Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season
Abstract
1. Introduction
2. Results
2.1. Biometric and Yield Parameters in Response to Different Greenhouse Irradiance Conditions
2.2. Macronutrients Accumulation in Response to Greenhouse Irradiance Conditions
2.3. SPAD Index, Chlorophyll Fluorescence Emission and Leaf Mass Area (LMA) in Response to Greenhouse Irradiance Conditions
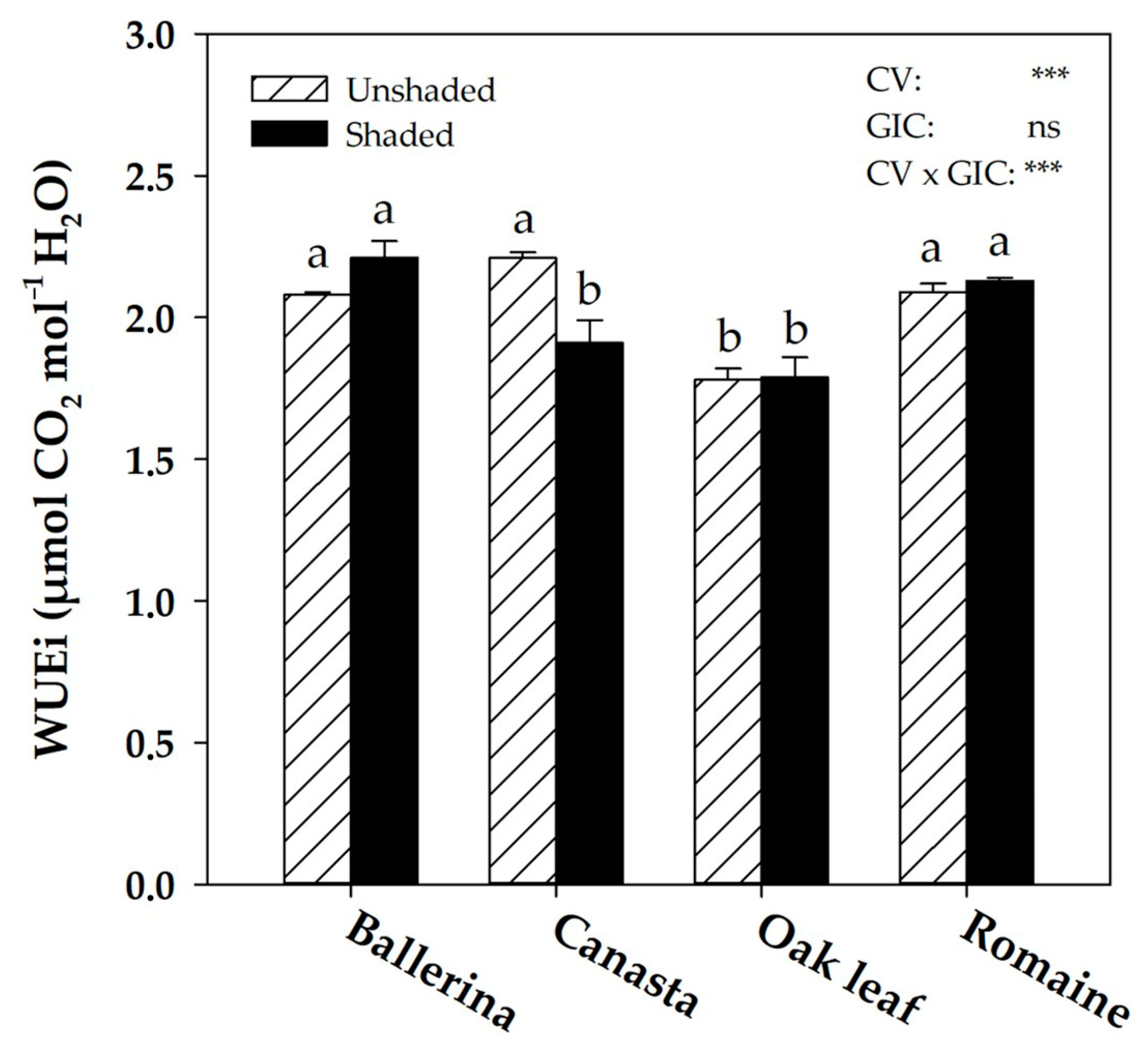
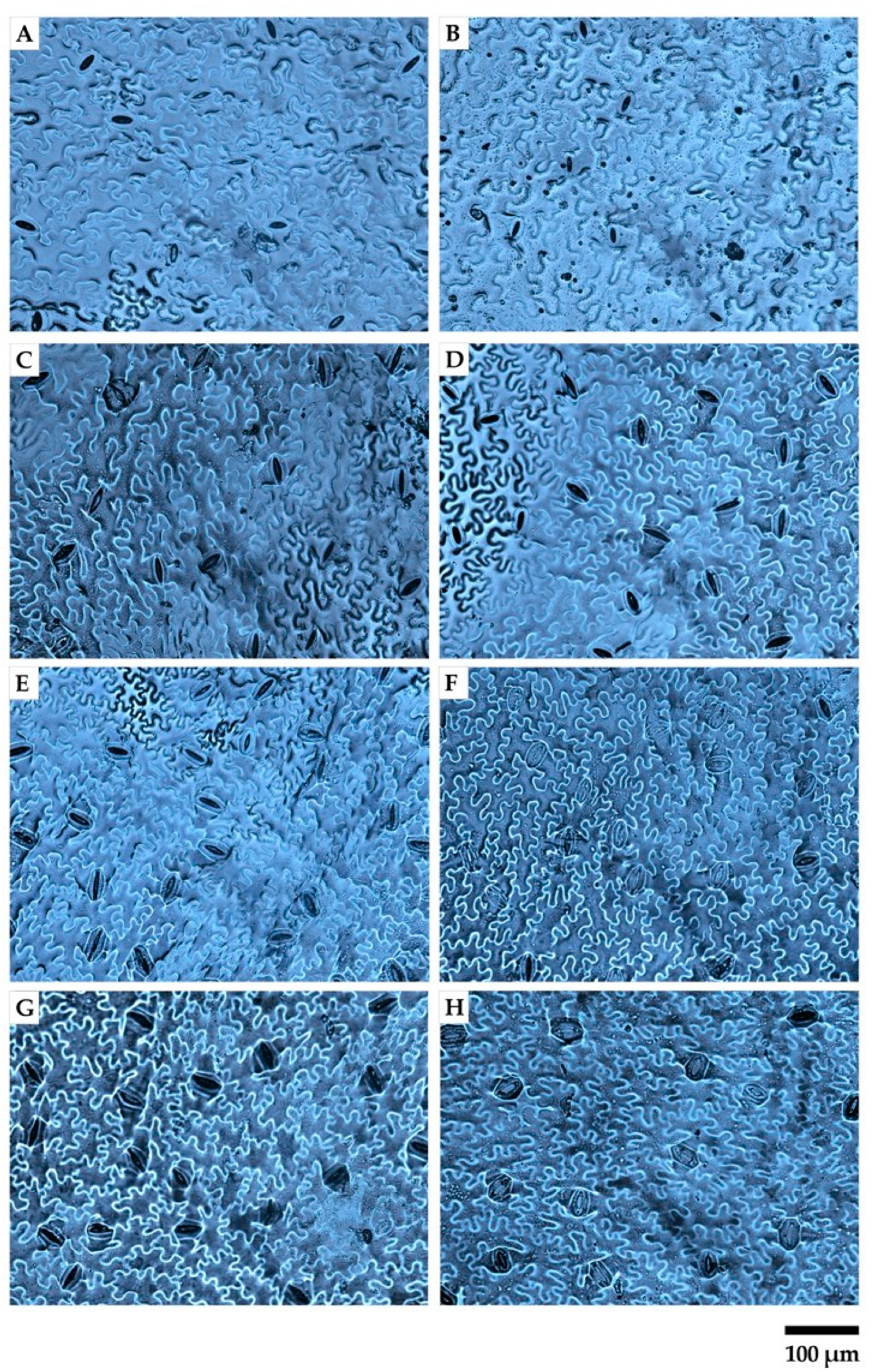
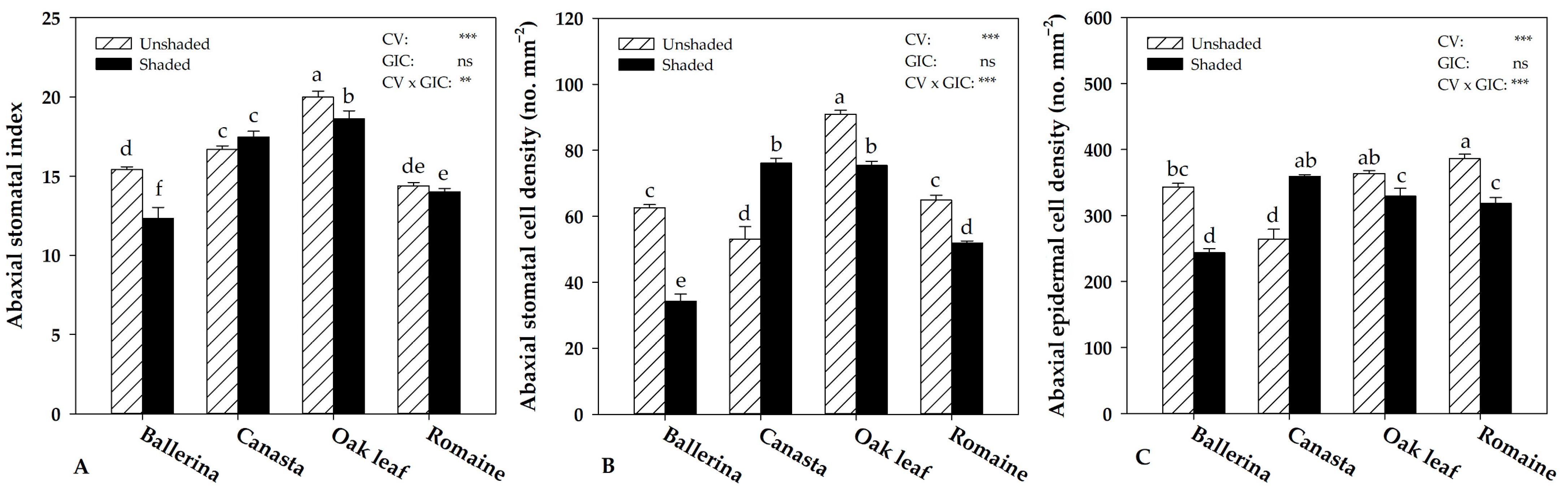
2.4. Instantaneous Water Use Efficiency and Morpho-Anatomical Leaf Traits in Response to Greenhouse Irradiance Conditions
2.5. Leaf Pigments and Total Ascorbic Acid Accumulation in Response to Greenhouse Irradiance Conditions
3. Discussion
3.1. Leaf Morpho-Anatomical Adaptations and Productivity of Lettuce under Excessive Irradiance and Heat Conditions
3.2. Fluorescence, Total Ascorbic Acid and Carotenoids Content of Lettuce under Excessive Irradiance and Heat Conditions
3.3. Leaf Ions Accumulation of Lettuce under Excessive Irradiance and Heat Conditions
4. Materials and Methods
4.1. Experimental Design, Plant Material and Growth Conditions
4.2. Growth, Yield and Sampling
4.3. Plant Growth Index and Soil Plant Analysis Development (SPAD) Index
4.4. Leaf Gas Exchange and Maximum Quantum Efficiency of Photosystem II
4.5. Total Nitrogen and Minerals Determination
4.6. Morpho-Anatomical Leaf Traits Determination
4.7. Total Ascorbic Acid and Leaf Pigments Determination
4.8. Statistics
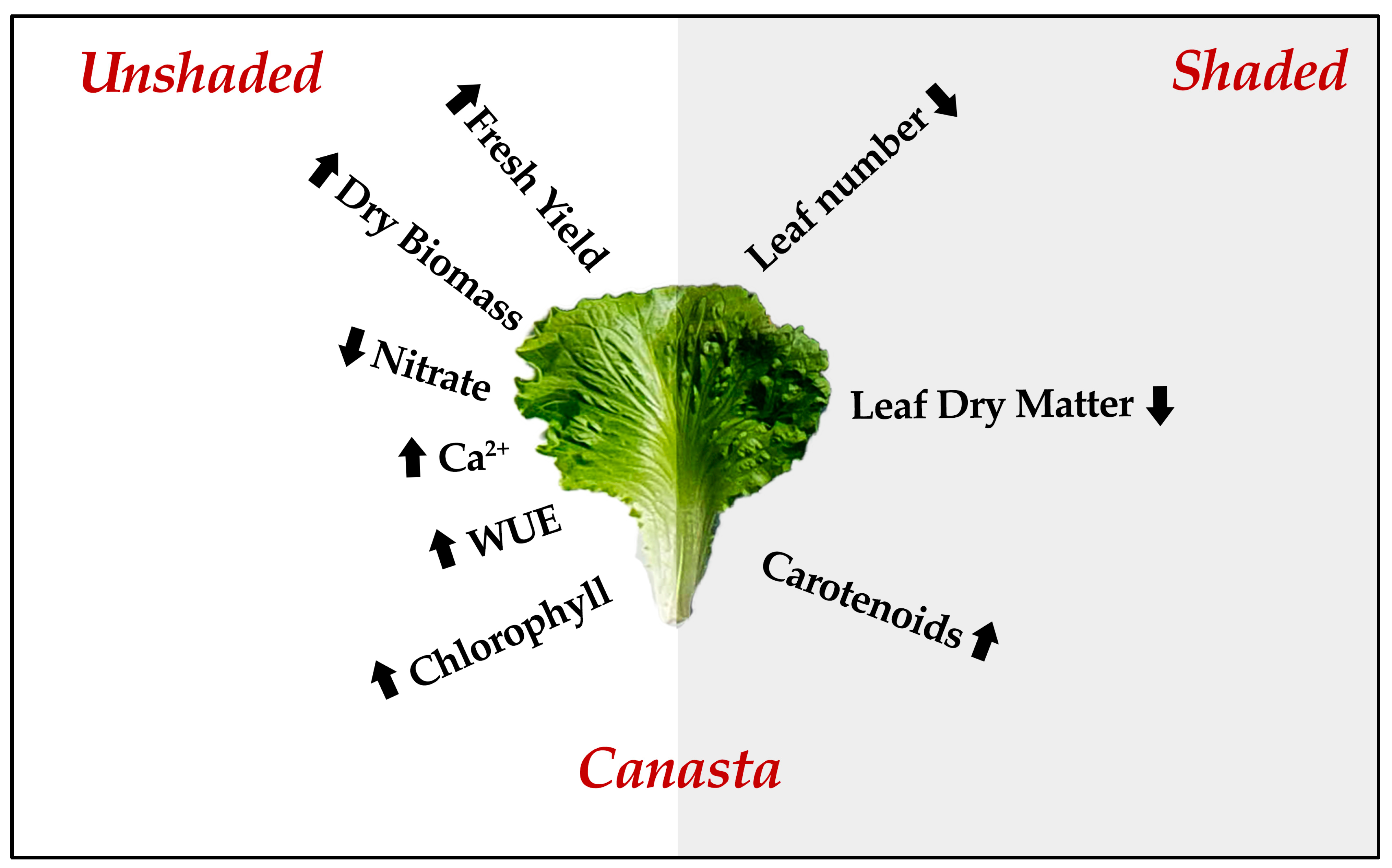
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.; Triantafyllidis, V.; Papasavvas, A.; Salahas, G.; Patakas, A. Yield and nutritional quality of greenhouse lettuce as affected by shading and cultivation season. Emir. J. Food Agric. 2013, 25, 974–979. [Google Scholar] [CrossRef]
- USDA (United State Departement of Agricolture). Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169247/nutrients (accessed on 30 March 2021).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Hunter, B.L. Enhancing Out-of-Season Production of Tomatoes and Lettuce Using High Tunnels; Utah State University: Logan, UT, USA, 2010. [Google Scholar]
- Ilić, Z.S.; Milenković, L.; Stanojević, L.; Cvetković, D.; Fallik, E. Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci. Hortic. 2012, 139, 90–95. [Google Scholar] [CrossRef]
- Meena, R.K.; Vashisth, A.; Singh, R.; Singh, B.; Manjaih, K.M. Study on change in microenvironment under different colour shade nets and its impact on yield of spinach (Spinacia oleracea L.). J. Agrometeorol. 2014, 16, 104–111. [Google Scholar]
