Fine Mapping of the Gene Controlling the Fruit Skin Hairiness of Prunus persica and Its Uses for MAS in Progenies
Abstract
1. Introduction
2. Results
2.1. Genotyping with NGS
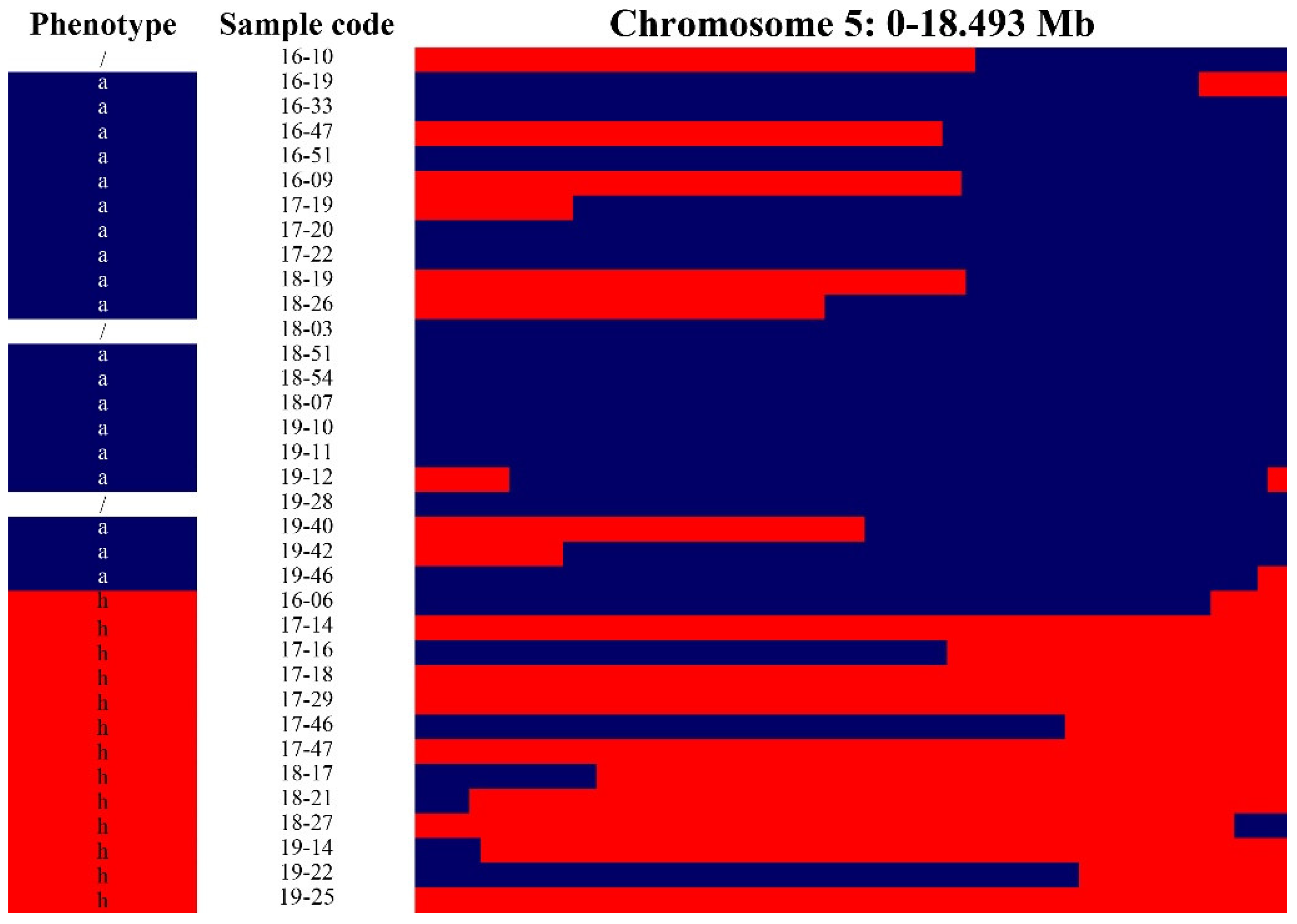
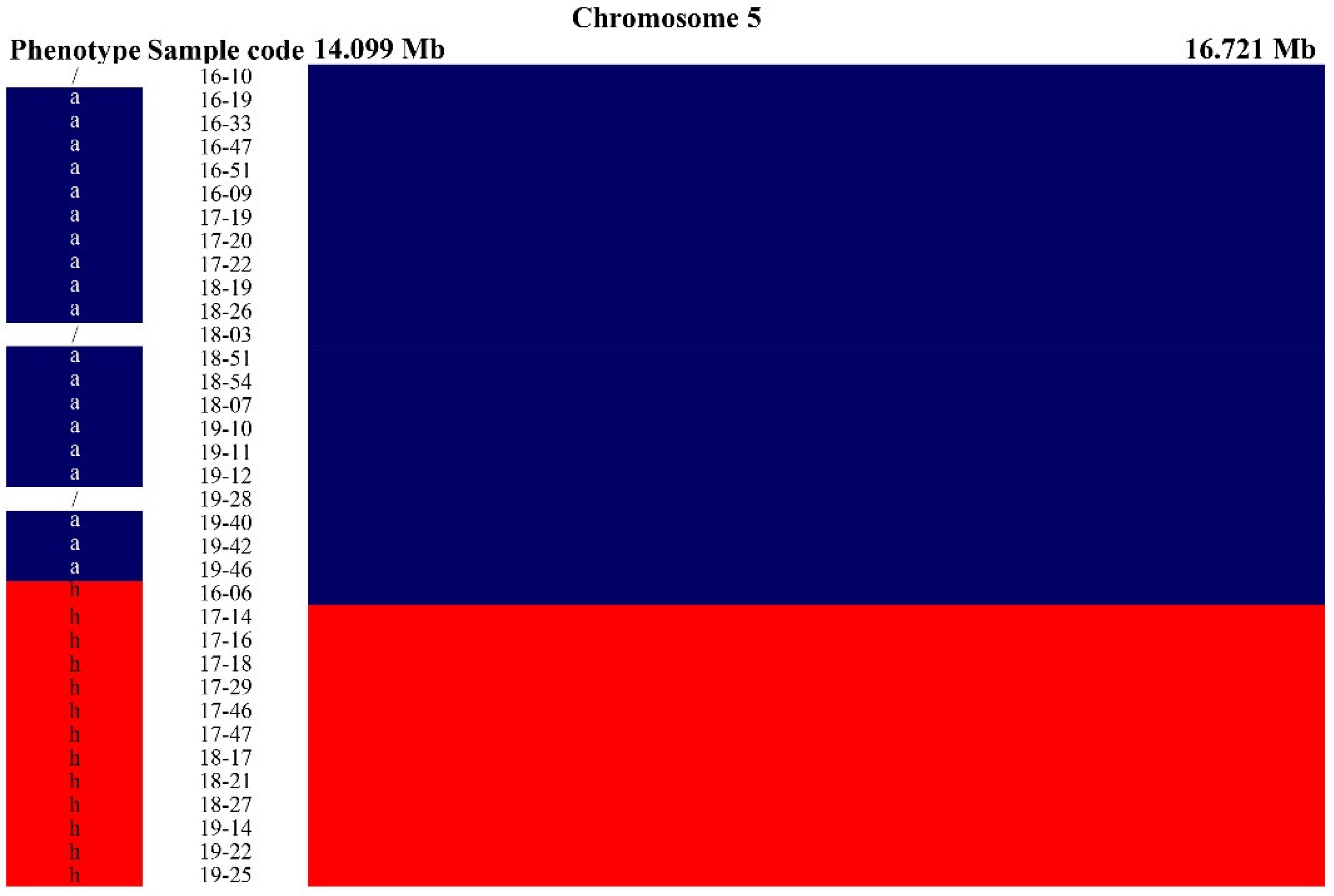
2.2. Mapping and Fine-Mapping of Hairiness/Non-Hairiness Based on Introgression Lines
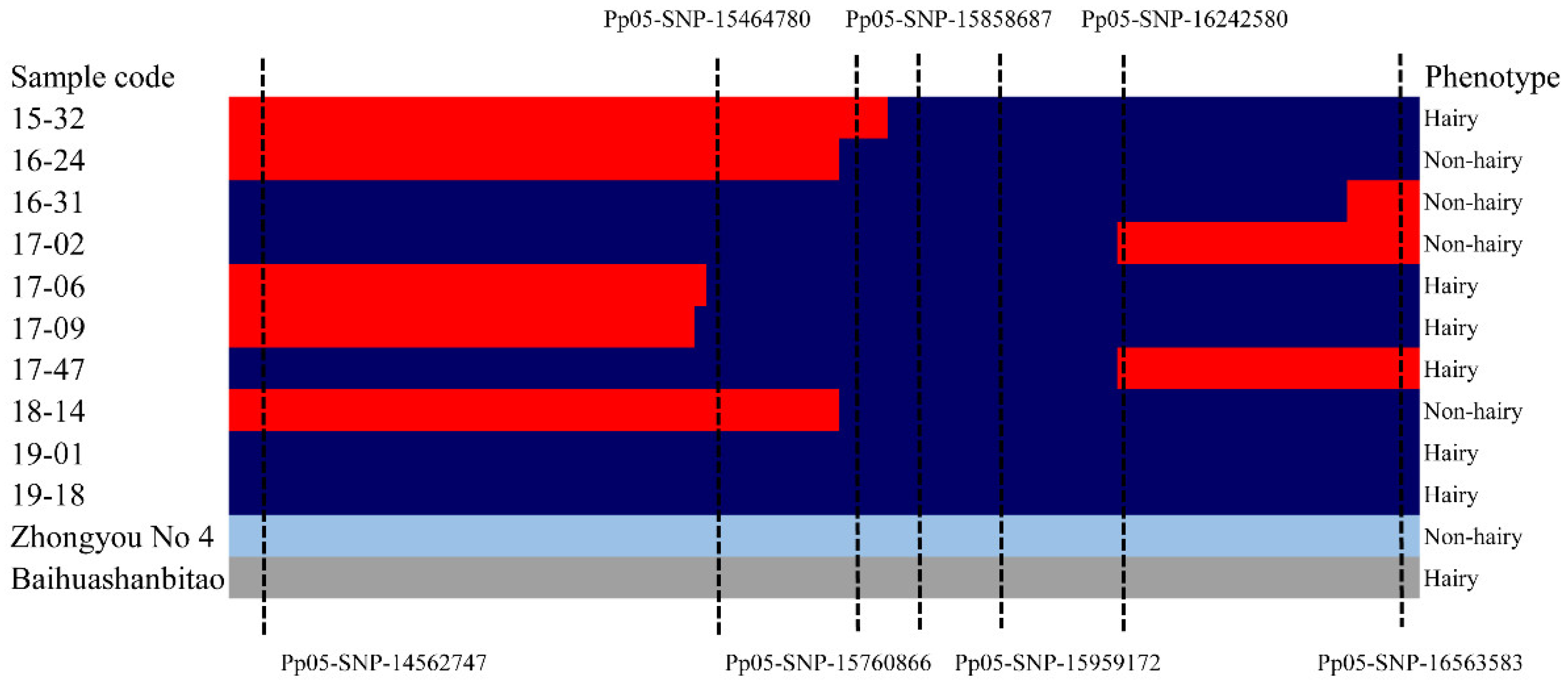
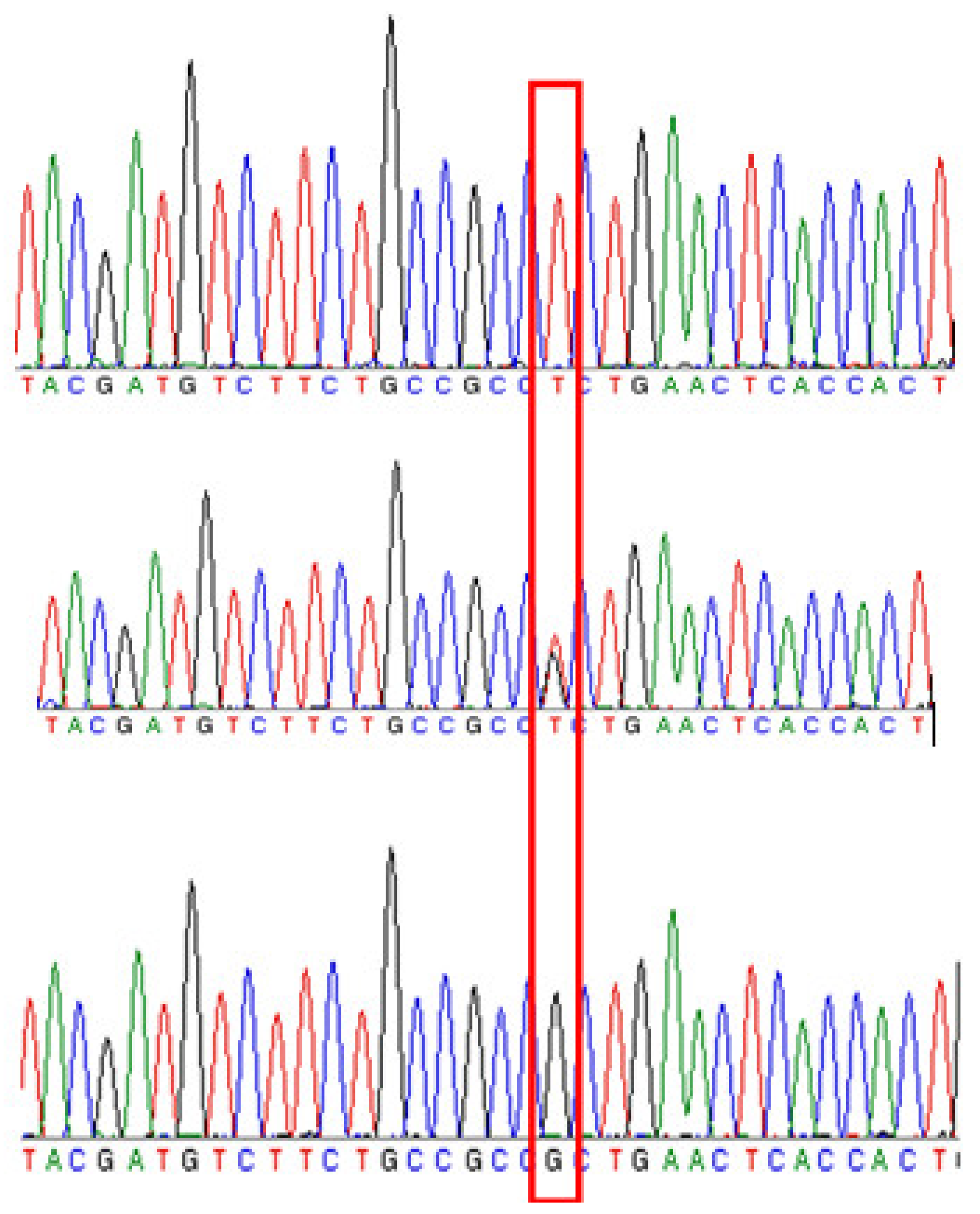
2.3. Candidate Gene Confirmation Based on the Resequencing Data of Two Parents and Other Cultivars (Lines)
2.4. MAS of Hairiness/Non-Hairiness in Peach Breeding
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Extraction
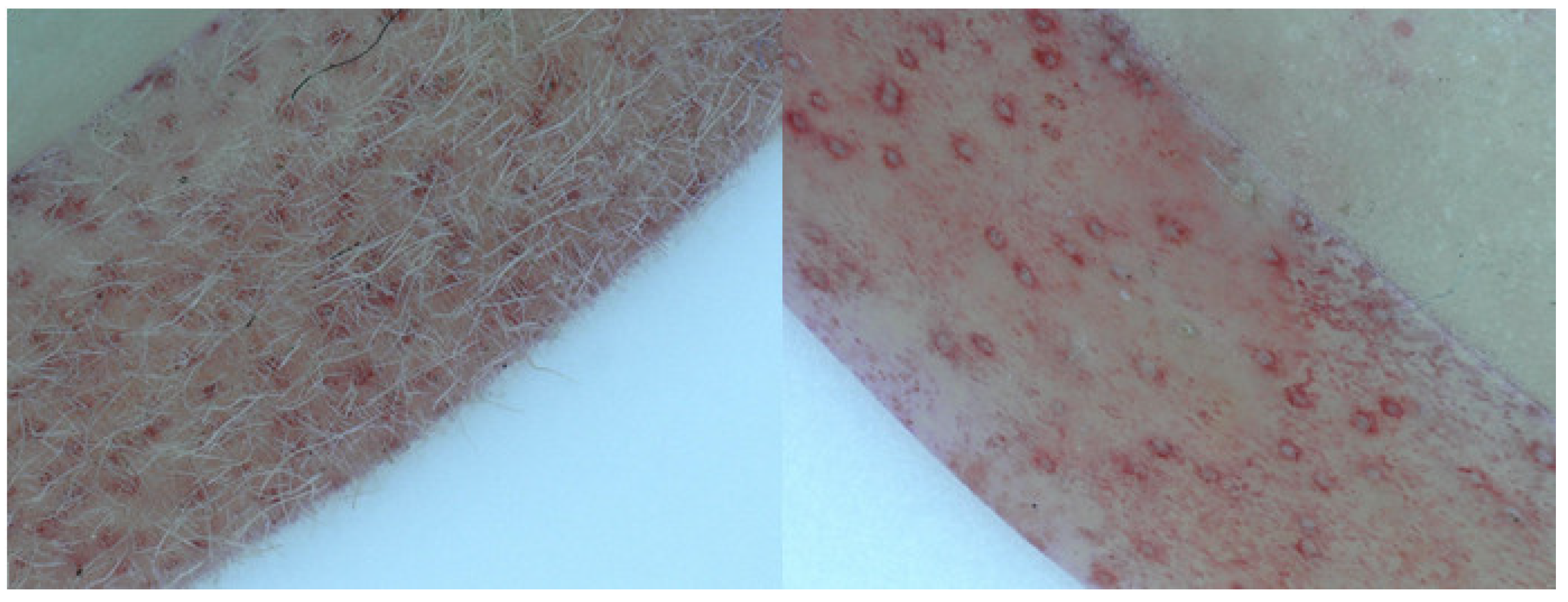
4.3. Phenotyping
4.4. NGS Genotyping and Data Analysis
4.5. Introgression Line for Mapping
4.6. Primer Design, PCR Amplification, and SNP Genotyping
4.7. Completely Linked Markers in the Fine-Mapping Region
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. FAOSTAT-FAO’s Online Statistical Database; FAO: Rome, Italy, 2018. [Google Scholar]
- Staff, T.P.O. A unique mutation in a myb gene cosegregates with the nectarine phenotype in peach. PLoS ONE 2014, 9, e90574. [Google Scholar]
- Creller, M.A.; Werner, D.J. Characterizing the Novel Fruit Surface Morphology of ‘Marina’ Peach Using Scanning Electron Microscopy. J. Am. Soc. Hortic. Sci. 1996, 121, 198–203. [Google Scholar] [CrossRef]
