Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Aqueous Enzymatic Extraction
2.4. Determination of Chemical Composition of Pumpkin Seed Oil Using Fatty Acid Methyl Ester/Gas Chromatographic-Mass Spectrometric (FAME/GC/MS) Method
2.5. Determination of Antioxidant Activities
2.5.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.5.2. 2,2′-Azino-Bis-3-Ethylbenzthiazoline-6-Sulphonic Acid (ABTS) Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Ferric Thiocyanate (FTC) Assay
2.6. Determination of Anti-Aging Activities
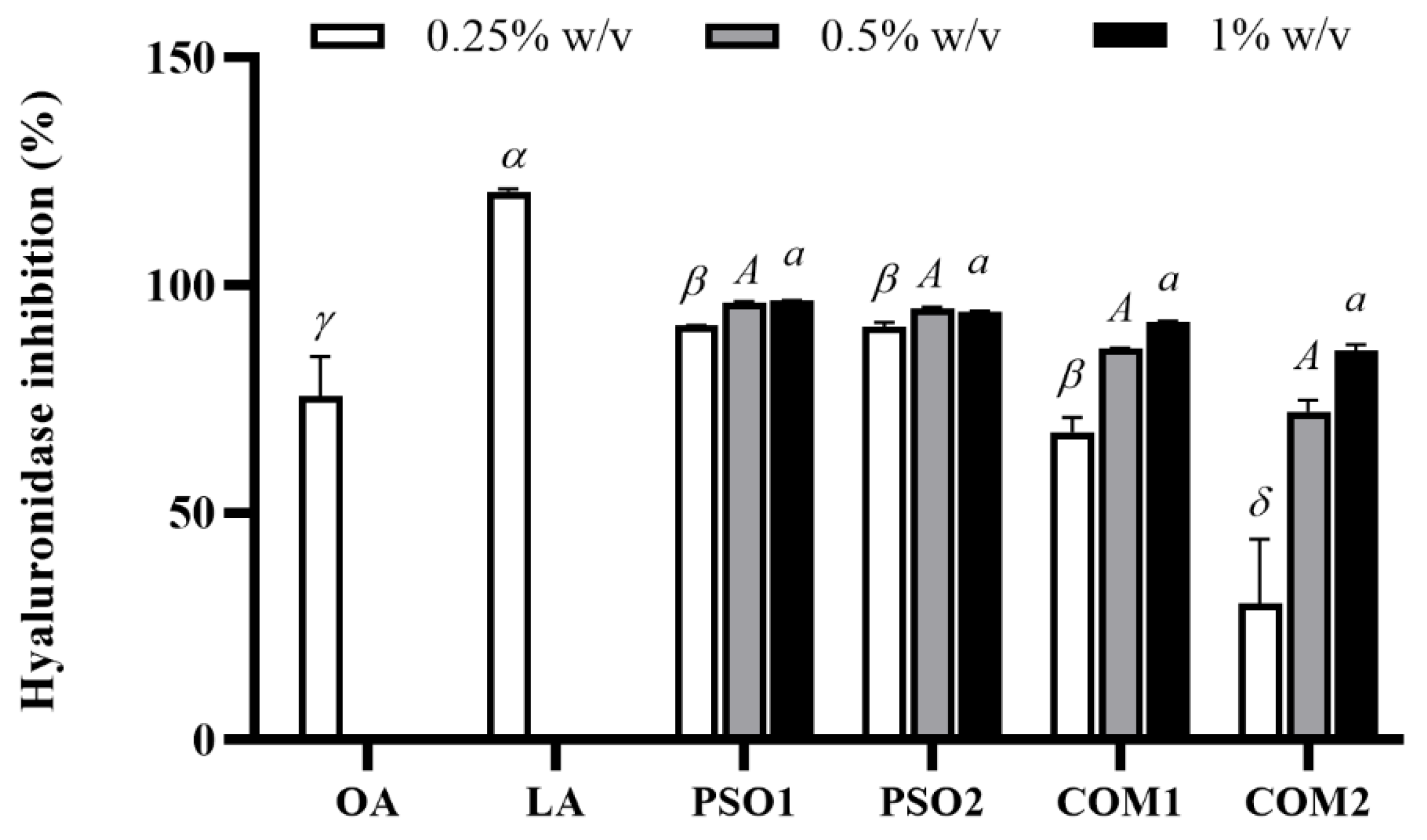
2.6.1. Anti-Hyaluronidase Activity
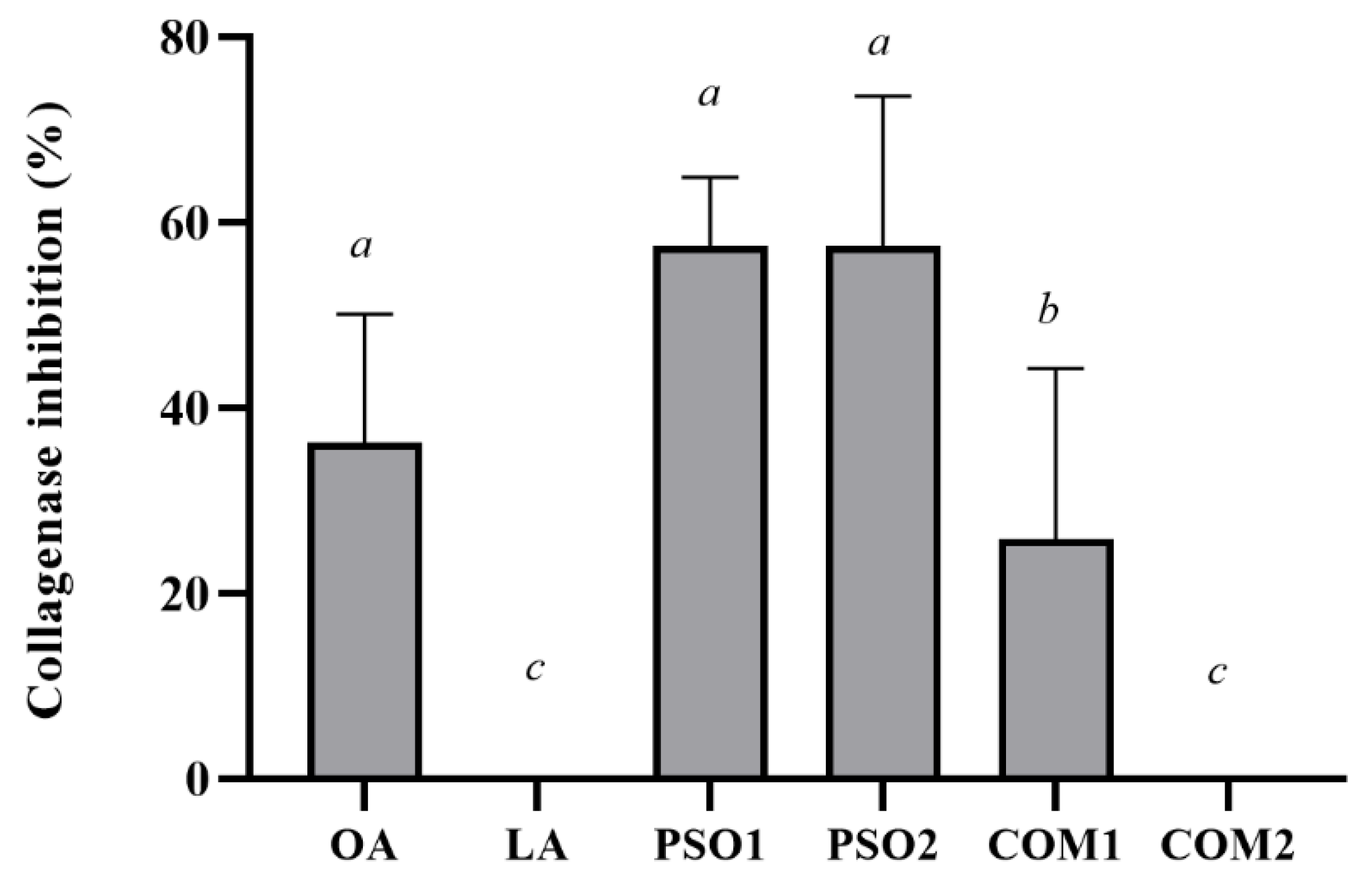
2.6.2. Anti-Collagenase Activity
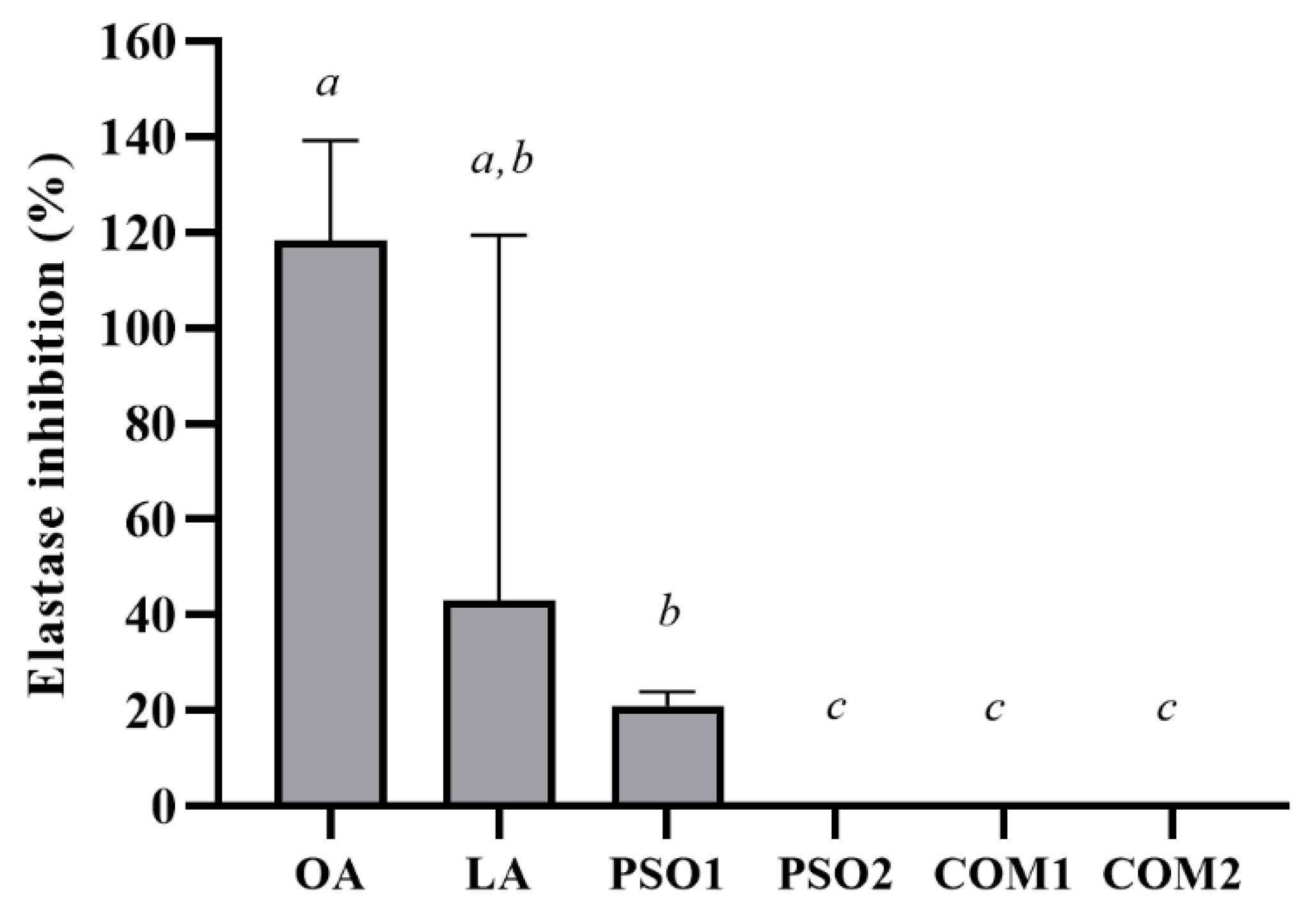
2.6.3. Anti-Elastase Activity
2.7. Determination of Anti-Tyrosinase Activities
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pumpkin Seed Oil Character
3.2. Chemical Composition of Pumpkin Seed Oil
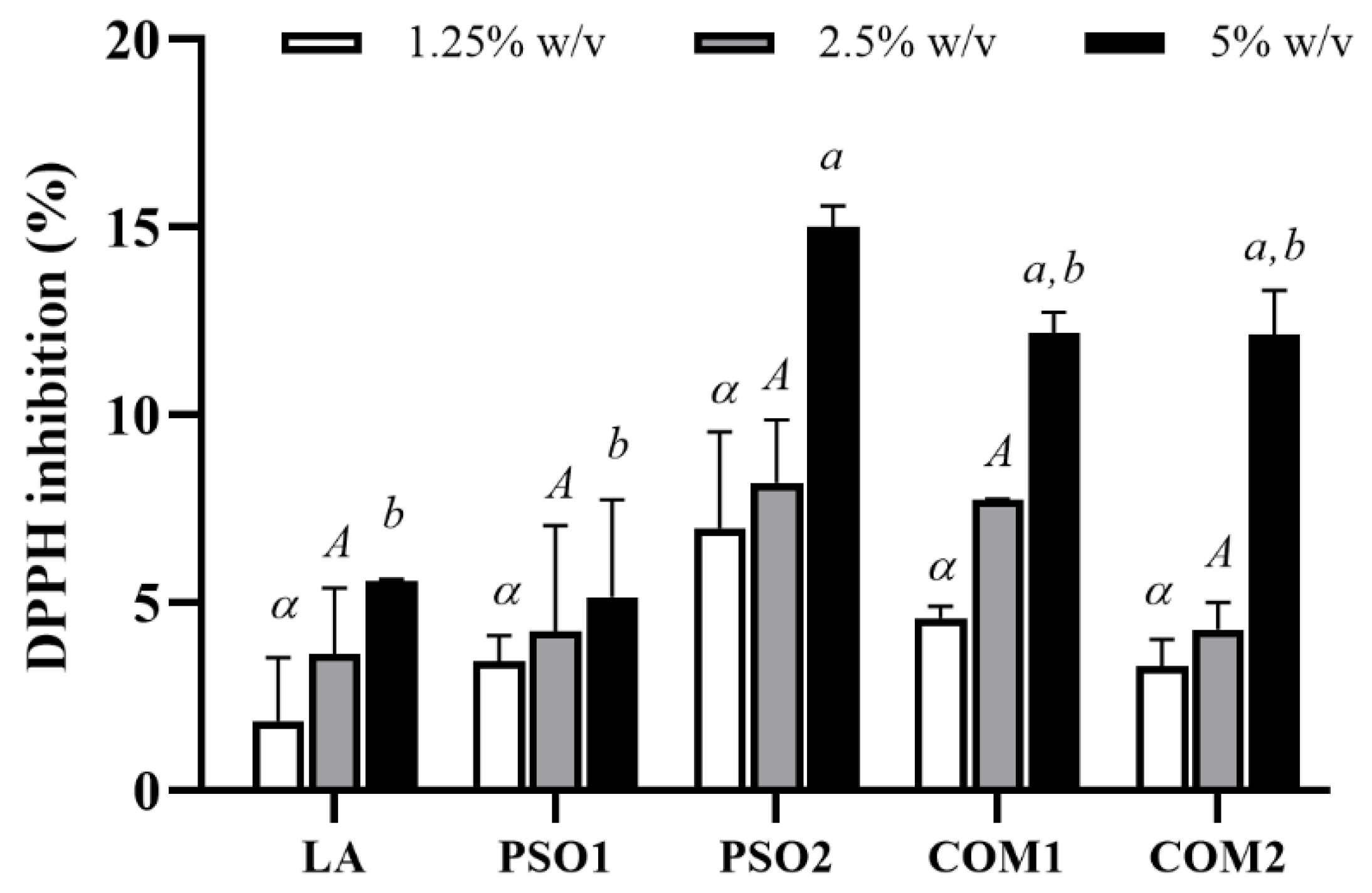
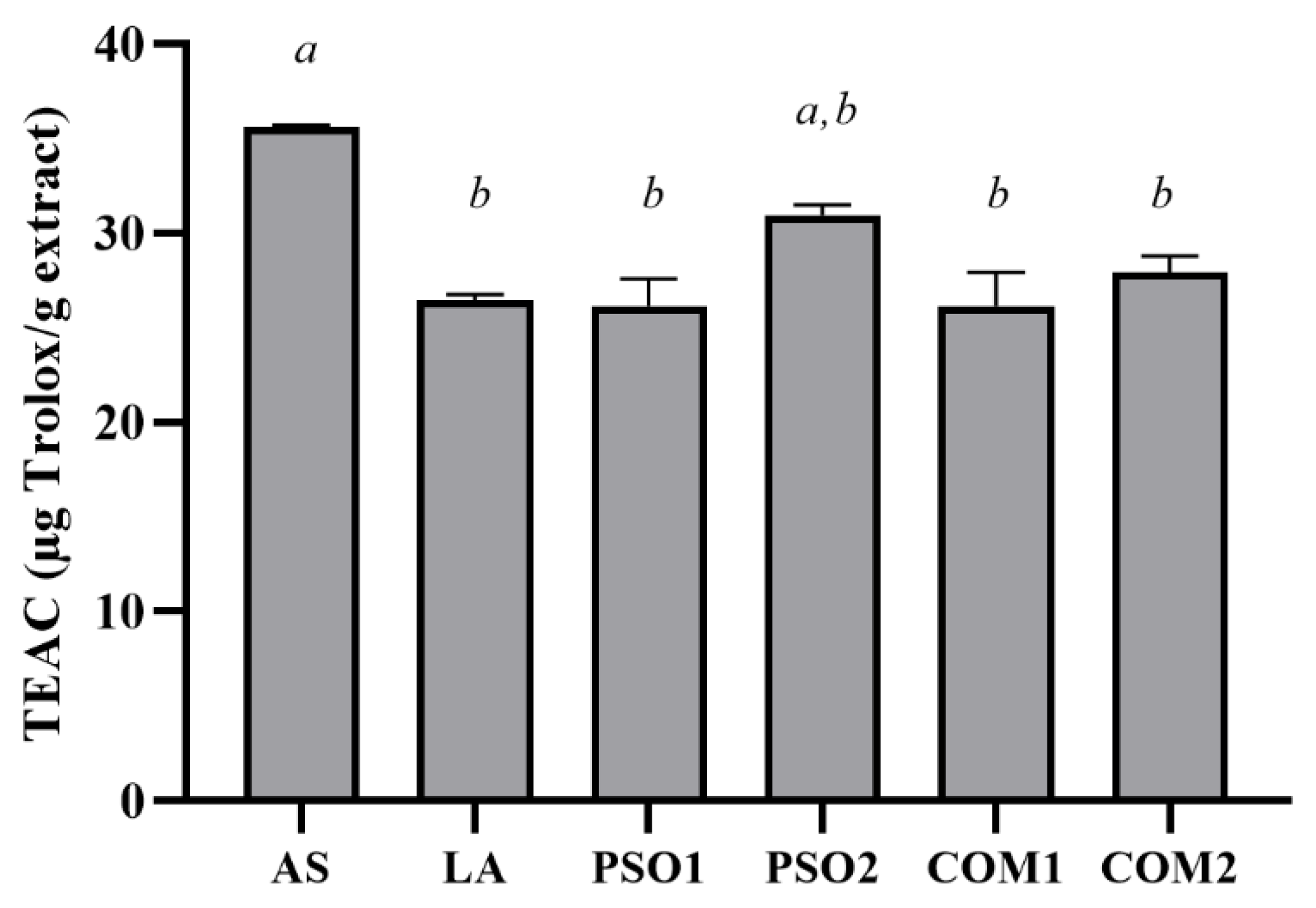
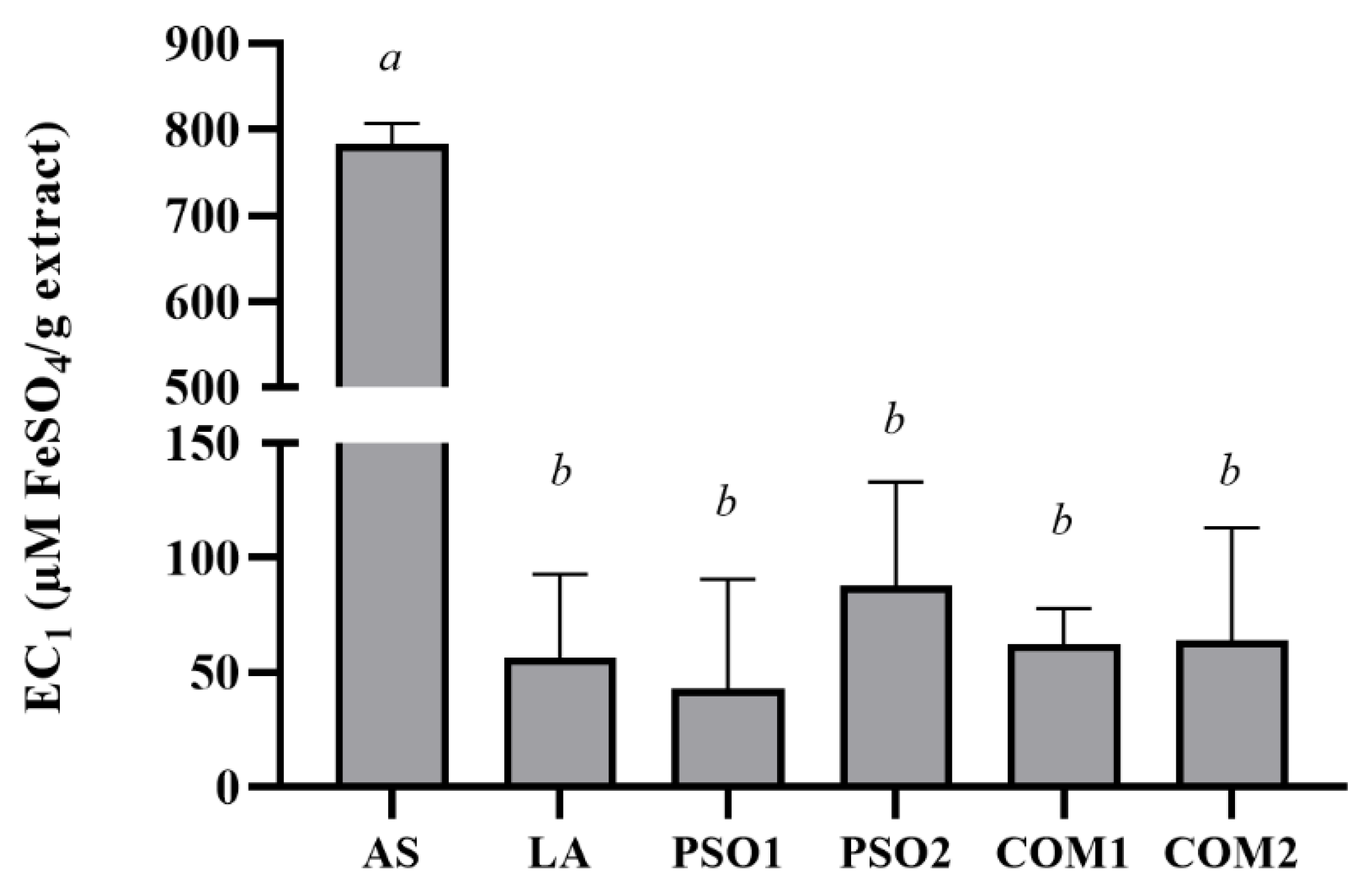
3.3. Antioxidant Activities of Pumpkin Seed Oil
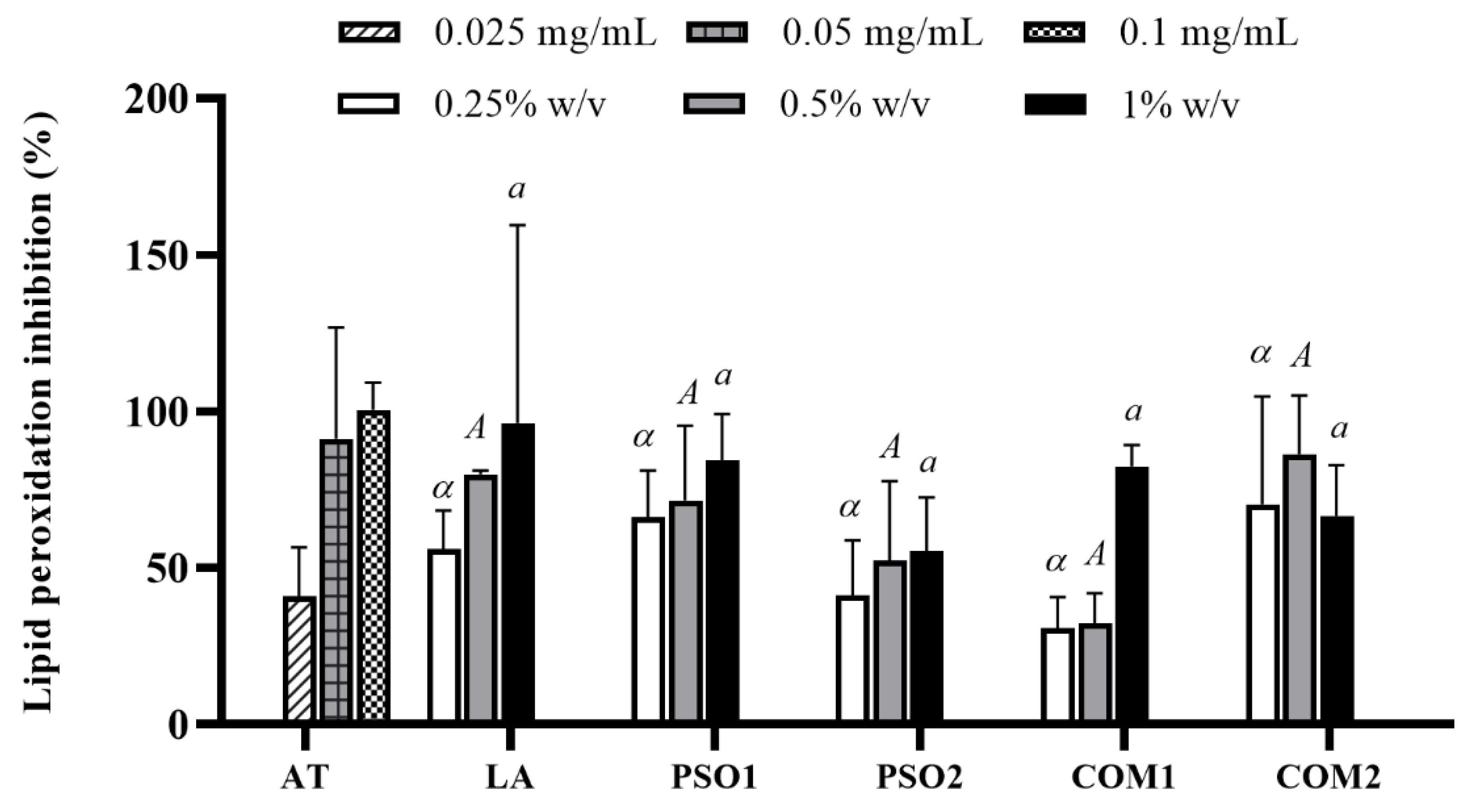
3.4. Anti-Aging Activity of Pumpkin Seed Oil
3.5. Anti-Tyrosinase Activity of Pumpkin Seed Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran. J. Basic Med. Sci. 2017, 20, 798–805. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Huang, W.-C.; Liu, C.-C.; Wang, M.-F.; Ho, C.-S.; Huang, W.-P.; Hou, C.-C.; Chuang, H.-L.; Huang, C.-C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef]
- Glew, R.H.; Glew, R.S.; Chuang, L.T.; Huang, Y.S.; Millson, M.; Constans, D.; Vanderjagt, D.J. Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita spp) and Cyperus esculentus Nuts in the Republic of Niger. Plant Foods Hum. Nutr. 2006, 61, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef]