- Flaishman, M.A.; Peles, Y.; Dahan, Y.; Milo-Cochavi, S.; Frieman, A.; Naor, A. Differential response of cell-cycle and cell-expansion regulators to heat stress in apple (Malus domestica) fruitlets. Plant Sci. 2015, 233, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ilić, S.Z.; Milenković, L.; Dimitrijević, A.; Stanojević, L.; Cvetković, D.; Kevrešan, F.E.; Mastilović, J. Light modification by color nets improve quality of lettuce from summer production. Sci. Hortic. 2017, 226, 389–397. [Google Scholar] [CrossRef]
- Lafta, A.; Sandoya, G.; Mou, B. Genetic Variation and Genotype by Environment Interaction for Heat Tolerance in Crisphead Lettuce. HortScience 2020, 56, 126–135. [Google Scholar] [CrossRef]
- Al-Said, F.; Hadley, P.; Pearson, S.; Khan, M.M.; Iqbal, Q. Effect of high temperature and exposure duration on stem elongation of iceberg lettuce. Pak. J. Agric. Sci. 2018, 55, 95–101. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, T.; Guo, S.; Liu, R.; Li, M. Leaf anatomy, photosynthesis and chlorophyll fluorescence of lettuce as influenced by arbuscular mycorrhizal fungi under high temperature stress. Sci. Hortic. 2021, 280, 109933. [Google Scholar] [CrossRef]
- Formisano, L.; El-Nakhel, C.; Corrado, G.; De Pascale, S.; Rouphael, Y. Biochemical, physiological, and productive response of greenhouse vegetables to suboptimal growth environment induced by insect nets. Biology 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front. Plant Sci. 2021, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Mistriotis, A.; Eleftherakis, D. Mechanical behaviour and properties of agricultural nets. Part II: Analysis of the performance of the main categories of agricultural nets. Polym. Test. 2007, 26, 970–984. [Google Scholar] [CrossRef]
- Shahak, Y. Photoselective netting: An overview of the concept, r and d and practical implementation in agriculture. Acta Hortic. 2014, 1015, 155–162. [Google Scholar] [CrossRef]
- Semida, W.M.; Ammar, M.S.; El-Sawah, N.A. Effects of Shade Level and Microenvironment on Vegetative Growth, Physiological and Biochemical Characteristics of Transplanted Cucumber (Cucumis sativus). Arch. Agric. Environ. Sci. 2017, 2, 361–368. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y.; Tang, J. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirb. Agric. 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant. Cell Environ. 