- Janick, J.; Moore, J. Peach In: Advances in Fruit Breeding; Lafayette, W., Ed.; Purdue University: West Lafayette, IN, USA, 1975. [Google Scholar]
- Alessandro, B.; Mara, V.; Filippo, D.F.; Angelo, R.; Carla, G.; Guido, M.; Gabriella, P.; Pietro, T. Different expression of Pp-LTP1 and accumulation of Pru p 3 in fruits of two Prunus persica L. Batsch Genotypes 2006, 171, 106–113. [Google Scholar]
- Carnés, J.; Fernandez-Caldas, E.; Gallego, M.T.; Ferre, A.; Cuesta-Herranz, J. Pru p 3 (LTP) content in peach extracts. Allergy 2002, 57, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Liu, D.; Song, W.; Liu, W.; Zhang, A.; Li, S. Genetic diversity and ecogeographical phylogenetic relationships among peach and nectarine cultivars based on simple sequence repeat (SSR) markers. J. Am. Soc. Hortic. Sci. 2006, 131, 513–521. [Google Scholar] [CrossRef]
- Ma, R.; Yu, M.; Tang, X.; Guo, H.; Zhou, J.; Zhao, M. Advances in nectarine breeding. J. Fruit Sci. 2000, 17, 214–219. [Google Scholar]
- Chaparro, J.X.; Werner, D.J.; O’Malley, D.; Sederoff, R.R. Targeted mapping and linkage analysis of morphological isozyme, and RAPD markers in peach. Theor. Appl. Genet. 1994, 87, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.A. The JH Hale peach as a parent in peach crosses. Proc. Natl. Acad. Sci. USA 1932, 29, 131–136. [Google Scholar]