- Montesano, D.; Blasi, F.; Simonetti, M.S.; Santini, A.; Cossignani, L. Chemical and Nutritional Characterization of Seed Oil from Cucurbita maxima L. (var. Berrettina) Pumpkin. Foods 2018, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, P.; Martha, R. Review of Cucurbita pepo (Pumpkin) its Phytochemistry and Pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Medjakovic, S.; Hobiger, S.; Ardjomand-Woelkart, K.; Bucar, F.; Jungbauer, A. Pumpkin seed extract: Cell growth inhibition of hyperplastic and cancer cells, independent of steroid hormone receptors. Fitoterapia 2016, 110, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Al Juhaimi, F.; Özcan, M.M. Effect of cold press and soxhlet extraction systems on fatty acid, tocopherol contents, and phenolic compounds of various grape seed oils. J. Food Process. Preserv. 2018, 42, e13417. [Google Scholar] [CrossRef] [Green Version]
- Cuco, R.P.; Cardozo-Filho, L.; da Silva, C. Simultaneous extraction of seed oil and active compounds from peel of pumpkin (Cucurbita maxima) using pressurized carbon dioxide as solvent. J. Supercrit. Fluids 2019, 143, 8–15. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G.; Gai, F.; Longato, E.; Amarowicz, R. Antioxidative activities and phenolic compounds of pumpkin (Cucurbita pepo) seeds and amaranth (Amaranthus caudatus) grain extracts. Nat. Prod. Res. 2017, 31, 2178–2182. [Google Scholar] [CrossRef]
