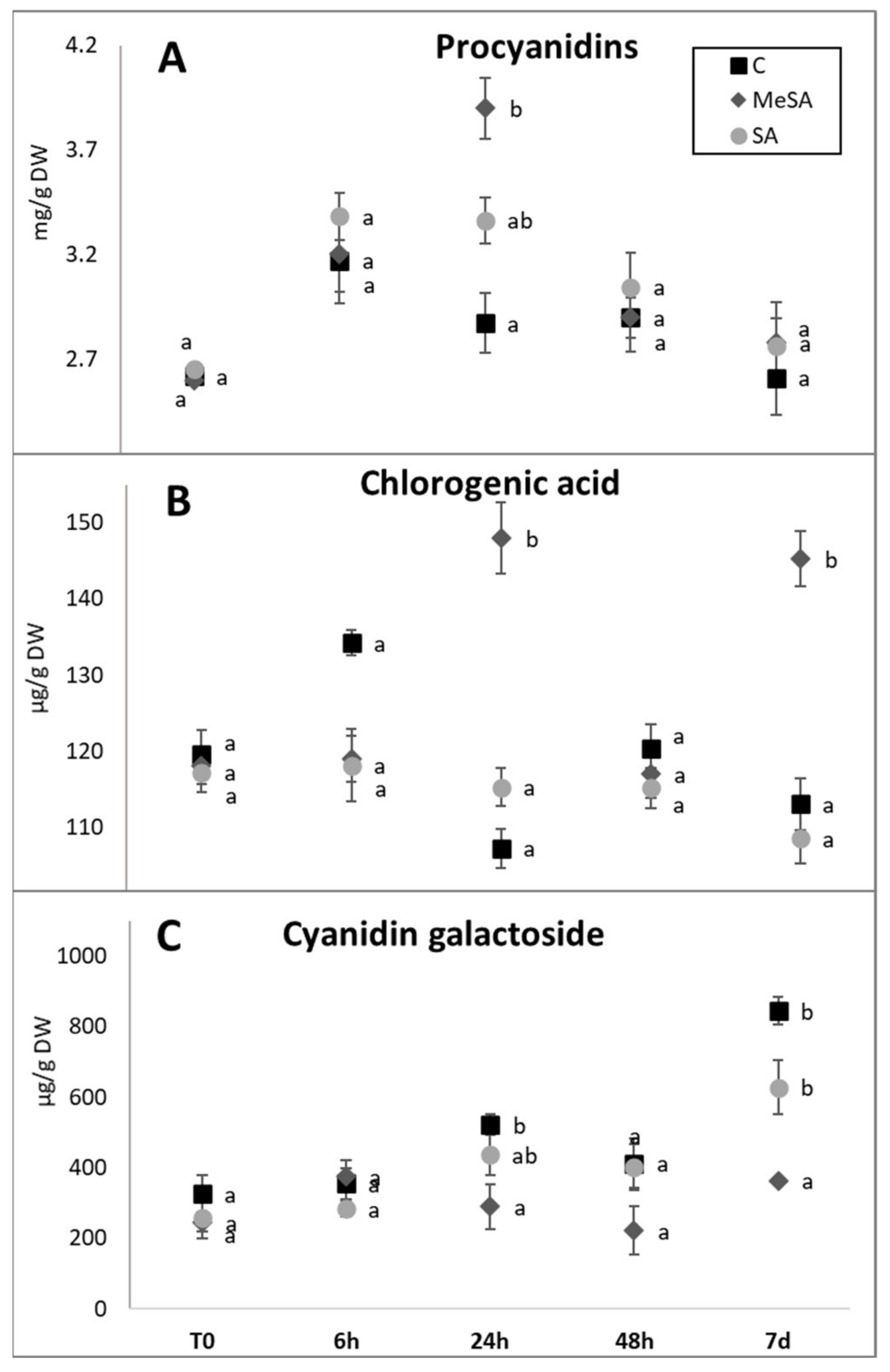
2.2. Effects of MeSA and SA on Accumulation of Phenolic Compounds in the Apple Peel
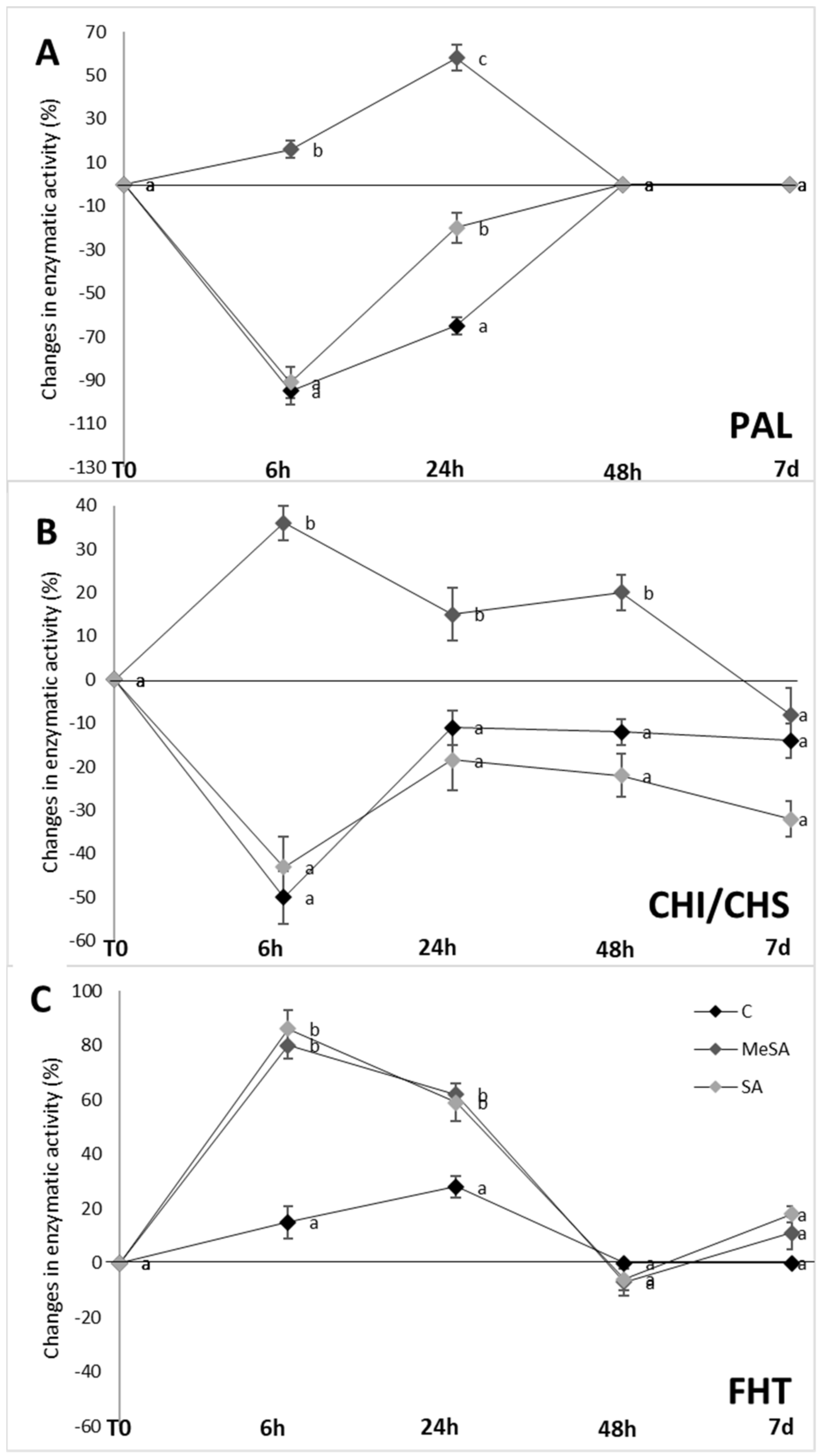
2.3. Enzymatic Activities
3. Materials and Methods
3.1. Plant Material
3.2. Treatments and Sampling
3.3. Fruit Quality and Colour Development
3.4. Chemicals and Standards
3.5. Extraction and HPLC-MS Analyses
3.6. Sample Preparation for Enzyme Activity Assays
3.7. Crude Extracts Preparation
3.8. Enzyme Assays
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| FHT | flavanone-3β-hydroxylase |
| HCA | hydroxycinnamic acids |
| HPLC | high-performance liquid chromatography |
| MeSA | methyl salicylic acid |
| PAL | phenylalanine ammonia-lyase |
| SA | salicylic acid |
References
- Raskin, I. Salicylate, a new plant hormone. Plant Physiol. 1992, 99, 799. [Google Scholar] [CrossRef] [Green Version]
- Shulaev, V.; Silvermann, P.; Raskin, I. Methyl salicylate–An airborne signal in pathogen resistance. Nature 1997, 385, 718–721. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Effect of salicylic acid treatments on storage life of peach fruits cv. ‘Flordaking’. Pak. J. Bot. 2012, 44, 119–124. [Google Scholar]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef]
- Huang, R.-H.; Liu, J.-H.; Lu, Y.-M.; Xia, R.-X. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharv. Biol. Technol. 2008, 47, 168–175. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Loyola-Vargas, V.M. Effect of acetylsalicylic acid on secondary metabolism of Catharanthus roseus tumor suspension cultures. Plant Cell Rep. 1997, 16, 287–290. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.; Karimi, E. Involvement of salicylic acid on antioxidant and anticancer properties, anthocyanin production and chalcone synthase activity in ginger (Zingiber officinale Roscoe) varieties. Int. J. Mol. Sci. 2012, 13, 14828–14844. [Google Scholar] [CrossRef]