- Dirlewanger, E.; Pronier, V.; Parvery, C.; Rothan, C.; Guye, A.; Monet, R. Genetic linkage map of peach [Prunus persica (L.) Batsch] using morphological and molecular markers. Theor. Appl. Genet. 1998, 97, 888–895. [Google Scholar] [CrossRef]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Cosson, P.; Boudehri, K.; Renaud, C.; Capdeville, G.; Tauzin, Y.; Laigret, F.; Moing, A. Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet. Genomes 2006, 3, 1–13. [Google Scholar] [CrossRef]
- Dantec, L.; Cardinet, G.; Bonet, J.; Fouché, M.; Boudehri, K.; Monfort, A.; Poëssel, J.L.; Moing, A.; Dirlewanger, E. Development and mapping of peach candidate genes involved in fruit quality and their transferability and potential use in other Rosaceae species. Tree Genet. Genomes 2010, 6, 995–1012. [Google Scholar] [CrossRef]
- Cao, K.; Zhou, Z.; Wang, Q.; Guo, J.; Zhao, P.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wang, X. Genome-wide association study of 12 agronomic traits in peach. Nat. Commun. 2016, 7, 13246. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.M.; Pardeshi, V.C.; Kadoo, N.Y.; Ghorpade, P.B.; Jana, M.M.; Gupta, V.S. Development of genomic simple sequence repeat markers for linseed using next-generation sequencing technology. Mol. Breed. 2012, 30, 597–606. [Google Scholar] [CrossRef]
- Aranzana, M.J.; Illa, E.; Howad, W.; Arús, P. A first insight into peach [Prunus persica (L.) Batsch] SNP variability. Tree Genet. Genomes 2012, 8, 1359–1369. [Google Scholar] [CrossRef]
- Li, H.; Keightley, D.N. The domestication of plants in China: Ecogeographical considerations. In The Origins of Chinese Civilization; University of California Press: Berkeley, CA, USA, 1983; Volume 1, pp. 21–64. [Google Scholar]