- Lohani, U.C.; Fallahi, P.; Muthukumarappan, K. Comparison of Ethyl Acetate with Hexane for Oil Extraction from Various Oilseeds. J. Am. Oil Chem. Soc. 2015, 92, 743–754. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Latif, S.; Diosady, L.L.; Anwar, F. Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur. J. Lipid Sci. Technol. 2008, 110, 887–892. [Google Scholar] [CrossRef]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Man, Y.B.C. Enzyme-Assisted Aqueous Extraction of Kalahari Melon Seed Oil: Optimization Using Response Surface Methodology. J. Am. Oil Chem. Soc. 2009, 86, 1235–1240. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Z.G.; Wang, X.; Han, J.Y.; Zhang, B.; Fu, Y.J.; Zhao, C.J. Application of cavitation system to accelerate aqueous enzymatic extraction of seed oil from Cucurbita pepo L. and evaluation of hypoglycemic effect. Food Chem. 2016, 212, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Konopka, I.; Roszkowska, B.; Czaplicki, S.; Tańska, M. Optimization of Pumpkin Oil Recovery by Using Aqueous Enzymatic Extraction and Comparison of the Quality of the Obtained Oil with the Quality of Cold-Pressed Oil. Food Technol. Biotechnol. 2016, 54, 413–420. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today. 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary screening of some tropical plants for anti-tyrosinase activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Zeitoun, H.; Michael-Jubeli, R.; El Khoury, R.; Baillet-Guffroy, A.; Tfayli, A.; Salameh, D.; Lteif, R. Skin lightening effect of natural extracts coming from Senegal botanical biodiversity. Int. J. Dermatol. 