2020, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Burkart, S. Photosynthesis and high light stress. Wild 1999, 25, 3–16. [Google Scholar]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Ruban, A.V. Plants in light. Commun. Integr. Biol. 2009, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Banaś, A.K.; Aggarwal, C.; Łabuz, J.; Sztatelman, O.; Gabryś, H. Blue light signalling in chloroplast movements. J. Exp. Bot. 2012, 63, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 97–139. ISBN 978-3-319-93592-8. [Google Scholar]
- Bartoli, G.; Bottega, S.; Spano, C. Morpho-anatomical and physiological traits of Agrostis castellana living in an active geothermal alteration field. Biology 2015, 70, 744–752. [Google Scholar] [CrossRef]
- Schymanski, S.J.; Or, D.; Zwieniecki, M. Stomatal Control and Leaf Thermal and Hydraulic Capacitances under Rapid Environmental Fluctuations. PLoS ONE 2013, 8, e54231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, J.; Zhao, T.H.; Cao, Y.; Wang, G.; Sun, B.; Yan, X.; Guo, W.; Li, M.H. The smaller the leaf is, the faster the leaf water loses in a temperate forest. Front. Plant Sci. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pathare, V.S.; Koteyeva, N.; Cousins, A.B. Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C4 grasses. New Phytol. 2020, 225, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Chaves, M.M.; Costa, J.M.; Zarrouk, O.; Pinheiro, C.; Lopes, C.M.; Pereira, J.S. Controlling stomatal aperture in semi-arid regions—The dilemma of saving water or being cool? Plant Sci. 2016, 251, 54–64. [Google Scholar] [CrossRef]
- Muir, C.D. Light and growth form interact to shape stomatal ratio among British angiosperms. New Phytol. 2017, 218, 242–252. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Rana, G. Water use efficiency of crops cultivated in the Mediterranean region: Review and analysis. Eur. J. Agron. 2008, 28, 493–507. [Google Scholar] [CrossRef]
- Tabit Shaban, N.; Tzvetkova, N.; Cherkez, R.; Parvanova, P. Evaluation of response of lettuce (Lactuca sativa L.) to temperature and light stress. Acta Agrobot. 2016, 69. [Google Scholar] [CrossRef]
- Lourdes, R.; Hipol, B.; Dionisio-Sese, M.L. Impact of Light Variation on the Antioxidant Properties of Red Lettuce. Electron. J. Biol. 2014, 10, 28–34. [Google Scholar]
- Trojak, M.; Skowron, E. Role of anthocyanins in high-light stress response. World Sci. News 2017, 81, 150–168. [Google Scholar]
- Hassanien, R.H.E.; Li, M. Influences of greenhouse-integrated semi-transparent photovoltaics on microclimate and lettuce growth. Int. J. Agric. Biol. Eng. 2017, 10, 11–22. [Google Scholar] [CrossRef]