- Aranzana, M.J.; Abbassi, E.K.; Howad, W.; Arús, P. Genetic variation, population structure and linkage disequilibrium in peach commercial varieties. BMC Genet. 2010, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Retrotransposon-Induced Mutations in Grape Skin Color. Science 2004, 304, 982. [Google Scholar] [CrossRef]
- Hollender, C.A.; Pascal, T.; Tabb, A.; Hadiarto, T.; Dardick, C. Loss of a highly conserved sterile alpha motif domain gene (weep) results in pendulous branch growth in peach trees. Proc. Natl. Acad. Sci. USA 2018, 115, 201704515. [Google Scholar] [CrossRef]
- Dardick, C.; Callahan, A.; Horn, R.; Ruiz, K.B.; Zhebentyayeva, T.; Hollender, C.; Whitaker, M.; Abbott, A.; Scorza, R. PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family f-ound in diverse plants species. Plant J. 2013, 75, 618–630. [Google Scholar] [CrossRef]
- Hollender, C.A.; Hadiarto, T.; Srinivasan, C.; Scorza, R.; Dardick, C. A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol. 2016, 210, 227–239. [Google Scholar] [CrossRef]
- Pan, L.; Zeng, W.; Niu, L.; Lu, Z.; Wang, X.; Liu, H.; Cui, G.; Zhu, Y.; Chu, J.; Li, W. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015, 66, 7031–7044. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horvath, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Lewinsohn, E.; Rosati, C. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef]