2020, 59, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-Reductase, IL-6 Secretion, and Oxidation Process of Equisetum debile Roxb. ex Vaucher Extract as Functional Food and Nutraceuticals Ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Paradee, N.; Howes, M.J.; Utama-ang, N.; Chaikitwattna, A.; Hider, R.; Srichairatanakool, S. A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human hepatoma (HuH7) cells. Phytother. Res. 2019, 33, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Saeio, K.; Chaiyana, W.; Okonogi, S. Antityrosinase and antioxidant activities of essential oils of edible Thai plants. Drug Discov. Ther. 2011, 5, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Osawa, T.; Namiki, M. A Novel Type of Antioxidant Isolated from Leaf Wax of Eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Chaiyana, W.; Sirithunyalug, J.; Somwongin, S.; Punyoyai, C.; Laothaweerungsawat, N.; Marsup, P.; Neimkhum, W.; Yawootti, A. Enhancement of the Antioxidant, Anti-Tyrosinase, and Anti-Hyaluronidase Activity of Morus alba L. Leaf Extract by Pulsed Electric Field Extraction. Molecules 2020, 25, 2212. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Laosirisathian, N.; Saenjum, C.; Sirithunyalug, J.; Eitssayeam, S.; Sirithunyalug, B.; Chaiyana, W. The Chemical Composition, Antioxidant and Anti-Tyrosinase Activities, and Irritation Properties of Sripanya Punica granatum Peel Extract. Cosmetics 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Indrianingsih, A.W.; Rosyida, V.T.; Apriyana, W.; Hayati, S.N.; Nisa, K.; Darsih, C.; Kusumaningrum, A.; Ratih, D.; Indirayati, N. Comparisons of antioxidant activities of two varieties of pumpkin (Cucurbita moschata and Cucurbita maxima) extracts. In Proceedings of the 2nd International Conference on Natural Products and Bioresource Sciences, Tangerang, Indonesia, 1–2 November 2018. [Google Scholar]