- Araki, Y.; Inoue, S.; Murakami, K. Effect of shading on growth and quality of summer spinach. In Proceedings of the Acta Horticulturae; Lee, J.-M., Watada, A.E., Gross, K.C., Lee, S.-K., Eds.; International Society for Horticultural Science: Seoul, Korea, 1999; Volume 483, pp. 105–110. [Google Scholar]
- Stagnari, F.; Galieni, A.; Pisante, M. Shading and nitrogen management affect quality, safety and yield of greenhouse-grown leaf lettuce. Sci. Hortic. 2015, 192, 70–79. [Google Scholar] [CrossRef]
- Wolff, X.Y.; Coltman, R.R. Productivity Under Shade in Hawaii of Five Crops Grown as Vegetables in the Tropics. J. Am. Soc. Hortic. Sci. 2019, 115, 175–181. [Google Scholar] [CrossRef]
- Lake, J.A.; Quick, W.P.; Beerling, D.J.; Woodward, F.I. Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef] [PubMed]
- Coupe, S.A.; Palmer, B.G.; Lake, J.A.; Overy, S.A.; Oxborough, K.; Woodward, F.I.; Gray, J.E.; Quick, W.P. Systemic signalling of environmental cues in Arabidopsis leaves. J. Exp. Bot. 2006, 57, 329–341. [Google Scholar] [CrossRef]
- Miyazawa, S.I.; Livingston, N.J.; Turpin, D.H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides). J. Exp. Bot. 2006, 57, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Ann. Bot. 2016, 118, 1127–1138. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Y.Y.; Zhao, X.; Yu, H.J.; Shi, K.; Yu, J.Q. Impact of light variation on development of photoprotection, antioxidants, and nutritional value in Lactuca sativa L. J. Agric. Food Chem. 2009, 57, 5494–5500. [Google Scholar] [CrossRef]
- Gerganova, M.; Popova, A.V.; Stanoeva, D.; Velitchkova, M. Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol. Biochem. 2016, 104, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Manojlović, M. Color Shade Nets Improve Vegetables Quality at Harvest and Maintain Quality during Storage. Contemp. Agric. 2018, 67, 9–19. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Troise, A.D.; Vitaglione, P.; De Pascale, S. Reducing Energy Requirements in Future Bioregenerative Life Support Systems (BLSSs): Performance and Bioactive Composition of Diverse Lettuce Genotypes Grown Under Optimal and Suboptimal Light Conditions. Front. Plant. Sci. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Reinink, K.; Groenwold, R.; Bootsma, A. Genotypical differences in nitrate content in Lactuca sativa L. and related species and correlation with dry matter content. Euphytica 1987, 36, 11–18. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. In Handbook of Photosynthesis; Taylor & Francis Group LLC: Milton, UK, 2005; Volume 2, pp. 123–148. [Google Scholar]