- Hadian-Delijou, M.; Esna-Ashari, M.; Sarikhani, H. Effect of pre-and post-harvest salicylic acid treatments on quality and antioxidant properties of ‘Red delicious’ apples during cold storage. Adv. Hortic. Sci. 2017, 31, 31–38. [Google Scholar]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.S.; Liu, X.Y.; Guo, X.M.; Wang, G.X.; Li, L.P. Effect of pre-harvest salicylic acid spray treatment on fruit quality and antioxidant capacity of raspberry during post-harvest storage. Food Sci. 2010, 10, 69. [Google Scholar]
- Wang, Z.; Ma, L.; Zhang, X.; Xu, L.; Cao, J.; Jiang, W. The effect of exogenous salicylic acid on antioxidant activity, bioactive compounds and antioxidant system in apricot fruit. Sci. Hortic. 2015, 181, 113–120. [Google Scholar] [CrossRef]
- Pila, N.; Gol, N.B.; Rao, T.R. Effect of post harvest treatments on physicochemical characteristics and shelf-life of tomato (Lycopersicun esculentum Mill.) fruits during storage. Agric. Environ. Sci. 2010, 9, 470–479. [Google Scholar]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre-and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Joyce, D.; Wearing, H.; Coates, L.; Terry, L. Effects of phosphonate and salicylic acid treatments on anthracnose disease development and ripening of ‘Kensington Pride’ mango fruit. Aust. J. Exper. Agric. 2001, 41, 805–813. [Google Scholar]
- Bizjak, J.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Changes in primary metabolites and polyphenols in the peel of ‘Braeburn’ apples (Malus domestica Borkh.) during advanced maturation. J. Agric. Food Chem. 2013, 61, 10283–10292. [Google Scholar] [CrossRef]
- Bizjak, J.; Weber, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Alam, Z.; Stich, K.; Halbwirth, H.; Veberic, R. Influence of Phostrade Ca on color development and anthocyanin content of ‘Braeburn’ apple (Malus domestica Borkh.). HortScience 2013, 48, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Mo, Y.; Gong, D.; Liang, G.; Han, R.; Xie, J.; Li, W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agric. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Supapvanich, S.; Mitsang, P.; Youryon, P.; Techavuthiporn, C.; Boonyaritthongchai, P.; Tepsorn, R. Postharvest quality maintenance and bioactive compounds enhancement in ‘Taaptimjaan’ wax apple during short-term storage by salicylic acid immersion. Hortic. Environ. Biotechnol. 2018, 59, 373–381. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M. Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J. Plant Nutrit. Soil Sci. 2009, 172, 270–276. [Google Scholar] [CrossRef]
- Yıldırım, E.; Dursun, A. In Effect of Foliar Salicylic Acid Applications on Plant Growth and Yield of Tomato under Greenhouse Conditions. In Proceedings of the International Symposium on Strategies Towards Sustainability of Protected Cultivation in Mild Winter Climate, Antalya, Turkey, 6–11 April 2008; Volume 807, pp. 395–400. [Google Scholar]
- Kazemi, M. Effect of foliar application with salicylic acid and methyl jasmonate on growth, flowering, yield and fruit quality of tomato. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 154–158. [Google Scholar]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [Green Version]
- Campos, Â.D.; Ferreira, A.G.; Hampe, M.M.V.; Antunes, I.F.; Brancão, N.; Silveira, E.P.; da Silva, J.B.; Osório, V.A. Induction of chalcone synthase and phenylalanine ammonia-lyase by salicylic acid and Colletotrichum lindemuthianum in common bean. Braz. J. Plant Physiol. 2003, 15, 129–134. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Wang, H.; Huang, L. Complementary action of jasmonic acid on salicylic acid in mediating fungal elicitor-induced flavonol glycoside accumulation of Ginkgo biloba cells. Plant Cell Environ. 2009, 32, 960–967. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Janisiewicz, W.J.; Nichols, B.; Jurick II, W.M.; Chen, P. Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharv. Biol. Technol. 2017, 127, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Mayr, U.; Treutter, D.; Santos-Buelga, C.; Bauer, H.; Feucht, W. Developmental changes in the phenol concentrations of ‘Golden delicious’ apple fruits and leaves. Phytochemistry 1995, 38, 1151–1155. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Bukhat, S.; Manzoor, H.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhao, W.L.; Gao, X.W. Communication between plants: Induced resistance in poplar seedlings following herbivore infestation, mechanical wounding, and volatile treatment of the neighbors. Entomol. Exp. Appl. 2013, 149, 110–117. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Gacnik, S.; Veberic, R.; Marinovic, S.; Halbwirth, H.; Mikulic-Petkovsek, M. Effect of pre-harvest treatments with salicylic and methyl salicylic acid on the chemical profile and activity of some phenylpropanoid pathway related enzymes in apple leaves. Sci. Hortic. 2021, 277, 109794. [Google Scholar] [CrossRef]
- Halbwirth, H.; Waldner, I.; Miosic, S.; Ibanez, M.; Costa, G.; Stich, K. Measuring flavonoid enzyme activities in tissues of fruit species. J. Agric. Food Chem. 2009, 57, 4983–4987. [Google Scholar] [CrossRef]
- Dellus, V.; Heller, W.; Sandermann, H., Jr.; Scalbert, A. Dihydroflavonol 4-reductase activity in lignocellulosic tissues. Phytochemistry 1997, 45, 1415–1418. [Google Scholar] [CrossRef]
- Sandermann, H.; Strominger, J.L. Purification and properties of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. J. Biol. Chem. 1972, 247, 5123–5131. [Google Scholar] [CrossRef]

| Phenolic Group | Treatment | Content (g/kg Dry Weight) | ||||
|---|---|---|---|---|---|---|
| Before Spraying | After Spraying | |||||
| 6 h | 24 h | 48 h | 7 days | |||
| Flavanols | Control | 3.17 ± 0.03 a | 3.88 ± 0.19 a | 3.48 ± 0.17 a | 3.53 ± 0.40 a | 3.37 ± 0.22 a |
| MeSA | 3.12 ± 0.01 a | 4.07 ± 0.58 a | 4.65 ± 0.50 b | 3.70 ± 0.31 a | 3.36 ± 0.15 a | |
| SA | 3.15 ± 0.02 a | 3.83 ± 0.24 a | 4.03 ± 0.12 ab | 3.56 ± 0.12 a | 3.22 ± 0.32 a | |
| Flavonols b | Control | 1.25 ± 0.08 a | 1.23 ± 0.07 a | 1.60 ± 0.22 a | 1.25 ± 0.04 a | 1.05 ± 0.02 a |
| MeSA | 0.91 ± 0.07 a | 1.30 ± 0.12 a | 1.46 ± 0.17 a | 1.08 ± 0.12 a | 1.40 ± 0.07 b | |
| SA | 1.02 ± 0.13 a | 1.40 ± 0.11 a | 1.78 ± 0.10 a | 1.12 ± 0.10 a | 1.05 ± 0.02 a | |