- Ramak, P.; Mahboubi, M. The beneficial effects of pumpkin (Cucurbita pepo L.) seed oil for health condition of men. Food Rev. Int. 2019, 35, 166–176. [Google Scholar] [CrossRef]
- Attah, J.C.; Ibemesi, J.A. Solvent extraction of the oils of rubber, melon, pumpkin and oil bean seeds. J. Am. Oil Chem. Soc. 1990, 67, 25–27. [Google Scholar] [CrossRef]
- Hrabovski, N.; Sinadinović-Fišer, S.; Nikolovski, B.; Sovilj, M.; Borota, O. Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Technol. 2012, 114, 1204–1211. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Ojeda-Amador, R.M.; Gomez-Alonso, S.; Salvador, M.D.; Fregapane, G.; Hamdi, S. Cucurbita maxima pumpkin seed oil: From the chemical properties to the different extracting techniques. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Fruhwirth, G.O.; Hermetter, A. Seeds and oil of the Styrian oil pumpkin: Components and biological activities. Eur. J. Lipid Sci. Technol. 2007, 109, 1128–1140. [Google Scholar] [CrossRef]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Souza, M.A.; Ferro, E.A.; Favoreto, S., Jr.; Pena, J.D. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Applequist, W.L.; Avula, B.; Schaneberg, B.T.; Wang, Y.H.; Khan, I.A. Comparative fatty acid content of seeds of four Cucurbita species grown in a common (shared) garden. J. Food Compos. Anal. 2006, 19, 606–611. [Google Scholar] [CrossRef]
- Simpson, B.W.; McLeod, C.M.; George, D.L. Selection for high linoleic acid content in sunflower (Helianthus annuus L.). Aust. J. Exp. Agric. 1989, 29, 233–239. [Google Scholar] [CrossRef]