- Pathak, J.; Ahmed, H.; Kumari, N.; Pandey, A.; Rajneesh; Sinha, R.P. Role of Calcium and Potassium in Amelioration of Environmental Stress in Plants. Prot. Chem. Agents Amelior. Plant Abiotic Stress 2020, 535–562. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Excitation spectra for Photosystem I and Photosystem II in chloroplasts and the spectral characteristics of the distribution of quanta between the two photosystems. BBA Bioenerg. 1975, 408, 297–305. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Part 2. CHemical and Microbiological Properties. Agronomy Monograph 9; Black, C.A., Evans, D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are Key Regulators of Drought Stress Tolerance in Tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kováčik, J. Basic physiology and biochemistry in environmental/experimental plant studies: How to quantify and interpret metabolites correctly. Environ. Pollut. 2021, 286, 117192. [Google Scholar] [CrossRef] [PubMed]


| Source of Variance | Leaf Number | Leaf Area | Fresh Yield | Dry Biomass | Leaf Dry Matter |
|---|---|---|---|---|---|
| (No. plant−1) | (cm2) | (g plant−1) | (g plant−1) | (%) | |
| Cultivar (CV) | |||||
| ‘Ballerina’ | 33.39 ± 0.57 b | 3729 ± 119 b | 222.7 ± 7.67 c | 13.15 ± 0.09 c | 5.94 ± 0.22 a |
| ‘Canasta’ | 30.67 ± 0.61 c | 3829 ± 66 b | 273.2 ± 5.82 a | 13.83 ± 0.54 b | 5.05 ± 0.10 b |
| ‘Oak leaf’ | 31.17 ± 0.61 c | 2331 ± 246 c | 173.1 ± 9.21 d | 8.30 ± 0.16 d | 4.84 ± 0.20 c |
| ‘Romaine’ | 38.67 ± 1.70 a | 4204 ± 168 a | 241.3 ± 7.08 b | 14.59 ± 0.31 a | 6.07 ± 0.18 a |
| *** | *** | *** | *** | *** | |
| Greenhouse Irradiance Conditions (GIC) | |||||
| Unshaded | 34.08 ± 1.48 | 3269 ± 264 | 217.7 ± 14.4 | 12.69 ± 0.85 | 5.84 ± 0.18 |
| Shaded | 32.86 ± 0.69 | 3777 ± 186 | 237.4 ± 8.04 | 12.24 ± 0.68 | 5.12 ± 0.16 |
| t-Test | ns | ns | ns | ns | * |
| CV × GIC | |||||
| ‘Ballerina’ × Unshaded | 32.33 ± 0.58 c | 3472 ± 69 d | 206.2 ± 4.02 e | 13.24 ± 0.18 b | 6.42 ± 0.05 a |
| ‘Ballerina’ × Shaded | 34.44 ± 0.40 b | 3986 ± 14 b | 239.2 ± 2.51 c | 13.05 ± 0.03 b | 5.46 ± 0.06 bc |
| ‘Canasta’ × Unshaded | 31.89 ± 0.11 c | 3950 ± 47 bc | 285.7 ± 1.80 a | 14.97 ± 0.29 a | 5.24 ± 0.08 c |
| ‘Canasta’ × Shaded | 29.44 ± 0.59 d | 3709 ± 72 cd | 260.7 ± 3.00 b | 12.69 ± 0.25 b | 4.87 ± 0.07 d |
| ‘Oak leaf’ × Unshaded | 29.89 ± 0.29 d | 1802 ± 108 f | 152.6 ± 0.83 g | 8.04 ± 0.17 c | 5.27 ± 0.08 c |