- Adami, M.; Franceschi, P.D.; Brandi, F.; Liverani, A.; Giovannini, D.; Rosati, C.; Dondini, L.; Tartarini, S. Identifying a carotenoid cleavage dioxygenase (ccd4) gene controlling yellow/white fruit flesh color of peach. Plant Mol. Biol. Rep. 2013, 31, 1166–1175. [Google Scholar] [CrossRef]
- Falchi, R.; Vendramin, E.; Zanon, L.; Scalabrin, S.; Cipriani, G.; Verde, I.; Vizzotto, G.; Morgante, M. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013, 76, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ma, R.; Gao, L.; Zhang, J.; Zhang, A.; Zhang, X.; Ren, F.; Zhang, W.; Liao, L.; Yang, Q. A 1.7-mb chromosomal inversion downstream of a ppofp1 gene is responsible for flat fruit shape in peach. Plant Biotechnol. J. 2020, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Shi, F.; Jones, A.D.; Marks, M.D.; Howe, G.A. Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 2010, 61, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.D.; Shim, E.J.; Jun, S.J.; Park, Y.D. Development of SNP Molecular Markers Related to Seed-hair Characteristic Based on EST Sequences in Carrot. Korean J. Hortic. Sci. Technol. 2013, 31, 80–88. [Google Scholar] [CrossRef][Green Version]
- Balcke, G.U.; Bennewitz, S.; Bergau, N.; Athmer, B.; Henning, A.; Majovsky, P.; Jiménez-Gómez, J.M.; Hoehenwarter, W.; Tissier, A. Multi-Omics of Tomato Glandular Trichomes Reveals Distinct Features of Central Carbon Metabolism Supporting High Productivity of Specialized Metabolites. Plant Cell 2017, 29, 960–983. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Miner, D.P.; Larson, M.; McDowell, E.; Gang, D.; Wilkerson, C.; Last, R.L. Studies of a Biochemical Factory: Tomato Trichome Deep Expressed Sequence Tag Sequencing and Proteomics. Plant Physiol. 2010, 153, 1212–1223. [Google Scholar] [CrossRef]
- Martin, C.; Glover, B.J. Functional aspects of cell patterning in aerial epidermis. Curr. Opin. Plant Biol. 2007, 10, 70–82. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zhao, L.; Gao, Z.; Li, X.; Xie, H.; Ni, J.; Gan, K.; Wu, S.; Ye, Z. Selection of Pru p 3 hypoallergenic peach and nectarine varieties. Allergy 2020, 75, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.T.; Zhang, F.; Lloyd, A.M. GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 2000, 156, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.; Weng, S.; Zhang, D.; Zhang, Y.; Shi, C. Characterization and fine mapping of the glabrous leaf and hull mutants (gl1) in rice (Oryza sativa L.). Plant Cell Rep. 2010, 29, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Serra, O.; Donoso, J.M.; Picañol, R.; Batlle, I.; Howad, W.; Eduardo, I.; Arús, P. Marker-assisted introgression (MAI) of almond genes into the peach background: A fast method to mine and integrate novel variation from exotic sources in long intergeneration species. Tree Genet. Genomes 2016, 12, 96. [Google Scholar] [CrossRef]