- Farag, M.A.; Elimam, D.M.; Afifi, S.M. Outgoing and potential trends of the omega-3 rich linseed oil quality characteristics and rancidity management: A comprehensive review for maximizing its food and nutraceutical applications. Trends Food Sci. Technol. 2021, 114, 292–309. [Google Scholar] [CrossRef]
- Siano, F.; Straccia, M.C.; Paolucci, M.; Fasulo, G.; Boscaino, F.; Volpe, M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 2016, 96, 1730–1735. [Google Scholar] [CrossRef]
- Akin, G.; Arslan, F.N.; Karuk Elmasa, S.N.; Yilmaz, I. Cold-pressed pumpkin seed (Cucurbita pepo L.) oils from the central Anatolia region of Turkey: Characterization of phytosterols, squalene, tocols, phenolic acids, carotenoids and fatty acid bioactive compounds. Grasas Y Aceites 2018, 69, e232. [Google Scholar] [CrossRef]
- Rabrenović, B.B.; Dimić, E.B.; Novaković, M.M.; Tešević, V.V.; Basić, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Libo, W.; Yaqin, X.; Yu, Y.; Xin, S. Aqueous enzymatic extraction of pumpkin seed oil and its physical-chemical properties. Trans. Chin. Soc. Agric. Eng. 2011, 10, 068. [Google Scholar]
- Yu, L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001, 49, 3452–3456. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Prommaban, A.; Utama-ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Porter, J.B.; Srichairatanakool, S. Phytosterol, Lipid and Phenolic Composition, and Biological Activities of Guava Seed Oil. Molecules 2020, 25, 2474. [Google Scholar] [CrossRef] [PubMed]
- Jurgita, K.; Judita, Č.E.; Elvyra, J.; Honorata, D.; Dovilė, L. Antioxidant Activity and other Quality Parameters of Cold Pressing Pumpkin Seed Oil. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Boujemaa, I.; El Bernoussi, S.; Harhar, H.; Tabyaoui, M. The influence of the species on the quality, chemical composition and antioxidant activity of pumpkin seed oil. Oilseeds Fats Crops Lipids 2020, 27, 40. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Liu, W.; Li, J.; Efferth, T. Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresour. Technol. 2010, 101, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Gehrcke, M.; Barbieri, A.V.; Zborowski, V.A.; Beck, R.C.R.; Nogueira, C.W.; Cruz, L. Pomegranate seed oil nanoemulsions improve the photostability and in vivo antinociceptive effect of a non-steroidal anti-inflammatory drug. Colloids Surf. B Biointerfaces 2016, 144, 214–221. [Google Scholar] [CrossRef]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A Potent Tyrosinase Inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with Anti-Melanogenesis Properties in α-MSH and IBMX-Induced B16F10 Melanoma Cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikul, P.; Sripisut, T.; Chanpirom, S.; Sathirachawan, K.; Ditthawuthikul, N. Melanogenesis Inhibitory and Antioxidant Effects of Camellia oleifera Seed Oil. Adv. Pharm. Bull. 2017, 7, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.X.; Duan, F.F.; Jia, S.S.; Cheng, F.R.; Yuan, K. Antioxidant and Tyrosinase Inhibitory Activities of Seed Oils from Torreya grandis Fort. ex Lindl. BioMed. Res. Int. 2018, 2018, 5314320. [Google Scholar] [CrossRef] [Green Version]

| Fatty Acid Composition (%) | Pumpkin Seed Oil | |||
|---|---|---|---|---|
| PSO1 | PSO2 | COM1 | COM2 | |
| Unsaturated fatty acid | ||||
| Palmitoleic acid (C16:1) | 0.09 | 0.14 | 0.08 | 0.09 |
| Oleic acid (C18:1 omega-9) | 31.22 | 37.45 | 33.39 | 28.64 |
| Cis linoleic acid (C18:2 omega-6) | 39.09 | 37.63 | 48.00 | 51.74 |