| ‘Oak leaf’ × Shaded | 32.44 ± 0.40 c | 2860 ± 101 e | 193.7 ± 0.50 f | 8.57 ± 0.17 c | 4.42 ± 0.10 e |
| ‘Romaine’ × Unshaded | 42.22 ± 1.31 a | 3854 ± 132 bc | 226.4 ± 5.05 d | 14.53 ± 0.61 a | 6.41 ± 0.13 a |
| ‘Romaine’ × Shaded | 35.11 ± 0.11 b | 4555 ± 6 a | 256.2 ± 1.60 b | 14.64 ± 0.31 a | 5.72 ± 0.14 b |
| *** | *** | *** | *** | ** |
| Source of Variance | Total N | NO3 | P | K | Ca | Mg | S | Na |
|---|---|---|---|---|---|---|---|---|
| (%) | (mg kg−1 FW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | |
| Cultivar (CV) | ||||||||
| ‘Ballerina’ | 3.32 ± 0.10 c | 2017 ± 69 b | 4.24 ± 0.17 c | 41.25 ± 0.66 c | 11.11 ± 0.77 a | 3.68 ± 0.09 b | 1.48 ± 0.13 a | 2.11 ± 0.07 b |
| ‘Canasta’ | 3.90 ± 0.10 a | 2279 ± 69 a | 5.03 ± 0.41 a | 39.17 ± 0.64 d | 8.27 ± 1.14 b | 3.22 ± 0.19 c | 1.63 ± 0.09 a | 1.40 ± 0.12 c |
| ‘Oak leaf’ | 3.65 ± 0.12 b | 2214 ± 50 a | 4.74 ± 0.19 b | 52.56 ± 3.31 a | 10.40 ± 0.42 a | 3.16 ± 0.28 c | 1.25 ± 0.06 b | 2.07 ± 0.11 b |
| ‘Romaine’ | 3.40 ± 0.03 c | 1744 ± 39 c | 4.19 ± 0.12 c | 45.45 ± 1.37 b | 10.80 ± 0.60 a | 4.22 ± 0.16 a | 1.08 ± 0.07 b | 3.57 ± 0.14 a |
| *** | *** | *** | *** | *** | *** | *** | *** | |
| Greenhouse Irradiance Conditions (GIC) | ||||||||
| Unshaded | 3.41 ± 0.07 | 1963 ± 56 | 4.07 ± 0.07 | 41.71 ± 0.86 | 11.66 ± 0.27 | 3.40 ± 0.17 | 1.50 ± 0.09 | 2.30 ± 0.21 |
| Shaded | 3.72 ± 0.09 | 2165 ± 77 | 5.03 ± 0.18 | 47.50 ± 2.37 | 8.63 ± 0.53 | 3.74 ± 0.18 | 1.21 ± 0.07 | 2.27 ± 0.28 |
| t-Test | * | * | *** | * | *** | ns | * | ns |
| CV × GIC | ||||||||
| ‘Ballerina’ × Unshaded | 3.12 ± 0.06 d | 1877 ± 24 c | 3.87 ± 0.11 e | 40.33 ± 1.15 def | 12.78 ± 0.28 a | 3.56 ± 0.05 b | 1.74 ± 0.11 a | 2.25 ± 0.02 b |
| ‘Ballerina’ × Shaded | 3.53 ± 0.06 c | 2156 ± 60 b | 4.61 ± 0.07 c | 42.16 ± 0.18 de | 9.45 ± 0.38 c | 3.79 ± 0.15 b | 1.21 ± 0.06 b | 1.98 ± 0.05 bc |
| ‘Canasta’ × Unshaded | 3.72 ± 0.10 b | 2143 ± 60 b | 4.13 ± 0.01 de | 38.64 ± 0.89 f | 10.70 ± 0.26 bc | 3.45 ± 0.28 bc | 1.80 ± 0.06 a | 1.67 ± 0.07 c |
| ‘Canasta’ × Shaded | 4.08 ± 0.06 a | 2416 ± 39 a | 5.93 ± 0.16 a | 39.69 ± 1.00 ef | 5.83 ± 0.67 d | 2.98 ± 0.20 cd | 1.45 ± 0.11 b | 1.14 ± 0.02 d |
| ‘Oak leaf’ × Unshaded | 3.40 ± 0.05 c | 2104 ± 13 b | 4.33 ± 0.13 cd | 45.29 ± 1.27 c | 11.20 ± 0.28 b | 2.59 ± 0.17 d | 1.24 ± 0.08 b | 1.85 ± 0.07 c |
| ‘Oak leaf’ × Shaded | 3.90 ± 0.05 ab | 2324 ± 4 a | 5.14 ± 0.07 b | 59.84 ± 0.38 a | 9.60 ± 0.39 c | 3.73 ± 0.17 b | 1.26 ± 0.12 b | 2.29 ± 0.09 b |
| ‘Romaine’ × Unshaded | 3.41 ± 0.06 c | 1727 ± 82 d | 3.94 ± 0.06 e | 42.58 ± 0.52 d | 11.96 ± 0.41 ab | 3.98 ± 0.10 ab | 1.22 ± 0.06 b | 3.44 ± 0.19 a |