- Robinson, J.T. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- VerdeI, G.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Paris, R.; Aramini, V.; Gazza, L.; Rossini, L. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 225. [Google Scholar] [CrossRef]
| Physical Position | Primer Sequence (5’- -3’) | Product Size (bp) | SNP Type | |
|---|---|---|---|---|
| Pp05-SNP-14562747 | GGATTAGTGGAGTGGGACAGC | AAGCATCCAGCCAAAACACC | 710 | G/T |
| Pp05-SNP-15464780 | GAAGAGCAAACTTGGCAGTCC | TTTTGAACCACTTGGGAAGC | 763 | C/T |
| Pp05-SNP-15760886 | TGACGATTTGAGAGATTGATCG | TTTTTGTGGGAAGAGGAAGG | 778 | C/T |
| Pp05-SNP-15858687 | ACAGCGTTCGGCTATGAACC | TTTCTTGGGAGTTTTGTGTGC | 866 | A/G |
| Pp05-SNP-15959172 | CTCTTACGACCAAGAACCAACC | GTTGGTGGAGTGGGAGAAGC | 815 | C/T |
| Pp05-SNP-16242580 | ACTGGTGGTTTGTTGGTTGG | TTTCCATACATGTCTTAAAGGTTC | 633 | C/G |
| Pp05-SNP-16563677 | ACGCAATTGGCAGTTACACC | CCACCAGAGGCTAGAGTTGC | 600 | T/C |
| Cultivars (Lines) | Phenotype | Genotype |
|---|---|---|
| Zhongyou No. 4 | Non-hairy | T/T |
| Baihuashanbitao | Hairy | G/G |
| 10–7 | Hairy | T/G |
| 96–51 | Hairy | T/G |
| Bairuyu | Hairy | G/G |
| ludong-2-04 | Non-hairy | T/T |
| P7-12-03 | Non-hairy | T/T |
| Shanza-02 | Hairy | T/G |
| Shuipingzhi | Hairy | T/G |
| Weni2 | Hairy | G/G |
| yb144 | Hairy | T/G |
| Zhongpan No. 01 | Hairy | T/G |
| Zhongtao No. 05 | Hairy | T/G |
| Zhongyou No. 08 | Non-hairy | T/T |
| Zhongyou No. 13 | Non-hairy | T/T |