| Alpha linolenic acid (C18:3 omega-3) | 0.14 | 0.03 | 0.28 | 0.22 |
| Eicosenoic acid (C20:1 omega-9) | 0.09 | 0.08 | 0.06 | 0.05 |
| Docosahexaenoic acid (C22:6 omega-3) | 0.01 | ND | ND | ND |
| Total monounsaturated fatty acid | 31.40 | 37.67 | 33.53 | 28.78 |
| Total polyunsaturated fatty acid | 39.24 | 37.66 | 48.28 | 51.96 |
| Saturated fatty acid | ||||
| Lauric acid (C12:0) | 0.01 | 0.02 | ND | ND |
| Myristic acid (C14:0) | 0.10 | 0.19 | 0.08 | 0.09 |
| Palmitic acid (C16:0) | 19.08 | 13.47 | 12.17 | 12.36 |
| Heptadecanoic acid (C17:0) | ND | ND | 0.05 | 0.08 |
| Stearic acid (C18:0) | 9.37 | 10.98 | 5.25 | 6.05 |
| Arachidic acid (C20:0) | 0.37 | 0.91 | 0.31 | 0.35 |
| Behenic acid (C22:0) | 0.07 | 0.20 | 0.08 | 0.09 |
| Lignoceric acid (C24:0) | 0.03 | 0.12 | 0.10 | 0.09 |
| Total saturated fatty acid | 29.03 | 25.89 | 18.04 | 19.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prommaban, A.; Kuanchoom, R.; Seepuan, N.; Chaiyana, W. Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants 2021, 10, 1582. https://doi.org/10.3390/plants10081582
Prommaban A, Kuanchoom R, Seepuan N, Chaiyana W. Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants. 2021; 10(8):1582. https://doi.org/10.3390/plants10081582
Chicago/Turabian StylePrommaban, Adchara, Ratthida Kuanchoom, Natthidaporn Seepuan, and Wantida Chaiyana. 2021. "Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction" Plants 10, no. 8: 1582. https://doi.org/10.3390/plants10081582
APA StylePrommaban, A., Kuanchoom, R., Seepuan, N., & Chaiyana, W. (2021). Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants, 10(8), 1582. https://doi.org/10.3390/plants10081582