| ‘Romaine’ × Shaded | 3.38 ± 0.02 c | 1762 ± 22 cd | 4.44 ± 0.07 c | 48.32 ± 0.95 b | 9.64 ± 0.51 c | 4.47 ± 0.24 a | 0.94 ± 0.05 c | 3.69 ± 0.20 a |
| ** | * | *** | *** | ** | ** | * | ** |
| Source of Variance | SPAD | Fluorescence | LMA | ||
|---|---|---|---|---|---|
| 8 DAT | 14 DAT | 21 DAT | Fv/Fm Ratio | (g DW m−2) | |
| Cultivar (CV) | |||||
| ‘Ballerina’ | 27.73 ± 0.85 a | 31.81 ± 0.28 b | 35.51 ± 0.59 a | 0.79 ± 0.02 ab | 47.88 ± 1.06 a |
| ‘Canasta’ | 27.53 ± 1.09 a | 31.97 ± 0.70 b | 35.82 ± 0.14 a | 0.79 ± 0.02 a | 37.63 ± 1.76 c |
| ‘Oak leaf’ | 20.79 ± 0.73 b | 22.85 ± 0.57 c | 22.47 ± 1.07 c | 0.78 ± 0.02 b | 26.19 ± 2.20 d |
| ‘Romaine’ | 28.23 ± 0.53 a | 33.40 ± 0.38 a | 34.05 ± 0.35 b | 0.80 ± 0.02 a | 42.60 ± 1.90 b |
| *** | *** | *** | * | *** | |
| Greenhouse Irradiance Conditions (GIC) | |||||
| Unshaded | 27.77 ± 0.97 | 30.88 ± 1.21 | 32.22 ± 1.31 | 0.75 ± 0.00 | 42.01 ± 2.32 |
| Shaded | 24.37 ± 0.93 | 29.13 ± 1.34 | 31.70 ± 2.05 | 0.83 ± 0.00 | 35.14 ± 2.65 |
| t-Test | * | ns | ns | *** | *** |
| CV × GIC | |||||
| ‘Ballerina’ × Unshaded | 29.52 ± 0.52 a | 32.34 ± 0.21 bc | 34.25 ± 0.29 c | 0.74 ± 0.00 | 49.88 ± 1.22 |
| ‘Ballerina’ × Shaded | 25.94 ± 0.34 c | 31.28 ± 0.28 cd | 36.77 ± 0.22 a | 0.84 ± 0.01 | 45.87 ± 0.34 |
| ‘Canasta’ × Unshaded | 29.88 ± 0.47 a | 33.40 ± 0.37 ab | 35.60 ± 0.18 b | 0.76 ± 0.01 | 41.47 ± 0.53 |
| ‘Canasta’ × Shaded | 25.18 ± 0.41 c | 30.54 ± 0.54 d | 36.05 ± 0.09 ab | 0.83 ± 0.01 | 33.79 ± 0.72 |
| ‘Oak leaf’ × Unshaded | 22.35 ± 0.45 d | 24.04 ± 0.23 e | 24.82 ± 0.38 d | 0.73 ± 0.01 | 30.48 ± 2.37 |
| ‘Oak leaf’ × Shaded | 19.23 ± 0.25 e | 21.66 ± 0.43 f | 20.12 ± 0.20 e | 0.82 ± 0.00 | 21.91 ± 0.36 |
| ‘Romaine’ × Unshaded | 29.33 ± 0.38 a | 33.74 ± 0.69 a | 34.22 ± 0.52 c | 0.76 ± 0.01 | 46.21 ± 2.14 |
| ‘Romaine’ × Shaded | 27.12 ± 0.17 b | 33.06 ± 0.38 ab | 33.88 ± 0.55 c | 0.84 ± 0.00 | 38.99 ± 0.67 |
| * | * | *** | ns | ns | |
| Source of Variance | TAA | Chlorophyll a | Chlorophyll b | Total Chlorophylls | Carotenoids |
|---|---|---|---|---|---|
| (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | |
| Cultivar (CV) | |||||
| ‘Ballerina’ | 10.02 ± 0.72 b | 15.06 ± 0.50 ab | 9.17 ± 0.25 a | 24.23 ± 0.75 a | 5.98 ± 0.35 b |
| ‘Canasta’ | 13.67 ± 0.47 a | 15.61 ± 0.42 a | 9.09 ± 0.69 a | 24.70 ± 1.06 a | 7.07 ± 0.25 a |
| ‘Oak leaf’ | 7.39 ± 0.71 c | 13.97 ± 0.32 b | 8.09 ± 0.28 b | 22.06 ± 0.58 b | 7.01 ± 0.16 a |
| ‘Romaine’ | 6.25 ± 0.52 c | 11.74 ± 0.23 c | 6.67 ± 0.20 c | 18.41 ± 0.37 c | 5.99 ± 0.14 b |