| Zhongyou No. 20 | Non-hairy | T/T |
| Segregation Populations | Individuals | Hairy | Non-Hairy | Accuracy | |
|---|---|---|---|---|---|
| Zhongyou No. 8 (non-hairy) | Beijingduan (hairy) | 30 | 13 | 17 | 100% |
| 09-bei8-25 (hairy) | Zhongtaobaiyu (hairy) | 196 | 196 | 0 | 100% |
| Zhongtao No. 5 (hairy) | Zhongyou No. 15 (non-hairy) | 15 | 0 | 15 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Pan, L.; Wei, B.; Niu, L.; Cui, G.; Wang, L.; Zeng, W.; Wang, Z. Fine Mapping of the Gene Controlling the Fruit Skin Hairiness of Prunus persica and Its Uses for MAS in Progenies. Plants 2021, 10, 1433. https://doi.org/10.3390/plants10071433
Lu Z, Pan L, Wei B, Niu L, Cui G, Wang L, Zeng W, Wang Z. Fine Mapping of the Gene Controlling the Fruit Skin Hairiness of Prunus persica and Its Uses for MAS in Progenies. Plants. 2021; 10(7):1433. https://doi.org/10.3390/plants10071433
Chicago/Turabian StyleLu, Zhenhua, Lei Pan, Bin Wei, Liang Niu, Guochao Cui, Luwei Wang, Wenfang Zeng, and Zhiqiang Wang. 2021. "Fine Mapping of the Gene Controlling the Fruit Skin Hairiness of Prunus persica and Its Uses for MAS in Progenies" Plants 10, no. 7: 1433. https://doi.org/10.3390/plants10071433
APA StyleLu, Z., Pan, L., Wei, B., Niu, L., Cui, G., Wang, L., Zeng, W., & Wang, Z. (2021). Fine Mapping of the Gene Controlling the Fruit Skin Hairiness of Prunus persica and Its Uses for MAS in Progenies. Plants, 10(7), 1433. https://doi.org/10.3390/plants10071433