| Hydroxycinnamic | Control | 0.18 ± 0.01 a | 0.20 ± 0.00 a | 0.19 ± 0.02 a | 0.18 ± 0.01 a | 0.17 ± 0.01 a |
| acids | MeSA | 0.16 ± 0.01 a | 0.18 ±0.02 a | 0.23 ± 0.01 b | 0.18 ± 0.01 a | 0.22 ± 0.01 b |
| SA | 0.16 ± 0.01 a | 0.19 ± 0.00 a | 0.17 ± 0.00 a | 0.17 ± 0.00 a | 0.17 ± 0.01 a | |
| Dihydrochalcones | Control | 1.61 ± 0.08 a | 1.70 ± 0.05 a | 2.15 ± 0.20 a | 1.81 ± 0.02 a | 1.90 ± 0.05 a |
| MeSA | 1.52 ± 0.16 a | 1.90 ± 0.19 a | 2.13 ± 0.17 a | 1.79 ± 0.08 a | 1.69 ± 0.10 a | |
| SA | 1.70 ± 0.14 a | 2.03 ± 0.02 a | 2.24 ± 0.08 a | 1.77 ± 0.12 a | 1.71 ± 0.02 a | |
| Anthocyanins | Control | 0.34 ± 0.05 a | 0.43 ± 0.09 a | 0.55 ± 0.03 b | 0.44 ± 0.07 a | 0.90 ± 0.04 b |
| MeSA | 0.26 ± 0.03 a | 0.27 ± 0.05 a | 0.31 ± 0.05 a | 0.24 ± 0.07 a | 0.39 ± 0.07 a | |
| SA | 0.28 ± 0.06 a | 0.47 ± 0.03 a | 0.46 ± 0.06 ab | 0.43 ± 0.07 a | 0.67 ± 0.08 b | |
| Total phenolics | Control | 6.56 ± 0.22 a | 7.44 ±0.20 a | 8.64 ± 0.60 a | 7.21 ± 0.42 a | 7.55 ± 0.21 a |
| MeSA | 5.97 ± 0.22 a | 7.73 ± 0.88 a | 7.81 ± 0.28 a | 6.98 ± 0.14 a | 6.84 ± 0.18 a | |
| SA | 6.30 ± 0.33 a | 7.91 ± 0.12 a | 7.67 ± 0.32 a | 7.04 ± 0.17 a | 6.82 ± 0.23 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacnik, S.; Veberič, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulič-Petkovšek, M. Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants 2021, 10, 1807. https://doi.org/10.3390/plants10091807
Gacnik S, Veberič R, Hudina M, Marinovic S, Halbwirth H, Mikulič-Petkovšek M. Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants. 2021; 10(9):1807. https://doi.org/10.3390/plants10091807
Chicago/Turabian StyleGacnik, Sasa, Robert Veberič, Metka Hudina, Silvija Marinovic, Heidi Halbwirth, and Maja Mikulič-Petkovšek. 2021. "Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest" Plants 10, no. 9: 1807. https://doi.org/10.3390/plants10091807
APA StyleGacnik, S., Veberič, R., Hudina, M., Marinovic, S., Halbwirth, H., & Mikulič-Petkovšek, M. (2021). Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants, 10(9), 1807. https://doi.org/10.3390/plants10091807