| *** | *** | *** | *** | *** | |
| Greenhouse Irradiance Conditions (GIC) | |||||
| Unshaded | 9.25 ± 0.66 | 14.03 ± 0.52 | 8.61 ± 0.42 | 22.64 ± 0.93 | 6.07 ± 0.19 |
| Shaded | 9.42 ± 1.18 | 14.16 ± 0.51 | 7.90 ± 0.36 | 22.06 ± 0.85 | 6.96 ± 0.18 |
| t-Test | ns | ns | ns | ns | ** |
| CV × GIC | |||||
| ‘Ballerina’ × Unshaded | 8.49 ± 0.28 c | 13.96 ± 0.16 b | 8.64 ± 0.19 bc | 22.60 ± 0.34 b | 5.21 ± 0.10 e |
| ‘Ballerina’ × Shaded | 11.56 ± 0.39 b | 16.15 ± 0.18 a | 9.70 ± 0.04 ab | 25.85 ± 0.22 a | 6.74 ± 0.07 bc |
| ‘Canasta’ × Unshaded | 12.81 ± 0.29 ab | 16.39 ± 0.48 a | 10.57 ± 0.36 a | 26.96 ± 0.69 a | 6.58 ± 0.18 c |
| ‘Canasta’ × Shaded | 14.52 ± 0.54 a | 14.84 ± 0.23 ab | 7.61 ± 0.19 cd | 22.45 ± 0.28 b | 7.57 ± 0.22 a |
| ‘Oak leaf’ × Unshaded | 8.65 ± 0.41 c | 13.99 ± 0.60 b | 8.40 ± 0.44 bc | 22.39 ± 1.03 b | 6.68 ± 0.06 bc |
| ‘Oak leaf’ × Shaded | 6.13 ± 0.87 cd | 13.94 ± 0.39 b | 7.79 ± 0.31 cd | 21.73 ± 0.70 b | 7.34 ± 0.14 ab |
| ‘Romaine’ × Unshaded | 7.04 ± 0.10 cd | 11.78 ± 0.23 c | 6.84 ± 0.21 d | 18.61 ± 0.02 c | 5.80 ± 0.18 de |
| ‘Romaine’ × Shaded | 5.46 ± 0.84 d | 11.71 ± 0.46 c | 6.49 ± 0.35 d | 18.21 ± 0.81 c | 6.18 ± 0.16 cd |
| *** | ** | *** | *** | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formisano, L.; Ciriello, M.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; Cristofano, F.; Duri, L.G.; Giordano, M.; Rouphael, Y.; De Pascale, S. Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season. Plants 2021, 10, 1179. https://doi.org/10.3390/plants10061179
Formisano L, Ciriello M, Cirillo V, Pannico A, El-Nakhel C, Cristofano F, Duri LG, Giordano M, Rouphael Y, De Pascale S. Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season. Plants. 2021; 10(6):1179. https://doi.org/10.3390/plants10061179
Chicago/Turabian StyleFormisano, Luigi, Michele Ciriello, Valerio Cirillo, Antonio Pannico, Christophe El-Nakhel, Francesco Cristofano, Luigi Giuseppe Duri, Maria Giordano, Youssef Rouphael, and Stefania De Pascale. 2021. "Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season" Plants 10, no. 6: 1179. https://doi.org/10.3390/plants10061179
APA StyleFormisano, L., Ciriello, M., Cirillo, V., Pannico, A., El-Nakhel, C., Cristofano, F., Duri, L. G., Giordano, M., Rouphael, Y., & De Pascale, S. (2021). Divergent Leaf Morpho-Physiological and Anatomical Adaptations of Four Lettuce Cultivars in Response to Different Greenhouse Irradiance Levels in Early Summer Season. Plants, 10(6), 1179. https://doi.org/10.3390/plants10061179











